Abstract
On March 11, 2020 the World Health Organization (WHO) upgraded the status of the coronavirus disease 2019 (COVID-19) outbreak from epidemic to a global pandemic. This infection is caused by a novel coronavirus, SARS-CoV-2. Several rapid diagnostic tests have been developed at an astonishing pace; however, COVID-19 requires more highly specific rapid point-of-care diagnostic tests. This review describes the currently available testing approaches, as well as the available test assays including the Xpert® Xpress SARS-CoV-2 test (takes ~45 min) and Abbott ID COVID-19 test (5 min) as easy to use point-of-care tests for diagnosis of novel COVID-19 that have so far received the US Food and Drug Administration emergency use authorizations clearance. This review is correct as of the date published and will be updated as more diagnostic tests come to light.
Keywords: Coronavirus, COVID-19, diagnosis, genome sequencing, SARS-CoV-2, Point-of-Care test
Introduction
Infectious diseases are still a major threat in the world today 1. The diagnosis and real-time tracking of both emerging 2 and re-emerging infectious diseases must be reliable, fast, and affordable. Healthcare systems use different approaches to make infectious diseases diagnoses, such as clinical assessment (signs and symptoms), microscopy, microbiological cultures, radiology, molecular techniques – classical or real-time polymerase chain reaction (PCR), genome sequencing (detection presence of nucleic acid), serology (detection of the pathogens or host antibodies), artificial intelligence and machine learning 3. In modern medical diagnostics, whole or targeted genome sequencing or detection technologies are indispensable. In the event of novel pathogens, such as coronavirus, these technologies offer the shortest turnaround time (TAT) for results to be reported. Coupling comprehensive clinical evaluation, triaging, genomic-based diagnostics, and epidemiology to innovative digital disease detection raises the possibility of an open, global, pathogen surveillance system.
In the current case of the coronavirus global pandemic 2019, healthcare systems have the challenge of massive testing, isolating (quarantine) those suspected of being exposed to the virus, re-testing (at the end of the viral incubation period), treating and managing those who turn out to be positive, as well as real-time tracing of their contacts. The diagnosis of a novel infectious disease such as COVID-19 furthermore presents a challenge of studying and understanding its pathogenesis before developing rapid test assays to be used to subsequently diagnose cases as the disease continues to infect more people. In the development of such a test, aspects such as sensitivity, specificity, false positive and false negative rates of a diagnostic test have to be meticulously considered. As this is a great undertaking requiring high scientific expertise, laboratory and financial resources, some nations especially low-income settings have had to rely on such support from developed nations to make modifications at a country level so that they reach their healthcare needs 4.
The current COVID-19 outbreak has confronted scientists with an unprecedented infectious disease challenge that demands the highest level of sharing of infectious disease-related information as such infectious disease outbreaks are becoming less confined by geographical or climatic boundaries 5, 6. Data sharing ensures that other nations use available facts and minimize wastage of time and resources. Another lesson learnt from this pandemic is that nations should also end secrecy in public health decision-making especially amidst suspected disease outbreaks and promote global cooperation 5. Globally, governments should be ready to share outbreak information on acquisition, transmission, and treatment as well as make efforts to fight the spreading of ‘fake news’ related to outbreaks. Nations with networks of high-containment biological laboratories must guarantee safety of all agents in their custody to prevent their potential deployment as bioweapons.
This review briefly describes the currently available testing approaches, as well as selected two easy to use point-of-care test assays, including the Xpert ® Xpress SARS-CoV-2 and Abbott ID COVID-19 tests, for diagnosis of novel COVID-19 infection. At the date of this publication, the US Food and Drug Administration (FDA) has granted emergency use authorizations clearance for 32 tests, as seen in Table 1. A routinely updated list of FDA approved tests can be accessed here.
Table 1. Coronavirus disease 2019 (COVID-19) diagnostic tests that have currently received Emergency Use Authorizations (EUA) by the US Food and Drug Administration.
| Date EUA
issued |
Manufacturer | Diagnostic name | Principle action |
|---|---|---|---|
| 04/08/2020 | DiaCarta, Inc | QuantiVirus SARS-CoV-2 Test kit | qRT-PCR for N, Orf1ab and E genes |
| 04/08/2020 | Becton, Dickinson & Company | BD SARS-CoV-2Reagents for BD
MAX System |
Multiplex qRT-PCR for N1 and N2 regions,
and the human RNase P gene |
| 04/07/2020 | InBios International, Inc | Smart Detect SARS-CoV-2 rRT-PCR
Kit |
Multiplex qRT-PCR for N, Orf1ab and E genes |
| 04/06/2020 | Gnomegen LLC | Gnomegen COVID-19 RT-Digital PCR
Detection Kit |
Real-time RT-digital PCR for N1 and N2
regions, and the human RNase P gene |
| 04/03/2020 | Co-Diagnostics, Inc. | Logix Smart Coronavirus Disease
2019 (COVID-19) Kit |
rRT-PCR for RdRp gene |
| 04/03/2020 | ScienCell Research Laboratories | ScienCell SARS-CoV-2 Coronavirus
Real-time RT-PCR (RT-qPCR) Detection Kit |
rRT-PCR for N and Human RPP30 genes |
| 04/03/2020 | Luminex Corporation | ARIES SARS-CoV-2 Assay | Multiplex qRT-PCR for ORF1ab, E gene, and N gene |
| 04/02/2020 | Becton, Dickinson & Company (BD) | BioGX SARS-CoV-2 Reagents for BD
MAX System |
Multiplexed qRT-PCR for N1 and RNase P |
| 04/01/2020 | Ipsum Diagnostics, LLC | COV-19 IDx assay | Multiplexed qRT-PCR for N and RNase P |
| 04/01/2020 | Cellex Inc. | qSARS-CoV-2 IgG/IgM Rapid Test | IgM and IgG antibodies against SARSCoV-2 |
| 03/30/2020 | QIAGEN GmbH | QIAstat-Dx Respiratory SARS-CoV-2
Panel |
Multiplexed qRT-PCR for Orf1b poly gene
(Rdrp gene) and E gene |
| 03/30/2020 | NeuMoDx Molecular, Inc. | NeuMoDx SARS-CoV-2 Assay | Multiplexed qRT-PCR for Nsp2 gene and N
gene |
| 03/27/2020 | Luminex Molecular Diagnostics,
Inc. |
NxTAG CoV Extended Panel Assay | Multiplex qRT-PCR for ORF1ab, and E gene |
| 03/27/2020 | Abbott Diagnostics Scarborough,
Inc. |
ID NOW COVID-19 | rRT-PCR for; RdRp gene |
| 03/26/2020 | BGI Genomics Co. Ltd | Real-Time Fluorescent RT-PCR Kit for
Detecting SARS-2019-nCoV |
Multiplex qRT-PCR for N gene, E gene, and
RdRp gene |
| 03/25/2020 | Avellino Lab USA, Inc. | AvellinoCoV2 test | rRT-PCR for N gene |
| 03/24/2020 | PerkinElmer, Inc. | PerkinElmer New Coronavirus Nucleic
Acid Detection Kit |
Multiplex qRT-PCR for ORF1ab and N genes |
| 03/23/2020 | Mesa Biotech Inc. | Accula SARS-Cov-2 Test | rRT-PCR for N gene |
| 03/23/2020 | BioFire Defense, LLC | BioFire COVID-19 Test | Nested multiplex real-time RT-PCR for
ORF1ab, ORF8 |
| 03/20/2020 | Cepheid | Xpert Xpress SARS-CoV-2 test | Multiplex qRT-PCR for E and N2 genes |
| 03/20/2020 | Primerdesign Ltd. | Primerdesign Ltd COVID-19 genesig
Real-Time PCR assay |
rRT-PCR for RdRp gene |
| 03/19/2020 | GenMark Diagnostics, Inc. | ePlex SARS-CoV-2 Test | rRT-PCR for N gene |
| 03/19/2020 | DiaSorin Molecular LLC | Simplexa COVID-19 Direct assay | Multiplex qRT-PCR for ORF1ab and S genes |
| 03/18/2020 | Abbott Molecular | Abbott RealTime SARS-CoV-2 assay | Multiplex qRT-PCR for RdRp and N genes |
| 03/17/2020 | Quest Diagnostics Infectious
Disease, Inc. |
Quest SARS-CoV-2 rRT-PCR | rRT-PCR for N gene (N1 and N3 genes) |
| 03/17/2020 | Quidel Corporation | Lyra SARS-CoV-2 Assay | rRT-PCR for ORF1ab |
| 03/16/2020 | Laboratory Corporation of America
(LabCorp) |
COVID-19 RT-PCR Test | rRT-PCR for N gene |
| 03/16/2020 | Hologic, Inc. | Panther Fusion SARS-CoV-2 | rRT-PCR for RdRp gene |
| 03/13/2020 | Thermo Fisher Scientific, Inc. | TaqPath COVID-19 Combo Kit | Multiplex qRT-PCR for ORF1ab, N gene, S
gene, MS2 |
| 03/12/2020 | Roche Molecular Systems, Inc.
(RMS) |
cobas SARS-CoV-2 | Multiplex qRT-PCR for ORF-1a and E-gene |
| 02/29/2020 | Wadsworth Center, New York State
Department of Public Health's (CDC) |
New York SARS-CoV-2 Real-time
Reverse Transcriptase (RT)-PCR Diagnostic Panel |
rRT-PCR for N gene |
| 02/04/2020 | Centers for Disease Control and
Prevention's (CDC) |
CDC 2019-nCoV Real-Time RT-PCR
Diagnostic Panel (CDC) |
Multiplex qRT-PCR for N1, N2, and RP genes
of the virus |
Molecular assays to diagnose COVID-19
The manifestation of the COVID-19 infection is highly nonspecific and presents respiratory symptoms, fever, cough, dyspnoea, and viral pneumonia 7. Thus, diagnostic tests specific to this infection are urgently required to confirm suspected cases, screen patients, and conduct virus surveillance. In this scenario, a rapid, robust, and cost-efficient device that can be used anywhere and which does not necessarily require a trained technician to operate 8 (i.e. at point-of-care) is crucial and urgently needed for the detection of COVID-19 9. Several assays that detect SARS-CoV-2 have been developed so far, including Rapid IgM-IgG Combined Antibody Test For Coronavirus (RayBiotech Life), 2019-nCoV IgG/IgM Antibody Detection Kit (MyBioSource, Inc.), qSARS-CoV-2 IgG/IgM Rapid Test (Cellex, Inc.), ARIES SARS-CoV-2 Assay (Luminex Corporation), SGTi-flex COVID-19 IgM/IgG (Sugentech, Inc), while still under development is the ultrasensitive, rapid, portable coronavirus SARS-CoV-2 nucleic acid sequence detection system that uses nanobiosensor-based aptamer technology 10 are currently under development. These are both in-house and commercially available. Some assays detect only the novel form of virus and some may also detect other viral strains (e.g. SARS-CoV) that are genetically similar to SARS-CoV-2 11. More about status of evaluation of SARS-COV-2 molecular diagnostics tests can be found here and here. This includes the viral molecular target utilised in the test, country of manufacturer and its regulatory status.
Virological culture to diagnose COVID-19
Emerging and re-emerging pathogens are global challenges for public health 12, 13. Laboratory biosafety requirements related to COVID-19 virus include the availability of biosafety requirements for viral cultures and further manipulations. The World Health Organisation (WHO) recommends that all procedures must be performed based on risk assessment and only by highly trained personnel with demonstrated capability in strict observance to all relevant protocols at all times 14. Non-propagative diagnostic laboratory work, such as viral genome sequencing and nucleic acid amplification tests, must be conducted at containment facilities and procedures equivalent to biosafety level 2 (BSL-2) while propagative work that involves coronavirus culture, isolation, animal inoculation or neutralization assays must be done at a high-biocontainment laboratory with inward directional airflow (minimum BSL-3) 14. Viral cultures are not recommended for routine diagnosis and must be carried in a minimum of BSL-3 facility or BSL-4 14. However, SARS-COV-2 virus isolation in cell cultures is critical to obtain isolates for characterization and to support the development of vaccines, therapeutic agents 15 and new or better diagnostic tests.
SARS-CoV-2 is isolated and propagated in primary monkey cells and cell lines such as the kidney Vero-E6, LLC-MK2, Human hepatoma cell line Huh7, human airway epithelial cells, and Vero-E6/TMPRSS2 (Transmembrane Serine Protease 2) 16. Not all countries or jurisdictions have the facilities to perform virological culture tests for COVID-19. This is due to several reasons such as the required level of technical expertise required for the tests and biosafety requirements. Therefore, in such cases, these regions, e.g. American Samoa, have had to ship clinical samples from suspected individuals to either the US CDC laboratory in Atlanta Georgia 17 or WHO reference testing laboratories in France, United Kingdom, China, Japan, Singapore, Australia, Thailand, India, USA, South Africa, Senegal, Russian Federation, Germany, and The Netherlands 18. This increases the TAT for the diagnosis even when they are shipped as expedited consignments.
In addition to virological culture, serological tests are currently under development and these could enable diagnosis of COVID-19 especially in patients for whom acute and convalescent paired samples are available. These are drawn approximately 2 weeks apart to monitor any significant changes in antibody titers of the patients. However, development of these types of tests is currently challenging due to a lack of knowledge about the antibody response elicited from the SARS-CoV-2 infection in humans, including the question concerning the antigenic differences between SARS-CoV-2 and SARS-CoV 19. In addition, these serological tests may face a challenge of cross-reactivity with other coronaviruses 18. However, the FDA has offered emergency use authorization of the first antibody-based test for COVID-19 that detects antibodies in the one’s blood, rather than for the virus in the nose or throat samples. This test is only done at certified laboratories and even though it takes 15 to 20 minutes to get a result after sample collection, it is not a bedside test 20.
Point-of-care tests to diagnose COVID-19
Point-of-care testing means that results are delivered to patients in the patient care setting, such as hospitals, urgent care centers and medical emergency rooms, instead of samples being sent to a testing laboratory. Real-time PCR, also known as quantitative PCR, is commonly used in molecular point-of-care testing. It can amplify more than one genomic target and in the case of COVID-19 and these can be ORF1ab of coronaviruses occupy about two thirds of their genomes. It encodes the replicase polyprotein and is translated from ORF1a and ORF1b, E-gene (envelope protein) and N-gene (the nucleocapsid protein) 21 and RNA-dependent RNA polymerase (RdRp) that is an essential viral enzyme, which replicates positive-strand RNA viral genome. For more specificity, some molecular diagnostic assays amplify more than one molecular target in the virus to minimize chances of all three targets mutating and hence being missed by the diagnostic test 21. During the development of disease testing assays, US manufacturers can apply for emergency clinical use authorization from FDA, and in the European Union manufacturers can obtain "Conformité Européene" which literally means "European Conformity", marking them for in vitro diagnostic use.
On March 21, 2020, the US FDA granted emergency use authorization to a rapid, point-of-care diagnostic test designed to detect COVID-19 infection 22. This test, Xpert ® Xpress SARS-CoV-2, was developed by Cepheid (Sunnyvale, California, USA) to detect SARS-CoV-2 in approximately 45 minutes, following clinical specimen collection from a nasopharyngeal swab, nasal wash or aspirate. The Xpert ® Xpress SARS-CoV-2 test cartridge is designed to detect nucleic acid from SARS-CoV-2 via real-time PCR 23. Xpert ® Xpress SARS-CoV-2 detects two targets, E and N2 genes 24. Furthermore, GeneXpert infinity automated systems do not require users to have special training to perform testing. The Xpert is capable of running 24/7, with many systems already doing so today for other infectious diseases, such as tuberculosis (Xpert ® MTB/RIF) 25, Chlamydia trachomatis and Neisseria gonorrhoeae (Xpert ® CT/NG) 26, methicillin-resistant Staphylococcus aureus (Xpert ® MRSA) 27, Group A Streptococcus (Xpert ® Xpress Strep A) 28 and Ebola (Xpert ® Ebola) 29.
A second FDA approved test is Abbott ID NOW™ COVID-19, which runs on the Abbott ID NOW™ platform – a lightweight box that can sit in a variety of locations. Positive results can be detected in as little as 5 minutes while negative results take 13 minutes. This test applies molecular technology targeting the COVID-19 RdRp gene 30. In addition, the above two COVID-19 molecular diagnostic tests are designed for near patient testing in a variety of healthcare environments using a range of sample types such as throat, nasal, nasopharyngeal and oropharyngeal swabs and this facilitates effective patient management.
As the number of confirmed cases of COVID-19 rises rapidly throughout the world, more and more nations continue to require reliable testing. A rapid, inexpensive, and easy-to-use point-of-care diagnostic device integrated with a smartphone could reduce transportation needs, lower the risk of spreading infection, alleviate the strain on the healthcare system, and mitigate the cost of testing for both individuals and governments 31. Unfortunately, scaling up production of available COVID-19 diagnostics, as well as personal protective equipment, remain an ongoing challenge in the fight against this disease. A list of other diagnostics under development and testing are listed here 32. The WHO prequalification team activated the emergency use listing (EUL) for candidates in vitro diagnostics to detect SARS-CoV-2. There is currently a total of 30 different diagnostic tests at the time of this publication 33. Since SARS-CoV-2 diagnostics development and approval are rapidly changing, this information is updated weekly at the WHO portal. As governments around the world are being encouraged by WHO to rapidly test a very large percentage of their populations to fight coronavirus 34, these tests will be critical in meeting this demand.
Whole-genome sequencing for COVID-19
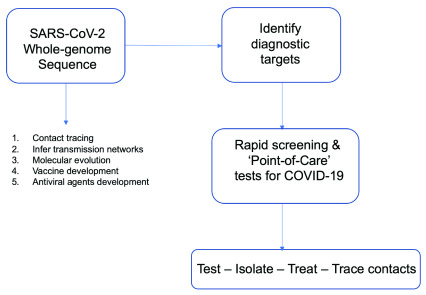
Following the emergence of SARS-CoV-2, genomic analyses continue to play a key role in the public health response by informing the design of appropriate molecular diagnostics and corroborating epidemiological efforts to trace contacts 35. In order to better understand the spread of this pandemic and design better interventions, whole genome sequencing of the virus from a range of clinical presentations of the disease in different parts of the world must occur ( Figure 1). It has been suggested from rapid data sharing using publicly available sequence data platforms that this pandemic was a point-source outbreak 35, 36. Therefore, researchers need more data from the whole genomes of SARS-CoV-2 including viral transcriptome 37. All these have the potential to reveal further insights into the biology of this emergent pathogen 35. Nanopore MinION sequencing technology has been used to sequence SARS-CoV-2 genomes 38, 39. Many of these genomes have been successfully uploaded to Global Initiative on Sharing All Influenza Data - GISAID by the WHO reference laboratories globally and Nextstrain. Other public repositories that have a number of these sequences are GenBank and the Sequence Read Archive of the US National Center for Biotechnology.
Figure 1. Utility of SARS-CoV-2 whole-genome sequencing.
The future of COVID-19 rapid point-of-care tests
Currently with more than 2.6 million infected individuals in 185 sovereign states and 183,000 deaths globally 40 at the time of writing, the COVID-19 pandemic may see deployment of COVID-19 rapid non-invasive point-of-care tests at civil airports and national borders to screen individuals and avoid imported cases of the infection. This would be similar to mitigation of aircraft hijackings and in-flight sabotage using mandatory civil aviation security procedures, including strict security screening for passengers and baggage at all commercial airports 41. Currently, screening for COVID-19 is performed by checking for high fever in individuals coming through airports and border controls using thermal screening guns. A nanosensor diagnostic platform is in development to test for this virus, which will replace thermal screening guns and be more specific. This technology, which will be in the form of a hand-held device, promises to give results specific for this virus within one minute, by detecting the nucleocapsid protein specific for this virus using nanosensor and aptamer technology 21.
Conclusions
To minimise the current COVID-19 pandemic related pressure on health systems globally, especially arising from ramping up testing capacity quickly, readily accessible, cheap, easy to use and interpret point-of-care diagnostic tests with high sensitivity must be developed, produced and distributed widely.
Data availability
No data is associated with this article.
Funding Statement
GM is supported through the DELTAS Africa Initiative [DEL-15-011 to THRiVE-2]. The DELTAS Africa Initiative is an independent funding scheme of the African Academy of Sciences (AAS)’s Alliance for Accelerating Excellence in Science in Africa (AESA) and supported by the New Partnership for Africa’s Development Planning and Coordinating Agency (NEPAD Agency) with funding from the Wellcome Trust [107742/Z/15/Z] and the UK government. The views expressed in this publication are those of the author(s) and not necessarily those of AAS, NEPAD Agency, Wellcome Trust or the UK government. GM also is supported through the Grand Challenges Africa programme [GCA/AMR/rnd2/058]. Grand Challenges Africa is a programme of the African Academy of Sciences (AAS) implemented through the Alliance for Accelerating Excellence in Science in Africa (AESA) platform, an initiative of the AAS and the African Union Development Agency (AUDA-NEPAD). GC Africa is supported by the Bill & Melinda Gates Foundation (BMGF) and The African Academy of Sciences and partners. The views expressed herein are those of the author(s) and not necessarily those of the AAS and her partners.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; peer review: 2 approved]
References
- 1. Mongan AE, Tuda JSB, Runtuwene LR: Portable sequencer in the fight against infectious disease. J Hum Genet. 2020;65(1):35–40. 10.1038/s10038-019-0675-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gardy JL, Loman NJ: Towards a genomics-informed, real-time, global pathogen surveillance system. Nat Rev Genet. 2018;19(1):9–20. 10.1038/nrg.2017.88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jiang F, Jiang Y, Zhi H, et al. : Artificial intelligence in healthcare: past, present and future. Stroke Vasc Neurol. 2017;2(4):230–243. 10.1136/svn-2017-000101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ayebare RR, Flick R, Okware S, et al. : Adoption of COVID-19 triage strategies for low-income settings. Lancet Respir Med. 2020;8(4):e22. 10.1016/S2213-2600(20)30114-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Coronavirus: three things all governments and their science advisers must do now. Nature. 2020;579(7799):319–320. 10.1038/d41586-020-00772-4 [DOI] [PubMed] [Google Scholar]
- 6. Peters A: The global proliferation of high-containment biological laboratories: understanding the phenomenon and its implications. Rev Sci Tech. 2018;37(3):857–883. 10.20506/37.3.2892 [DOI] [PubMed] [Google Scholar]
- 7. Huang C, Wang Y, Li X, et al. : Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nguyen T, Zoëga Andreasen S, Wolff A, et al. : From Lab on a Chip to Point of Care Devices: The Role of Open Source Microcontrollers. Micromachines (Basel). 2018;9(8): pii: E403. 10.3390/mi9080403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nguyen T, Duong Bang D, Wolff A: 2019 Novel Coronavirus Disease (COVID-19): Paving the Road for Rapid Detection and Point-of-Care Diagnostics. Micromachines (Basel). 2020;11(3): pii: E306. 10.3390/mi11030306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. rapid microbiology: Pinpoint’s Low-Cost Handheld Covid-19 Aptamer-based Diagnostic Device in Development. Accessed April 8, 2020. Reference Source [Google Scholar]
- 11. National laboratories. Accessed March 22, 2020. Reference Source [Google Scholar]
- 12. Zhu N, Zhang D, Wang W, et al. : A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. 10.1056/NEJMoa2001017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gao GF: From “A”IV to “Z”IKV: Attacks from Emerging and Re-emerging Pathogens. Cell. 2018;172(6):1157–1159. 10.1016/j.cell.2018.02.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. World Health Organization: Laboratory testing for coronavirus disease 2019 (COVID-19) in suspected human cases: interim guidance, 2 March 2020. World Health Organization;2020. Reference Source [Google Scholar]
- 15. Loeffelholz MJ, Tang YW: Laboratory diagnosis of emerging human coronavirus infections - the state of the art. Emerg Microbes Infect. 2020;9(1):747–756. 10.1080/22221751.2020.1745095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Matsuyama S, Nao N, Shirato K, et al. : Enhanced isolation of SARS-CoV-2 by TMPRSS2-expressing cells. Proc Natl Acad Sci. 2020;117(13):7001–7003. 10.1073/pnas.2002589117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. American Samoa’s coronavirus conundrum: No way to test. Accessed April 8, 2020. Reference Source [Google Scholar]
- 18. who-reference-laboratories-providing-confirmatory-testing-for-covid-19.pdf. Accessed March 22, 2020. Reference Source [Google Scholar]
- 19. Lv H, Wu NC, Tsang OTY, et al. : Cross-reactive antibody response between SARS-CoV-2 and SARS-CoV infections. bioRxiv. 2020. 10.1101/2020.03.15.993097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wetsman N: FDA authorizes first antibody-based test for COVID-19. The Verge.Accessed April 8, 2020. Reference Source [Google Scholar]
- 21. rapidmicrobiology: Coronavirus (SARS-CoV-2): Test Kits to Detect the Causative Agent of COVID-19. Accessed April 2, 2020. Reference Source [Google Scholar]
- 22. Point-of-Care COVID-19 Test Gets FDA Authorization. HCPLive.Accessed March 22, 2020. Reference Source [Google Scholar]
- 23.download.pdf. https://www.fda.gov/media/136314/download. Accessed March 22, 2020. [Google Scholar]
- 24.download.pdf. https://www.fda.gov/media/136315/download. Accessed April 9, 2020. [Google Scholar]
- 25. Steingart KR, Sohn H, Schiller I, et al. : Xpert ® MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev. 2013; (1): CD009593. 10.1002/14651858.CD009593.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gaydos CA, Van Der Pol B, Jett-Goheen M, et al. : Performance of the Cepheid CT/NG Xpert Rapid PCR Test for Detection of Chlamydia trachomatis and Neisseria gonorrhoeae. J Clin Microbiol. 2013;51(6):1666–1672. 10.1128/JCM.03461-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Spencer DH, Sellenriek P, Burnham CAD: Validation and implementation of the GeneXpert MRSA/SA blood culture assay in a pediatric setting. Am J Clin Pathol. 2011;136(5):690–694. 10.1309/AJCP07UGYOKBVVNC [DOI] [PubMed] [Google Scholar]
- 28. Xpert-Xpress-Strep-A-ENGLISH-Package-Insert-301-6574-Rev-C.pdf. Accessed April 8, 2020. Reference Source [Google Scholar]
- 29.download.pdf. https://www.fda.gov/media/91944/download. Accessed April 8, 2020. [Google Scholar]
- 30. ID NOW TM COVID-19. Alere is now Abbott. Accessed April 2, 2020. Reference Source [Google Scholar]
- 31. Yang T, Wang YC, Shen CF, et al. : Point-of-Care RNA-Based Diagnostic Device for COVID-19. Diagnostics (Basel). 2020;10(3): pii: E165. 10.3390/diagnostics10030165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sheridan C: Fast, portable tests come online to curb coronavirus pandemic. Nat Biotechnol. 2020. 10.1038/d41587-020-00010-2 [DOI] [PubMed] [Google Scholar]
- 33. https://www.who.int/diagnostics_laboratory/200407_eul_covid19_ivd_update.pdf?ua=1 [Google Scholar]
- 34. Governments Must Ramp Up COVID-19 Testing, Says WHO: The Scientist Magazine®. Accessed April 9, 2020. Reference Source [Google Scholar]
- 35. Taiaroa G, Rawlinson D, Featherstone L, et al. : Direct RNA sequencing and early evolution of SARS-CoV-2. bioRxiv. 2020; 2020.03.05.976167. 10.1101/2020.03.05.976167 [DOI] [Google Scholar]
- 36. Hadfield J, Megill C, Bell SM, et al. : Nextstrain: real-time tracking of pathogen evolution. Bioinformatics. 2018;34(23):4121–4123. 10.1093/bioinformatics/bty407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kim D, Lee JY, Yang JS, et al. : The architecture of SARS-CoV-2 transcriptome. bioRxiv. 2020; 2020.03.12.988865. 10.1101/2020.03.12.988865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhou P, Yang XL, Wang XG, et al. : A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. 10.1038/s41586-020-2012-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. COVID-19: Community News. Oxford Nanopore Technologies. Accessed March 22, 2020. Reference Source [Google Scholar]
- 40. COVID-19 Map. Johns Hopkins Coronavirus Resource Center. Accessed April 8, 2020. Reference Source [Google Scholar]
- 41. Blalock G, Kadiyali V, Simon DH: The Impact of Post-9/11 Airport Security Measures on the Demand for Air Travel. 42. 10.2139/ssrn.677563 [DOI] [Google Scholar]