Abstract
Objective:
To address the independent role of peptidylarginine deiminase type 2 and 4 (PAD2 and PAD4, respectively) in generating rheumatoid arthritis (RA) autoantigens by using a system that mimics intracellular citrullination in the RA joint.
Methods:
293T cells expressing PAD2, PAD4 or mock transfected cells were used as targets in cytotoxic assays using lymphokine-activated killer cells, cytotoxic YT cell granule contents, or purified human perforin. Protein citrullination and autoantigen production were determined by immunoblotting using the anti-modified citrulline-Senshu method and RA sera (n=30), respectively.
Results:
RA sera recognized at least three categories of autoantigens in PAD-expressing target cells killed by the cytotoxic lymphocyte granule–induced death pathway. These include autoantigens targeted in their native form, as citrullinated antigens, and antigens cleaved by cytotoxic proteases (e.g. granzymes). Interestingly, although target cells expressing PAD2 or PAD4 showed prominent hypercitrullination of a broad range of proteins during cytotoxic granule-induced cell damage, autoantibodies in RA only targeted a very limited number of antigens in hypercitrullinated cells. Furthermore, RA sera also showed distinct specificities to autoantigens generated by PAD2 or PAD4.
Conclusion:
The cytotoxic granule-induced death pathway has the capacity to modify antigens by inducing hypercitrullination and antigen cleavage in target cells. Interestingly, among a large number of citrullinated proteins generated by PAD2 and PAD4 in cells, only few of these proteins are likely involved in the production of autoantibodies in RA.
Introduction
The finding that a significant number of patients with rheumatoid arthritis (RA) have antibodies to citrullinated proteins (known as ACPAs) has fueled the notion that dysregulated citrullination is important for RA pathogenesis (1). This hypothesis has sparked interest in understanding the mechanisms that drive citrullination in RA with the goal of identifying pathogenic pathways and new therapeutic targets in this disease. Citrullination is the enzymatic deimination of arginine residues to citrulline (1), mediated by the peptidylarginine deiminases (PADs). In particular, the finding that PAD2 and PAD4 are detected in rheumatoid synovial tissue and fluid has suggested that these enzymes are responsible for generating the prominent citrullination found in the RA joint (2). Moreover, it has focused interest on understanding the independent role of these PADs in the production of citrullinated antigens targeted in RA. Using recombinant enzymes, initial studies have suggested that PAD2 and PAD4 have distinct specificity and efficiency in generating citrullinated RA autoantigen (3, 4), and in generating citrullinated epitopes targeted by RA autoantibodies (5). Nevertheless, further studies have suggested that despite these potential differences, both enzymes citrullinate similar substrates leading to similar recognition by RA autoantibodies (6).
The study of PADs in vitro, however, has potential caveats that may affect autoantigen citrullination. In contrast to citrullination in cells that is efficiently activated under physiological calcium and redox conditions (3, 7, 8), in vitro citrullination in extracellular fluids (9, 10), using purified components (3, 4, 6), or PADs released from cells (9, 11), requires the addition of reducing agents (dithiothreitol or reduced glutathione) and/or supraphysiological amounts of calcium (2–10 mM) (3, 4, 6, 9, 10), which do not co-exist in vivo. Under these artificial conditions in which recombinant PADs are hyperactivated and the substrates partially denatured by reducing agents, it is possible that stringent conditions required for PAD specificity are affected. Similarly, proteins may be aberrantly citrullinated, potentially producing epitopes that may not exist in citrullinated proteins found in the RA joint.
Synovial fluid cells from patients with RA contain a unique pattern of citrullination that includes proteins spanning the range of molecular weights, termed hypercitrullination (7). This process is reproduced during cell death induced by killer cells (via perforin) and the membrane attack complex (MAC) of complement, which are membranolytic pathways active in the RA joint and of importance in RA pathogenesis (7). To address the independent role of PAD2 and PAD4 in generating intracellular RA autoantigens, we developed a system that mimics hypercitrullination in the RA joint by using perforin-induced cell damage and target cells expressing PAD2 or PAD4.
Material and Methods
RA serum.
Sera from 30 patients with ACPA-positive RA were obtained from a convenience cohort. All individuals provided informed consent as approved by the Johns Hopkins Institutional Review Board.
Cytotoxic assays and immunoblot analysis.
Detailed descriptions are available in the online supplementary methods.
Results
PAD2 and PAD4 generate distinct patterns of cellular hypercitrullination in response to cytotoxic cell death.
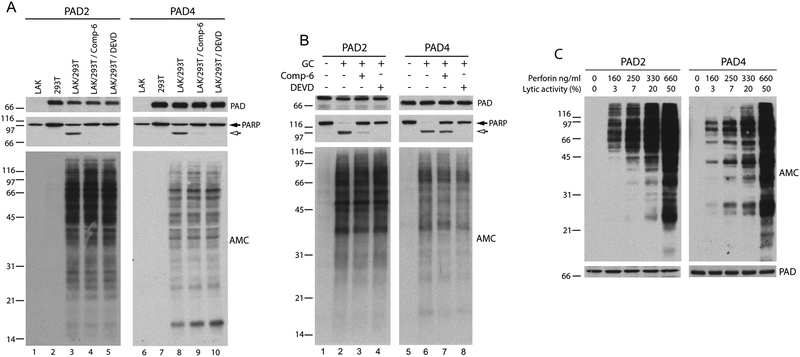
To define independent patterns of cellular hypercitrullination induced by PAD2 and PAD4 in response to cytotoxic lymphocyte–induced cell death, 293T cells, which are ordinarily PAD-negative (7), were transfected to express equivalent amounts of PAD2 or PAD4 and used as targets in cytotoxic assays. Similar to previous studies using neutrophils that endogenously express PADs (7), 293T cells expressing PADs exhibited prominent hypercitrullination during cell death induced by lymphokine-activated killer (LAK) cells (Figure 1A, lower panel, lanes 3 and 8). In contrast to primary cells, however, this approach offers the unique opportunity to express individual PADs and study their distinct cellular citrullination activity in response to effector pathways found in the RA joint. Indeed, different efficiencies and patterns of citrullination were observed depending on the PAD isoform activated in the target cells. Activation of PAD2 induced the most prominent citrullination, with strong preference for substrates above 31kDa (Figure 1A, lower panel, lane 3). In contrast, activation of PAD4 induced a less prominent but more widespread pattern of substrate citrullination, targeting molecules across a broader range of molecular weights (Figure 1A, lower panel, lane 8).
Figure 1.
Cytotoxic lymphocyte granule-mediated cell death and perforin induce hypercitrullination in target cells expressing PAD2 or PAD4. (A and B) 293T cells expressing PAD2 (lanes 2–5 in A and 1–4 in B) or PAD4 (lanes 7–10 in A and 5–8 in B) were pre-incubated in the absence or presence of inhibitors of granzyme B [compound 6 (Comp-6)] or effector caspases (z-DEVD-FMK), followed by co-incubation in the absence or presence of LAK cells (A) or YT granule contents (GC) (B). In A, LAK cells were incubated alone as controls (lanes 1 and 6). After terminating the reactions, the samples were analyzed by immunoblotting using anti-PAD2, anti-PAD4 (upper panel), and anti-PARP (middle panel) antibodies. Global citrullination was detected by anti-modified citrulline (AMC) immunoblotting (lower panel). Filled and unfilled arrows denote intact PARP and its apoptotic fragment, respectively. (C) 293T cells expressing PAD2 or PAD4 were incubated in the presence of increasing amounts of human purified perforin. The lytic activity of each perforin concentration is indicated. After terminating the reactions, the samples were electrophoresed and the proteins were visualized by immunoblotting using AMC, anti-PAD2 (left panel) or anti-PAD4 (right panel) antibodies. The experiments were performed on at least two separate occasions, with similar results.
Since hypercitrullination induced in neutrophils killed by LAKs is independent of apoptosis and driven by perforin (7), killing assays were performed in the presence of cell permeable inhibitors of granzyme B (compound-6) or effector caspases (z-DEVD-FMK), which efficiently prevent cytotoxic cell-mediated apoptosis (12, 13). While the inhibitors blocked the apoptosis-induced cleavage of poly(ADP-ribose) polymerase 1 (PARP) (Figure 1A, middle panel, lanes 4–5 and 9–10), neither inhibitor affected PAD2- or PAD4-mediated hypercitrullination induced by cytotoxic cells (Figure 1A, lower panel, lanes 4–5 and 9–10). Similar results were obtained using purified granule contents from the cytotoxic cell line YT (13), which contain perforin and granzyme B, confirming that the induction of hypercitrullination in target cells is mediated through the cytotoxic lymphocyte granule pathway, but independent of granzyme B or caspases (Figure 1B).
Since cytotoxic cells kill target cells by apoptosis rather than lysis, sublytic concentrations of the pore forming protein perforin (i.e. defined as 5 to < 30% induction of cell lysis) are thought to be physiologically relevant (14). Because sublytic concentrations of perforin vary by cell type and perforin preparation, we defined lysis conditions by incubating PAD-expressing 293T cells with increasing amounts of purified perforin from sublytic (3–20% lysis) to lytic (50% lysis) concentrations (Figure 1C). Strikingly, even the lowest sublytic amount of perforin tested (equivalent to 3% lysis) was sufficient to initiate protein citrullination, a process that became more prominent with increasing perforin concentrations (generating 7–20% lysis). At lytic concentrations (i.e. 50% lysis), perforin induced massive citrullination. Physiologically relevant sublytic amounts of perforin are therefore sufficient to induce rapid and prominent activation of PAD2 and PAD4 in target cells. Moreover, the data supports the use of PAD-expressing 293T cells as a viable system to dissect the individual role of PAD isoforms in the generation of hypercitrullinated proteins caused by cytotoxic cell damage.
Autoantibodies in RA target a limited number of antigens in hypercitrullinated cells.
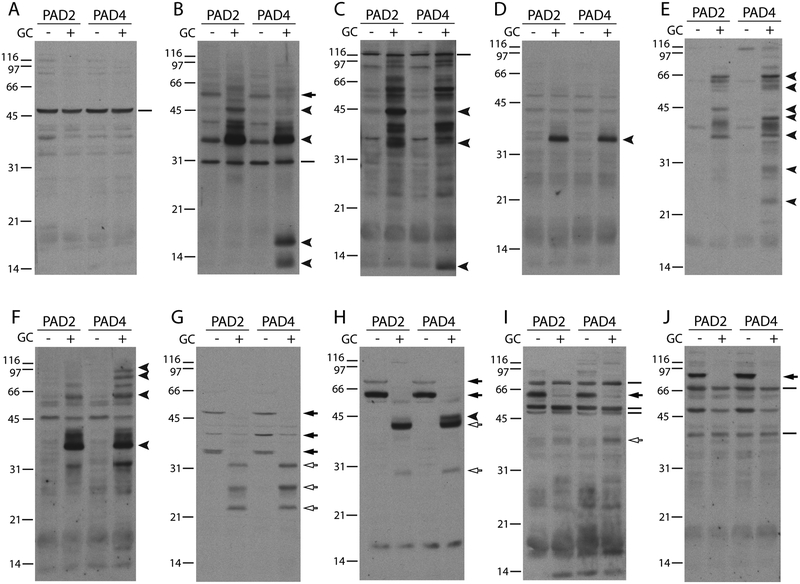
During cellular hypercitrullination induced by membranolytic damage, several dozen proteins are modified by the PAD enzymes (7, 8). However, whether autoantibodies exist for each citrullinated molecule or whether only few citrullinated antigens are targeted in RA is unclear. To define the spectrum of citrullinated antigens targeted by autoantibodies in hypercitrullinated cells, we used immunoblotting to screen RA sera (n = 30) for antibodies to antigens present in lysates from PAD-expressing 293T cells exposed to cytotoxic granule contents. Unlike cytotoxic assays using LAK cells, this approach allowed for the study of cytotoxic lymphocyte granule-induced hypercitrullination without the protein background from the killer cells. Surprisingly, despite the extensive hypercitrullination observed in these cells following treatment with granule contents (Figure 1B), only a limited number of these proteins were recognized by RA patient autoantibodies (Figure 2). Although the overall pattern of antigen recognition was unique between individuals, the autoantigens appeared to fall into three different categories with some being targeted by multiple different RA sera (Figure 2). The first group of antigens includes those whose immunoreactivity was not affected by target cell killing, possibly denoting autoantigens targeted in their native form (Figure 2A–C and I–J). The second subset includes antigens whose recognition is enhanced by PAD activation in killed cells, likely representing citrullinated autoantigens targeted by ACPAs (Figure 2B–F and H). In the third subset, autoantibody reactivity was more consistent with binding to antigens cleaved by proteases present in the cytotoxic granules (i.e. granzymes), which is marked by loss of a higher molecular weight band in unkilled cells and reciprocal recognition of smaller molecular weight bands in killed cells that correspond to cleavage fragments (Figure 2G–J). Thus, while the novel patterns of immunoreactivity might be primarily attributed to citrullination, it is likely that the complex patterns of antigens detected by RA sera result from the combination of citrullination and protein cleavage.
Figure 2.
Cytotoxic granule-mediated target cell death induces novel patterns of RA autoantigens in cells expressing PAD2 and PAD4. (A-J) 293T cells expressing PAD2 or PAD4 were incubated in the presence (+) or absence (–) of cytotoxic granule contents (GC). After 3 hrs at 37°C, the samples were electrophoresed and immunoblotted using human RA sera. Each panel shows the analysis of one individual RA serum. Solid lines mark antigens that did not change upon target cell killing, filled arrowheads denote antigens present only in hypercitrullinated dying cells, filled arrows mark antigens detected in control cells that disappeared following killing with generation of reciprocal antigen fragments of lower molecular weight (unfilled arrows). The data shown are representative from the analysis of 30 individual RA serum.
PAD2 and PAD4 generate distinct patterns of citrullinated autoantigens.
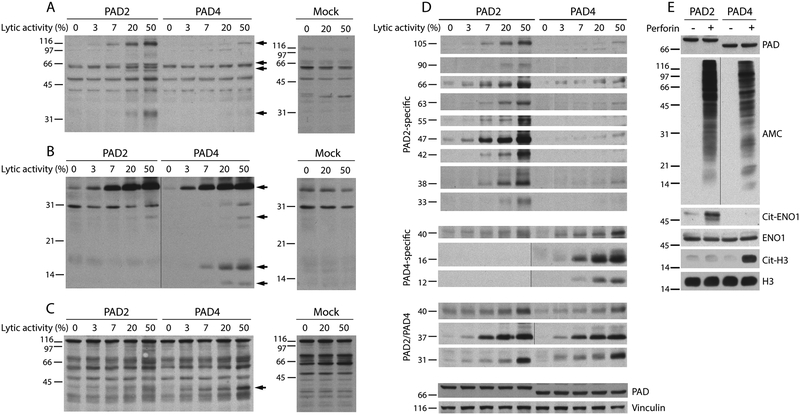
To ensure that novel autoantigens would only be generated as result of hyperactivation of PADs, but not by proteolytic cleavage, RA sera were further screened against lysates from PAD-expressing 293T cells exposed to increasing amounts of purified perforin. For this analysis, we selected RA the sera that recognized patterns compatible with protein citrullination, like those shown in Figure 2B–F. Similar to cells killed with cytotoxic granule contents (Figure 2), we observed recognition of a limited number of autoantigens by RA sera (Figure 3A–C and supplementary figure 1) despite the presence of global hypercitrullination in the target cells (Figure 1C).
Figure 3.
PAD2 and PAD4 generate distinct patterns of citrullinated autoantigens. 293T cells expressing PAD2, PAD4 or mock transfected cells were incubated with increasing amounts perforin (the lytic activity is indicated). After 3 hrs at 37°C, the samples were electrophoresed and immunoblotted using human RA sera. (A-C) The filled arrows mark antigens generated during cellular hypercitrullination by PAD2 and/or PAD4. The data shown are from three representative RA sera out of the 12 individual RA sera tested. (D) Antigens generated by PAD2 or PAD4 during perforin-induced hypercitrullination in panels A-C and in supplementary Figure 1 were arranged according to their molecular weight and PAD specificity. The data summarize patterns of antigen recognition by nine individual RA sera. Detection of PADs and vinculin was used as loading controls. (E) 293T cells expressing PAD2 or PAD4 were incubated in the presence (+) or absence (–) of lytic amounts of perforin (50% lysis). After 3 hrs at 37°C, native and citrullinated histone H3 (H3 and cit-H3, respectively) were detected by immunoblotting and α-enolase (ENO1) was purified by immunoprecipitation (IP). The purified immune complexes were divided in two and analyzed by immunoblotting. Anti-ENO1 antibodies (also used for IP) and mouse anti-rabbit IgG light chain were used to detect ENO1. Citrullinated ENO1 (Cit-ENO1) was detected by AMC. PAD expression and global citrullination were visualized by immunoblotting using anti-PAD2, anti-PAD4 and AMC antibodies. The black vertical line marks panels in which intervening lanes containing irrelevant data was spliced out.
To gain further insights into the patterns of RA autoantigens generated by PAD2 and PAD4, individual antigens detected by RA sera (Figure 3A–C and supplementary Figure 1) were classified according to their molecular weight and PAD specificity (Figure 3D). Three clear patterns of autoantigens were noted: (i) antigens that were only generated by PAD2, (ii) antigens generated only by PAD4, and (iii) antigens that were generated by both PAD2 and PAD4. In this regard, it is intriguing that some RA sera only targeted antigens generated by PAD2 (Figure 3A and supplementary Figure 1A and B) or by PAD4 (Figure 3C). This specificity is not explained by differences in the activation of the PAD isoforms, because we also found sera that similarly detected antigens in both PAD2 and PAD4 expressing cells (Figure 3B and supplementary figure 1C–F). Importantly, similar patterns of antigen recognition were not reproduced using mock-transfected cells exposed to perforin (Figure 3A–C and supplementary Figure 1), which confirmed that the production of autoantigens was fully dependent on PAD activation.
In addition, we addressed PAD specificity against two important citrullinated autoantigens in RA, α-enolase (ENO1) and histone H3 (Figure 3E). In regard to PAD specificity, citrullination of ENO1 or histone H3 was specific for cells expressing PAD2 or PAD4, respectively (Figure 3E). These data importantly contrast with the finding that purified ENO1 and histone H3 are citrullinated in vitro by both PADs (6), underscoring fundamental differences between cellular and in vitro citrullination using purified proteins.
Discussion
Citrullination is a normal process across multiple tissues in humans. In a recent study, citrullination was detected in more than 200 proteins in different healthy human tissues, including proteins that are well characterized targets of autoantibodies in RA (i.e. citrullinated fibrinogen alpha chain, collagen, and vimentin) (15). Considering that citrullination is a physiologic process, it is unclear why this modification becomes a target of an abnormal immune response in RA, and why antibodies to citrullinated proteins are associated with joint damage while sparing other organ systems highly enriched in citrullinated proteins, such as the nervous and digestive systems, among many others (15). In this study, we found that not all citrullinated proteins generated by PADs are recognized by autoantibodies in RA. Instead, RA sera target a very limited number of proteins across a broad spectrum of cellular antigens modified by PADs. This unique specificity of ACPAs may explain why these antibodies have no effector function on other tissues containing citrullinated proteins, and suggests that the specific targets of ACPAs are likely generated and enriched in target tissues in RA.
The data also support the idea that citrullination is not the only determinant of antigen recognition by RA antibodies. This must be the case since, otherwise, every citrullinated protein in hypercitrullinated cells would be similarly detected by RA sera. Using synthetic citrullinated peptides, in vitro citrullinated purified proteins and recombinant monoclonal ACPAs, a recent study suggested that antigen recognition by ACPAs is dependent on amino acid motifs containing citrulline residues rather than the more extended tertiary protein structure (16). If these motifs are only found in a limited number of citrullinated proteins, this model could explain, at the molecular level, how only a restricted set of antigens in hypercitrullinated cells is recognized by antibodies in RA. In this scenario, however, it may also be expected that ACPA recognition of unique motifs shared by few citrullinated proteins should generate homogeneous patterns of antigen detection among patients with RA. Intriguingly, we found a broad diversity of antigen recognition by RA sera. Some sera detected multiple bands (Figure 3A–B and supplementary Figure 1A, B and F), while others were specific for a single antigen generated by PADs (Figure 3C and supplementary Figure 1C–E). These findings are difficult to explain by a single model in which common citrullinated motifs are targeted by ACPAs. Instead, the data suggest the existence of RA antibodies with restricted macromolecular specificity to antigens generated by PADs in cells.
Since PAD2 mainly has a cytoplasmic distribution while PAD4 is predominantly nuclear (17), distinct patterns of citrullination generated in cells may be explained by substrate accessibility. Nevertheless, the finding that RA sera can clearly distinguish between cellular antigens generated by PAD2 and PAD4 raises interesting mechanistic questions about whether there are distinct roles for these enzymes in RA pathogenesis. Despite the observed robust hypercitrullination induced using lytic amounts of perforin in cells expressing these enzymes (Figure 1C), we identified RA sera that only detected antigens generated either by activation of PAD2 (Figure 3A and supplementary Figure 1A and B) or PAD4 (Figure 3C). These findings are not adequately explained by current models of citrullination in RA, which focus on non-specific citrullination of antigens by PADs and high cross-reactivity among ACPAs (6, 16). Instead, these data suggest that although citrullination is a common target of the immune response in RA, it is possible that different mechanisms may define the set of citrullinated antigens targeted by individual patients with this disease. Thus, depending on genetic and environmental factors, patients with RA may generate antibodies specifically targeting one or more antigens generated by PAD2, PAD4, or both, likely from a pre-determined group of potentially immunogenic citrullinated proteins. This set of proteins may correspond to novel non-physiologic targets of PADs that only become citrullinated under pathological conditions in RA target tissues (1, 7, 8).
Unexpectedly, the data also revealed that similar to other systemic autoimmune diseases (13), there is a set of autoantigens in RA that are modified through cleavage by killer proteases from cytotoxic cells. Indeed, a recent study demonstrated that granzyme B-mediated cleavage of PAD4 increased its recognition by CD4+ T cells from patients with RA (18). This finding reinforces the importance of posttranslational modifications in the lack of tolerance to autoantigens in RA and suggests that the cytotoxic-lymphocyte granule pathway may generate autoantigens by different mechanism, such as citrullination and proteolysis.
In summary, these data show that antibodies in RA target a limited number of proteins in cytotoxic granule-induced hypercitrullinated cells, with marked heterogeneity in antigen specificity among individual patients with RA. Moreover, although PAD2 and PAD4 both have the capacity to generate citrullinated autoantigens, the immune response in some RA patients can distinguish between antigens generated by each enzyme. Interestingly, while these studies are limited to intracellular antigens, it is important to note that ordinarily PADs have limited activity in extracellular fluids (9, 10), suggesting that significant citrullination of released intracellular antigens is unlikely to occur extracellularly. Thus, hypercitrullinated proteins from dying cells are likely the major source of intracellular citrullinated antigens found in the RA joint. This study highlights potential discrepancies in the study of citrullinated autoantigens using in vitro versus cellular approaches and underscores the need for physiologic strategies to study the antigenic drivers of the ACPA response in RA.
Supplementary Material
Grant Support:
Funding for this project was provided by the Jerome L. Greene Foundation and National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) at the National Institutes of Health (NIH) [grant number R01 AR069569]. The content of this paper is solely the responsibility of the author and does not represent the official views of the NIAMS or the NIH.
Financial Interests:
ED and FA are authors on licensed patent no. 8,975,033, entitled “Human autoantibodies specific for PAD3 which are cross-reactive with PAD4 and their use in the diagnosis and treatment of rheumatoid arthritis and related diseases” and on provisional patient no. 62/481,158 entitled “Anti-PAD2 antibody for treating and evaluating rheumatoid arthritis”. FA serves as consultant for Bristol-Myers Squibb, has received a grant from Medimmune, and personal fees from Celgene, outside of this submitted work. ED has received a grant from Pfizer, Celgene, and Medimmune and personal fees from Celgene, outside of this submitted work. The remaining authors declare no competing interests.
References
- 1.Darrah E, Andrade F. Rheumatoid arthritis and citrullination. Curr Opin Rheumatol. 2018;30:72–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Foulquier C, Sebbag M, Clavel C, Chapuy-Regaud S, Al BR, Mechin MC, et al. Peptidyl arginine deiminase type 2 (PAD-2) and PAD-4 but not PAD-1, PAD-3, and PAD-6 are expressed in rheumatoid arthritis synovium in close association with tissue inflammation. Arthritis Rheum. 2007;56:3541–53. [DOI] [PubMed] [Google Scholar]
- 3.Darrah E, Rosen A, Giles JT, Andrade F. Peptidylarginine deiminase 2, 3 and 4 have distinct specificities against cellular substrates: novel insights into autoantigen selection in rheumatoid arthritis. Ann Rheum Dis. 2012;71:92–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Assohou-Luty C, Raijmakers R, Benckhuijsen WE, Stammen-Vogelzangs J, de RA, van Veelen PA, et al. The human peptidylarginine deiminases type 2 and type 4 have distinct substrate specificities. Biochim Biophys Acta. 2014;1844:829–36. [DOI] [PubMed] [Google Scholar]
- 5.Blachere NE, Parveen S, Frank MO, Dill BD, Molina H, Orange DE. High-Titer Rheumatoid Arthritis Antibodies Preferentially Bind Fibrinogen Citrullinated by Peptidylarginine Deiminase 4. Arthritis Rheumatol. 2017;69:986–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Damgaard D, Bawadekar M, Senolt L, Stensballe A, Shelef MA, Nielsen CH. Relative efficiencies of peptidylarginine deiminase 2 and 4 in generating target sites for anti-citrullinated protein antibodies in fibrinogen, alpha-enolase and histone H3. PLoS One. 2018;13:e0203214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Romero V, Fert-Bober J, Nigrovic PA, Darrah E, Haque UJ, Lee DM, et al. Immune-mediated pore-forming pathways induce cellular hypercitrullination and generate citrullinated autoantigens in rheumatoid arthritis. Sci Transl Med. 2013;5:209ra150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Konig MF, Abusleme L, Reinholdt J, Palmer RJ, Teles RP, Sampson K, et al. Aggregatibacter actinomycetemcomitans-induced hypercitrullination links periodontal infection to autoimmunity in rheumatoid arthritis. Sci Transl Med. 2016;8:369ra176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spengler J, Lugonja B, Jimmy YA, Zubarev RA, Creese AJ, Pearson MJ, et al. Release of Active Peptidyl Arginine Deiminases by Neutrophils Can Explain Production of Extracellular Citrullinated Autoantigens in Rheumatoid Arthritis Synovial Fluid. Arthritis Rheumatol. 2015;67:3135–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Damgaard D, Bjorn ME, Steffensen MA, Pruijn GJ, Nielsen CH. Reduced glutathione as a physiological co-activator in the activation of peptidylarginine deiminase. Arthritis Res Ther. 2016;18:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou Y, Chen B, Mittereder N, Chaerkady R, Strain M, An LL, et al. Spontaneous Secretion of the Citrullination Enzyme PAD2 and Cell Surface Exposure of PAD4 by Neutrophils. Front Immunol. 2017;8:1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Romero V, Fellows E, Jenne DE, Andrade F. Cleavage of La protein by granzyme H induces cytoplasmic translocation and interferes with La-mediated HCV-IRES translational activity. Cell Death Differ. 2009;16:340–8. [DOI] [PubMed] [Google Scholar]
- 13.Casciola-Rosen L, Andrade F, Ulanet D, Wong WB, Rosen A. Cleavage by granzyme B is strongly predictive of autoantigen status: Implications for initiation of autoimmunity. Journal of Experimental Medicine. 1999;190:815–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Metkar SS, Menaa C, Pardo J, Wang B, Wallich R, Freudenberg M, et al. Human and mouse granzyme A induce a proinflammatory cytokine response. Immunity. 2008;29:720–33. [DOI] [PubMed] [Google Scholar]
- 15.Lee CY, Wang D, Wilhelm M, Zolg DP, Schmidt T, Schnatbaum K, et al. Mining the Human Tissue Proteome for Protein Citrullination. Mol Cell Proteomics. 2018;17:1378–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steen J, Forsstrom B, Sahlstrom P, Odowd V, Israelsson L, Krishnamurthy A, et al. Recognition of Amino Acid Motifs, Rather Than Specific Proteins, by Human Plasma Cell-Derived Monoclonal Antibodies to Posttranslationally Modified Proteins in Rheumatoid Arthritis. Arthritis Rheumatol. 2019;71:196–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakashima K, Hagiwara T, Yamada M. Nuclear localization of peptidylarginine deiminase V and histone deimination in granulocytes. J Biol Chem. 2002;277:49562–8. [DOI] [PubMed] [Google Scholar]
- 18.Darrah E, Kim A, Zhang X, Boronina T, Cole RN, Fava A, et al. Proteolysis by Granzyme B Enhances Presentation of Autoantigenic Peptidylarginine Deiminase 4 Epitopes in Rheumatoid Arthritis. J Proteome Res. 2017;16:355–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.