Abstract
Background:
Optimal treatment for distal radius fractures (DRFs) in older adults remains uncertain. No randomized trials comparing the most frequently used treatments in this population have been conducted. Surgical treatment rates vary widely, though the sustained benefits of surgery are uncertain.
Methods:
The Wrist and Radius Injury Surgical Trial (WRIST), randomized, multicenter trial, enrolled 304 adults age 60 years and older with isolated, unstable DRFs at 24 institutions. Patients who wanted surgery (n=187) were randomized to internal fixation with volar plate (VLPS), external fixation (EFP), or percutaneous pinning; patients who preferred conservative management (n=117) received casting. The primary outcome was the 12-month Michigan Hand Outcomes Questionnaire (MHQ) Summary score. Secondary outcomes included MHQ domain scores and radiographic parameters.
Results:
At 12 months, there were no differences by treatment in primary or the majority of secondary outcomes. Twelve-month MHQ Summary scores differed between VLPS and EFP by 3 points (97.5% CL: −6.0, 11.5) and between VLPS and pinning by −0.14(−9.2,8.9). However, at 6 weeks, mean MHQ Summary score for VLPS was greater than EFP by 19(p<0.001), pinning by 11(p<0.001) and casting by 7(p=0.03). VLPS participants demonstrated significantly better radiologic alignment throughout the follow-up period, though there was no relationship between any outcome and radiographic alignment. Malunion was experienced by 48% of casting participants.
Conclusions:
Recovery was fastest for VLPS participants and slowest for EFP participants according to most measures, but by 12 months there were no meaningful differences in outcomes. Casting participants experienced satisfactory results despite loss of radiologic alignment.
INTRODUCTION
Distal radius fractures (DRFs) hinder quality of life (QOL) and independence of older adults worldwide. Reported incidence varies considerably with rates from approximately 450/100,000 among women age 70-79 years in the UK and US to over 1000/100,000 among Finnish and Swedish women over 80 years.1-4 Despite over 200 years of experience treating DRFs, there is disagreement regarding the best treatment for older adults.5 In this context, the use of more costly internal fixation, most often with volar locking plate systems (VLPS), has increased. Studies in Finland, Korea, the Netherlands, Sweden, and the US show dramatic increases in internal fixation and wide variations in its use based on physician age or professional society affiliation, geographic location, or facility type.6-14
In previous randomized controlled trials of older patients, VLPS resulted in better radiographic alignment compared to external fixation, pinning, and casting. This may not lead to better functional or patient-reported outcomes, however. For example, Marcheix et al. and Martinez-Mendez et al. demonstrated better motion in patients treated with VLPS compared to those treated with pinning or casting, but other studies reported no significant differences.15-19 These conflicting results are echoed in our systematic review.20 Even patients are ambivalent in their choice. Decision models, including our time trade-off survey of adults age 65 years and older, demonstrate that older patients place nearly identical values on VLPS and casting.21,22
Despite this uncertainty, a clinical trial to derive Level-1 evidence by comparing the most common treatments among older adults has never been conducted. The 24-center Wrist and Radius Injury Surgical Trial (WRIST), an ambitious international and collaborative randomized trial, compared VLPS to external fixation ± pin fixation (EFP), closed reduction+percutaneous pinning (CRPP), and closed reduction+casting for treating unstable DRFs in older patients. We hypothesize that 12 months after surgery, VLPS will facilitate better patient-reported outcomes (PROs), quality of life (QOL), and functional and radiological outcomes and faster recovery than other treatments.
METHODS
Study Design and Eligibility Criteria
Michigan Medicine was the Coordinating Center for WRIST. Participants were screened at 24 health systems in Canada, Singapore, and the US from April 10, 2012 through December 31, 2016. (See Table, Supplemental Digital Content 1, which shows Participating institutions and primary practice locations.) Patients who were 60 years or older and not residing in a nursing home or other institutional setting were screened for eligibility. The surgical and observation groups had identical eligibility criteria: isolated fractures (concomitant ulnar styloid fracture was allowed) with displacement warranting surgical intervention (Arbeitsgemeinschaft für Osteosynthesefragen (AO) type A2, A3, C1, or C2, and meeting 1 of the following radiographic criteria post-reduction: dorsal angulation>10°, radial inclination<15°, or radial shortening>3mm). All fractures were amenable to treatment with all 3 surgical treatment options. Patients with open fractures, bilateral fractures, prior DRF to the same wrist, or additional serious trauma were ineligible. Other exclusion criteria were neurologic conditions affecting upper extremity sensation or movement, comorbid conditions prohibiting surgery, serious neurologic or psychiatric conditions precluding informed consent, and inability to complete study questionnaires and follow directions in English (or Chinese in Singapore).
The WRIST protocol was approved by the institutional review board at the Michigan Medicine and at all sites. A Data Safety and Monitoring Board (DSMB) appointed by the National Institute for Arthritis and Musculoskeletal and Skin Diseases oversaw this study.
Randomization and Blinding
Randomization, prepared by the study statistician prior to the start of enrollment, was stratified by study site using random block sizes of 3, 6, and 9. After obtaining written informed and surgical consent, surgical participants were randomized to receive VLPS, EFP, or CRPP via a secure website.23. Every effort was made to blind participants to treatment until after surgery. However, participants were sometimes informed the treatment assignment by clinic staff during surgery date confirmation phone calls or preoperatively by members of the anaesthesiology team. It was not possible to blind surgeons. Assessors were aware of treatment type when external fixator or pins were still present and VLPS was always present on radiographs.
Interventions
Randomized participants were treated with one of three standard-of-care procedures: open reduction and internal fixation with VLPS, closed reduction and external fixation with a bridging fixator with or without supplemental k-wire fixation, or closed reduction and k-wire fixation. All surgeons were fellowship trained hand surgeons and were familiar with and proficient for each procedure. Implant/fixator brand and the use of tourniquet, deep vein thrombosis prophylaxis, or prophylactic antibiotics were left to the discretion of the treating surgeon. We collected these variables along with surgical time and complications. Participants in the observation group were not randomized but managed with casting. Surgeons had equipoise regarding the three surgical procedures but an unstable DRF in an older adult will almost inevitably collapse without surgical intervention. However, recognizing that there will always be patients who prefer nonsurgical management, we followed these participants, who met all inclusion/exclusion criteria but did not want to undergo surgery, as an observation group. Follow-up care for all study arms, including hand therapy, was per institutional standard.
Data Collection and Outcome Measures
Members of the research team not involved with patient care performed assessments at enrollment, 2 weeks, 6 weeks, 3 months, 6 months, and 12 months after final fracture manipulation – emergency department reduction for casting participants or surgery for randomized participants. The primary outcome was the legacy Michigan Hand Outcomes Questionnaire (MHQ) Summary score at 12-month assessment.24,25
Participants completed the full MHQ at the 6-week through 12-month assessments. Only the MHQ Pain domain was completed at enrollment and the 2-week assessment because patients are immobilized at these times. The SF-36 was completed at enrollment and all follow-up visits. At enrollment, participants provided demographic information and completed the Self-Administered Comorbidity Checklist.26 Participants were asked to report pre-injury activity level using the Rapid Assessment of Physical Activity (RAPA).27,28 Grip and lateral pinch strength and wrist motion were measured at the 6-week through 12-month assessments. Providers completed the Complication Checklist for Distal Radius Fracture at all follow-up assessments.29 Posteroanterior, oblique, and lateral view radiographs were obtained at the enrollment through final assessment. Digital copies of radiographs were sent to the Coordinating Center; radial height, radial inclination, volar/dorsal tilt, and ulnar deviation were measured.
Statistical Analysis
The primary analytic method was intention-to-treat. All available data were used without imputations for missing values. We assessed differences in baseline characteristics and the extent of missingness in outcome variables across study arms. The primary analysis compared 12-month MHQ Summary score by the 3 randomized arms using a linear mixed-effects model based on data from 6 weeks through 12 months with random effects for study sites and for participants nested within study sites.30 Primary exposure variables were two indicators for EFP and CRPP to estimate the difference between EFP and VLPS and CRPP and VLPS. To compare rate of recovery across treatment groups, we graphically explored the outcomes data over time and included in the model time indicators and time by group interaction terms. We separately performed covariate-adjusted analyses including the casting arm.31,32 The model included 3 study arm indicators, log-transformed time (days since surgery or fracture), time by study arm interactions, baseline pain, age, race, smoking status, RAPA score, and random intercepts and slopes. Similar analyses were performed for secondary outcomes. We reported the rates of refusal to receive the randomized assignment, intraoperative crossovers, and postoperative crossovers by arm. Sensitivity analyses were performed as-treated. In these analyses, preoperative or intraoperative crossover outcomes were analysed by the treatment received, and if a participant changed treatment groups during the follow-up period, post-crossover outcomes were excluded. Relationships between PROs and radiographic measures were assessed with Spearman correlation. All statistical analyses were done using Stata 15.1 (College Station, TX).
We designed WRIST to have 80% power to detect a meaningful difference of 8 points (0.41 standardized effect size) in MHQ Summary score with a 0.025 level test at 12 months between VLPS and each of CRPP and EFP and proposed enrolling 152 participants per group over a 30-month enrollment period assuming 15% attrition and an intra-site correlation of 0.01. Twenty-four months after enrollment began, owing to slow enrollment, the DSMB advised futility assessment by conducting a conditional power analysis based on the observed effect size and observed enrollment rate at the interim with 73 participants randomized.33 Assuming a minimum of 141 participants (47/group) randomized over 4 years, conditional power to detect the differences observed at the interim of 17 and 7 points in mean MHQ Summary scores between the 2 pairs of surgical groups of interest were 94% and 26%, respectively, with 0.025 level tests and observed within-group standard deviations. Based on this result, the DSMB approved the study to continue.
RESULTS
We screened 2,190 DRF patients for inclusion; 65% (1,423) were ineligible. (Figure 1) Of 767 eligible patients, 60% (463) declined enrollment. A total of 304 participants (40% of eligible patients, 14% of screened patients) were enrolled in the study; 187 (62%) were randomized to receive one of the three surgical procedures (VLPS:65; EFP:64; CRPP:58) and 117 (38%) opted for casting. Eight casting participants were later found to be ineligible and were excluded from analysis, leaving 109 in analytic cohort for the casting group. Participants’ mean age was 71 years, 84% (256) were female, and 82% (249) were white. (Table 1) This is similar to patients who declined enrollment (mean 70 years, 87% female), though declining patients were significantly more likely to be white (93% white; p<0.0001). Randomized participants were similar across surgical arms in all measured baseline characteristics. Casting participants were significantly older than randomized participants (mean 76 vs 68 years; p<0.0001) and also reported less pain at baseline compared to the randomized group (56 vs. 65 points;p<0.001; 0-100, 0=no pain).
Figure 1. CONSORT Diagram.
*ineligible fractures include non-displaced fractures, open fractures, and those that are not amenable to treatment with all three surgical methods. **Non-community dwelling patients include those who reside in a nursing home or other institutional setting. *** These were cases where the patient was too anxious, upset, or angry for the surgeon to feel comfortable broaching the subject of a clinical trial or other circumstances where the surgeon felt for some other reason that discussing the trial was inappropriate.
Table 1.
Baseline demographic and clinical characteristics by study arm
| Randomized Participants | |||||
|---|---|---|---|---|---|
| VLPS | External Fixation |
Percutaneous Pinning |
Casting | ||
| Enrolled, n | 65 | 64 | 58 | 109 | p-valueb |
| Female, n (%) | 55 (85%) | 59 (92%) | 49 (84%) | 93 (85%) | 0.57 |
| Age, mean (SD) | 67 (6.2) | 70 (8.4) | 68 (7.0) | 76 (10) | <0.001 |
| median (range)a | 66 (59-85) | 68 (59-92) | 67 (58-84) | 75 (59-97) | |
| Race, n (%) | |||||
| Asian | 1 (2%) | 3 (3%) | 4 (7%) | 16 (15%) | 0.10 |
| Black | 3 (5%) | 6 (9%) | 2 (3%) | 6 (6%) | |
| White | 58 (92%) | 54 (84%) | 52 (90%) | 85 (78%) | |
| other | 1 (2%) | 1 (3%) | 0 | 2 (2%) | |
| missing | 2 (3%) | 0 | 0 | 0 | |
| Education, n (%) | |||||
| High school diploma/GED or less | 21 (34%) | 18 (29%) | 23 (40%) | 47 (44%) | 0.63 |
| Vocational school/associate’s degree/ some college | 19 (31%) | 18 (29%) | 20 (35%) | 30 (28%) | |
| Bachelor’s degree+ | 21 (34%) | 27 (43%) | 14 (25%) | 31 (29%) | |
| missing | 4 (6%) | 1 (2%) | 1 (2%) | 1 (1%) | |
| Employment – baseline, n (%) | |||||
| Full-time | 13 (21%) | 12 (19%) | 11 (20%) | 9 (9%) | 0.27 |
| Part-time | 7 (11%) | 8 (13%) | 7 (13%) | 12 (12%) | |
| Retired | 36 (57%) | 38 (61%) | 36 (65%) | 72 (73%) | |
| Receiving disability | 2 (3%) | 3 (4%) | 0 | 2 (2%) | |
| Unemployed | 5 (8%) | 1 (2%) | 1 (2%) | 4 (4%) | |
| missing | 2 (3%) | 2 (3%) | 3 (6%) | 10 (9%) | |
| Household Income, n (%) | |||||
| <$20,000 | 10 (18%) | 13 (22%) | 8 (16%) | 31 (32%) | 0.23 |
| $20,000 - $39,000 | 15 (26%) | 14 (24%) | 13 (25%) | 32 (33%) | |
| $40,000 - $59,999 | 12 (21%) | 15 (25%) | 9 (18%) | 14 (14%) | |
| $60,000+ | 20 (35%) | 17 (29%) | 21 (41%) | 21 (21%) | |
| missing | 8 (13%) | 5 (8%) | 7 (13%) | 11 (10%) | |
| RAPA Functional Status – pre-injury, n (%) | |||||
| Sedentary | 8 (13%) | 7 (11%) | 3 (6%) | 16 (15%) | 0.12 |
| Under-active | 27 (42%) | 30 (47%) | 29 (50%) | 62 (57%) | |
| Active | 29 (45%) | 27 (42%) | 26 (43%) | 30 (28%) | |
| missing | 1 (2%) | 0 | 0 | 1 (1%) | |
| Smoking status, n (%) | |||||
| Never | 28 (44%) | 40 (63%) | 31 (53%) | 56 (51%) | 0.37 |
| Former, <10 years | 4 (6%) | 1 (2%) | 4 (7%) | 2 (2%) | |
| Former, 10+ years | 24 (38%) | 17 (27%) | 17 (29%) | 42 (39%) | |
| Current | 8 (13%) | 6 (9%) | 6 (10%) | 9 (8%) | |
| Missing | 1 (2%) | 0 | 0 | 0 | |
| No. comorbidities, mean (SD) | 3.1 (2.2) | 3.6 (2.5) | 3.4 (2.1) | 3.8 (2.7) | 0.62 |
| median (range) | 3 (0-11) | 3 (0-12) | 4 (0-10) | 3 (0-12) | |
| AO class, n (%) | |||||
| A1 | 0 | 1 (2%) | 0 | 1 (1%) | 0.77 |
| A2 | 33 (52%) | 26 (42%) | 30 (57%) | 40 (44%) | |
| A3 | 4 (6%) | 9 (14%) | 7 (9%) | 12 (13%) | |
| C1 | 3 (5%) | 3 (5%) | 3 (4%) | 10 (11%) | |
| C2 | 20 (31%) | 20 (35%) | 16 (30%) | 25 (28%) | |
| C3 | 1 (2%) | 1 (2%) | 0 | 2 (2%) | |
| Missing | 4 (6%) | 4 (6%) | 2 (3%) | 19 (17%) | |
| Ulnar styloid fracture, n (%) | |||||
| Yes | 27 (42%) | 31 (48%) | 19 (33%) | 47 (43%) | 0.11 |
| No | 35 (54%) | 29 (45%) | 37 (64%) | 41 (38%) | |
| Missing | 3 (5%) | 4 (6%) | 2 (3%) | 21 (19%) | |
| Fracture to enrollment, days | |||||
| mean (SD) | 5.0 (3.8) | 4.9 (3.6) | 5.5 (3.9) | 7.8 (5.2) | <0.0001 |
| median (range) | 4 (0-14) | 4 (0-17) | 4 (0-16) | 7 (0-22) | |
In the early phases of the study 5 participants under age 60 years were enrolled
Comparisons between the combined randomized arms vs. casting group, based on chi-square tests for categorical variables and t-tests for continuous variables.
Casting participants missed 12-month assessment more often (casting:48%; VLPS:15%; EFP:23%; CRPP:26%; p<0.0001). Participants reporting more baseline pain (p=0.04) and current smokers (p=0.001) were also more likely to miss 12-month assessments, whereas participants with higher pre-injury activity level (p=0.02) were less likely. As a result, four-arm analyses were adjusted for smoking status in addition to pre-specified covariates.
Surgery
Randomized participants underwent surgery a mean 8.6 days after fracture (range 0-23 days). (Table 2) Intraoperative complications occurred only twice (broken drill tip and thin skin tearing, both VLPS). Additionally, 1 EFP and 2 VLPS participants were admitted postoperatively for shortness of breath. The protocol allowed surgeons to change treatment intraoperatively if satisfactory reduction could not be achieved or postoperatively if fracture reduction was not maintained. Nine crossovers occurred in each of the EFP and casting groups (14% and 8%, respectively) and 10 in CRPP (17%). The crossovers included a total of 8 participants who refused their randomized procedures. (See Table, Supplemental Digital Content 2, which shows crossovers during the study period).
Table 2.
Surgery Details
| VLPSa (n=65) | External Fixationa (n=63) |
Percutaneous Pinninga (n=58) |
p-valuec | |
|---|---|---|---|---|
| Fracture to surgery, days | ||||
| mean (SD) | 8.2 (4.7) | 8.7 (4.8) | 8.9 (5.1) | 0.90 |
| median (range) | 8.0 (1-22) | 8.0 (1-23) | 7.5 (0-20) | |
| Prophylactic antibiotic use, n (%) | ||||
| Yes | 56 (86%) | 56 (89%) | 8 (14%) | 0.37 |
| No | 6 (9%) | 4 (6%) | 47 (81%) | |
| missing | 3 (5%) | 3 (5%) | 3 (5%) | |
| Deep vein thrombosis prophylaxis, n (%) | ||||
| Yes | 10 (15%) | 7 (11%) | 7 (12%) | 0.75 |
| No | 52 (80%) | 53 (84%) | 48 (83%) | |
| missing | 3 (5%) | 3 (5%) | 3 (5%) | |
| Tourniquet use, n (%) | ||||
| Yes | 60 (92%) | 53 (84%) | 27 (47%) | <0.001 |
| No | 1 (2%) | 5 (8%) | 27 (47%) | |
| missing | 3 (5%) | 3 (5%) | 4 (7%) | |
| Tourniquet used, time, minutesb | ||||
| mean (SD) | 55 (26.8) | 40 (15.4) | 32 (21.7) | <0.001 |
| median (range) | 46 (17-160) | 40 (13-75) | 25 (11-84) | |
| Procedure time, minutes | ||||
| mean (SD) | 68 (34.3) | 54 (23.3) | 41 (38.5) | <0.001 |
| median (range) | 61 (22-210) | 52 (16-128) | 31 (8-200) | |
| Surgeon-rated difficulty of procedurec | ||||
| mean (SD) | 4.5 (2.2) | 4.5 (2.0) | 3.8 (2.3) | 0.09 |
| median (range) | 4 (1-9) | 4 (1-9) | 3 (1-8) | |
| Surgeon-rated quality of reductiond | ||||
| mean (SD) | 8.1 (1.5) | 7.4 (1.6) | 7.6 (1.5) | 0.008 |
| median (range) | 9 (2-10) | 8 (4-10) | 8 (3-10) |
Total number of ITT cohort participants were 65, 64 and 58 for VLPS, eternal fixation and percutaneous pinning groups, respectively, but one external fixation participant received casting after a hip fracture (see eTable 3)
among participants for whom a tourniquet was used
1-10 with 10 being the most difficult
1-10 with 10 being the best reduction
Comparisons across the three surgical groups, based on chi-square test or Fisher’s exact tests for categorical variables and Kruskal-Wallis tests for continuous variables.
Primary Outcome
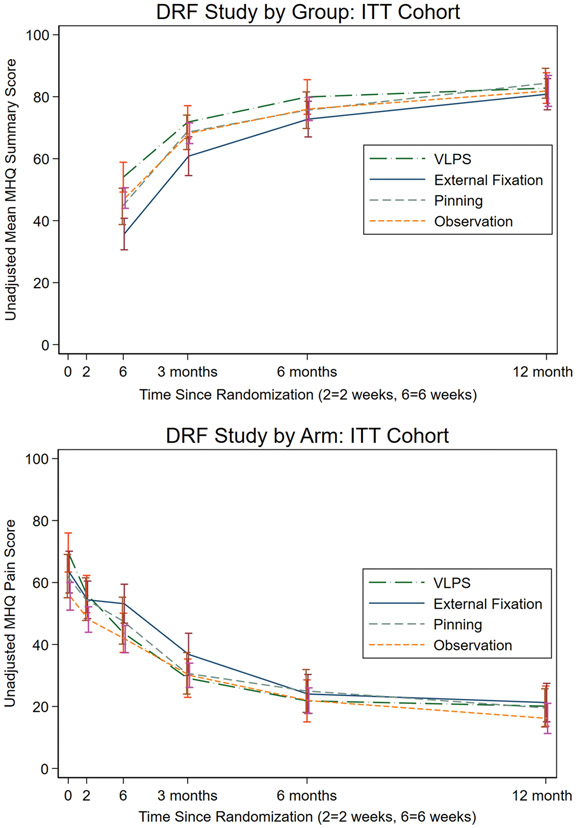
The MHQ was completed at least once by 96% of randomized participants. Among the three randomized surgical arms, we did not find significant difference in 12-month MHQ Summary score by treatment: the difference between VLPS and EFP was 2.7 points (97.5%CL=−6.0,11.5) and between VLPS and CRPP was −0.14 points (−9.2,8.9) (Table 3, Figure 2a) (See Table, Supplemental Digital Content 3, which shows primary and secondary outcomes by study arm and assessment time.) (See Figure, Supplemental Digital Content 4, which shows Box plots showing distribution of data by study arm: (above) Unadjusted mean MHQ Summary score by study arm and (below) Unadjusted mean MHQ Pain score by study arm.) (See Table, Supplemental Digital Content 5, which shows Linear mixed-effects model of the primary outcome, MHQ Summary score). There were differences early in the study, however. In analyses including the casting group, mean scores at six-weeks were higher in VLPS than EFP by 19.1 points (99%CL=11.4, 26.7), CRPP by 10.7 points (2.8,18.5), and casting by 5.9 points (−1.7,13.4). Additionally, compared with EFP, 6-week MHQ Summary score means were higher in CRPP by 8.4 points (0.6,16.2) and in casting by 13.2 points (6.0,20.4). Differences across arms in MHQ Summary scores got smaller over the follow-up time. Until there were no differences in 12-month predicted marginal mean MHQ Summary scores across the 4 groups (chisq(3)=0.58p=0.90).
Table 3.
Unadjusted primary and secondary outcomes by study arm and assessment time
| Randomized Participants | ||||||
|---|---|---|---|---|---|---|
| n | VLPS | External Fixation |
Percutaneous Pinning |
Casting | ||
| MHQa | ||||||
| Summary score | 6 weeks† | 273 | 54 (49, 59) | 36 (31, 41) | 45 (39, 50) | 47 (44, 51) |
| 12 months | 203 | 83 (78, 88) | 81 (76, 86) | 84 (80, 89) | 82 (77, 87) | |
| Pain score | enrollment‡ | 293 | 70 (63, 76) | 63 (57, 70) | 62 (55, 69) | 56 (51, 60) |
| 2 weeks | 268 | 56 (50, 62) | 54 (48, 60) | 55 (48, 62) | 48 (44, 52) | |
| 6 weeks† | 273 | 44 (37, 50) | 53 (47, 59) | 48 (40, 55) | 42 (37, 46) | |
| 12 months | 203 | 20 (14, 27) | 21 (15, 27) | 20 (13, 26) | 16 (11, 21) | |
| Function score | 6 weeks‡ | 273 | 53 (48, 58) | 34 (28, 39) | 40 (34, 47) | 43 (39, 47) |
| 12 months | 203 | 79 (73, 85) | 77 (72, 82) | 78 (72, 84) | 76 (70, 82) | |
| ADL score | 6 weeks‡ | 273 | 48 (41, 55) | 24 (18, 30) | 30 (22, 37) | 32 (28, 37) |
| 12 months | 203 | 84 (79, 90) | 83 (77, 88) | 88 (84, 92) | 85 (79, 91) | |
| Work score | 6 weeks† | 273 | 46 (39, 52) | 28 (21, 35) | 40 (32, 49) | 38 (33, 43) |
| 12 months | 203 | 79 (72, 87) | 82 (75, 89) | 84 (77, 90) | 83 (77, 88) | |
| Aesthetics score | 6 weeks† | 273 | 68 (61, 74) | 51 (44, 59) | 65 (58, 72) | 70 (65, 74) |
| 12 months | 203 | 86 (81, 91) | 83 (77, 89) | 85 (78, 92) | 83 (78, 89) | |
| Satisfaction score | 6 weeks‡ | 273 | 54 (47, 61) | 30 (24, 36) | 40 (33, 48) | 43 (38, 48) |
| 12 months | 203 | 79 (72, 87) | 76 (69, 83) | 80 (73, 88) | 78 (71, 85) | |
| Short Form 36b | ||||||
| Physical Component score | enrollment | 292 | 34 (31, 36) | 33 (30, 35) | 36 (33, 39) | 35 (33, 37) |
| 2 weeks | 268 | 36 (34, 38) | 33 (31, 35) | 35 (33, 38) | 37 (35, 39) | |
| 6 weeks† | 268 | 41 (39, 43) | 36 (34, 38) | 40 (37, 43) | 39 (37, 41) | |
| 12 months | 202 | 46 (43, 49) | 46 (43, 49) | 48 (44, 51) | 47 (44, 50) | |
| Mental Component score | enrollment | 292 | 48 (45, 52) | 50 (46, 53) | 51 (48, 55) | 49 (47, 52) |
| 2 weeks | 268 | 48 (44, 51) | 49 (45, 52) | 50 (45, 54) | 49 (47, 52) | |
| 6 weeks | 269 | 52 (49, 55) | 48 (44, 52) | 51 (47, 54) | 52 (49, 55) | |
| 12 months | 202 | 54 (51, 56) | 53 (50, 57) | 55 (53, 58) | 57 (55, 59) | |
| Grip strength, | 6 weeks‡ | 214 | 34 (28, 40) | 18 (11, 26) | 22 (15, 28) | 21 (16, 26) |
| 12 months | 186 | 84 (79, 90) | 73 (65, 80) | 82 (77, 86) | 80 (74, 85) | |
| Key pinch strength, % | 6 weeks‡ | 216 | 61 (55, 66) | 36 (27, 46) | 40 (32, 49) | 46 (40, 52) |
| 12 months† | 188 | 93 (87, 98) | 86 (82, 91) | 91 (86, 95) | 86 (82, 90) | |
| Flexion, % | 6 weeks† | 219 | 56 (49, 63) | 39 (29, 49) | 45 (37, 53) | 52 (47, 58) |
| 12 months | 188 | 87 (82, 91) | 86 (80, 91) | 85 (78, 92) | 80 (74, 87) | |
| Extension, % | 6 weeks‡ | 217 | 57 (50, 64) | 27 (17, 37) | 32 (23, 41) | 50 (43, 56) |
| 12 months | 189 | 96 (90, 102) | 87 (81, 92) | 90 (85, 95) | 97 (90, 103) | |
| Radial deviation, % | 6 weeks‡ | 215 | 61 (53, 70) | 30 (18, 43) | 47 (37, 57) | 64 (52, 76) |
| 12 months‡ | 189 | 92 (82, 102) | 100 (88, 113) | 89 (77, 101) | 115 (102, 129) | |
| Ulnar deviation, % | 6 weeks‡ | 216 | 67 (58, 76) | 42 (29, 54) | 45 (35, 55) | 61 (50, 72) |
| 12 months | 189 | 91 (84, 99) | 84 (79, 90) | 92 (82, 101) | 89 (69, 108) | |
| Pronation, % | 6 weeks | 230 | 88 (82, 94) | 78 (65, 91) | 83 (73, 93) | 87 (80, 93) |
| 12 months† | 188 | 99 (98, 100) | 96 (94, 98) | 99 (98, 100) | 96 (92, 100) | |
| Supination, % | 6 weeks | 229 | 82 (71, 92) | 64 (50, 78) | 61 (49, 72) | 73 (64, 82) |
| 12 months | 188 | 99 (93, 105) | 94 (90, 99) | 100 (94, 106) | 93 (88, 97) | |
| Volar/dorsal tilt c,° | enrollment‡ | 249 | −19 (−23, −5) | −11 (−15, −8) | −13 (−16, −9) | −9 (−12, −7) |
| 2 weeks‡ | 233 | 5 (3, 6) | −1 (−4, 4) | 1 (−2, 3) | −12 (−14, −8) | |
| 6 weeks‡ | 239 | 5 (3, 7) | −1 (−3, −2) | 1 (−1, 4) | −12 (−15, −9) | |
| 12 months‡ | 137 | 3 (0, 6) | −1 (−5, 2) | 2 (−1, 4) | −11 (−16, −7) | |
| Ulnar variance, mm | Enrollment | 242 | 2.9 (2.2, 3.7) | 2.0 (1.2, 2.8) | 2.1 (1.3, 2.9) | 2.3 (1.7, 2.9) |
| 2 weeks‡ | 230 | 0.6 (−0.1, 1.3) | 1.8 (1.3, 2.4) | 1.7 (1.1, 2.2) | 3.3 (2.5, 4.0) | |
| 6 weeks‡ | 237 | 1.3 (0.4, 2.2) | 2.0 (1.4, 2.6) | 2.8 (2.2, 3.4) | 3.6 (2.8, 4.4) | |
| 12 months† | 135 | 1.4 (0.7, 2.0) | 3.2 (1.6, 4.8) | 2.1 (1.6, 2.7) | 2.9 (2.1, 3.8) | |
| Radial inclination, ° | Enrollment | 251 | 16 (14, 17) | 16 (14, 18) | 16 (15, 18) | 17 (15, 18) |
| 2 weeks‡ | 235 | 21 (19, 22) | 21 (19, 22) | 23 (20, 23) | 14 (14, 17) | |
| 6 weeks‡ | 243 | 22 (20, 23) | 20 (19, 21) | 22 (21, 24) | 15 (14, 17) | |
| 12 months† | 139 | 22 (20, 23) | 20 (18, 21) | 21 (19, 23) | 17 (15, 20) | |
| Radial height, mm | enrollment | 292 | 8.1 (7.3, 8.9) | 8.5 (7.2, 9.8) | 8.3 (7.5, 9.0) | 8.8 (8.1, 9.5) |
| 2 weeks‡ | 230 | 10.8 (10.0, 11.5) | 10.4 (9.5, 11.4) | 10.9 (10.1, 11.7) | 7.9 (7.2, 8.7) | |
| 6 weeks‡ | 235 | 11.4 (10.5, 12.3) | 10.4 (9.4, 11.4) | 10.1 (9.1, 11.0) | 8.5 (7.5, 9.4) | |
| 12 months† | 135 | 11.1 (10.1, 12.1) | 9.8 (8.9, 10.8) | 10.8 (9.8, 11.8) | 9.1 (7.9, 10.4) | |
All cell values are mean (95% Confidence Limit), and all hand outcomes are specific to injured side (hand or wrist), except Work subdomain which has one score for both hands. Grip strength and key pinch strength are % injured hand out of uninjured hand, and for other functional measures are % injured wrist out of uninjured wrist.
Abbreviation: MHQ=Michigan Hand Outcomes; ADL=activities of daily living.
p<0.01 from unadjusted comparison across 4 arms using ANOVA
p<0.001 from unadjusted comparison across 4 arms using ANOVA
MHQ Summary and domain scores, except pain, range 0-100, with 100 indicating no hand disability. Pain scores also range 0-100 but 0 indicates no pain
SF-36 score range 0-100, with 100 indicating the best quality of life
volar tilt values are recorded as positive numbers and dorsal tilt values are recorded as negative numbers
Figure 2:
a: Unadjusted mean MHQ Summary score by study arm; b: Unadjusted mean MHQ Pain score by study arm
Secondary Outcomes
No differences were seen in 12-month MHQ Pain score across the 4 groups (chisq(3) =5.49,p=0.14). At 6 weeks, although not statistically significant, VLPS participants reported less pain than other surgical arms where the model-based predicted mean Pain score was lower than EFP by 7 points (99%CL=−14.0,0.8) and lower than CRPP by 5 points (−12.9,2.2). (Figure 2b) (See Figure, Supplemental Digital Content 4). At 6 weeks, in 4-arm analysis, VLPS participants scored significantly higher than the other arms on the Function, Satisfaction, and ADL domains. For the Work and Aesthetics domains, EFP participants scored significantly worse than all other arms. No difference in SF-36 score was found across arms at any follow-up time.
At 6 weeks, VLPS participants regained significantly more grip (chis(3)=22.3;p<0.001) and lateral pinch strength ((chis(3)=26.6;p<0.001) than all other arms. At 12 months, VLPS participants continued to demonstrate recovery of significantly more grip strength (as percent of the uninjured hand) with higher predicted mean than EFP by 11%(99%CL=0.9,21.5) and casting by 14%(3.1,24.2), but no difference was seen from pinning (5.9%, 99%CL=−4.8,16.5). And there were no significant differences across arms in pinch strength at 12 months (chis(3)=3.58,p=0.31). In all 4 groups, neither grip nor pinch strength reached means over 95% of the uninjured hand at 12 months. Finally, VLPS participants regained significantly more motion (compared to the uninjured wrist) at 6 weeks, but there were no significant differences at 12 months. The exception was radial deviation; casting participants’ mean injured wrist radial deviation was 115% of the uninjured wrist, most likely due to collapse of the radial column. This was 23% more than CRPP (p=0.007) and 21% more than VLPS (p=0.009), but no different than EFP (p=0.13).
At 12-month assessment, casting participants demonstrated significantly lower radial height (8.7 vs 10.8mm;p<0.0001) and inclination (17° vs 21°;p<0.0001) and significantly more volar/dorsal tilt (16° vs 8°;p<0.0001) and ulnar variance (3 vs 2mm;p=0.03). These values (except for tilt) are within normal range28,29 and the differences are likely not clinically significant. Correlation between radiologic outcomes and MHQ Function or Satisfaction was low in the combined data at 6 and 12 months. (See Table, Supplemental Digital Content 6, which shows non-parametric correlation between continuous variables and selected outcomes.)
Fracture malunion was experienced by all study participants (VLPS:6%, EFP:16%, CRPP:9%, casting:48%) Twenty-nine participants (25%) randomized to EFP or CRPP experienced pin site infections. There were no differences in infection occurrence (p=0.92) or severity (p=0.28) based on treatment. There was also 1 VLPS wound infection treated with oral antibiotics. VLPS hardware was removed from 3 participants due to tendon irritation. A more in-depth exploration of WRIST complications has been previously published.34
DISCUSSION
Regardless of treatment, 12 months after surgery or fracture, participants generally reported satisfactory hand outcomes, high QOL, and acceptable strength and motion. VLPS participants recovered the most hand strength and had significantly better patient-reported function at early follow-up times, but by 12-month assessment, any difference had disappeared. In fact, our casting participants, of whom almost 50% experienced malunion, had PROs that were indistinguishable from VLPS participants as soon as 6 weeks after fracture. There are 4 systematic reviews examining DRF treatment in older adults, and together they have compared a myriad of treatments. Our results are similar to our previous systematic review; casting resulted in suboptimal radiographic alignment, but at final follow-up assessment there were no clinical or statistically significant differences in motion or PROs.20 This is further supported by two meta-analyses of surgical versus nonsurgical treatment in older patients that found no differences in pain, DASH score, strength, or motion despite significantly better alignment in surgically-treated fractures.35,36
Because of the documented reluctance of older adults to be involved in surgical clinical trials recruitment was challenging.37,38 We estimated a 50% refusal rate and a 15% ineligibility rate. In practice, 65% of screened patients were ineligible and another 60% of eligible patients declined enrollment. Because the final sample size was lower than expected, type II error is a possibility. However, we note that the observed differences in MHQ Summary scores at the primary endpoint of 12 months were much smaller than the a priori determined clinically meaningful difference of 8 points.39 Another limitation is that in this study, casting arm participants were different from randomized participants in baseline age and pain resulting in a potential selection bias. To mitigate this potential bias, we used best statistical practices and used all longitudinally assessed data and adjusted for variables related to missingness.
Participants were treated at 24 different sites and surgery was performed by nearly 40 different surgeons. Because of the trial’s size, we did not attempt to standardize intraoperative practices, postoperative care or hand therapy. This gives our results increased generalizability. Furthermore, the results are immediately applicable to practice because there are no adjustments required from the “clinical trial efficacy environment” to standard practice.
WRIST is unique among randomized trials of DRF treatment in patients age 60 years and over. Previous randomized studies among this population have compared VLPS with casting, external fixation, or pinning, but none have compared all three surgical treatments.15-19 Furthermore, WRIST is the only multicenter study and has the largest sample size. We found that all DRF treatments provide acceptable pain relief and, ultimately, satisfactory outcomes. Frequent pin site infections make CRPP and EFP less appealing. EFP participants also scored significantly worse on most PROs up to 6 months after surgery. The benefits of VLPS over casting are less clear. VLPS participants reported significantly better function, including ability to perform ADLs, earlier in the follow-up period than the other groups. However, as soon as 6 weeks there were no significant differences in function or PROs between VLPS and casting, even with nearly half of casting participants experiencing malunion. Furthermore, casting participants avoid the risks associated with VLPS. Although VLPS patients were less likely to experience complications than other surgical groups, 1 wound infection occurred and 2 participants had unplanned postoperative hospital admissions because of anaesthesia complications. On the other hand, nearly all (97%) VLPS participants were out of splints by 6-week assessment whereas over 75% of casting participants remained immobilized. It is our conclusion that VLPS and casting are both acceptable treatments for unstable DRFs in patients age 60 years and older. However, activity level and independent living vary greatly among patients in this age group. Treatment recommendations must be made with patient values and goals in mind. Thus, the differences between DRF treatments are not reflected in the final outcomes, but rather in the recovery process, primarily surgery and its inherent risks versus extended immobilization.
Supplementary Material
Table, Supplemental Digital Content 1. Participating institutions and primary practice locations
Table, Supplemental Digital Content 2. Crossovers during the study period
Table, Supplemental Digital Content 3. Primary and secondary outcomes by study arm and assessment time
Figure, Supplemental Digital Content 4. Box plots showing distribution of data by study arm: (above) Unadjusted mean MHQ Summary score by study arm and (below) Unadjusted mean MHQ Pain score by study arm
Table, Supplemental Digital Content 5. Linear mixed-effects model of the primary outcome, MHQ Summary score.
Table , Supplemental Digital Content 6. Non-parametric correlation between continuous variables and selected outcomes
Acknowledgments
Financial Disclosure Statement: The authors have the following to disclose: Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases and the National Institute on Aging of the National Institutes of Health under Award Number R01 AR062066 and by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number 2 K24-AR053120-06. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors have no conflicts of interest to disclose.
Footnotes
The WRIST Group - Michigan Medicine (Coordinating Center): Kevin C. Chung, MD, MS (Principal Investigator); H. Myra Kim, ScD (Study Biostatistician); Steven C. Haase, MD; Jeffrey N. Lawton, MD; John R. Lien, MD; Adeyiza O. Momoh, MD; Kagan Ozer, MD; Erika D. Sears, MD, MS; Jennifer F. Waljee, MD, MPH; Matthew S. Brown, MD; Hoyune E. Cho, MD; Brett F. Michelotti, MD; Sunitha Malay, MPH (Study Coordinator); Melissa J. Shauver, MPH (Study Coordinator). Beth Israel Deaconess Medical Center: Tamara D. Rozental, MD (Co-Investigator); Paul T. Appleton, MD; Edward K. Rodriguez, MD, PhD; Laura N. Deschamps, DO; Lindsay Mattfolk, BA; Katiri Wagner. Brigham and Women’s Hospital: Philip Blazar, MD (Co-Investigator); Brandon E. Earp, MD; W. Emerson Floyd; Dexter L. Louie, BS. Duke Health: Fraser J. Leversedge, MD (Co-Investigator); Marc J. Richard, MD; David, S. Ruch, MD; Suzanne Finley, CRC; Cameron Howe, CRC; Maria Manson; Janna Whitfield, BS. Fraser Health Authority: Bertrand H. Perey, MD (Co-Investigator); Kelly Apostle, MD, FRCSC; Dory Boyer, MD, FRCSC; Farhad Moola, MD, FRCSC; Trevor Stone, MD, FRCSC; Darius Viskontas, MD, FRCSC; Mauri Zomar, CCRP; Karyn Moon; Raely Moon. HealthPartners Institute for Education and Research: Loree K. Kalliainen, MD, MA (Co-Investigator, now at University of North Carolina Health Care ); Christina M. Ward, MD (Co-Investigator); James W. Fletcher, MD; Cherrie A. Heinrich, MD; Katharine S. Pico, MD; Ashish Y. Mahajan, MD; Brian W. Hill, MD; Sandy Vang, BA. Johns Hopkins Medicine: Dawn M. Laporte, MD (Co-Investigator); Erik A. Hasenboehler, MD; Scott D. Lifchez, MD; Greg M. Osgood, MD; Babar Shafiq, MD, MS; Jaimie T. Shores, MD; Vaishali Laljani. Kettering Health Network: H. Brent Bamberger, DO (Co-Investigator); Timothy W. Harman, DO; David W. Martineau, MD; Carla Robinson, PA-C, MPAS; Brandi Palmer, MS, PC, CCRP. London Health Sciences Centre: Ruby Grewal, MD, MS (Co-Investigator); Ken A. Faber, MD; Joy C. MacDermid, PhD (Study Epidemiologist); Kate Kelly, MSc, MPH; Katrina Munro; Joshua I. Vincent, PT, PhD. Massachusetts General Hospital: David Ring, MD, PhD (Co-Investigator, now at University of Texas Health Austin); Jesse B. Jupiter, MD, MA; Abigail Finger, BA; Jillian S. Gruber, MD; Rajesh K. Reddy; Taylor M. Pong; Emily R. Thornton, BSc. Mayo Clinic: David G. Dennison, MD (Co-Investigator); Sanjeev Kakar, MD; Marco Rizzo, MD; Alexander Y. Shin, MD; Tyson L. Scrabeck, CCRP. The MetroHealth System: Kyle Chepla, MD (Co-Investigator); Kevin Malone, MD; Harry A. Hoyen, MD; Blaine Todd Bafus, MD; Roderick B. Jordan, MD; Bram Kaufman, MD; Ali Totonchil, MD; Dana R. Hromyak, BS, RRT; Lisa Humbert, RN. National University of Singapore: Sandeep Sebastin, MCh (Co-Investigator), Sally Tay. Northwell Health: Kate W. Nellans, MD, MPH (Co-Investigator); Sara L. Merwin, MPH. Norton Healthcare: Ethan W. Blackburn, MD (Co-Investigator); Sandra J. Hanlin, APRN, NP-C; Barbara Patterson, BSN, CCRC. OrthoCarolina Research Institute: R. Glenn Gaston, MD (Co-Investigator); R. Christopher Cadderdon, MD; Erika Gordon Gantt, MD; John S. Gaul, MD; Daniel R. Lewis, MD; Bryan J. Loeffler, MD; Lois K. Osier, MD; Paul C. Perlik, MD; W. Alan Ward, MD; Benjamin Connell, BA, CCRC; Pricilla Haug, BA, CCRC; Caleb Michalek, BS, CCRC. Pan Am Clinic/University of Manitoba: Tod A. Clark, MD, MSc, FRCSC(Co-Investigator); Sheila McRae, MSc, PhD. University of Connecticut Health: Jennifer Moriatis Wolf, MD (Co-Investigator, now at University of Chicago Medicine); Craig M. Rodner, MD; Katy Coyle, RN. University of Oklahoma Medicine: Thomas P. Lehman, MD, PT (Co-Investigator); Yuri C. Lansinger, MD; Gavin D. O’Mahony, MD; Kathy Carl, BA, CCRP; Janet Wells. University of Pennsylvania Health System: David J. Bozentka, MD (Co-Investigator); L. Scott Levin, MD; David P. Steinberg, MD; Annamarie D. Horan, PhD; Denise Knox, BS; Kara Napolitano, BS. University of Pittsburgh Medical Center: John Fowler, MD (Co-Investigator); Robert Goitz, MD; Cathy A. Naccarelli; Joelle Tighe. University of Rochester: Warren C. Hammert, MD, DDS (Co-Investigator); Allison W. McIntyre, MPH; Krista L. Noble; Kaili Waldrick. University of Washington Medicine: Jeffery B. Friedrich, MD (Co-Investigator); David Bowman; Angela Wilson. Wake Forest Baptist Health: Zhongyu Li, MD, PhD (Co-Investigator); L. Andrew Koman, MD; Benjamin R. Graves, MD; Beth P. Smith, PhD; Debra Bullard.
Trial Registration: ClinicalTrials.gov NCT01589692
REFERENCES
- 1.Baron JA, Karagas M, Barrett J, et al. Basic epidemiology of fractures of the upper and lower limb among Americans over 65 years of age. Epidemiol. 1996;7(6):612–618. [DOI] [PubMed] [Google Scholar]
- 2.Brogren E, Petranek M, Atroshi I. Incidence and characteristics of distal radius fractures in a southern Swedish region. BMC Musculoskelet Dis. 2007;8:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flinkkila T, Sirnio K, Hippi M, et al. Epidemiology and seasonal variation of distal radius fractures in Oulu, Finland. Osteoporos Int. 2011;22(8):2307–2312. [DOI] [PubMed] [Google Scholar]
- 4.Stirling ERB, Johnson NA, Dias JJ. Epidemiology of distal radius fractures in a geographically defined adult population. J Hand Surg Eur Vol. 2018;43(9):974–982. [DOI] [PubMed] [Google Scholar]
- 5.Bruce KK, Merenstein DJ, Narvaez MV, et al. Lack of Agreement on Distal Radius Fracture Treatment. J Am Board Fam Med. 2016;29(2):218–225. [DOI] [PubMed] [Google Scholar]
- 6.Childs S, Mann T, Dahl J, et al. Differences in the Treatment of Distal Radius Fractures by Hand Fellowship Trained Surgeons: A Study of ABOS Candidate Data. J Hand Surg Am. 2017;42(2):e91–e97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chung KC, Shauver MJ, Yin H. The relationship between ASSH membership and the treatment of distal radius fracture in the United States Medicare population. J Hand Surg Am. 2011;36(8):1288–1293. [DOI] [PubMed] [Google Scholar]
- 8.Chung KC, Shauver MJ, Yin H, Kim HM, Baser O, Birkmeyer JD. Variations in the use of internal fixation for distal radial fracture in the United States medicare population. J Bone Joint Surg Am. 2011;93(23):2154–2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hevonkorpi TP, Launonen AP, Huttunen TT, Kannus P, Niemi S, Mattila VM. Incidence of distal radius fracture surgery in Finns aged 50 years or more between 1998 and 2016 - too many patients are yet operated on? BMC Musculoskelet Disord. 2018;19(1):70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jo YH, Lee BG, Kim JH, et al. National Surgical Trends for Distal Radius Fractures in Korea. J Korean Med Sci. 2017;32(7):1181–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neuhaus V, Bot AG, Guitton TG, Ring DC. Influence of surgeon, patient and radiographic factors on distal radius fracture treatment. J Hand Surg Eur Vol. 2015;40(8):796–804. [DOI] [PubMed] [Google Scholar]
- 12.Walenkamp MM, Mulders MA, Goslings JC, Westert GP, Schep NW. Analysis of variation in the surgical treatment of patients with distal radial fractures in the Netherlands. J Hand Surg Eur Vol. 2016;42(1):39–44. [DOI] [PubMed] [Google Scholar]
- 13.Waljee JF, Zhong L, Shauver MJ, Chung KC. The influence of surgeon age on distal radius fracture treatment in the United States: a population-based study. J Hand Surg Am. 2014;39(5):844–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilcke MK, Hammarberg H, Adolphson PY. Epidemiology and changed surgical treatment methods for fractures of the distal radius: a registry analysis of 42,583 patients in Stockholm County, Sweden, 2004-2010. Acta Orthop. 2013;84(3):292–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arora R, Lutz M, Deml C, Krappinger D, Haug L, Gabl M. A prospective randomized trial comparing nonoperative treatment with volar locking plate fixation for displaced and unstable distal radial fractures in patients sixty-five years of age and older. J Bone Joint Surg Am. D2011;93(23):2146–2153. [DOI] [PubMed] [Google Scholar]
- 16.Hollevoet N, Vanhoutie T, Vanhove W, Verdonk R. Percutaneous K-wire fixation versus palmar plating with locking screws for Colles' fractures. Acta Orthop Belg. 2011;77(2):180–187. [PubMed] [Google Scholar]
- 17.Marcheix PS, Dotzis A, Benko PE, Siegler J, Arnaud JP, Charissoux JL. Extension fractures of the distal radius in patients older than 50: a prospective randomized study comparing fixation using mixed pins or a palmar fixed-angle plate. J Hand Surg Eur Vol. 2010;35(8):646–651. [DOI] [PubMed] [Google Scholar]
- 18.Martinez-Mendez D, Lizaur-Utrilla A, de-Juan-Herrero J. Intra-articular distal radius fractures in elderly: a randomized prospective study of casting versus volar plating. J Hand Surg Eur Vol. 2017;43(2):142–147. [DOI] [PubMed] [Google Scholar]
- 19.Navarro CM, Ahrengart L, Tornqvist H, Ponzer S. Volar Locking Plate or External Fixation With Optional Addition of K-Wires for Dorsally Displaced Distal Radius Fractures: A Randomized Controlled Study. J Orthop Trauma. 2016;30(4):201–224. [DOI] [PubMed] [Google Scholar]
- 20.Diaz-Garcia RJ, Oda T, Shauver MJ, Chung KC. A systematic review of outcomes and complications of treating unstable distal radius fractures in the elderly. J Hand Surg Am. 2011;36(5):824–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shauver MJ, Clapham PJ, Chung KC. An economic analysis of outcomes and complications of treating distal radius fractures in the elderly. J Hand Surg Am. 2011;36(12):1912–1918 e1913. [DOI] [PubMed] [Google Scholar]
- 22.Koenig KM, Davis GC, Grove MR, Tosteson AN, Koval KJ. Is early internal fixation preferred to cast treatment for well-reduced unstable distal radial fractures? J Bone Joint Surg Am. 2009;91(9):2086–2093. [DOI] [PubMed] [Google Scholar]
- 23.Treatment Assignment Tool - University of Michigan. 2014; https://michrapps.med.umich.edu/tatum/#. Accessed March 9, 2016.
- 24.Chung KC, Pillsbury MS, Walters MR, Hayward RA. Reliability and validity testing of the Michigan Hand Outcomes Questionnaire. J Hand Surg Am. 1998;23(4):575–587. [DOI] [PubMed] [Google Scholar]
- 25.Kotsis SV, Lau FH, Chung KC. Responsiveness of the Michigan Hand Outcomes Questionnaire and physical measurements in outcome studies of distal radius fracture treatment. J Hand Surg Am. 2007;32(1):84–90. [DOI] [PubMed] [Google Scholar]
- 26.Sangha O, Stucki G, Liang MH, Fossel AH, Katz JN. The Self-Administered Comorbidity Questionnaire: a new method to assess comorbidity for clinical and health services research. Arthritis Rheum. 2003;49(2):156–163. [DOI] [PubMed] [Google Scholar]
- 27.LoGerfo JP. Rapid Assessment of Physical Activity. Seattle, WA: University of Washington Health Promotion Research Center; 2006. [Google Scholar]
- 28.Topolski TD, LoGerfo J, Patrick DL, Williams B, Walwick J, Patrick MB. The Rapid Assessment of Physical Activity (RAPA) among older adults. Prev Chronic Dis. 2006;3(4):A118. [PMC free article] [PubMed] [Google Scholar]
- 29.McKay SD, MacDermid JC, Roth JH, Richards RS. Assessment of complications of distal radius fractures and development of a complication checklist. J Hand Surg Am. 2001;26(5):916–922. [DOI] [PubMed] [Google Scholar]
- 30.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- 31.Schmoor C, Gall C, Stampf S, Graf E. Correction of confounding bias in non-randomized studies by appropriate weighting. Biom J. 2011;53(2):369–387. [DOI] [PubMed] [Google Scholar]
- 32.Streeter AJ, Lin NX, Crathorne L, et al. Adjusting for unmeasured confounding in nonrandomized longitudinal studies: a methodological review. J Clin Epidemiol. 2017;87:23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li G, Shih WJ, Xie T, Lu J. A sample size adjustment procedure for clinical trials based on conditional power. Biostatistics. 2002;3(2):277–287. [DOI] [PubMed] [Google Scholar]
- 34.Chung KC, Malay S, Shauver MJ, Kim HM. Assessment of Distal Radius Fracture Complications Among Adults 60 Years or Older: A Secondary Analysis of the WRIST Randomized Clinical Trial. JAMA Netw Open. 2019;2(1):e187053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen Y, Chen X, Li Z, Yan H, Zhou F, Gao W. Safety and Efficacy of Operative Versus Nonsurgical Management of Distal Radius Fractures in Elderly Patients: A Systematic Review and Meta-analysis. J Hand Surg Am. 2016;41(3):404–413. [DOI] [PubMed] [Google Scholar]
- 36.Ju JH, Jin GZ, Li GX, Hu HY, Hou RX. Comparison of treatment outcomes between nonsurgical and surgical treatment of distal radius fracture in elderly: a systematic review and meta-analysis. Langenbecks Arch Surg. 2015;400(7):767–779. [DOI] [PubMed] [Google Scholar]
- 37.Cassidy EL, Baird E, Sheikh JI. Recruitment and retention of elderly patients in clinical trials: issues and strategies. Am J Geriatr Psychiatry. 2001;9(2):136–140. [PubMed] [Google Scholar]
- 38.Macias FM, Ramsay RE, Rowan AJ. Recruitment and retention in clinical trials of the elderly. Int Rev Neurobiol. 2007;81:265–272. [DOI] [PubMed] [Google Scholar]
- 39.Shauver MJ, Chung KC. The minimal clinically important difference of the Michigan hand outcomes questionnaire. J Hand Surg Am. 2009;34(3):509–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table, Supplemental Digital Content 1. Participating institutions and primary practice locations
Table, Supplemental Digital Content 2. Crossovers during the study period
Table, Supplemental Digital Content 3. Primary and secondary outcomes by study arm and assessment time
Figure, Supplemental Digital Content 4. Box plots showing distribution of data by study arm: (above) Unadjusted mean MHQ Summary score by study arm and (below) Unadjusted mean MHQ Pain score by study arm
Table, Supplemental Digital Content 5. Linear mixed-effects model of the primary outcome, MHQ Summary score.
Table , Supplemental Digital Content 6. Non-parametric correlation between continuous variables and selected outcomes