Abstract
Social vigilance is a behavioral strategy commonly used in adverse or changing social environments. In animals, a combination of avoidance and vigilance allows an individual to evade potentially dangerous confrontations while monitoring the social environment to identify favorable changes. However, prolonged use of this behavioral strategy in humans is associated with increased risk for anxiety disorders, a major burden for human health. Elucidating the mechanisms of social vigilance in animals could provide important clues for new treatment strategies for social anxiety. Importantly, during adolescence the prevalence of social anxiety increases significantly. We hypothesize that many of the actions typically characterized as anxiety behaviors begin to emerge during this time as strategies for navigating more complex social structures. Here we consider how the social environment and the pubertal transition shape neural circuits that modulate social vigilance, focusing on the bed nucleus of the stria terminalis and prefrontal cortex. The emergence of gonadal hormone secretion during adolescence has important effects on the function and structure of these circuits, and may play a role in the emergence of a notable sex difference in anxiety rates across adolescence. However, the significance of these changes in the context of anxiety is still uncertain, as not enough studies are sufficiently powered to evaluate sex as a biological variable. We conclude that greater integration between human and animal models will aid the development of more effective strategies for treating social anxiety.
Graphical Abstract
We hypothesize that behaviors characterized as social anxiety can function as strategies for navigating adverse social environments. The social environment and the pubertal transition shape neural circuits that modulate social vigilance, including the bed nucleus of the stria terminalis and prefrontal cortex. Gonadal hormone secretion during adolescence may play a role in the emergence of sex differences in anxiety rates across adolescence.
1. Introduction
Anxiety disorders are the most commonly diagnosed mental illness, with twenty percent of adults experiencing an anxiety disorder within their lifetime. Available treatments such as benzodiazepines (Cassano et al., 2002) and selective serotonin reuptake inhibitors (Baldwin et al., 2011) are widely prescribed, but many patients do not experience remission using these therapeutics. Identification of underlying mechanisms contributing to anxiety could lead to novel approaches for individuals who do not respond to existing treatments. Studies of behavior related to anxiety in non-human animals have already yielded important discoveries of relevant brain circuits and neurochemical systems (Cryan & Holmes, 2005; Davis et al., 2010; Calhoon & Tye, 2015). Most of the approaches used to identify these systems include tasks that involve exploration of novel environments, in the absence of social cues. Different behavioral strategies could be engaged in social contexts, which may have special relevance for social anxiety.
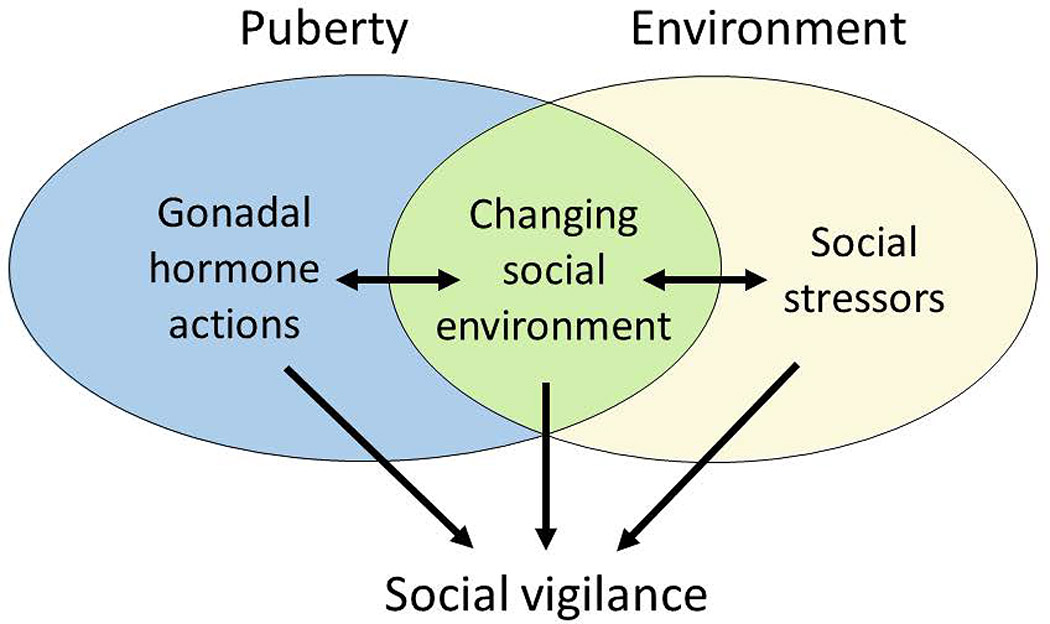
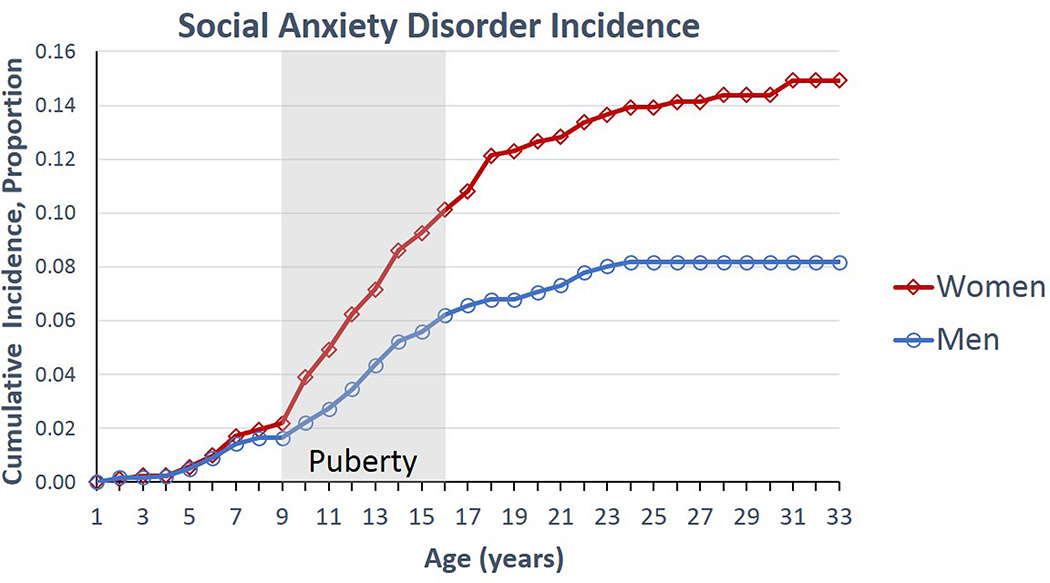
For example, social avoidance and social vigilance are two important components of social anxiety. Social avoidance refers to behavioral withdrawal in a novel social context and may be a protective response to avoid aggressive individuals. Prior research has focused on social avoidance, perhaps due to its role in preventing normal functioning in humans. In this review, we consider the role of social vigilance, which consists of increased monitoring of social cues, often while simultaneously avoiding social contexts. Understanding the underlying mechanisms of social vigilance could provide new insights into how social anxiety disorder develops. Based on work in non-human animals, we propose that vigilance is a coping strategy that increases in adverse or changing social environments (Fig. 1). Leading risk factors for social anxiety disorders are tied to either adverse or changing social environments. In particular, we will review studies in animals showing that social vigilance may be effective for exploiting opportunities in a changing social environment. However, in humans prolonged expression of social vigilance may be problematic, leading to increased risk for anxiety disorders (Silvers et al., 2017). Identifying the underlying mechanisms of social vigilance may help explain why rates of anxiety disorders are higher in women than in men (Hollingworth et al., 2010; Wesselhoeft et al., 2015). There are likely multiple factors contributing to this sex difference involving an interplay of biological, cultural, and experiential factors (Altemus et al., 2014). However, an important clue comes from demographic data showing that sex differences in the prevalence of social anxiety emerge during adolescence (Fig. 2) (Beesdo et al., 2007; Wesselhoeft et al., 2015). This period is characterized by physiological sexual differentiation and dynamic changes in the social environment, such as moving into a new social group. Social interactions become more salient (Walker et al., 2017), with some evidence in humans that this effect is more pronounced in girls (Guyer et al., 2009). The pubertal transition triggers changes in brain structure and function through gonadal hormone dependent (Schulz & Sisk, 2016) and independent (Paul et al., 2018) mechanisms. These changes may modulate susceptibility to social anxiety (Davey et al., 2008). Additionally, stressful social interactions during adolescence can have exaggerated effects on brain structure and function (Romeo, 2017; Rowson et al., 2019). Historically, animal models for studying mechanisms related to anxiety were strongly biased towards males. Increasing representation of females in animal models of anxiety is likely to provide key insights into sex differences in the prevalence of anxiety (Shansky & Woolley, 2016; McCarthy et al., 2017).
Figure 1.
During puberty gonadal hormones shape brain development as an individual adapts to a changing social environment. A dynamic social environment can introduce challenging social conditions. Evidence in non-human animals suggest that social vigilance may be a strategy for coping with adverse social environments while waiting for better opportunities in the social environment.
Figure 2.
Lifetime cumulative incidence estimates for social anxiety disorder. Figure redrawn with permission based on data from Beesdo et al. (2007).
In this review, we examine studies in animal model systems that show how social vigilance allows individuals to avoid confrontations yet capitalize on beneficial changes in the social environment. Mechanistically, we focus on the role of the bed nucleus of the stria terminalis, a component of the extended amygdala that is receiving increased attention for its role in modulating anxiety in both human and non-human animals. Intriguingly, in mice social opportunity is associated with increased engagement of the frontal cortex (Williamson, Klein, et al., 2019). The prefrontal cortex undergoes major structural and functional changes during adolescence, a period during which anxiety rates increase at a faster rate in women than in men. Throughout this review, we consider how adolescent development shapes social vigilance and anxiety. Our review touches on tantalizing clues that may contribute to new perspectives on the development of anxiety disorders, but also reveals major gaps in understanding.
2. Social environment can regulate the timing of puberty
A major theme of this review is how adolescence shapes how an individual copes with the social environment. However, there is strong evidence that the timing of pubertal development is highly sensitive to the social environment itself. Low social status delays puberty in marmosets (Abbott & Hearn, 1978; Ginther et al., 2002), rhesus monkeys (Bercovitch, 1993) and baboons (Onyango et al., 2013). Mechanistic studies in naked mole rats suggest that socially induced delay in puberty is mediated by decreased gonadotropin action. Hypothalamic RFamide-related peptide-3 (RFRP-3) is a potent inhibitory modulator of GnRH action (Zhou et al., 2013) and is elevated in subordinate naked mole rats while exogenous RFRP-3 treatment inhibited sexual and dominance behaviors (Peragine et al., 2017). The removal of the dominant female triggers ovarian cycles in subordinate females within a week (Margulis et al., 1995). Less dramatic transitions have been observed in mice (Koyama & Kamimura, 1999; Williamson, Lee, et al., 2019). Within 3 minutes of the removal of a dominant male, subdominant male mice responded with increases in aggressive behavior and within 1 hr show increased GnRH mRNA levels in the medial preoptic area (Williamson et al., 2017). Similar changes occur in male cichlid fish after acquiring a new territory (Burmeister et al., 2005; Maruska & Fernald, 2018). Socially ascending male mice also had increased immediate early gene expression in hypothalamic and limbic brain regions linked to the modulation of social behavior, including infralimbic and prelimbic regions of frontal cortex (Williamson, Klein, et al., 2019). Animal studies show how the social environment can cause changes in brain activity and pubertal timing. Correlational data from humans echo these.
In humans, associations between the social environment and pubertal timing are complex. On the one hand, there is a well-replicated finding that adverse social experiences such as harsh parenting and maltreatment (including physical and sexual abuse) are linked to accelerated pubertal onset, particularly for females (Belsky et al., 2007; Boynton-Jarrett et al., 2013; Noll et al., 2017). Early menarche has been linked to increased risk of anxiety and depression (Mendle, 2014; Colich et al., 2019). In contrast, experiences of deprivation such as poverty and food insecurity are linked to delayed pubertal maturation (Sumner et al., 2019). Currently, the mechanisms through which the social environment modulates the timing of puberty in humans are unclear. However, the effects of exposure to violence are thought to be mediated by threat-response systems, whereas the delay of puberty observed in food insecurity is theorized to be a response to limited bioenergetic resources (Ellis et al., 2009). Social status impacts the timing of puberty by regulating gonadotropin function, and in some cases opportunities in the social environment trigger rapid development. How are these opportunities detected? In the next section, we consider social vigilance as a strategy to remain cognizant of changes in the environment while avoiding social conflicts.
3. Social anxiety and vigilance as behavioral strategies for adverse social environments
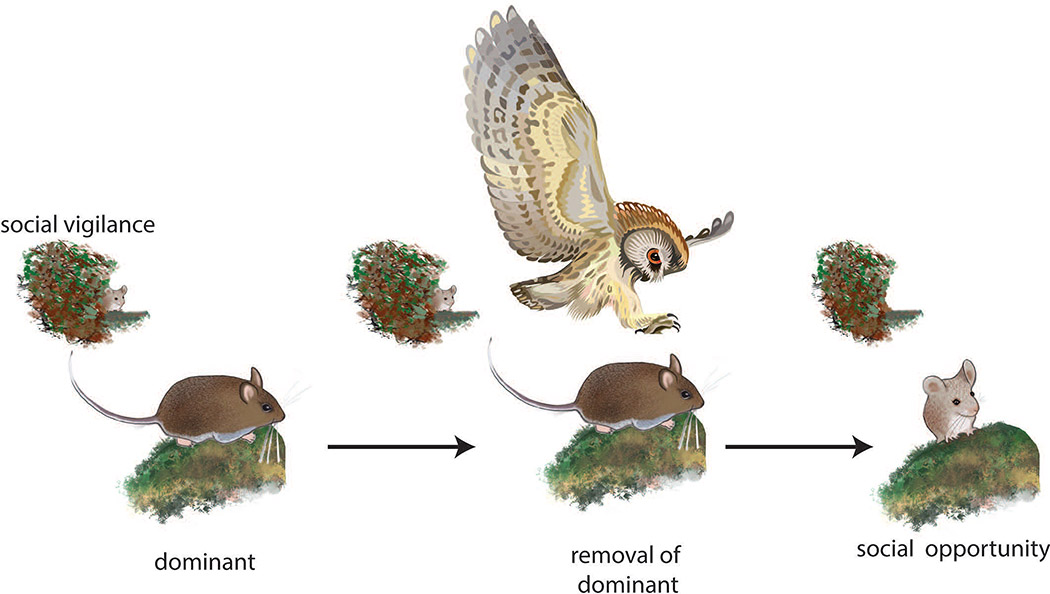
Exposure to adverse social contexts induces evolutionarily conserved behavioral and physiological responses. One of the most robust observations is that individuals that lose aggressive encounters avoid novel social contexts, a behavior referred to as social avoidance. The phenotype has been observed in rodents (Blanchard et al., 2001; Huhman, 2006), birds (Carere et al., 2001), and primates (Shively et al., 1997). This response may allow individuals to avoid engaging in energetically costly and potentially dangerous contests with little opportunity of winning (Neat et al., 1998). This can be an effective short-term strategy, but social avoidance does not provide an obvious route for enhancing social status. Social vigilance, in which individuals avoid yet monitor unfamiliar social contexts, may be a key behavioral mechanism that allows individuals to seize opportunities when the social environment changes (Fig. 3). Social vigilance can be quantified as orienting behavior in a laboratory social interaction test when a focal animal orients towards, but avoids, an unfamiliar individual confined to a small cage (Duque-Wilckens et al., 2018; Williams et al., 2018). If orienting behavior is not observed when the stimulus animal is absent, this demonstrates the social nature of the response. Social vigilance can take other forms, such as the “stretch-attend” posture, which consists of orienting towards a threat while maintaining a crouched posture that reduces visibility. Stretch-attend postures are evoked by social threats (McCann & Huhman, 2012; Morrison et al., 2012) and predator cues (Hubbard et al., 2004). Vigilance can also take the form of visual scanning, during which an individual forgoes other activities such as feeding. This form of social vigilance is elevated in low status individuals of avian (Ekman, 1987), marsupial (Blumstein et al., 2001), and primate (Shepherd et al., 2006) species. Social vigilance usually coincides with social avoidance, but social avoidance can occur in the absence of social vigilance. These distinct behavioral phenotypes may be driven by distinct mechanisms (see section 5).
Figure 3:
Hypothesized use of social vigilance by lower social status individuals. Data from rodents and fish suggest that lower status individuals avoid and monitor the activities of more dominant individuals. If a dominant individual is removed through predation or illness, information gained through social vigilance allows individuals to exploit new opportunities in the social environment. Mouse drawings by Natalia Duque-Wilckens.
In humans, individuals with high trait anxiety initially react more quickly to aversive images, suggesting an enhanced state of vigilance (Mogg et al., 2004). Importantly, increased vigilance combined with social avoidance are key components of behavioral inhibition (Kagan et al., 1987), a temperamental predisposition in humans that appears early in development as reticence to approach unfamiliar people or objects (Henderson et al., 2015). Behavioral inhibition is the strongest predictor for onset of social anxiety disorders in adulthood (Clauss & Blackford, 2012). Importantly, behavioral inhibition only translates into later anxiety disorders in the presence of additional risk factors, such as adverse social experiences including victimization by peers or low social status within one’s peer group (Rubin et al., 2009).
Socioeconomic status is a proxy measure of social position in human society, and is linked to behavioral, cognitive, and neural indices of vigilance to social threat in childhood (Chen & Matthews, 2001; Boyce et al., 2012), adolescence (Inderbitzen et al., 1997; Chen et al., 2004), and adulthood (Gianaros et al., 2008; Kraus et al., 2011; Cundiff et al., 2016). Importantly, some evidence suggests that low socioeconomic status may be associated with heightened vigilance to only social and not non-social threats (Hostinar et al., 2017). Furthermore, loneliness (i.e., subjective social isolation) is associated with enhanced rather than decreased monitoring of social cues (Gardner et al., 2005; Vanhalst et al., 2017). In this way, human social vigilance closely resembles the combination of social attention and avoidance observed in rodents exposed to social stress. It has been theorized that enhanced social monitoring may operate as a strategy for coping with and improving one’s social status (Pickett & Gardner, 2005). Taken together, these findings underscore the critical need to assess whether social vigilance has a strategic role for coping with low social status. Interestingly, adolescent development is frequently characterized by new opportunities to transition from lower to higher social status.
4. Social status and adolescent development
We hypothesize that social vigilance could be an important behavioral strategy used during adolescence to cope with lower social status, as it is common for adolescents to have lower social status than older, more established adults. In rhesus macaques, older adults easily gained social dominance in a new social group by social posturing, which led to avoidance by the smaller/younger members (Bernstein & Mason, 1963). In stable social groups, older adults have the advantage of developed relationships of peer support against younger challengers (Gartlan, 1968). Age is also a good predictor of social status in rodents. Analyses of a large group (30–50) of rats in a laboratory colony found that increased age was a better predictor of social status than body weight (Macdonald et al., 1995). Similar results were observed in colonies of rats living outdoors. Younger males only increased social status after older males had succumbed to predation (Adams & Boice, 1983). A key contributing mechanism to the effects of experience may be the winner effect. The winner effect is a phenomenon wherein males that win an aggressive encounter show higher levels of aggression in future encounters and an increased likelihood of winning future contests (Franz, McLean, Tung, Altmann, & Alberts, 2015; Lehner, Rutte, & Taborsky, 2011; Oyegbile & Marler, 2005; Marler & Trainor, in press). In males, the experience of winning an aggressive encounter is coupled with a temporary spike in testosterone levels in the winning male, after the victor has been determined (Wingfield & Wada, 1989; Oyegbile & Marler, 2005). Males deprived of this post-winning testosterone spike do not show future increases in aggressive behavior (Trainor et al., 2004). It is important to note that these studies focus exclusively on adult males. Adolescents have less time to win aggressive encounters than adults, and thus less time to build up the positive reinforcement of aggressive behavior associated with the winner effect. Thus, less experience, lower aggression levels, and reduced social support are possible factors that can put an adolescent animal at a social disadvantage compared to already established adults. We hypothesize that observation of social dynamics through social vigilance and other behaviors could be a key behavioral strategy used by adolescents as they try to eke out the foundations of their own social status. Watching and waiting for a social opportunity, as documented in studies of cichlid fish (Burmeister et al., 2005; Maruska & Fernald, 2018) and mice (Williamson, Klein, et al., 2019; Williamson et al., 2017), could be an important strategy for adolescents. However, exaggerated social vigilance expression could contribute to increased risk for anxiety disorders (Clauss & Blackford, 2012).
5. The bed nucleus of the stria terminalis (BNST) as a modulator of social vigilance
The BNST is a key component of neural circuits modulating behavioral responses to threat (Trainor et al., 2004). The BNST is ideally suited to modulate social vigilance because of its strong connections with the social behavior network (O’Connell & Hofmann, 2011) as well as extended amygdala circuits that modulate responses to threat. Our understanding of how the BNST controls behavioral responses to threats has evolved over time. Early work suggested a dissociation between the BNST and the central nucleus of the amygdala, with the BNST modulating responses to more diffuse threats and the central nucleus of the amygdala being more important for more defined threats (e.g. a conditioned cue) (Walker & Davis, 1997). More recent data suggest more overlap in function, and imaging studies often show coordinated responses between BNST and central nucleus of the amygdala (Davis et al., 2010; Daniel & Rainnie, 2016). Imaging data from hundreds of rhesus monkeys (Oler et al., 2010) and human participants (Yassa et al., 2012; Avery et al., 2016) also show that threat exposure increases activity within the BNST. It has been hypothesized that the BNST may assign a valence to ambiguous social contexts based on prior experience (Lebow & Chen, 2016). Data from the California mouse social defeat model are consistent with this idea (Duque-Wilckens et al. 2018).
Studies on the California mice point to the BNST as a key modulator of social vigilance (Duque-Wilckens et al., 2018). In this monogamous species, both males and females are aggressive, which allows for the study of social stress in both sexes (Steinman & Trainor, 2017). In adult females but not males, social stress increased expression of brain derived neurotrophic factor within the BNST and infusion of a tyrosine kinase B receptor (TrkB, the main receptor for brain-derived neurotropic factor) antagonist into the BNST restored normal social approach behavior in stressed females (Greenberg et al., 2014). These results suggest that social stress may induce synaptic plasticity within the BNST. In the BNST, studies of male rodents show that repeated stressors can enhance excitatory neurotransmission in some cell types (Dabrowska et al., 2013) while reducing excitability in other cell types (McElligott et al., 2010). The BDNF findings in California mice suggest that there could be important sex differences in stress-induced synaptic plasticity. A likely candidate pathway is the oxytocin system.
In adult female but not male California mice, social stress has long-term effects on oxytocin neurons within the ventral BNST (Steinman et al., 2016). Oxytocin is usually assumed to be produced within the hypothalamus, but oxytocin-producing neurons are present in the BNST of mice (Nasanbuyan et al., 2018), rats (DiBenedictis et al., 2017), prairie voles (Kelley et al., 2018), and marmosets (Wang et al., 1997). Social stress has enduring effects on the reactivity of oxytocin neurons. Ten weeks after a final stress exposure, females had more oxytocin/c-fos colocalizations than controls following exposure to novel environment, even in the absence of social cues (Steinman et al., 2016). This suggests that stress enhances the reactivity of BNST oxytocin neurons in novel environments. Intriguingly, when tested in a novel environment, infusion of an oxytocin receptor antagonist into the anteromedial BNST reduced social vigilance in stressed females and increased social approach (Duque-Wilckens et al., 2018). Impressively, a systemic injection of oxytocin receptor antagonist had identical results. To achieve the same effect with a selective serotonin reuptake inhibitor, 4 weeks of daily treatment was required (Greenberg et al., 2014). Although oxytocin is normally considered a neuropeptide that enhances social approach, many studies show that oxytocin can induce social avoidance and anxiety (Eckstein et al., 2014; Beery, 2015), especially in females. Together these results suggest that oxytocin enhances the salience of both positive and aversive social interactions (Shamay-Tsoory & Abu-Akel, 2016). Context-dependent effects of oxytocin may be mediated by distinct neural circuits that promote either social approach or social vigilance (Steinman et al., 2019). Indeed, stress-induced vigilance can be observed in the absence of alterations in social approach (Newman et al., 2019), and reduced social approach can be observed in the absence of social vigilance (Williams and Trainor, unpublished). A major unanswered question is why social stress does not affect oxytocin neurons in males as it does in females. In adult California mice, gonadal hormones are not a critical mechanism driving sex differences (Trainor et al., 2011, 2013). However, both male and female juvenile California mice exhibit stress-induced social vigilance (Wright and Trainor, unpublished). Studies in male adolescent male C57Bl6/J mice show that social stress reduces social approach (Iñiguez et al., 2014, 2016) and increases vigilance (S. Iñiguez, personal communication). Interestingly, populations of “unsusceptible” mice, which do not exhibit decreased social approach, are routinely observed in adult male C57Bl6/J (Krishnan et al., 2007; Cao et al., 2010; Bagot et al., 2015) but have not been reported for adolescent mice. These results implicate adolescence as a key time window during which neural circuits of social vigilance may be reprogramed.
Knowledge of BNST structure and function during adolescent development is sparse. Several subregions of the BNST are larger in males compared to females (Allen & Gorski, 1990; Campi et al., 2013; Morishita et al., 2017) and some show sex differences in chemoarchitecture (Bamshad et al., 1993; Juntti et al., 2010; Gegenhuber & Tollkuhn, 2019). These sex differences in the neuroanatomy likely contribute to sex-dependent reproductive behaviors (Juntti et al., 2010) and learning patterns (Bangasser et al., 2005; Bangasser & Shors, 2008). Rodent studies show that post-natal testosterone exposure increases the size of some subregions of BNST (del Abril et al., 1987) and increases vasopressin production (Han & De Vries, 2003). However, additional sexual differentiation may occur later in life. A study of postmortem human brain samples showed that sex differences in the size of the BNST were not present in samples from children, but were present in adults (Chung et al., 2002). To date, human neuroimaging studies have not examined developmental changes in the BNST. However, cross-sectional studies have reported that during puberty amygdala volumes tend to increase in boys and decrease in girls (Vijayakumar et al., 2018). Cross-sectional studies have not detected associations between gonadal hormones and amygdala volumes, but a longitudinal study showed that boys with greater increases in testosterone during adolescence had larger increases in amygdala volume (Wierenga et al., 2018). In cross-sectional studies, age and testosterone are confounded during adolescence, so longitudinal studies represent a more powerful approach for detecting associations between gonadal hormones and brain development. Changes in amygdala anatomy during puberty correspond with sex differences in functional properties of medial amygdala neurons that emerge during puberty in mice (Bergan et al., 2014). While there are still significant gaps in knowledge, it appears that adolescence could be a key period for maturation of the BNST and amygdala.
Consistent with this idea, work in mice shows that in both males and females, gonadal hormones modulate the size and chemoarchitecture of the posterior BNST during puberty (Morishita et al., 2017). It is unknown whether any of these sex differences contribute to social vigilance or anxiety. Work in rats suggests that the BNST seems to be more reactive to social contexts during adolescent development than in adulthood (Saalfield & Spear, 2019), at least in males. For example interacting with an unfamiliar male generated stronger c-fos responses in the BNST and central nucleus of the amygdala in adolescent male rats compared to adult male rats (Varlinskaya et al., 2013). The BNST is also responsive to social threats in adolescent monkeys (Fox et al., 2008), but comparable data on BNST function are not available for adolescent rodents or humans. More data comparing male and female behavior and brain function during adolescence could be provide important insights, as emerging data indicate that significant reorganization of neural circuits can occur during this period. For example, major synaptic reorganization has been described in the prefrontal cortex (Delevich, Thomas, & Wilbrecht, 2019).
6. Executive Control of Anxiety-related Behavior
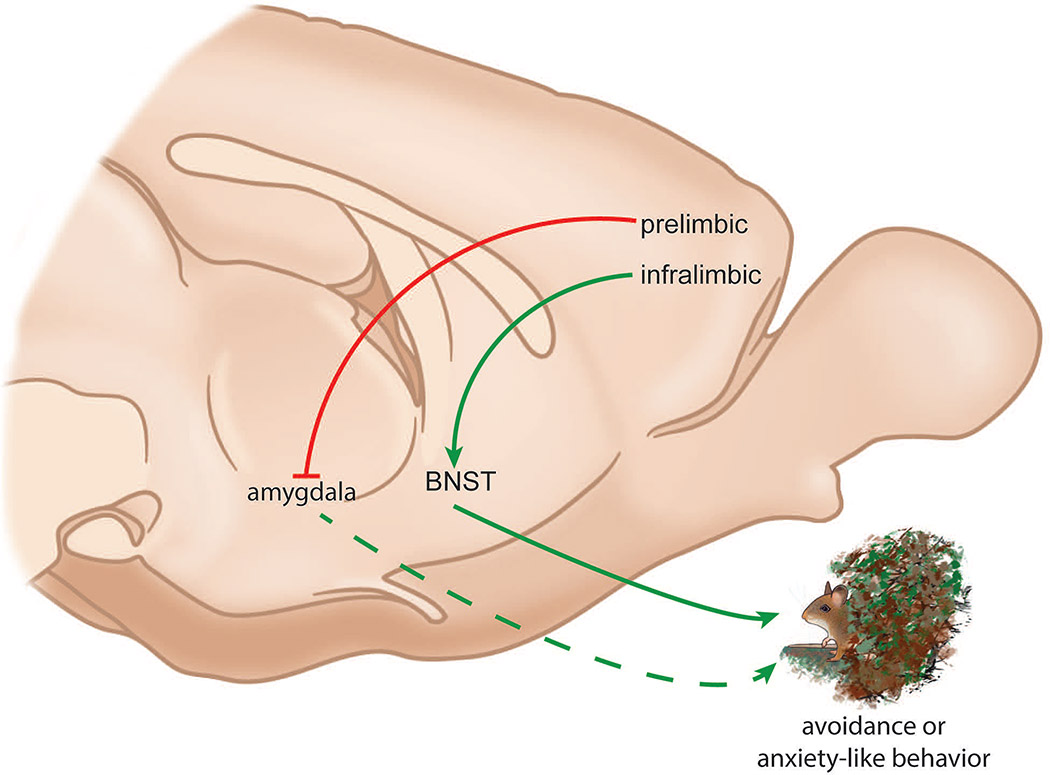
Similar to the BNST, the prefrontal cortex undergoes major changes during adolescent development. Rodent studies show that the number of synapses in the frontal cortex decreases in both males and females during adolescence (Drzewiecki et al., 2016), an effect that is accelerated by gonadal hormones in females (Piekarski, Boivin, et al., 2017). Fewer synapses may contribute to decreases in gray matter observed during adolescence by human imaging studies (Lenroot & Giedd, 2006; Vijayakumar et al., 2018). The decline in synapse number is associated with an increase in stability among remaining synapses (Pattwell et al., 2016a). Changes in synaptic plasticity in the prefrontal cortex could impact anxiety-related behaviors such as social vigilance (Fig. 4).
Figure 4:
Simplified model for interactions between the frontal cortex and bed nucleus of the stria terminalis (BNST). Excitatory neurons in the infralimbic cortex project to the BNST, which plays an important role in driving anxiety-related behaviors. In contrast the prelimbic cortex exerts inhibitory input on the amygdala, which in turn may reduce anxiety-related behaviors. Mouse drawings by Natalia Duque-Wilckens.
Anatomical tracing studies in rodents show that medial prefrontal cortex has direct connections with the BNST both from its prelimbic (Room et al., 1985; Chiba et al., 2001; Vertes, 2004; Radley et al., 2009) and infralimbic regions (Room et al., 1985; Hurley et al., 1991; Chiba et al., 2001; Vertes, 2004). Emerging data suggest that the more dorsal prelimbic cortex and the more ventral infralimbic cortex have distinct effects on behavioral responses to threat (Calhoon & Tye, 2015). When mice explore anxiogenic environments such as the open arms of an elevated plus maze, neural activity within the ventral prelimbic cortex increases (Adhikari et al., 2010, 2011). These neurons receive input from the ventral hippocampal neurons, and optogenetic inhibition of these inputs increased exploration (Padilla-Coreano et al., 2016). These data are generally consistent with the human imaging studies reporting increased activity in frontal cortex activity in response to aversive contexts. A blind spot in the literature is over-reliance on data from male rodents, even though there is growing evidence that stress-induced plasticity in frontal cortex function can be sex-specific (Gruene et al., 2015; Baratta et al., 2019). Interestingly, ventral hippocampus also has strong connections with ventral BNST (Cullinan et al., 1993), which also can drive anxiogenic states (Jennings et al., 2013). Ventral BNST contains oxytocin neurons that become more reactive in females following social defeat (Steinman et al., 2016). Suppression of oxytocin synthesis in the ventral BNST also reduces stress-induced vigilance in females (Duque-Wilkens et al. in preparation), suggesting that ventral BNST is an important node for anxiety-related behaviors in social contexts. Overall, these findings suggest that a circuit encompassing the ventral hippocampus, prelimbic cortex, and ventral BNST is important for generating anxiety-related behaviors in threatening contexts. As mentioned above, the BNST also receives input from the infralimbic cortex. Interestingly, several lines of evidence suggest infralimbic cortex reduces behavioral responses to threat. For example, increased activity in the infralimbic cortex is important for the extinction of conditioned fear responses (Milad & Quirk, 2002; Chang et al., 2010; Holmes et al., 2012). Infralimbic cortex has strong functional connections with amygdala (Kim et al., 2011) and these projections are essential for effective extinction of fear responses (Sierra-Mercado et al., 2011; Bloodgood et al., 2018). Currently, the functional effects of infralimbic projections to BNST have not been examined, so further study of the impact of anxiety or threat on neural activity within the frontal cortex is needed (Park & Moghaddam, 2017). This is especially true for understanding frontal cortex function in humans.
Human imaging studies have reported contrasting results regarding the relationship between responses of the prefrontal cortex and vigilance to threat. One line of evidence linked increased vigilance and anxiety to reduced activity in lateral or medial prefrontal cortex (Bishop et al., 2004; Bishop, 2009). Among children diagnosed with anxiety disorders, individuals reacting more quickly to aversive images had reduced functional connectivity between medial prefrontal cortex and amygdala (Price et al., 2016). Reduced functional connectivity between medial prefrontal cortex and amygdala while viewing aversive images was also observed in youth who had experienced maternal deprivation (Gee et al., 2013), which is associated with increased anxiety. In contrast, children rated higher for behavioral inhibition (Fu et al., 2017) or trait anxiety (Telzer et al., 2008) had higher levels of activity in the dorsolateral prefrontal cortex (but not mediolateral prefrontal cortex) in tasks that require attention orienting away from aversive images. Transcranial direct current stimulation directed towards dorsolateral prefrontal cortex reduced vigilance towards aversive images in healthy volunteers (Ironside et al., 2016). Similarly, adults diagnosed with posttraumatic stress disorder showed increased activity in ventrolateral prefrontal cortex in response to aversive images (Adenauer et al., 2010). One problem for resolving these apparently contrasting results is that some studies do not report imaging results from all subregions of the prefrontal cortex (dorsomedial, ventrolateral, etc.). This makes it more difficult to assess subregion-specific responses during vigilance, as well as compare with rodent studies that distinguish between infralimbic and prelimbic cortex. A related question is whether the social environment modulates how the frontal cortex regulates vigilance and other anxiety-related behaviors.
Curiously, there is a strong increase in activity in prelimbic cortex of a subordinate male mouse when a dominant is removed (Wang et al., 2011). Experimental enhancement of excitatory neurotransmission in subordinate male mice increased aggressive behavior. This suggests that disruptions in the social environment may be anxiogenic, even when an individual has an opportunity to compete for higher social status and suggests that the prefrontal cortex regulation of vigilance behavior could be dependent on the social status of the individual. Imaging data from humans also implicates a role for prelimbic cortex in social contexts, as a meta-analysis showed that dorsomedial (prelimbic) prefrontal cortex was consistently linked with adolescent decision-making in social contexts (van Hoorn et al., 2019). These findings highlight another gap in the preclinical literature, the sparse knowledge of how prelimbic or infralimbic cortex affects anxiety-related behavior during adolescence. Adolescence is a time when individuals start to establish themselves as a competitive member of a social group. This is met with a large range of behavioral changes as well as functional and anatomical changes within the frontal cortex (Drzewiecki et al., 2016; Piekarski, Johnson, et al., 2017), yet only a few studies have considered how these changes affect behavior (Pattwell et al., 2016b). Similarly, there are opportunities to consider how changing social environments affect prefrontal cortex in females, as recent data show that social hierarchies can be studied in female mice housed in more naturalistic conditions (Williamson, Klein et al. 2019).
The data discussed give some insight into potential pathways for vigilance activation but shed very little light on how vigilance could be suppressed in inappropriate contexts. This is an area ripe for future research and at this time the authors have no knowledge of research into executive inhibition of BNST. Future animal studies on this topic should also endeavor to include females, because all of the neurophysiological studies reviewed in this section focused on male mice or rats.
7. Conclusions
Here we reviewed evidence for a novel hypothesis that social vigilance could function as a behavioral strategy for improving one’s social status. Studies in rodents indicate that stressful social environments can induce social vigilance, which is mediated in part by the BNST. We also reviewed evidence in mice and fish showing that subordinate individuals could detect changes in their social environment within minutes, and respond by engaging the reproductive axis and frontal cortex. These findings suggest that social vigilance may be an important strategy for detecting changes in the social environment. Imaging studies focusing on anxiety disorders in humans have also linked vigilance and avoidance in the dot-probe tasks, but the extent to which these associations apply to more real-world conditions is less clear. Greater implementation of more ethological approaches such as social interaction tasks or ecological momentary assessments to capture naturalistic variation in daily social interactions could be informative. New methods for quantifying the BNST in human imaging studies provide an opportunity to test whether BNST activity tracks social vigilance, as would be predicted from animal models. Adolescence may be an ideal period for interventions aiming to alter behavioral, cognitive, social or neurobiological features of social anxiety given that puberty is a period of dynamic reorganization across these levels.
The science of adolescence is, in itself, in a period of adolescence. The number of research articles on brain function in adolescence accelerated from less than 70 in the year 2000 to more than 700 in 2018. Although we know that adolescence is a period of sexual differentiation, fundamental questions about the mechanisms that contribute to sex differences in social anxiety remain unanswered. Most human neuroimaging studies have been underpowered to assess sex differences. Although some studies incorporate measures of gonadal hormones, few assess hormone levels longitudinally, which is a more effective analytical approach. Mechanistic studies in animal models systems do not fare much better, even after the implementation of “sex as a biological variable” policies in the United States. Only a few groups have rigorously investigated changes in brain structure and function in adolescent males and females. Thus, there is a major gap in the literature addressing the life history time point when sex differences in anxiety disorders emerge. Another potential barrier to progress is the limited attention to participants’ social status and their social mobility (upward or downward). Incorporating measures of participants’ current social context or prior social history could be an important approach for accounting for variability in other neuroendocrine and behavioral variables. Gathering more detailed data on the social environment in clinical populations could help leverage new animal models for assessing sex differences in how social stressors impact the brain (Piekarski, Johnson, et al., 2017). So far most of these approaches have been applied primarily in adults (but see Bourke & Neigh, 2011). Greater integration between human and animal model studies could facilitate the development of more effective strategies for treating social anxiety.
Acknowledgements
The authors thank A. Fox and E. Boorman for helpful conversations. BCT was supported by NIH R01 MH103322. There are no new data in this manuscript.
List of Abbreviations
- BNST
bed nucleus of the stria terminalis
Footnotes
Conflict of Interest Statement
The authors declare no conflict of interest.
Data Availability Statement:
No new data has been used
References
- Abbott DH & Hearn JP (1978) Physical, hormonal and behavioural aspects of sexual development in the marmoset monkey, Callithrix jacchus. J. Reprod. Fertil, 53, 155–166. [DOI] [PubMed] [Google Scholar]
- Adams N & Boice R (1983) A longitudinal study of dominance in an outdoor colony of domestic rats. Journal of Comparative Psychology, 97, 24–33. [Google Scholar]
- Adenauer H, Pinösch S, Catani C, Gola H, Keil J, Kissler J, & Neuner F (2010) Early processing of threat cues in posttraumatic stress disorder-evidence for a cortical vigilance-avoidance reaction. Biol. Psychiatry, 68, 451–458. [DOI] [PubMed] [Google Scholar]
- Adhikari A, Topiwala MA, & Gordon JA (2010) Synchronized Activity between the Ventral Hippocampus and the Medial Prefrontal Cortex during Anxiety. Neuron, 65, 257–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adhikari A, Topiwala MA, & Gordon JA (2011) Single Units in the Medial Prefrontal Cortex with Anxiety-Related Firing Patterns Are Preferentially Influenced by Ventral Hippocampal Activity. Neuron, 71, 898–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen LS & Gorski RA (1990) Sex difference in the bed nucleus of the stria terminalis of the human brain. J. Comp. Neurol, 302, 697–706. [DOI] [PubMed] [Google Scholar]
- Altemus M, Sarvaiya N, & Neill Epperson C (2014) Sex differences in anxiety and depression clinical perspectives. Front Neuroendocrinol, 35, 320–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery SN, Clauss JA, & Blackford JU (2016) The human BNST: functional role in anxiety and addiction. Neuropsychopharmacology, 41, 126–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagot RC, Parise EM, Peña CJ, Zhang H-X, Maze I, Chaudhury D, Persaud B, Cachope R, Bolaños-Guzmán CA, Cheer JF, Deisseroth K, Han M-H, & Nestler EJ (2015) Ventral hippocampal afferents to the nucleus accumbens regulate susceptibility to depression. Nature Communications, 6, 7062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin D, Woods R, Lawson R, & Taylor D (2011) Efficacy of drug treatments for generalised anxiety disorder: systematic review and meta-analysis. BMJ, 342, d1199. [DOI] [PubMed] [Google Scholar]
- Bamshad M, Novak MA, & Devries GJ (1993) Sex and Species-Differences in the Vasopressin Innervation of Sexually Naive and Parental Prairie Voles, Microtus-Ochrogaster and Meadow Voles, Microtus-Pennsylvanicus. Journal of Neuroendocrinology, 5, 247–255. [DOI] [PubMed] [Google Scholar]
- Bangasser DA, Santollo J, & Shors TJ (2005) The bed nucleus of the stria terminalis is critically involved in enhancing associative learning after stressful experience. Behav. Neurosci, 119, 1459–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA & Shors TJ (2008) The Bed Nucleus of the Stria Terminalis Modulates Learning after Stress in Masculinized But Not Cycling Females. J. Neurosci, 28, 6383–6387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baratta MV, Gruene TM, Dolzani SD, Chun LE, Maier SF, & Shansky RM (2019) Controllable stress elicits circuit-specific patterns of prefrontal plasticity in males, but not females. Brain Struct Funct, 224, 1831–1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beery AK (2015) Antisocial oxytocin: complex effects on social behavior. Curr Opin Behav Sci, 6, 174–182. [Google Scholar]
- Beesdo K, Bittner A, Pine DS, Stein MB, Höfler M, Lieb R, & Wittchen H-U (2007) Incidence of social anxiety disorder and the consistent risk for secondary depression in the first three decades of life. Arch. Gen. Psychiatry, 64, 903–912. [DOI] [PubMed] [Google Scholar]
- Belsky J, Steinberg LD, Houts RM, Friedman SL, DeHart G, Cauffman E, Roisman GI, Halpern-Felsher BL, Susman E, & NICHD Early Child Care Research Network (2007) Family rearing antecedents of pubertal timing. Child Dev, 78, 1302–1321. [DOI] [PubMed] [Google Scholar]
- Bercovitch FB (1993) Dominance rank and reproductive maturation in male rhesus macaques (Macaca mulatta). J. Reprod. Fertil, 99, 113–120. [DOI] [PubMed] [Google Scholar]
- Bergan JF, Ben-Shaul Y, & Dulac C (2014) Sex-specific processing of social cues in the medial amygdala. Elife, 3, e02743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein IS & Mason WA (1963) Group formation by rhesus monkeys. Animal Behaviour, 11, 28–31. [Google Scholar]
- Bishop S, Duncan J, Brett M, & Lawrence AD (2004) Prefrontal cortical function and anxiety: controlling attention to threat-related stimuli. Nat Neurosci, 7, 184–188. [DOI] [PubMed] [Google Scholar]
- Bishop SJ (2009) Trait anxiety and impoverished prefrontal control of attention. Nature Neuroscience, 12, 92–98. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, McKittrick CR, & Blanchard DC (2001) Animal models of social stress: effects on behavior and brain neurochemical systems. Physiology & Behavior, 73, 261–271. [DOI] [PubMed] [Google Scholar]
- Bloodgood DW, Sugam JA, Holmes A, & Kash TL (2018) Fear extinction requires infralimbic cortex projections to the basolateral amygdala. Transl Psychiatry, 8, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumstein DT, Daniel JC, & Evans CS (2001) Yellow-Footed Rock-Wallaby Group Size Effects Reflect A Trade-Off. Ethology, 107, 655–664. [Google Scholar]
- Bourke CH & Neigh GN (2011) Behavioral effects of chronic adolescent stress are sustained and sexually dimorphic. Hormones and Behavior, 60, 112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce WT, Obradovic J, Bush NR, Stamperdahl J, Kim YS, & Adler N (2012) Social stratification, classroom climate, and the behavioral adaptation of kindergarten children. Proc. Natl. Acad. Sci. U.S.A, 109 Suppl 2, 17168–17173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boynton-Jarrett R, Wright RJ, Putnam FW, Hibert EL, Michels KB, Forman MR, & Rich-Edwards J (2013) Childhood Abuse and Age at Menarche. J Adolesc Health, 52, 241–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmeister SS, Jarvis ED, & Fernald RD (2005) Rapid Behavioral and Genomic Responses to Social Opportunity. PLoS Biology, 3, e363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoon GG & Tye KM (2015) Resolving the neural circuits of anxiety. Nat. Neurosci, 18, 1394–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campi KL, Jameson CE, & Trainor BC (2013) Sexual dimorphism in the brain of the monogamous california mouse (Peromyscus californicus). Brain, Behavior and Evolution, 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J-L, Covington HE, Friedman AK, Wilkinson MB, Walsh JJ, Cooper DC, Nestler EJ, & Han M-H (2010) Mesolimbic dopamine neurons in the brain reward circuit mediate susceptibility to social defeat and antidepressant action. J. Neurosci, 30, 16453–16458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carere C, Welink D, Drent PJ, Koolhaas JM, & Groothuis TGG (2001) Effect of social defeat in a territorial bird (Parus major) selected for different coping styles. Physiology & Behavior, 73, 427–433. [DOI] [PubMed] [Google Scholar]
- Cassano GB, Rossi NB, & Pini S (2002) Psychopharmacology of anxiety disorders. Dialogues Clin Neurosci, 4, 271–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Berke JD, & Maren S (2010) Single-Unit Activity in the Medial Prefrontal Cortex during Immediate and Delayed Extinction of Fear in Rats. PLOS ONE, 5, e11971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E, Langer DA, Raphaelson YE, & Matthews KA (2004) Socioeconomic status and health in adolescents: the role of stress interpretations. Child Dev, 75, 1039–1052. [DOI] [PubMed] [Google Scholar]
- Chen E & Matthews KA (2001) Cognitive appraisal biases: an approach to understanding the relation between socioeconomic status and cardiovascular reactivity in children. Ann Behav Med, 23, 101–111. [DOI] [PubMed] [Google Scholar]
- Chiba T, Kayahara T, & Nakano K (2001) Efferent projections of infralimbic and prelimbic areas of the medial prefrontal cortex in the Japanese monkey, Macaca fuscata. Brain Research, 888, 83–101. [DOI] [PubMed] [Google Scholar]
- Chung WC, De Vries GJ, & Swaab DF (2002) Sexual Differentiation of the Bed Nucleus of the Stria Terminalis in Humans May Extend into Adulthood | Journal of Neuroscience. J. Neurosci, 22, 1027–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clauss JA & Blackford JU (2012) Behavioral Inhibition and Risk for Developing Social Anxiety Disorder: A Meta-Analytic Study. Journal of the American Academy of Child & Adolescent Psychiatry, 51, 1066–1075.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colich NL, Platt JM, Keyes KM, Sumner JA, Allen NB, & McLaughlin KA (2019) Earlier age at menarche as a transdiagnostic mechanism linking childhood trauma with multiple forms of psychopathology in adolescent girls. Psychol Med, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF & Holmes A (2005) The ascent of mouse: advances in modelling human depression and anxiety. Nat Rev Drug Discov, 4, 775–790. [DOI] [PubMed] [Google Scholar]
- Cullinan WE, Herman JP, & Watson SJ (1993) Ventral subicular interaction with the hypothalamic paraventricular nucleus: Evidence for a relay in the bed nucleus of the stria terminalis. Journal of Comparative Neurology, 332, 1–20. [DOI] [PubMed] [Google Scholar]
- Cundiff JM, Smith TW, Baron CE, & Uchino BN (2016) Hierarchy and health: Physiological effects of interpersonal experiences associated with socioeconomic position. Health Psychol, 35, 356–365. [DOI] [PubMed] [Google Scholar]
- Dabrowska J, Hazra R, Guo J-D, Li C, DeWitt S, Xu J, Lombroso PJ, & Rainnie DG (2013) Striatal-Enriched Protein Tyrosine Phosphatase—STEPs Toward Understanding Chronic Stress-Induced Activation of Corticotrophin Releasing Factor Neurons in the Rat Bed Nucleus of the Stria Terminalis. Biological Psychiatry, Stress: Impact on Brain and Body, 74, 817–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel SE & Rainnie DG (2016) Stress modulation of opposing circuits in the bed nucleus of the stria terminalis. Neuropsychopharmacology, 41, 103–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey CG, Yücel M, & Allen NB (2008) The emergence of depression in adolescence: development of the prefrontal cortex and the representation of reward. Neurosci Biobehav Rev, 32, 1–19. [DOI] [PubMed] [Google Scholar]
- Davis M, Walker DL, Miles L, & Grillon C (2010) Phasic vs Sustained Fear in Rats and Humans: Role of the Extended Amygdala in Fear vs Anxiety. Neuropsychopharmacology, 35, 105–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Abril A, Segovia S, & Guillamon A (1987) The bed nucleus of the stria terminalis in the rat: regional sex differences controlled by gonadal steroids early after birth. Developmental Brain Research, 32, 295–300. [DOI] [PubMed] [Google Scholar]
- Delevich K, Thomas AW, & Wilbrecht L (2019) Adolescence and “late blooming” synapses of the prefrontal cortex. Cold Spring Harbor Symposia on Quantitative Biology, 83:37–43 [DOI] [PubMed] [Google Scholar]
- DiBenedictis BT, Nussbaum ER, Cheung HK, & Veenema AH (2017) Quantitative mapping reveals age and sex differences in vasopressin, but not oxytocin, immunoreactivity in the rat social behavior neural network. J. Comp. Neurol, 525, 2549–2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drzewiecki CM, Willing J, & Juraska JM (2016) Synaptic number changes in the medial prefrontal cortex across adolescence in male and female rats: A role for pubertal onset. Synapse, 70, 361–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque-Wilckens N, Steinman MQ, Busnelli M, Chini B, Yokoyama S, Pham M, Laredo SA, Hao R, Perkeybile AM, Minie VA, Tan PB, Bales KL, & Trainor BC (2018) Oxytocin Receptors in the Anteromedial Bed Nucleus of the Stria Terminalis Promote Stress-Induced Social Avoidance in Female California Mice. Biol Psychiatry, 83, 203–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckstein M, Scheele D, Weber KS, Stoffel-Wagner B, Maier W, & Hurlemann R (2014) Oxytocin facilitates the sensation of social stress. Hum Brain Mapp, 35, 4741–4750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekman Jan. Exposure and time use in willow tit flocks: the cost of subordination. Animal Behaviour. 1987; 35(2) 445–452. [Google Scholar]
- Ellis BJ, Figueredo AJ, Brumbach BH, & Schlomer GL (2009) Fundamental Dimensions of Environmental Risk: The Impact of Harsh versus Unpredictable Environments on the Evolution and Development of Life History Strategies. Hum Nat, 20, 204–268. [DOI] [PubMed] [Google Scholar]
- Fox AS, Shelton SE, Oakes TR, Davidson RJ, & Kalin NH (2008) Trait-Like Brain Activity during Adolescence Predicts Anxious Temperament in Primates. PLOS ONE, 3, e2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz M, McLean E, Tung J, Altmann J, & Alberts SC (2015) Self-organizing dominance hierarchies in a wild primate population. Proceedings of the Royal Society B: Biological Sciences, 282, 20151512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X, Taber-Thomas BC, & Pérez-Edgar K (2017) Frontolimbic functioning during threat-related attention: Relations to early behavioral inhibition and anxiety in children. Biological Psychology, 122, 98–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner WL, Pickett CL, Jefferis V, & Knowles M (2005) On the outside looking in: loneliness and social monitoring. Pers Soc Psychol Bull, 31, 1549–1560. [DOI] [PubMed] [Google Scholar]
- Gartlan JS (1968) Structure and function in primate society. Folia Primatol, 8, 89–120. [DOI] [PubMed] [Google Scholar]
- Gee DG, Gabard-Durnam LJ, Flannery J, Goff B, Humphreys KL, Telzer EH, Hare TA, Bookheimer SY, & Tottenham N (2013) Early developmental emergence of human amygdala-prefrontal connectivity after maternal deprivation. Proc. Natl. Acad. Sci. U.S.A, 110, 15638–15643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gegenhuber B & Tollkuhn J (2019) Signatures of sex: Sex differences in gene expression in the vertebrate brain. Wiley Interdisciplinary Reviews: Developmental Biology, 0, e348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Horenstein JA, Hariri AR, Sheu LK, Manuck SB, Matthews KA, & Cohen S (2008) Potential neural embedding of parental social standing. Soc Cogn Affect Neurosci, 3, 91–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginther AJ, Carlson AA, Ziegler TE, & Snowdon CT (2002) Neonatal and pubertal development in males of a cooperatively breeding primate, the cotton-top tamarin (Saguinus oedipus oedipus). Biol. Reprod, 66, 282–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg GD, Laman-Maharg A, Campi KL, Voigt H, Orr VN, Schaal L, & Trainor BC (2014) Sex differences in stress-induced social withdrawal: Role of brain derived neurotrophic factor in the bed nucleus of the stria terminalis. Frontiers in Behavioral Neuroscience, 7, 223 10.3389/fnbeh.2013.00223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruene TM, Roberts E, Thomas V, Ronzio A, & Shansky RM (2015) Sex-specific neuroanatomical correlates of fear expression in prefrontal-amygdala circuits. Biol. Psychiatry, 78, 186–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, McClure-Tone EB, Shiffrin ND, Pine DS, & Nelson EE (2009) Probing the neural correlates of anticipated peer evaluation in adolescence. Child Dev, 80, 1000–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han TM & De Vries GJ (2003) Organizational effects of testosterone, estradiol, and dihydrotestosterone on vasopressin mRNA expression in the bed nucleus of the stria terminalis. J. Neurobiology, 54, 502–510. [DOI] [PubMed] [Google Scholar]
- Henderson HA, Pine DS, & Fox NA (2015) Behavioral Inhibition and Developmental Risk: A Dual-Processing Perspective. Neuropsychopharmacology, 40, 207–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingworth SA, Burgess PM, & Whiteford HA (2010) Affective and anxiety disorders: prevalence, treatment and antidepressant medication use. Aust N Z J Psychiatry, 44, 513–519. [DOI] [PubMed] [Google Scholar]
- Holmes A, Fitzgerald PJ, MacPherson KP, DeBrouse L, Colacicco G, Flynn SM, Masneuf S, Pleil KE, Li C, Marcinkiewcz CA, Kash TL, Gunduz-Cinar O, & Camp M (2012) Chronic alcohol remodels prefrontal neurons and disrupts NMDAR-mediated fear extinction encoding. Nat Neurosci, 15, 1359–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostinar CE, Ross KM, Chan M, Chen E, & Miller GE (2017) Threat vigilance and socioeconomic disparities in metabolic health. Dev. Psychopathol, 29, 1721–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard DT, Blanchard DC, Yang M, Markham CM, Gervacio A, Chun-I L, & Blanchard RJ (2004) Development of defensive behavior and conditioning to cat odor in the rat. Physiology & Behavior, 80, 525–530. [DOI] [PubMed] [Google Scholar]
- Huhman Kim L. Social conflict models: Can they inform us about human psychopathology?. Hormones and Behavior. 2006; 50(4) 640–646. [DOI] [PubMed] [Google Scholar]
- Hurley KM, Herbert H, Moga MM, & Saper CB (1991) Efferent projections of the infralimbic cortex of the rat. Journal of Comparative Neurology, 308, 249–276. [DOI] [PubMed] [Google Scholar]
- Inderbitzen HM, Walters KS, & Bukowski AL (1997) The role of social anxiety in adolescent peer relations: differences among sociometric status groups and rejected subgroups. J Clin Child Psychol, 26, 338–348. [DOI] [PubMed] [Google Scholar]
- Iñiguez SD, Aubry A, Riggs LM, Alipio JB, Zanca RM, Flores-Ramirez FJ, Hernandez MA, Nieto SJ, Musheyev D, & Serrano PA (2016) Social defeat stress induces depression-like behavior and alters spine morphology in the hippocampus of adolescent male C57BL/6 mice. Neurobiology of Stress, 5, 54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iñiguez SD, Riggs LM, Nieto SJ, Dayrit G, Zamora NN, Shawhan KL, Cruz B, & Warren BL (2014) Social defeat stress induces a depression-like phenotype in adolescent male c57BL/6 mice. Stress, 17, 247–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ironside M, O’Shea J, Cowen PJ, & Harmer CJ (2016) Frontal Cortex Stimulation Reduces Vigilance to Threat: Implications for the Treatment of Depression and Anxiety. Biol. Psychiatry, 79, 823–830. [DOI] [PubMed] [Google Scholar]
- Jennings JH, Sparta DR, Stamatakis AM, Ung RL, Pleil KE, Kash TL, & Stuber GD (2013) Distinct extended amygdala circuits for divergent motivational states. Nature, 496, 224–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juntti SA, Tollkuhn J, Wu MV, Fraser EJ, Soderborg T, Tan S, Honda S-I, Harada N, & Shah NM (2010) The Androgen Receptor Governs the Execution, but Not Programming, of Male Sexual and Territorial Behaviors. Neuron, 66, 260–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan J, Reznick JS, & Snidman N (1987) The physiology and psychology of behavioral inhibition in children. Child Development, 58, 1459–1473. [PubMed] [Google Scholar]
- Kelley AM, Saunders AG, & Ophir AG (2018) Mechanistic substrates of a life history transition in male prairie voles: developmental plasticity in affiliation and aggression corresponds to nonapeptide neuronal function. Horm. Behav, 99, 14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Gee DG, Loucks RA, Davis FC, & Whalen PJ (2011) Anxiety dissociates dorsal and ventral medial prefrontal cortex functional connectivity with the amygdala at rest. Cereb. Cortex, 21, 1667–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama S & Kamimura S (1999) Lowered sperm motility in subordinate social status of mice. Physiol. Behav, 65, 665–669. [DOI] [PubMed] [Google Scholar]
- Kraus MW, Horberg EJ, Goetz JL, & Keltner D (2011) Social class rank, threat vigilance, and hostile reactivity. Pers Soc Psychol Bull, 37, 1376–1388. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Han M-H, Graham DL, Berton O, Renthal W, Russo SJ, LaPlant Q, Graham A, Lutter M, Lagace DC, Ghose S, Reister R, Tannous P, Green TA, Neve RL, Chakravarty S, Kumar A, Eisch AJ, Self DW, Lee FS, Tamminga CA, Cooper DC, Gershenfeld HK, & Nestler EJ (2007) Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell, 131, 391–404. [DOI] [PubMed] [Google Scholar]
- Lebow MA & Chen A (2016) Overshadowed by the amygdala: the bed nucleus of the stria terminalis emerges as key to psychiatric disorders. Mol. Psychiatry, 21, 450–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehner SR, Rutte C, & Taborsky M (2011) Rats Benefit from Winner and Loser Effects. Ethology, 117, 949–960. [Google Scholar]
- Lenroot RK & Giedd JN (2006) Brain development in children and adolescents: Insights from anatomical magnetic resonance imaging. Neuroscience & Biobehavioral Reviews, Methodological and Conceptual Advances in the Study of Brain-Behavior Dynamics: A Multivariate Lifespan Perspective, 30, 718–729. [DOI] [PubMed] [Google Scholar]
- Macdonald DW, Berdoy M, & Smith P (1995) Stability of Social Status in Wild Rats: Age and the Role of Settled Dominance. Behaviour, 132, 193–212. [Google Scholar]
- Margulis SW, Saltzman W, & Abbott DH (1995) Behavioral and hormonal changes in female naked mole-rats (Heterocephalus glaber) following removal of the breeding female from a colony. Horm Behav, 29, 227–247. [DOI] [PubMed] [Google Scholar]
- Marler CA & Trainor BC in press The challenge hypothesis revisited: focus on reproductive experience and neural mechanisms. Hormones and Behavior. [DOI] [PubMed] [Google Scholar]
- Maruska KP & Fernald RD (2018) Astatotilapia burtoni: A Model System for Analyzing the Neurobiology of Behavior. ACS Chem Neurosci, 9, 1951–1962. [DOI] [PubMed] [Google Scholar]
- McCann KE & Huhman KL (2012) The effect of escapable versus inescapable social defeat on conditioned defeat and social recognition in Syrian hamsters. Physiology & Behavior, 105, 493–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, Woolley CS, & Arnold AP (2017) Incorporating sex as a biological variable in neuroscience: what do we gain? Nat. Rev. Neurosci, 18, 707–708. [DOI] [PubMed] [Google Scholar]
- McElligott ZA, Klug JR, Nobis WP, Patel S, Grueter BA, Kash TL, & Winder DG (2010) Distinct forms of Gq-receptor-dependent plasticity of excitatory transmission in the BNST are differentially affected by stress. Proc. Natl. Acad. Sci. U.S.A, 107, 2271–2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendle J (2014) Why Puberty Matters for Psychopathology. Child Development Perspectives, 8, 218–222. [Google Scholar]
- Milad MR & Quirk GJ (2002) Neurons in medial prefrontal cortex signal memory for fear extinction. Nature, 420, 70–74. [DOI] [PubMed] [Google Scholar]
- Mogg K, Bradley BP, Miles F, & Dixon R (2004) Time course of attentional bias for threat scenes: Testing the vigilance-avoidance hypothesis. Cognition and Emotion, 18, 689–700. [Google Scholar]
- Morishita M, Maejima S, & Tsukahara S (2017) Gonadal Hormone-Dependent Sexual Differentiation of a Female-Biased Sexually Dimorphic Cell Group in the Principal Nucleus of the Bed Nucleus of the Stria Terminalis in Mice. Endocrinology, 158, 3512–3525. [DOI] [PubMed] [Google Scholar]
- Morrison KE, Curry DW, & Cooper MA (2012) Social status alters defeat-induced neural activation in Syrian hamsters. Neuroscience, 210, 168–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasanbuyan N, Yoshida M, Takayanagi Y, Inutsuka A, Nishimori K, Yamanaka A, & Onaka T (2018) Oxytocin-Oxytocin Receptor Systems Facilitate Social Defeat Posture in Male Mice. Endocrinology, 159, 763–775. [DOI] [PubMed] [Google Scholar]
- Neat FC, Taylor AC, & Huntingford FA (1998) Proximate costs of fighting in male cichlid fish: the role of injuries and energy metabolism. Animal Behaviour, 55, 875–882. [DOI] [PubMed] [Google Scholar]
- Newman EL, Covington HE, Suh J, Bicakci MB, Ressler KJ, DeBold JF, & Miczek KA (2019) Fighting females: Neural and behavioral consequences of social defeat stress in female mice. Biological Psychiatry,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noll JG, Trickett PK, Long JD, Negriff S, Susman EJ, Shalev I, Li JC, & Putnam FW (2017) Childhood Sexual Abuse and Early Timing of Puberty. J Adolesc Health, 60, 65–71. [DOI] [PubMed] [Google Scholar]
- O’Connell LA & Hofmann HA (2011) The Vertebrate mesolimbic reward system and social behavior network: A comparative synthesis. Journal of Comparative Neurology, 519, 3599–3639. [DOI] [PubMed] [Google Scholar]
- Oler JA, Fox AS, Shelton SE, Rogers J, Dyer TD, Davidson RJ, Shelledy W, Oakes TR, Blangero J, & Kalin NH (2010) Amygdalar and hippocampal substrates of anxious temperament differ in their heritability. Nature, 466, 864–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onyango PO, Gesquiere LR, Altmann J, & Alberts SC (2013) Puberty and dispersal in a wild primate population. Horm Behav, 64, 240–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyegbile TO & Marler CA (2005) Winning fights elevates testosterone levels in California mice and enhances future ability to win fights. Hormones and Behavior, 48, 259–267. [DOI] [PubMed] [Google Scholar]
- Padilla-Coreano N, Bolkan SS, Pierce GM, Blackman DR, Hardin WD, Garcia-Garcia AL, Spellman TJ, & Gordon JA (2016) Direct Ventral Hippocampal-Prefrontal Input Is Required for Anxiety-Related Neural Activity and Behavior. Neuron, 89, 857–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J & Moghaddam B (2017) Impact of anxiety on prefrontal cortex encoding of cognitive flexibility. Neuroscience, Cognitive Flexibility: Development, Disease, and Treatment, 345, 193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattwell SS, Liston C, Jing D, Ninan I, Yang RR, Witztum J, Murdock MH, Dincheva I, Bath KG, Casey BJ, Deisseroth K, & Lee FS (2016a) Dynamic changes in neural circuitry during adolescence are associated with persistent attenuation of fear memories. Nature Communications, 7, 11475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattwell SS, Liston C, Jing D, Ninan I, Yang RR, Witztum J, Murdock MH, Dincheva I, Bath KG, Casey BJ, Deisseroth K, & Lee FS (2016b) Dynamic changes in neural circuitry during adolescence are associated with persistent attenuation of fear memories. Nat Commun, 7, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul MJ, Probst CK, Brown LM, & de Vries GJ (2018) Dissociation of Puberty and Adolescent Social Development in a Seasonally Breeding Species. Current Biology, 28, 1116–1123.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peragine DE, Pokarowski M, Mendoza-Viveros L, Swift-Gallant A, Cheng H-YM, Bentley GE, & Holmes MM (2017) RFamide-related peptide-3 (RFRP-3) suppresses sexual maturation in a eusocial mammal. Proc. Natl. Acad. Sci. U.S.A, 114, 1207–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickett CL, & Gardner WL (2005) The social monitoring system: Enhanced sensitivity to social cues as an adaptive response to social exclusion In The social outcast: Ostracism, social exclusion, rejection, and bullying (pp. 213–226). Williams KD, Forgas JP, Von Hippel W (Eds) New York, NY: Psychology Press. [Google Scholar]
- Piekarski DJ, Boivin JR, & Wilbrecht L (2017) Ovarian Hormones Organize the Maturation of Inhibitory Neurotransmission in the Frontal Cortex at Puberty Onset in Female Mice. Curr. Biol, 27, 1735–1745.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piekarski DJ, Johnson CM, Boivin JR, Thomas AW, Lin WC, Delevich K, M Galarce E, & Wilbrecht L (2017) Does puberty mark a transition in sensitive periods for plasticity in the associative neocortex? Brain Res., 1654, 123–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price RB, Allen KB, Silk JS, Ladouceur CD, Ryan ND, Dahl RE, Forbes EE, & Siegle GJ (2016) Vigilance in the laboratory predicts avoidance in the real world: A dimensional analysis of neural, behavioral, and ecological momentary data in anxious youth. Developmental Cognitive Neuroscience, 19, 128–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Gosselink KL, & Sawchenko PE (2009) A Discrete GABAergic Relay Mediates Medial Prefrontal Cortical Inhibition of the Neuroendocrine Stress Response. J. Neurosci, 29, 7330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo RD (2017) The impact of stress on the structure of the adolescent brain: Implications for adolescent mental health. Brain Res, 1654, 185–191. [DOI] [PubMed] [Google Scholar]
- Room P, Russchen FT, Groenewegen HJ, & Lohman AHM (1985) Efferent connections of the prelimbic (area 32) and the infralimbic (area 25) cortices: An anterograde tracing study in the cat [WWW Document]. Journal of Comparative Neurology,. URL https://onlinelibrary.wiley.com/doi/abs/10.1002/cne.902420104 [DOI] [PubMed] [Google Scholar]
- Rowson SA, Bekhbat M, Kelly SD, Binder EB, Hyer MM, Shaw G, Bent MA, Hodes G, Tharp G, Weinshenker D, Qin Z, & Neigh GN (2019) Chronic adolescent stress sex-specifically alters the hippocampal transcriptome in adulthood. Neuropsychopharmacology, 44, 1207–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin KH, Coplan RJ, & Bowker JC (2009) Social Withdrawal in Childhood. Annu Rev Psychol, 60, 141–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saalfield J & Spear L (2019) Fos activation patterns related to acute ethanol and conditioned taste aversion in adolescent and adult rats. Alcohol,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz KM & Sisk CL (2016) The organizing actions of adolescent gonadal steroid hormones on brain and behavioral development. Neurosci Biobehav Rev, 70, 148–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamay-Tsoory S & Abu-Akel A (2016) The social salience hypothesis of oxytocin. Biol Psychiatry, 79, 197–202. [DOI] [PubMed] [Google Scholar]
- Shansky RM & Woolley CS (2016) Considering Sex as a Biological Variable Will Be Valuable for Neuroscience Research. J. Neurosci, 36, 11817–11822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd SV, Deaner RO, & Platt ML (2006) Social status gates social attention in monkeys. Current Biology, 16, R119–R120. [DOI] [PubMed] [Google Scholar]
- Shively CA, Laber-Laird K, & Anton RF (1997) Behavior and physiology of social stress and depression in female cynomolgus monkeys. Biological Psychiatry, 41, 871–882. [DOI] [PubMed] [Google Scholar]
- Sierra-Mercado D, Padilla-Coreano N, & Quirk GJ (2011) Dissociable Roles of Prelimbic and Infralimbic Cortices, Ventral Hippocampus, and Basolateral Amygdala in the Expression and Extinction of Conditioned Fear. Neuropsychopharmacol, 36, 529–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvers JA, Goff B, Gabard-Durnam LJ, Gee DG, Fareri DS, Caldera C, & Tottenham N (2017) Vigilance, the Amygdala, and Anxiety in Youths with a History of Institutional Care. Biol Psychiatry Cogn Neurosci Neuroimaging, 2, 493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman MQ, Duque-Wilckens N, Greenberg GD, Hao R, Campi KL, Laredo SA, Laman-Maharg A, Manning CE, Doig IE, Lopez EM, Walch K, Bales KL, & Trainor BC (2016) Sex-Specific Effects of Stress on Oxytocin Neurons Correspond With Responses to Intranasal Oxytocin. Biological Psychiatry, 80, 406–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman MQ, Duque-Wilckens N, & Trainor BC (2019) Complementary Neural Circuits for Divergent Effects of Oxytocin: Social Approach Versus Social Anxiety. Biol. Psychiatry, 85, 792–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman MQ & Trainor BC (2017) Sex differences in the effects of social defeat on brain and behavior in the California mouse: Insights from a monogamous rodent. Seminars in Cell and Developmental Biology, 61, 92–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner JA, Colich NL, Uddin M, Armstrong D, & McLaughlin KA (2019) Early Experiences of Threat, but Not Deprivation, Are Associated With Accelerated Biological Aging in Children and Adolescents. Biol. Psychiatry, 85, 268–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer EH, Mogg K, Bradley BP, Mai X, Ernst M, Pine DS, & Monk CS (2008) Relationship between trait anxiety, prefrontal cortex, and attention bias to angry faces in children and adolescents. Biological Psychology, 79, 216–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trainor BC, Bird IM, & Marler CA (2004) Opposing hormonal mechanisms of aggression revealed through short-lived testosterone manipulations and multiple winning experiences. Hormones and Behavior, 45, 115–121. [DOI] [PubMed] [Google Scholar]
- Trainor BC, Pride MC, Landeros RV, Knoblauch NW, Takahashi EY, Silva AL, & Crean KK (2011) Sex differences in social interaction behavior following social defeat stress in the monogamous California mouse (peromyscus californicus). PLoS ONE, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trainor BC, Takahashi EY, Campi KL, Florez SA, Greenberg GD, Laman-Maharg A, Laredo SA, Orr VN, Silva AL, & Steinman MQ (2013) Sex differences in stress-induced social withdrawal: Independence from adult gonadal hormones and inhibition of female phenotype by corncob bedding. Hormones and Behavior, 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hoorn J, Shablack H, Lindquist KA, & Telzer EH (2019) Incorporating the social context into neurocognitive models of adolescent decision-making: A neuroimaging meta-analysis. Neurosci Biobehav Rev, 101, 129–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhalst J, Gibb BE, & Prinstein MJ (2017) Lonely adolescents exhibit heightened sensitivity for facial cues of emotion. Cogn Emot, 31, 377–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Vogt BA, & Spear LP (2013) Social context induces two unique patterns of c-Fos expression in adolescent and adult rats. Dev Psychobiol, 55, 684–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP (2004) Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse, 51, 32–58. [DOI] [PubMed] [Google Scholar]
- Vijayakumar N, Op de Macks Z, Shirtcliff EA, & Pfeifer JH (2018) Puberty and the human brain: Insights into adolescent development. Neurosci Biobehav Rev, 92, 417–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DL & Davis M (1997) Double dissociation between the involvement of the bed nucleus of the stria terminalis and the central nucleus of the amygdala in startle increases produced by conditioned versus unconditioned fear. J. Neurosci, 17, 9375–9383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DM, Bell MR, Flores C, Gulley JM, Willing J, & Paul MJ (2017) Adolescence and Reward: Making Sense of Neural and Behavioral Changes Amid the Chaos. J. Neurosci, 37, 10855–10866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Zhu J, Zhu H, Zhang Q, Lin Z, & Hu H (2011) Bidirectional Control of Social Hierarchy by Synaptic Efficacy in Medial Prefrontal Cortex. Science, 334, 693–697. [DOI] [PubMed] [Google Scholar]
- Wang Z, Moody K, Newman JD, & Insel TR (1997) Vasopressin and oxytocin immunoreactive neurons and fibers in the forebrain of male and female common marmosets (Callithrix jacchus). Synapse, 27, 14–25. [DOI] [PubMed] [Google Scholar]
- Wesselhoeft R, Pedersen CB, Mortensen PB, Mors O, & Bilenberg N (2015) Gender-age interaction in incidence rates of childhood emotional disorders. Psychol Med, 45, 829–839. [DOI] [PubMed] [Google Scholar]
- Wierenga LM, Bos MGN, Schreuders E, Vd Kamp F, Peper JS, Tamnes CK, & Crone EA (2018) Unraveling age, puberty and testosterone effects on subcortical brain development across adolescence. Psychoneuroendocrinology, 91, 105–114. [DOI] [PubMed] [Google Scholar]
- Williams AV, Laman-Maharg A, Armstrong CV, Ramos-Maciel S, Minie VA, & Trainor BC (2018) Acute inhibition of kappa opioid receptors before stress blocks depression-like behaviors in California mice. Prog. Neuropsychopharmacol. Biol. Psychiatry, 86, 166–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson CM, Klein IS, Lee W, & Curley JP (2019) Immediate early gene activation throughout the brain is associated with dynamic changes in social context. Soc Neurosci, 14, 253–265. [DOI] [PubMed] [Google Scholar]
- Williamson CM, Lee W, Decasien AR, Lanham A, Romeo RD, & Curley JP (2019) Social hierarchy position in female mice is associated with plasma corticosterone levels and hypothalamic gene expression. bioRxiv, 529131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson CM, Romeo RD, & Curley JP (2017) Dynamic changes in social dominance and mPOA GnRH expression in male mice following social opportunity. Horm. Behav, 87, 80–88. [DOI] [PubMed] [Google Scholar]
- Wingfield JC & Wada M (1989) Changes in plasma levels of testosterone during male-male interactions in the song sparrow, Melospiza melodia: time course and specificity of response. J Comp Physiol A, 166, 189–194. [Google Scholar]
- Yassa MA, Hazlett RL, Stark CEL, & Hoehn-Saric R (2012) Functional MRI of the amygdala and bed nucleus of the stria terminalis during conditions of uncertainty in generalized anxiety disorder. J Psychiatr Res, 46, 1045–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S, Holmes MM, Forger NG, Goldman BD, Lovern MB, Caraty A, Kalló I, Faulkes CG, & Coen CW (2013) Socially regulated reproductive development: Analysis of GnRH-1 and kisspeptin neuronal systems in cooperatively breeding naked mole-rats (Heterocephalus glaber). Journal of Comparative Neurology, 521, 3003–3029. [DOI] [PubMed] [Google Scholar]