Abstract
Background:
Preterm infants face unique stress states in early life. Early-life stress has been associated with changes in cortisol reactivity and behavioral abnormalities later in childhood in non-preterm populations. The neonatal infant stressor scale (NISS) has been used to estimate infant stress in the neonatal intensive care unit (NICU), but has not been biomarker-validated. The relationship between NISS scores and salivary cortisol is unknown.
Objective:
To test the association between NISS scores and salivary cortisol in the NICU Hospital Exposures and Long-Term Health (NICU-HEALTH) preterm birth cohort.
Methods:
386 salivary cortisol specimens were collected from 125 NICU-HEALTH participants during the NICU hospitalization. NISS scores were calculated to represent the infant’s experience in the 6 hours prior to specimen collection. Adjusted mixed-effect regression models were used to assess the association between each NISS score and salivary cortisol.
Results:
Acute and total NISS scores were significantly associated with salivary cortisol level (p=0.002 and 0.05 respectively). The chronic NISS score was not associated with salivary cortisol levels. Caffeine treatment and postmenstrual age of the infant at time of exam were important covariates in all models.
Conclusion:
Acute and total NISS score are associated with salivary cortisol level in hospitalized moderately preterm infants.
Background
Over the past four decades, survival of preterm infants has improved dramatically(1). Neonatology has accordingly broadened focus from improving survival among preterm infants to reducing the significant neurobehavioral impairments commonly linked to preterm birth. However, even infants born in the moderate or late preterm period (28-36 weeks of gestation) have higher rates of behavioral, cognitive, and psychiatric deficits, poor emotional regulation, and suboptimal school performance compared to term-born peers(2). The specific mechanism by which preterm birth leads to life-long behavioral morbidity remains unknown, although alteration in cortical developmental trajectory, rather than focal brain injury following preterm birth, is implicated(3). Stress-related changes on neurodevelopmental outcome are a potentially modifiable factor influencing morbidity related to preterm birth. Identifying and mitigating stressful practices and events in the NICU is therefore important to preterm infant care. Accurate categorization of stress states in the NICU is central to elucidating the role of stress pathways in development.
Preterm infants experience physical and psychosocial stress during a critical developmental period analogous to the late second and third trimester periods of in utero development(4). Biologically active free cortisol enters cells by passive diffusion and can be measured in all bodily fluids. Salivary cortisol reflects levels of free cortisol in blood(32). Infants do not develop diurnal cortisol cycling until 44-48 weeks PMA(28), so unlike older children and adults single salivary cortisol measurements (rather than serial measurements) can be used in preterm infant populations to reflect recent stressful events(9). Associations between stress states and preterm infant cortisol measurements have been documented in small NICU-based studies with limited sample size(4-8). Existing studies also document increases in cortisol following painful procedures(9, 10) and decrements in cortisol level following social interventions and parental interaction(7, 11) – interventions protective from toxic stress in other pediatric populations(12).
Moreover, lasting alterations in childhood hypothalamic-pituitary-adrenal (HPA) axis function are associated with estimated NICU-based stress(4, 13). These long-term alterations in stress response are associated not only with changes in baseline cortisol and diurnal cortisol reactivity later in childhood(9, 13), but also with behavioral abnormalities including internalizing symptoms, attention, memory, and psychiatric problems(14, 15). Small, single center preliminary studies highlight the importance of investigating NICU-based stress as a contributing factor to long-term neurodevelopmental outcomes of preterm infants.
Clinical assessment of stress in preterm infants is difficult. Both physical exam findings such as cry and startle, and physiological signs such as pulse and respiratory rate are not specific indicators of stress in preterm infants. To address this problem, C.A. Newnham et al. developed the neonatal infant stressor scale (NISS)(16) to estimate infant stress in the neonatal intensive care unit (NICU). The NISS is a standardized measure of exposure to a range of NICU stressors based on prospective survey of stressful activities by bedside NICU staff. The survey tool, developed through interviews with neonatal intensive care providers at two institutions, aims to quantify stressful experiences of the neonate that are common during the NICU hospitalization. The tool assigns points to various care practices felt by the neonatal care team to be stressful, and classifies these care practices and specific medical events as “acute” or “chronic” stressors. Points are summed to an “acute” and “chronic” score, which can be reported independently or joined into a composite stress score.
Although used as a predictive measure in a number of studies focused on the relationship between NICU-based stress and developmental outcome(17, 18), the NISS has not previously been biomarker-validated. In the present study, we compared NISS scores with a concurrently collected biomarker of infant stress, salivary cortisol.
Methods
This study was conducted using the infrastructure of the Neonatal Intensive Care Unit Hospital Exposures and Long-Term Health (NICU-HEALTH) cohort(19). NICU-HEALTH is an ongoing longitudinal preterm birth cohort based in the premise that modifiable environmental exposures in the NICU contribute to adverse multi-organ system development in children born preterm. Moderately preterm infants born between 28-0/7 and 32-6/7 weeks of gestation without genetic or congenital anomalies and their mothers are recruited from an urban academic medical center level IV NICU in New York City. Recruitment began in 2011 and continues through multiple non-continuous enrollment phases to the present with goal enrollment of 400 infants. All participants in the current study were enrolled in Phase II of NICU-HEALTH, born March 2015 through December 2018. Longitudinal follow-up in NICU-HEALTH includes serial neurodevelopmental assessments from the NICU hospitalization through age 2. Follow-up through later childhood is coordinated through the Developmental Impact of NICU Exposures (DINE) cohort of the United States National Institutes of Health Environmental Influences on Child Health Outcomes (ECHO) program.
NISS
NISS scores were calculated daily as described in C.A. Newnham et al(16). Much NISS data was abstracted from the infant’s NICU medical record by the study team with event time stamps from the clinical ICU record. Some NISS data points, such as infant position change, respiratory equipment adjustment, removal from the incubator, and oropharyngeal or tracheal suctioning are not recorded in the infant’s medical record at our institution. For these variables, information was collected prospectively from the infant’s shift nurse on paper collection forms left at the bedside by the study staff. The form also included a field to record “other” events or changes in the infant’s care not otherwise captured in the medical record. Study staff entered all data into a REDCap data management system. All data representing the infant’s NICU experience in the six hours preceding saliva collection were used to calculate the acute, chronic, and total NISS included in this analysis per C.A. Newnham et al(16).
Saliva Collection
Saliva collection was performed for all participants in NICU-HEALTH at two time points on a single day each week during the NICU hospitalization by study staff. The time of saliva collection was recorded and used as the “start” of the 6-hour look-back for NISS score calculation as described above. A cotton swab (SalivaBio Infant Swab, Salimetrics) was placed in the infant’s mouth for 2-5 minutes until saturated with saliva. The swab was then centrifuged at 4000 rotations per minute for 30 minutes and extracted saliva was aliquoted to storage vials and frozen at −80°C pending batch analysis.
Salivary Cortisol Measurement
Thawed samples were centrifuged at 2,000×g at 10°C for 10 minutes to obtain a clear sample. Saliva specimens from NICU-HEALTH were sent frozen to the Technical University of Dresden for analysis. Cortisol was analyzed in duplicate using a commercially available chemiluminescence assay (IBL, Hamburg, Germany) with a cortisol-biotin conjugate as tracer (sensitivity of 0.43 nmol/L; batch-dependent intra- and inter-assay coefficients of variance range 4-10%)(20).
Statistical Analysis
Descriptive statistics were calculated for exposure variables, outcome variables, and covariates. Partially missing data was imputed using the MICE (Multivariate Imputation by Chained Equations) R package(21).
Three NISS exposure variables (acute, chronic, and total NISS) were calculated by summing appropriate NISS subscales. The calculation was based on the NICU Infant Stressor Record Sheet published as Appendix A in C.A. Newnham et al.(16). In this study, a single afternoon saliva specimen was compared to the NISS score representing the previous six hours of NICU care(9). The Shapiro–Wilk test was used to examine the normality of the outcome variable, the salivary cortisol concentration. Spearman correlation coefficients were used to examine the bivariate relationship between the acute NISS score and salivary cortisol, the chronic NISS score and salivary cortisol, and the total NISS score and salivary cortisol. Mixed-effects regression models were used to assess the association between each NISS score and salivary cortisol, adjusted for the fixed effect of covariates and the random effect of family as many of our study participants were siblings (twins or triplets). A cutoff of 2-tailed P = 0.05 was used to indicate significance. Potential covariates for adjusted modeling were selected from literature review based on their associations with stress response(22-26). The following were included as covariates in analyses due to known relevance: child sex (boy/girl), postmenstrual age (PMA), GA at birth, race/ethnicity, delivery type (vaginal/cesarean), birth weight small for GA (<10th percentile; yes/no), insurance type (Medicaid/private insurance), base deficit in the first 12-hours of life (a marker of severity of illness at birth), a scaled NICU morbidity score based on number of significant prematurity-related diagnoses at NICU discharge, after Stroustrup et al(27), and caffeine citrate treatment on the day of saliva collection (yes/no).
We additionally performed a sensitivity analysis. Because an infants’ HPA axis matures with development, the association between NISS exposure and salivary cortisol could vary by infants’ gestational age at birth and chronological age. In the sensitivity analysis, developmental age was represented by PMA which was considered as a categorical variable(28-29, 30-31, 32-33, 34-35, 36-37, and >38 weeks PMA). We modeled the association between NISS exposure and cortisol using mixed-effects regression models, adjusted for covariates as above, along with categorical PMA and an interaction term between NISS exposure and categorized PMA. We thereby computed the PMA-stratified coefficient between NISS exposure and salivary cortisol. All analyses were conducted using R v3.5.1 software.
Results
Three hundred eighty six salivary cortisol specimens matched to NISS scores taken from 125 NICU-HEALTH participants were included in the present analyses. Table 1 shows demographic and clinical characteristics of our patient population. The subset of NICU-HEALTH participants in whom salivary cortisol levels were available is similar to the entire NICU-HEALTH cohort, with the exception of the incidence of necrotizing enterocolitis, retinopathy of prematurity, and intraventricular hemorrhage. This is because saliva collection was added to the NICU-HEALTH study in phase II. Our NICU saw significant improvements in clinical outcome metrics for very low birth weight infants between the epoch coinciding with phase I enrolment and phase II enrolment.
Table 1:
Demographic and clinical characteristics of the entire NICU-HEALTH cohort compared to the subset used in the present analysis. Data are number (percent of cohort), average ± standard deviation, or median (interquartile range) as appropriate.
| NICU-HEALTH Cohort |
NISS Analysis Cohort |
|
|---|---|---|
| Number enrolled | 286 | 125 |
| Demographics | ||
| Female | 140 (49%) | 64 (47%) |
| Medicaid | 81 (29%) | 45 (33%) |
| White (non-hispanic) | 154 (54%) | 71 (52%) |
| Black (non-hispanic) | 57 (20%) | 26 (19%) |
| Asian (non-hispanic) | 21 (7.3%) | 12 (8.8%) |
| Mixed Race | 35 (12%) | 29 (21%) |
| Hispanic Ethnicity | 25 (8.7%) | 12 (8.8%) |
| Birth characteristics | ||
| Gestational Age (weeks) | 30.2 ± 2.0 | 30.8 ± 1.4 |
| Birth weight (grams) | 1,359 ± 365 | 1,445 ± 330 |
| Birth weight Z-score for GA | −0.16 ± 0.88 | −0.08 ± 0.93 |
| Small for Gestational Age | 33 (12%) | 17 (12%) |
| Multiple Gestation | 136 (48%) | 57 (42%) |
| Maternal age at delivery (years) | 33 ± 6 | 34 ± 7 |
| Vaginal Delivery | 123 (43%) | 42 (31%) |
| Base deficit at birth | 3.9 ± 3.1 | 3.6 ± 3.2 |
| Apgar score at 1 minute | 8 (7, 9) | 8 (7, 9) |
| Apgar score at 5 minutes | 9 (9, 9) | 9 (9, 9) |
| Morbidities of Prematurity | ||
| Bronchopulmonary dysplasia | 49 (18%) | 10 (7%) |
| Necrotizing enterocolitis | 7 (2.6%) | 1 (0.7%) |
| Retinopathy of prematurity stage 2-4 | 28 (10.1%) | 2 (1.5%) |
| Intraventricular hemorrhage grade II-IV | 25 (9.0%) | 5 (3.7%) |
| Biomarker Characteristics | ||
| Salivary cortisol (nmol/L) | 4.83 (3.05, 8.22) | 4.50 (2.86, 7.33) |
Sixteen out of 68 NISS subscales had missing data. Among those sixteen subscales, eight subscales were 100% missing as these data were not recorded in the medical record at our institution or able to be collected prospectively in a reliable fashion. These were: multiple attempts inserting a peripheral intravenous (IV) line or central catheter, removing an unwrapped infant from the incubator/bed, removing a wrapped infant from the incubator/bed, eye toilet, IV flushing, stomach aspiration via oro/nasogastric tube, attachment of monitor sensors, and application of cream to body. One subscale, “nursed in incubator” was universally coded “no” as it is not a practice pursued in our unit. We did not include these subscales in the present analysis. Six subscales had varied missing rates: eye examination (1%), suction of nose and mouth (19%), insertion of nasal continuous positive airway pressure (CPAP) tube (18%), insertion of Hudson Prong (15%), insertion of nasogastric tube (1%), and Hudson Prong CPAP (6%).
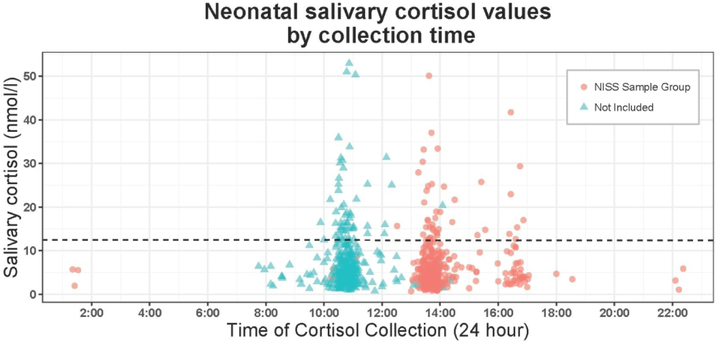
Prior to completing the planned study analyses, we evaluated whether salivary cortisol levels in our cohort were truly non-periodic (i.e., did not demonstrate diurnal variation), as expected based on existing literature (28). The lack of diurnal cortisol variation in our preterm population was confirmed (Figure 1).
Figure 1:
Salivary cortisol by time of specimen collection.
Diurnal cycling of cortisol does not begin until well after a preterm infant’s due date.(38) As salivary specimens collected as part of NICU-HEALTH are collected prior to 44 weeks PMA, it was not expected that cortisol values would vary by time of day of collection. This was confirmed; the regression line on the plot of cortisol level by time of collection is horizontal, demonstrating no association. For the present analysis, collections where events during the 6-hour period prior to collection could be confirmed were selected.
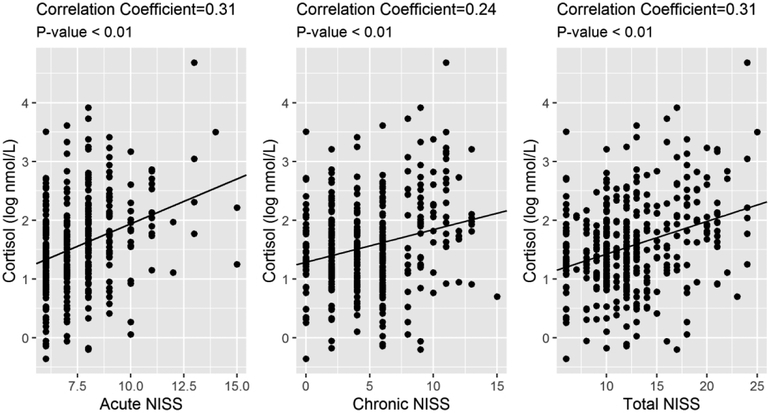
Normality test results show that NISS exposure variables (acute NISS, chronic NISS, and total NISS) and salivary cortisol concentrations were not normally distributed. The Spearman correlation analyses indicate that NISS exposure variables were significantly correlated with salivary cortisol (Figure 2; all P-values < 0.0001). In the following regression analysis, salivary cortisol is log 10 transformed to meet the assumptions of the regression model.
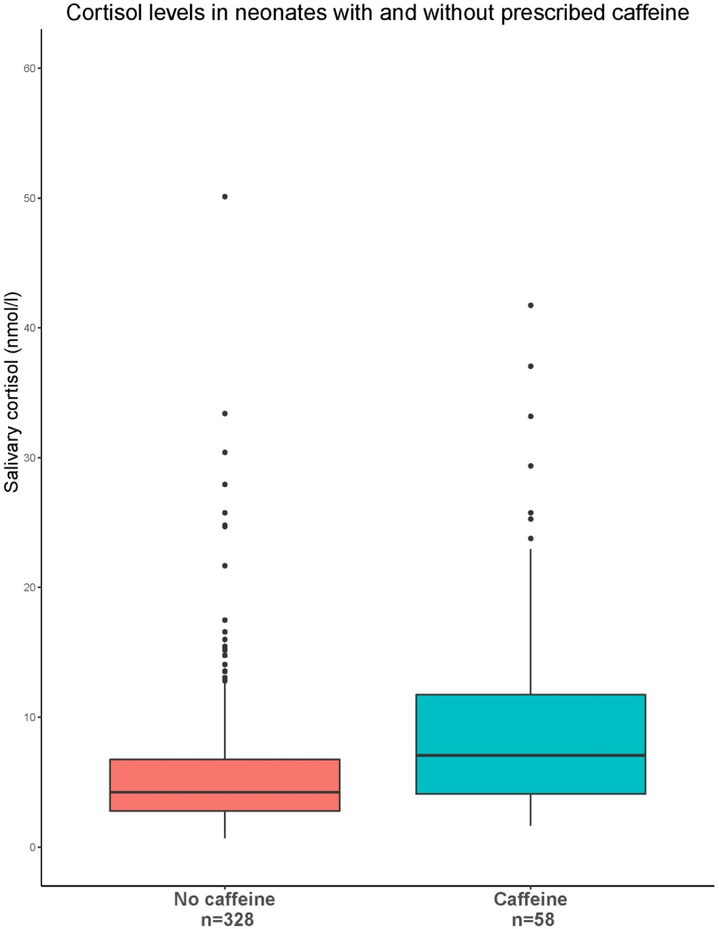
Figure 2:
Salivary cortisol for infants being treated with without caffeine treatment at the time of specimen collection. Caffeine has previously been shown to impact cortisol levels in adult populations(26). This was confirmed in NICU-HEALTH.
Table 2 and Figure 3 show the adjusted associations between each NISS score and salivary cortisol, respectively. The acute NISS scores were significantly associated with an increased level of salivary cortisol (β=0.10, P-value=0.0005), indicating that a 1-unit increase of acute NISS score was associated with 0.10 log nmol/L increase of cortisol in preterm infants’ salivary cortisol (Table 2). The association between chronic NISS score and salivary cortisol level was not significant (β=0.01, P-value=0.61) (Table 3). As the total NISS was predominantly calculated from the acute score, and as the association of the acute score with increased salivary cortisol was strong, the total NISS score was also significantly associated with salivary cortisol level (β=0.03, P-value=0.04), likely driven by the association between the acute score and salivary cortisol (Table 4).
Table 2.
Mixed-effects regression models predicting the association between acute, chronic, and total NISS and salivary cortisol concentration (log nmol/L).
| Acute NISS model | Chronic NISS model | Total NISS model | |||||
|---|---|---|---|---|---|---|---|
| β | 95% CI | β | 95% CI | β | 95% CI | ||
| Acute NISS | 0.09 | 0.03, 0.14 | -- | -- | -- | -- | |
| Chronic NISS | -- | -- | 0.01 | −0.02, 0.04 | -- | -- | |
| Total NISS | -- | -- | -- | -- | 0.03 | 0.0004, 0.05 | |
| Female sex | 0.04 | −0.18 0.25 | −0.02 | −0.24, 0.21 | 0.006 | −0.22, 0.23 | |
| Postmenstrual age | –0.08 | −0.13, −0.04 | −0.12 | −0.16, −0.07 | −0.10 | −0.14, −0.05 | |
| Birth weight z-score | 0.04 | −0.08, 0.17 | −0.01 | −0.14, 0.12 | −0.002 | −0.13, 0.13 | |
| Gestational Age | 0.08 | −0.01, 0.16 | 0.07 | −0.02, 0.15 | 0.07 | −0.02, 0.16 | |
| Race/ ethnicity | |||||||
| Non-Hispanic White | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | |
| Non-Hispanic Black | 0.15 | −0.14, 0.43 | 0.13 | −0.17, 0.43 | 0.16 | −0.13, 0.46 | |
| Hispanic | 0.02 | −0.35, 0.38 | −0.04 | −0.42, 0.34 | −0.03 | −0.41, 0.35 | |
| Other | 0.03 | −0.23, 0.29 | 0.03 | −0.25, 0.30 | 0.06 | −0.21, 0.33 | |
| Cesarean birth | 0.02 | −0.22, 0.26 | −0.01 | −0.25, 0.25 | −0.002 | −0.25, 0.25 | |
| Private insurance | 0.24 | 0.02, 0.46 | 0.24 | 0.01, 0.46 | 0.25 | 0.03, 0.47 | |
| Base excess at birth | 0.01 | −0.02, 0.04 | 0.01 | −0.01, 0.04 | 0.01 | −0.01, 0.04 | |
| NICU morbidity | 0.07 | −0.18, 0.33 | 0.10 | −0.17, 0.37 | 0.05 | −0.22, 0.32 | |
| Caffeine treatment | 0.38 | 0.12, 0.64 | 0.31 | 0.04, 0.59 | 0.28 | 0.01, 0.55 | |
| Antenatal steroid exposure | 0.15 | −0.23, 0.52 | 0.12 | −0.27, 0.51 | 0.10 | −0.28, 0.49 | |
| Postnatal steroid treatment | 1.14 | −0.38, 2.66 | 1.37 | −0.14, 2.89 | 1.22 | −0.31, 2.74 | |
Figure 3:
Association between acute, chronic, and total NISS score and salivary cortisol concentration
Table 3.
Mixed-effects regression model predicting the association between chronic NISS and salivary cortisol concentrations (log nmol/L).
| Variables | β | 95% CI | P-value | |
|---|---|---|---|---|
| Chronic NISS | 0.01 | −0.02, 0.04 | 0.61 | |
| Female Sex | −0.001 | −0.23, 0.23 | 0.99 | |
| Postmenstrual Age | −0.11 | −0.16, −0.07 | <0.001* | |
| Birth weight z-score | −0.02 | −0.15, 0.11 | 0.76 | |
| Gestational Age | 0.05 | −0.03, 0.14 | 0.26 | |
| Race/ ethnicity | ||||
| Non-Hispanic White | Ref. | Ref. | Ref. | |
| Non-Hispanic Black | 0.10 | −0.20, 0.40 | 0.58 | |
| Hispanic | 0.02 | −0.35, 0.41 | 0.92 | |
| Other | −0.01 | −0.26, 0.28 | 0.95 | |
| Cesarean birth | 0.01 | −0.25, 0.26 | 0.96 | |
| Private Insurance | 0.21 | −0.01, 0.44 | 0.07 | |
| Base excess | 0.01 | −0.02, 0.04 | 0.34 | |
| NICU morbidity | 0.11 | −0.16, 0.39 | 0.40 | |
| Caffeine Treatment | 0.32 | 0.05, 0.60 | 0.02* | |
Table 4.
Mixed-effects regression model predicting the association between total NISS and salivary cortisol concentrations (log nmol/L).
| Variables | β | 95% CI | P-value | |
|---|---|---|---|---|
| Total NISS | 0.03 | 0.002, 0.05 | 0.04* | |
| Female Sex | 0.01 | −0.21, 0.24 | 0.90 | |
| Postmenstrual Age | −0.09 | −0.14, −0.04 | <0.001* | |
| Birth weight z-score | −0.01 | −0.14, 0.12 | 0.83 | |
| Gestational Age | 0.06 | −0.02, 0.15 | 0.16 | |
| Race/ ethnicity | ||||
| Non-Hispanic White | Ref. | Ref. | Ref. | |
| Non-Hispanic Black | 0.14 | −0.16, 0.43 | 0.36 | |
| Hispanic | 0.04 | −0.33, 0.41 | 0.83 | |
| Others | 0.05 | −0.22, 0.31 | 0.73 | |
| Cesarean birth | −0.01 | −0.24, 0.26 | 0.94 | |
| Private Insurance | 0.22 | 0.003, 0.45 | 0.05 | |
| Base excess | 0.01 | −0.01, 0.04 | 0.34 | |
| NICU morbidity | 0.05 | −0.22, 0.33 | 0.68 | |
| Caffeine Treatment | 0.29 | 0.02, 0.56 | 0.04* | |
In all analyses, PMA and caffeine treatment were significant covariates in the association between NISS and salivary cortisol.
In sensitivity analysis, PMA did not significantly modify the association between NISS (acute, chronic, and total) and salivary cortisol except in analyses using data from our most mature participants. These significant results were likely skewed by the extremely small size of this group compared to the other gestational age groupings. The sensitivity analysis indicated that the association between NISS and salivary cortisol was robust across different age groups of premature infants.
Discussion
In this study we wanted to assess whether NICU-based stress estimated by the NISS correlates with salivary cortisol, a biomarker of infant stress response. The NISS was proposed as a non-invasive and practical way to estimate preterm infant cumulative stressors in the NICU(16). It has been used by multiple groups to estimate NICU-based stress, and NISS scores have been associated with neurodevelopmental outcomes in small studies(17, 18, 29-31). The NISS is derived from caregiver assessment of what a preterm infant may or may not find stressful. Although logical, it has not previously been validated with an accepted biomarker of infant stress response. As the NISS has not previously been linked to an accepted biomarker of infant stress response, however, the ability to draw conclusions from small studies of the relationship between the NISS and developmental outcomes has been limited. Without biomarker validation, it has not been clear whether infant stress, or some of the specific care practices assessed by the NISS, drive the demonstrated associations with neurodevelopment. To our knowledge, this is the first study to demonstrate that the NISS correlates with salivary cortisol.
Biologically active free cortisol enters cells by passive diffusion and can be measured in all bodily fluids. Salivary cortisol reflects levels of free cortisol in blood(32). Infants do not develop diurnal cortisol cycling until 44-48 weeks PMA(28), so unlike older children and adults single salivary cortisol measurements (rather than serial measurements) can be used in preterm infant populations to reflect recent stressful events(9). In this study, a single afternoon saliva specimen was compared to the NISS score representing the previous six hours of NICU care(9).
Early childhood stress can have a lasting impact on HPA responsiveness, producing abnormal cortisol profiles later in life(33, 34). In otherwise well children, prenatal and early childhood stress exposure has been associated with long-term alterations in brain structure seen on neuroimaging, as well as with adverse neurodevelopmental outcomes, including psychiatric disorders and cognitive impairments(34).
By comparing NISS to an accepted biomarker of stress response, our study provides important information about how the NISS should be used in clinical practice and research. The acute NISS score correlates well with salivary cortisol while the chronic NISS score does not. This result is expected based on our study design. We used a spot salivary cortisol measurement, known to reflect acute stress response over the previous 6 hours as our biomarker(9), not an average or cumulative measure of cortisol over time. Therefore, our study design allowed us to detect acute changes in cortisol level related to NISS scores, but may not have captured chronic stressors accurately. Support for this hypothesis can be found in the work of A.L. D’Agata et al.(35) who found that the number of stressful events in the previous 7 days correlated with skin cortisol in hospitalized preterm infants. In that study, skin cortisol was not associated with stressful events over a shorter timeframe, i.e. was associated with a modified chronic NISS scale but not associated with a modified acute NISS scale. That study, in contrast to ours, used the NISS as the “gold standard” measure to test a novel measure of infant stress, skin cortisol. Based on that work, skin cortisol likely reflects cumulative or chronic stress rather than acute stress better measured by the more acutely reactive salivary cortisol. Further studies of preterm infant stress using a validated biomarker of chronic preterm infant stress in a large population are needed to explore this possibility.
Alternatively, it could be that chronic states purported by the designers of the NISS to be stressful, such as the presence of CPAP, do not provoke a biological response in the infant. This would be important information for caregivers and families. If the chronic NISS scale cannot be biomarker validated, efforts to reduce infant stress in the NICU should focus on reduction of acute events.
This study also demonstrated the impact on caffeine therapy on preterm infant cortisol levels. Although caffeine therapy is ubiquitous for preterm infants, the impact of caffeine on cortisol levels has only previously been shown in adults(36). As caffeine has been previously associated with improvements in neurodevelopmental outcomes in preterm infants(37), our study demonstrates the need to include caffeine therapy as a covariate in studies of preterm infant cortisol levels and neurodevelopmental outcome.
Another important finding of this study are replication of the previously shown association between preterm infant cortisol level and PMA(38). The HPA axis which mediates cortisol production in response to stressful events begins to respond to environmental stimuli in the second trimester of in utero development and continues to mature through early childhood(39, 40). The importance of PMA in our analysis highlights the need to account for developmental stage in any study of stress during the dynamic preterm period.
Our study has a number of strengths. Our study population, hospitalized preterm infants in an academic medical center, is similar to the population in which the NISS was designed and tested. Our prospective data collection and extensive medical record abstraction for NICU-HEALTH is standardized and complete. Our dataset has a low rate of missing data. These analyses do have a few limitations, however. We could not compare salivary cortisol levels to the complete NISS as originally published, because we were not able to assess a small number of NISS variables in our unit. The gestational age of our study population is limited to the overall NICU-Health phase II cohort, which excludes extremely premature infants with GA < 28 weeks. Therefore, we cannot comment on the association of salivary cortisol level with NISS scores for preterm infants born before 28 weeks gestation. Similarly, we cannot comment on the relationship between caffeine exposure and salivary cortisol in preterm infants with born less than 28 weeks, those most regularly started on caffeine therapy. As our unit does not universally prescribe caffeine to infants born in the moderate preterm period and generally discontinues administration well before hospital discharge of these patients, only 58 salivary cortisol specimens in this study were obtained from infants actively receiving caffeine treatment. This limits our ability to generalize findings about the relationship between salivary cortisol levels and caffeine exposure. We focused our study on events occurring in the 6 hours leading up to saliva collection, reflecting the meaningful time period for salivary cortisol changes. It is possible, however, that a highly stressful event occurring just before this time window could lead to misclassification by NISS scores. Review of the medical records of NICU-HEALTH patients makes this unlikely, as although all NISS variables are not recorded completely outside of the 6 hour window, significant events (surgeries, major medical decompensation, etc.) are.
Demonstration of the association between the acute NISS and salivary cortisol provides support for the expanded use of the NISS in clinical and research endeavors. The lack of previous data linking the NISS to any biomarker of infant stress limited its use in both research and clinically oriented initiatives to better define and to reduce the impact of NICU-based stress on preterm infant development. Neonatal researchers and care providers may now chose the NISS as a non-invasive low-cost measure reflecting acute infant stress.
Conclusions
The acute NISS score for the 6 hour period prior to saliva collection is strongly associated with salivary cortisol level in our cohort of hospitalized moderately preterm infants. The total NISS score is also associated with increased salivary cortisol, although the chronic score is not. Treatment with caffeine and PMA significantly impact this association. Clinicians and researchers interested in the role of NICU-based stress could use the acute NISS score as a reasonable estimate of an infant’s acute stress response.
Acknowledgments
Funding and competing interests: NICU-HEALTH is supported by a cooperative agreement, UH3OD023320, from the National Institutes of Health for the Environmental Influences on Child Health Outcomes (ECHO) program. Additional past funding for this cohort came through pilot grants from the Passport Foundation, the Mount Sinai Children’s Environmental Health Center, a National Institute of Environmental Health Sciences (NIEHS) mentored award K23ES022268 to Dr. Stroustrup, and the primary phase of the ECHO program UG3OD02332. The study funders did not and will not have a role in or authority over study design; collection, management, analysis, and interpretation of data; writing of reports; or the decisions to submit reports for publication.
Appendix:
Sensitivity analysis to test the impact of PMA on the association between NISS and salivary cortisol.
Table 5.
The PMA-stratified association (β) between Acute, Chronic, and Total NISS and salivary cortisol concentrations (log nmol/L). Mixed-effects regression model was adjusted with covariates (sex, birth weight z-score, gestational age, race/ethnicity, delivery type, insurance type, base excess, NICU morbidity, and caffeine citrate treatment).
| Acute NISS | Chronic NISS | Total NISS | ||||
|---|---|---|---|---|---|---|
| PMA (weeks) | Stratified β | P-value | Stratified β | P-value | Stratified β | P-value |
| 1 = 28-29 (N=11) | −0.24 | 0.18 | 0.16 | 0.20 | 0.03 | 0.62 |
| 2 = 30-31 (N=47) | 0.07 | 0.25 | 0.03 | 0.31 | 0.04 | 0.24 |
| 3 = 32-33 (N=100) | 0.09 | 0.13 | 0.02 | 0.36 | 0.04 | 0.17 |
| 4 = 34-35 (N=156) | 0.12 | 0.08 | −0.005 | 0.70 | 0.02 | 0.32 |
| 5 = 36-37 (N=60) | 0.15 | 0.20 | −0.03 | 0.40 | 0.005 | 0.78 |
| 6 = >38 (N=12) | −0.20 | 0.41 | −0.31 | 0.04 | −0.24 | 0.05 |
| Contrast | Difference β | P-value | Difference β | P-value | Difference β | P-value |
| 1 versus 2 | −0.31 | 0.34 | 0.13 | 0.83 | −0.01 | 1.00 |
| 1 versus 3 | −0.33 | 0.25 | 0.14 | 0.78 | −0.01 | 1.00 |
| 1 versus 4 | −0.36 | 0.18 | 0.17 | 0.62 | 0.01 | 1.00 |
| 1 versus 5 | −0.39 | 0.23 | 0.19 | 0.52 | 0.02 | 1.00 |
| 1 versus 6 | −0.04 | 1.00 | 0.47 | 0.01 | 0.27 | 0.18 |
| 2 versus 3 | −0.02 | 0.99 | 0.01 | 1.00 | 0.0001 | 1.00 |
| 2 versus 4 | −0.05 | 0.98 | 0.04 | 0.90 | 0.02 | 0.99 |
| 2 versus 5 | −0.08 | 0.98 | 0.07 | 0.80 | 0.03 | 0.97 |
| 2 versus 6 | 0.27 | 0.92 | 0.34 | 0.01 | 0.28 | 0.01 |
| 3 versus 4 | −0.03 | 1.00 | 0.03 | 0.94 | 0.02 | 0.98 |
| 3 versus 5 | −0.06 | 0.99 | 0.05 | 0.85 | 0.03 | 0.96 |
| 3 versus 6 | 0.29 | 0.89 | 0.33 | 0.01 | 0.28 | 0.01 |
| 4 versus 5 | −0.03 | 1.00 | 0.03 | 0.99 | 0.01 | 1.00 |
| 4 versus 6 | 0.32 | 0.85 | 0.30 | 0.01 | 0.26 | 0.01 |
| 5 versus 6 | 0.35 | 0.83 | 0.27 | 0.05 | 0.25 | 0.04 |
Footnotes
Registration details: Phase I of NICU-HEALTH (called Hospital-Based Phthalate Exposure in Very Low Birth Weight Neonates) was registered with ClinicalTrials.gov NCT01420029 on August 19, 2011. NICU-HEALTH was registered with ClinicalTrials.gov NCT01963065 on October 16, 2013.
REFERENCES
- 1.Patel RM. Short- and Long-Term Outcomes for Extremely Preterm Infants. Am J Perinatol. 2016;33(3):318–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Jong M, Verhoeven M, van Baar AL. School outcome, cognitive functioning, and behaviour problems in moderate and late preterm children and adults: a review. Semin Fetal Neonatal Med. 2012;17(3):163–9. [DOI] [PubMed] [Google Scholar]
- 3.Kapellou O, Counsell SJ, Kennea N, et al. Abnormal cortical development after premature birth shown by altered allometric scaling of brain growth. PLoS Med. 2006;3(8):e265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith GC, Gutovich J, Smyser C, et al. Neonatal intensive care unit stress is associated with brain development in preterm infants. Ann Neurol. 2011;70(4):541–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Provenzi L, Fumagalli M, Sirgiovanni I, et al. Pain-related stress during the Neonatal Intensive Care Unit stay and SLC6A4 methylation in very preterm infants. Front Behav Neurosci. 2015;9:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rohan AJ. Pain-associated stressor exposure and neuroendocrine values for premature infants in neonatal intensive care. Dev Psychobiol. 2016;58(1):60–70. [DOI] [PubMed] [Google Scholar]
- 7.Schwilling D, Vogeser M, Kirchhoff F, et al. Live music reduces stress levels in very low-birthweight infants. Acta Paediatr. 2015;104(4):360–7. [DOI] [PubMed] [Google Scholar]
- 8.Srinath BK, Shah J, Kumar P, Shah PS. Kangaroo care by fathers and mothers: comparison of physiological and stress responses in preterm infants. J Perinatol. 2015. [DOI] [PubMed] [Google Scholar]
- 9.Morelius E, He HG, Shorey S. Salivary Cortisol Reactivity in Preterm Infants in Neonatal Intensive Care: An Integrative Review. Int J Environ Res Public Health. 2016;13(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Osman M, Elsharkawy A, Abdel-Hady H. Assessment of pain during application of nasal-continuous positive airway pressure and heated, humidified high-flow nasal cannulae in preterm infants. J Perinatol. 2015;35(4):263–7. [DOI] [PubMed] [Google Scholar]
- 11.Morelius E, Ortenstrand A, Theodorsson E, Frostell A. A randomised trial of continuous skin-to-skin contact after preterm birth and the effects on salivary cortisol, parental stress, depression, and breastfeeding. Early Hum Dev. 2015;91(1):63–70. [DOI] [PubMed] [Google Scholar]
- 12.Johnson SB, Riley AW, Granger DA, Riis J. The science of early life toxic stress for pediatric practice and advocacy. Pediatrics. 2013;131(2):319–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brummelte S, Chau CM, Cepeda IL, et al. Cortisol levels in former preterm children at school age are predicted by neonatal procedural pain-related stress. Psychoneuroendocrinology. 2015;51:151–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bagner DM, Sheinkopf SJ, Vohr BR, Lester BM. A preliminary study of cortisol reactivity and behavior problems in young children born premature. Dev Psychobiol. 2010;52(6):574–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quesada AA, Tristao RM, Pratesi R, Wolf OT. Hyper-responsiveness to acute stress, emotional problems and poorer memory in former preterm children. Stress. 2014;17(5):389–99. [DOI] [PubMed] [Google Scholar]
- 16.Newnham CA, Inder TE, Milgrom J. Measuring preterm cumulative stressors within the NICU: the Neonatal Infant Stressor Scale. Early Hum Dev. 2009;85(9):549–55. [DOI] [PubMed] [Google Scholar]
- 17.Cong X, Wu J, Vittner D, et al. The impact of cumulative pain/stress on neurobehavioral development of preterm infants in the NICU. Early Hum Dev. 2017;108:9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morag I, Rotem I, Frisch M, et al. Cumulative pain-related stress and developmental outcomes among low-risk preterm infants at one year corrected age. Early Hum Dev. 2017;109:1–5. [DOI] [PubMed] [Google Scholar]
- 19.Stroustrup A, Bragg JB, Spear EA, Aguiar A, Zimmerman E, Isler JR, Busgang SA, Curtin PC, Gennings C, Andra SS, Arora M. Cohort profile: the neonatal intensive care unit hospital exposures and long-term health (NICU-HEALTH) cohort, a prospective preterm birth cohort in New York City. BMJ Open 2019; in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kirschbaum C, Hellhammer D. Response variability of salivary cortisol under psychological stimulation. J Clin Chem Clin Biochem. 1989;27(4):237. [PubMed] [Google Scholar]
- 21.van Buuren S, Groothuis-Oudshoorn K. mice: Multivariate Imputation by Chained Equations in R. 2011. 2011;45(3):67. [Google Scholar]
- 22.Taylor A, Fisk NM, Glover V. Mode of delivery and subsequent stress response. Lancet. 2000;355(9198):120. [DOI] [PubMed] [Google Scholar]
- 23.Iwata S, Kinoshita M, Okamura H, et al. Intrauterine growth and the maturation process of adrenal function. PeerJ. 2019;7:e6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hajat A, Diez-Roux A, Franklin TG, et al. Socioeconomic and race/ethnic differences in daily salivary cortisol profiles: the multi-ethnic study of atherosclerosis. Psychoneuroendocrinology. 2010;35(6):932–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bolt RJ, Van Weissenbruch MM, Popp-Snijders C, Sweep FG, Lafeber HN, Delemarre-van de Waal HA. Maturity of the adrenal cortex in very preterm infants is related to gestational age. Pediatr Res. 2002;52(3):405–10. [DOI] [PubMed] [Google Scholar]
- 26.Lovallo WR, Whitsett TL, al'Absi M, Sung BH, Vincent AS, Wilson MF. Caffeine stimulation of cortisol secretion across the waking hours in relation to caffeine intake levels. Psychosom Med. 2005;67(5):734–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stroustrup A, Bragg JB, Busgang SA, et al. Sources of clinically significant neonatal intensive care unit phthalate exposure. J Expo Sci Environ Epidemiol. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stalder T, Baumler D, Miller R, Alexander N, Kliegel M, Kirschbaum C. The cortisol awakening response in infants: ontogeny and associations with development-related variables. Psychoneuroendocrinology. 2013;38(4):552–9. [DOI] [PubMed] [Google Scholar]
- 29.Cassiano RGM, Gaspardo CM, Linhares MBM. Temperament moderated by neonatal factors predicted behavioral problems in childhood: A prospective longitudinal study. Early Hum Dev. 2019;135:37–43. [DOI] [PubMed] [Google Scholar]
- 30.Gaspardo CM, Cassiano RGM, Gracioli SMA, Furini GCB, Linhares MBM. Effects of Neonatal Pain and Temperament on Attention Problems in Toddlers Born Preterm. J Pediatr Psychol. 2018;43(3):342–51. [DOI] [PubMed] [Google Scholar]
- 31.Gorzilio DM, Garrido E, Gaspardo CM, Martinez FE, Linhares MB. Neurobehavioral development prior to term-age of preterm infants and acute stressful events during neonatal hospitalization. Early Hum Dev. 2015;91(12):769–75. [DOI] [PubMed] [Google Scholar]
- 32.Kirschbaum C, Hellhammer DH. Salivary cortisol in psychoneuroendocrine research: recent developments and applications. Psychoneuroendocrinology. 1994;19(4):313–33. [DOI] [PubMed] [Google Scholar]
- 33.Morelius E, Ivars K, Gustafsson PA, Theodorsson E, Nelson N. Salivary cortisol circadian rhythm in infants at psychosocial risk showed more variations than previous studies of healthy full-term infants. Acta Paediatr. 2017;106(12):2060–1. [DOI] [PubMed] [Google Scholar]
- 34.McEwen BS, Gianaros PJ. Stress- and allostasis-induced brain plasticity. Annu Rev Med. 2011;62:431–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.D'Agata AL, Roberts MB, Ashmeade T, Dutra SVO, Kane B, Groer MW. Novel method of measuring chronic stress for preterm infants: Skin cortisol. Psychoneuroendocrinology. 2019;102:204–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lovallo WR, Farag NH, Vincent AS, Thomas TL, Wilson MF. Cortisol responses to mental stress, exercise, and meals following caffeine intake in men and women. Pharmacol Biochem Behav. 2006;83(3):441–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murner-Lavanchy IM, Doyle LW, Schmidt B, et al. Neurobehavioral Outcomes 11 Years After Neonatal Caffeine Therapy for Apnea of Prematurity. Pediatrics. 2018;141(5). [DOI] [PubMed] [Google Scholar]
- 38.Ivars K, Nelson N, Theodorsson A, Theodorsson E, Strom JO, Morelius E. Development of salivary cortisol circadian rhythm in preterm infants. PLoS One. 2017;12(8):e0182685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gitau R, Fisk NM, Glover V. Human fetal and maternal corticotrophin releasing hormone responses to acute stress. Arch Dis Child Fetal Neonatal Ed. 2004;89(1):F29–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McLaughlin KA, Sheridan MA, Tibu F, Fox NA, Zeanah CH, Nelson CA 3rd. Causal effects of the early caregiving environment on development of stress response systems in children. Proc Natl Acad Sci U S A. 2015;112(18):5637–42. [DOI] [PMC free article] [PubMed] [Google Scholar]