Abstract
Objective.
Growing evidence suggests increasing frequencies of autoimmunity and certain autoimmune diseases, but findings are limited by the lack of systematic data and evolving approaches and definitions. We investigated whether the prevalence of antinuclear antibodies (ANA), the most common biomarker of autoimmunity, changed over a recent 25-year span in the U.S.
Methods.
Serum ANA were measured by standard indirect immunofluorescence assays on HEp-2 cells in 14,211 participants ≥12 years old from the U.S. National Health and Nutrition Examination Survey, with approximately one-third from each of three time periods: 1988-1991, 1999-2004, and 2011-2012. We used logistic regression adjusted for sex, age, race/ethnicity, and survey-design variables to estimate changes in ANA prevalence across the periods.
Results.
The prevalence of ANA was 11.0% (CI=9.7-12.6%) in 1988-1991, 11.5% (CI=10.3-12.8%) in 1999-2004, and 15.9% (CI=14.3-17.6%) in 2011-2012 (trend P<0.0001), which corresponds to 22, 27, and 41 million affected individuals, respectively. Among adolescents (ages 12-19 years), ANA prevalence rose steeply, with odds ratios of 2.02 (CI=1.16-3.53) and 2.88 (CI=1.64-5.04) in the second and third time periods relative to the first (trend P<0.0001). ANA prevalence increased in both sexes (especially males), older adults (ages ≥50 years), and non-Hispanic whites. These increases were not explained by concurrent trends in obesity/overweight, smoking, or drinking.
Conclusion.
The prevalence of ANA in the U.S. has increased considerably in recent years. Additional studies to determine factors underlying these increases could elucidate causes of autoimmunity and enable development of preventative measures.
INTRODUCTION
Autoimmune diseases are a diverse group of disorders characterized by damaging immune responses to self-antigens and, for the most part, are of unknown etiology (1, 2). They are thought to impact 3-5% of the population, with rising rates noted several decades ago (3). Recent studies suggest continued increases for certain autoimmune diseases (4-6), but it is unclear whether these trends are due to changes in recognition and diagnosis, or are true temporal changes in incidence (7).
As the most common biomarker of autoimmunity, antinuclear antibodies (ANA) are observed in patients with many autoimmune diseases. ANA are also seen in the general population where they have been associated with demographic factors such as older age, female sex and parity (8, 9), genetic factors (10), and various environmental exposures, including chemicals, infections, and medications (11-13). To investigate whether autoimmunity is increasing over time in the U.S. population, we used data from the National Health and Nutrition Examination Survey (NHANES) to estimate the prevalence of ANA over a 25-year span from 1988 to 2012.
MATERIALS AND METHODS
Study population.
We measured ANA in 14,211 persons aged ≥12 years sampled from three NHANES time periods: 1988-1991 (4,727 persons), 1999-2004 (4,749 persons), and 2011-2012 (4,735 persons). The NHANES sampled nationally representative members of the noninstitutionalized U.S. population and provided weights to adjust for nonresponse and the probability of selection into each ANA subsample (14). All participants completed questionnaires and most provided blood specimens. Available data included demographics, health covariates, measured factors (e.g., height and weight), and constructed variables such as body mass index (BMI). The NHANES protocol was approved by the human subjects Institutional Review Board of the U.S. Centers for Disease Control and Prevention (CDC), and all participants gave written informed consent.
ANA assessment.
Serum samples were shipped with dry ice and stored at −80°C until evaluation by indirect immunofluorescence at a 1:80 dilution using the NOVA Lite HEp-2 ANA slide with DAPI kit (INOVA Diagnostics, San Diego, CA), with a highly specific fluorescein isothiocyanate (FITC)-conjugated secondary antibody (goat anti-human IgG). Images were captured via the NOVA View automated fluorescence microscope system (INOVA Diagnostics) and stored digitally. Immunofluorescence staining intensities were graded 0-4 compared to standard references (8). Values of 1-4 indicated ANA positivity; those graded 3 or 4 were further assessed by sequential ANA titers up to 1:1280 dilution. ANA patterns (including nuclear, cytoplasmic, or mitotic) were defined according to international consensus (15). All samples were assayed using the same methods in a single laboratory. Readings were made independently by at least two experienced evaluators (blinded to sample characteristics and time period), who agreed on >95% of the intensities and patterns; differences were resolved by consensus or adjudicated by a third blinded rater. Repeat testing of random samples showed >98% concordance.
Participant characteristics.
We considered sex, age, and race/ethnicity as correlates of ANA and possible explanatory variables or modifiers of ANA time trends. Age was categorized by decade for covariate adjustment and into three groups for stratification: adolescents (12-19 years), younger adults (20-49 years), or older adults (≥50 years). Race/ethnicity was categorized as non-Hispanic white, non-Hispanic black, Mexican-American, or other. Using previous covariate definitions (8), we also examined BMI, smoking exposure, alcohol use, poverty income ratio (PIR), and education. The NHANES includes limited data on autoimmune diseases, but self-reports of doctor-diagnosed thyroid disease were available for all participants ≥20 years old across the three time periods.
Statistical analysis.
A dichotomous response variable was created by treating an ANA grade of 0 as negative and grades 1-4 as positive. We estimated period-specific ANA prevalence overall and in subgroups defined by participant characteristics. Estimates and 95% confidence intervals (CIs) were derived from weighted logistic regression models for ANA positivity. The number of people with ANA in the population was estimated from period-specific weighted frequencies. For each period, we evaluated ANA associations with characteristic categories via prevalence odds ratios (ORs) and 95% CIs from weighted logistic models adjusted for sex, age, and race/ethnicity. The overall association of each characteristic with ANA was assessed by an F-test from a statistical contrast.
We investigated ANA time trends overall and in subgroups to explore trend modifiers. We fitted two logistic models to data from all three periods and both models adjusted for sex, age, and race/ethnicity. The first model included a categorical covariate for time period, from which ORs and 95% CIs were calculated to assess how ANA differed in the second and third periods relative to the first. The second model included a quantitative covariate for the time between period midpoints (0, 12, or 22 years) and ANA time trends were assessed by a χ2-test. These exploratory analyses did not formally test if ANA time trends differed across subgroups. Supplemental analyses examined time trends in thyroid disease and the association between thyroid disease and ANA.
All analyses were performed in SAS (version 9.4, Cary, NC, U.S.A.) and all accounted for the survey design variables (strata, clusters, and sampling weights). The sampling weights allow for population-representative estimates, adjusted for nonresponse and selection probabilities (14). We used the SurveyLogistic procedure to perform the logistic analyses, with domain statements to properly handle the sampling weights in subgroup analyses. Variance estimates (for the CIs) were obtained via the Taylor series method. Reported P values were 2-sided and unadjusted for multiple comparisons, though multiplying the P values by the number of comparisons would provide a conservative Bonferroni-type correction.
Ethics committee approval.
Written informed consent was obtained from all cases and this study was approved by the U.S. CDC research ethics board.
RESULTS
Participant characteristics and ANA prevalence.
Sample characteristics, for each period separately and combined, are shown in Table 1. Certain characteristics changed over time (e.g., smoking decreased, whereas obesity and drinking increased). A total of 1,976 (13.9%) of the 14,211 participants were ANA positive.
Table 1.
Unweighted ANA counts, sample sizes, and category percentages for selected characteristics by time period.
| Period 1: 1988-1991 |
Period 2: 1999-2004 |
Period 3: 2011-2012 |
Combined |
|||||
|---|---|---|---|---|---|---|---|---|
| No. of Participants: | No. of Participants: | No. of Participants: | No. of Participants: | |||||
| Characteristic a | ANA+ | Total (%) | ANA+ | Total (%) | ANA+ | Total (%) | ANA+ | Total (%) |
| Overall | 643 | 4,727 (100) | 580 | 4,749 (100) | 753 | 4,735 (100) | 1,976 | 14,211 (100) |
| Sex | ||||||||
| Male | 216 | 2,363 (50.0) | 169 | 2,282 (48.1) | 265 | 2,338 (49.4) | 650 | 6,983 (49.1) |
| Female | 427 | 2,364 (50.0) | 411 | 2,467 (52.0) | 488 | 2,397 (50.6) | 1,326 | 7,228 (50.9) |
| Age (years) | ||||||||
| Adolescent (12-19) | 45 | 676 (14.3) | 114 | 1,190 (25.1) | 102 | 843 (17.8) | 261 | 2,709 (19.1) |
| Younger Adult (20-49) | 248 | 2,218 (46.9) | 188 | 1,905 (40.1) | 275 | 2,080 (43.9) | 711 | 6,203 (43.7) |
| Older Adult ( ≥ 50 ) | 350 | 1,833 (38.8) | 278 | 1,654 (34.8) | 376 | 1,812 (38.3) | 1,004 | 5,299 (37.3) |
| Race/Ethnicity | ||||||||
| Non-Hispanic White | 252 | 2,060 (43.6) | 248 | 2,115 (44.5) | 269 | 1,669 (35.3) | 769 | 5,844 (41.1) |
| Non-Hispanic Black | 176 | 1,164 (24.6) | 137 | 993 (20.9) | 223 | 1,219 (25.7) | 536 | 3,376 (23.8) |
| Mexican-American | 196 | 1,354 (28.6) | 156 | 1,245 (26.2) | 69 | 543 (11.5) | 421 | 3,142 (22.1) |
| Other | 19 | 149 ( 3.2) | 39 | 396 ( 8.3) | 192 | 1,304 (27.5) | 250 | 1,849 (13.0) |
| Body Mass Index (BMI) | ||||||||
| Underweight/Healthy | 293 | 2,191 (46.5) | 238 | 1,880 (39.7) | 249 | 1,729 (37.1) | 780 | 5,800 (41.1) |
| Overweight | 197 | 1,483 (31.5) | 160 | 1,462 (30.9) | 224 | 1,361 (29.2) | 581 | 4,306 (30.5) |
| Obese | 150 | 1,036 (22.0) | 181 | 1,393 (29.4) | 264 | 1,571 (33.7) | 595 | 4,000 (28.4) |
| Smoking Exposure | ||||||||
| None | 88 | 411 ( 9.1) | 274 | 1,929 (40.9) | 446 | 2,612 (55.2) | 808 | 4,952 (35.4) |
| Second-Hand | 352 | 2,788 (61.6) | 212 | 1,752 (37.1) | 166 | 1,154 (24.4) | 730 | 5,694 (40.7) |
| Active | 164 | 1,326 (29.3) | 92 | 1,039 (22.0) | 140 | 967 (20.4) | 396 | 3,332 (23.8) |
| Alcohol Consumption | ||||||||
| None | 349 | 2,009 (53.0) | 166 | 1,050 (35.4) | 175 | 898 (29.3) | 690 | 3,957 (40.3) |
| Light | 104 | 834 (22.0) | 156 | 1,225 (41.3) | 230 | 1,349 (44.0) | 490 | 3,408 (34.7) |
| Moderate/Heavy | 93 | 948 (25.0) | 49 | 695 (23.4) | 110 | 818 (26.7) | 252 | 2,461 (25.1) |
Abbreviations: ANA = antinuclear antibodies; ANA+ = positive for ANA.
NOTE: Some groups were oversampled in certain cycles (e.g., adolescents in 1999-2004 and Asian-Americans in 2011-2012).
BMI was categorized as underweight/healthy, overweight, or obese using standard cut points of <25, 25 to <30, or ≥30 kg/m2 for persons ≥20 years old and by applying 2000 CDC growth chart percentiles of <85, 85 to <95, or ≥95 for persons 12-19 years old. Smoking exposure was based on current measured cotinine levels and classified as none (<0.05 ng/ml), second-hand (0.05 to 15 ng/ml), or active (>15 ng/ml). Alcohol consumption (available for ages ≥20 years) was based on the number of drinks in the past year and classified as none (<12 total), light (1-3 per week), or moderate/heavy (>3 per week).
Adjusting for the survey design variables, but not for covariates, yielded population-representative ANA prevalence estimates of 11.0% (CI=9.7%-12.6%) in 1988-1991, 11.5% (CI=10.3%-12.8%) in 1999-2004, and 15.9% (CI=14.3%-17.6%) in 2011-2012 (see Figure 1 for a visual display and Table 2 for numerical values). These estimates correspond to approximately 22 (CI=19-25), 27 (CI=23-30), and 41 (CI=34-48) million ANA-positive persons, respectively.
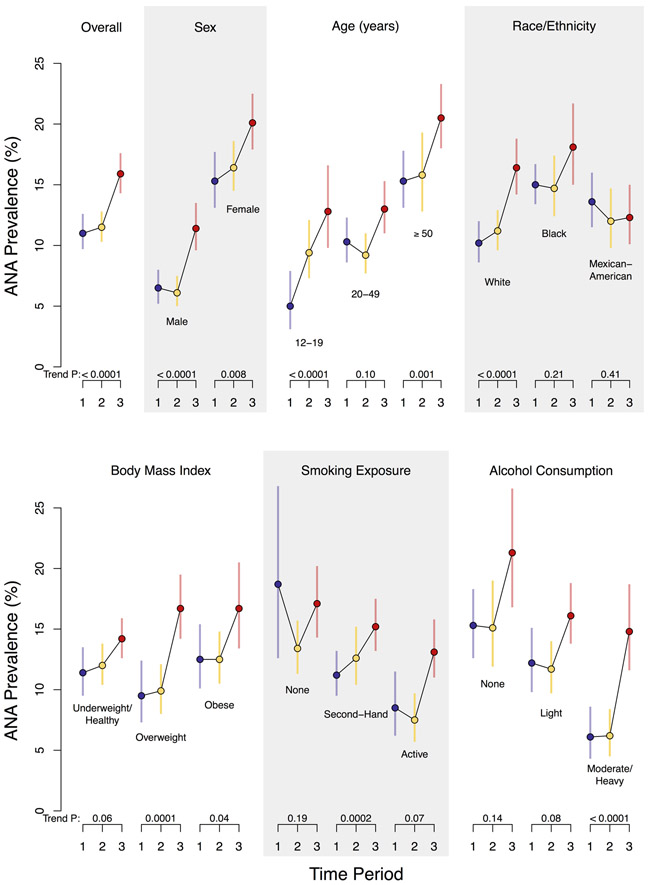
Figure 1. Prevalence of antinuclear antibodies (ANA) by time period in the U.S. population and selected subgroups.
Each circle represents a weighted estimate of ANA prevalence and the vertical bar denotes its 95% confidence interval, with blue coloring for Period 1 (1988-1991), yellow for Period 2 (1999-2004), and red for Period 3 (2011-2012). The estimates for the three periods are connected by black lines to visualize time trends. For each period, the prevalence estimate was derived from a logistic regression model for ANA positivity that adjusted for the survey design variables (strata, clusters, and sampling weights) and a single categorical covariate for the characteristic defining the subgroup. Participants with missing subgroup data (for BMI, smoking exposure, or alcohol consumption) were excluded from those analyses. The P value to assess the strength of evidence for an ANA time trend is displayed below each characteristic category and was derived from a logistic regression model that additionally adjusted for sex, age, and race/ethnicity.
Table 2.
Weighted estimates of ANA prevalence for selected characteristics by time period.
| Weighted Estimate of ANA Prevalence (95% CI) as a Percentage a | |||
|---|---|---|---|
| Characteristic b | Period 1: 1988-1991 | Period 2: 1999-2004 | Period 3: 2011-2012 |
| Overall | 11.0 ( 9.7 - 12.6) | 11.5 (10.3 - 12.8) | 15.9 (14.3 - 17.6) |
| Sex | |||
| Male | 6.5 ( 5.2 - 8.0) | 6.1 ( 5.0 - 7.5) | 11.4 ( 9.6 - 13.5) |
| Female | 15.3 (13.1 - 17.7) | 16.4 (14.5 - 18.6) | 20.1 (17.9 - 22.5) |
| Age (years) | |||
| Adolescent (12-19) | 5.0 ( 3.1 - 7.9) | 9.4 ( 7.3 - 12.1) | 12.8 ( 9.8 - 16.6) |
| Younger Adult (20-49) | 10.3 ( 8.6 - 12.3) | 9.2 ( 7.7 - 11.0) | 13.0 (11.0 - 15.3) |
| Older Adult ( ≥ 50 ) | 15.3 (13.1 - 17.8) | 15.8 (12.8 - 19.3) | 20.5 (18.0 - 23.3) |
| Race/Ethnicity | |||
| Non-Hispanic White | 10.2 ( 8.6 - 12.0) | 11.2 ( 9.6 - 12.9) | 16.4 (14.2 - 18.8) |
| Non-Hispanic Black | 15.0 (13.4 - 16.7) | 14.7 (12.4 - 17.4) | 18.1 (15.0 - 21.7) |
| Mexican-American | 13.6 (11.5 - 16.0) | 12.0 ( 9.8 - 14.7) | 12.3 (10.1 - 15.0) |
| Other | 12.0 ( 6.2 - 21.9) | 9.5 ( 6.9 - 13.0) | 14.0 (12.0 - 16.2) |
| Body Mass Index (BMI) | |||
| Underweight/Healthy | 11.4 ( 9.5 - 13.5) | 12.0 (10.4 - 13.8) | 14.2 (12.6 - 15.9) |
| Overweight | 9.5 ( 7.3 - 12.4) | 9.9 ( 8.0 - 12.1) | 16.7 (14.2 - 19.5) |
| Obese | 12.5 (10.1 - 15.4) | 12.5 (10.5 - 14.8) | 16.7 (13.4 - 20.5) |
| Smoking Exposure | |||
| None | 18.7 (12.6 - 26.8) | 13.4 (11.3 - 15.7) | 17.1 (14.3 - 20.2) |
| Second-Hand | 11.2 ( 9.5 - 13.2) | 12.6 (10.4 - 15.2) | 15.2 (13.2 - 17.5) |
| Active | 8.5 ( 6.2 - 11.5) | 7.5 ( 5.7 - 9.7) | 13.1 ( 10.9 - 15.8) |
| Alcohol Consumption | |||
| None | 15.3 (12.6 - 18.3) | 15.1 (11.9 - 19.0) | 21.3 (16.8 - 26.6) |
| Light | 12.2 ( 9.8 - 15.1) | 11.7 ( 9.7 - 14.0) | 16.1 (13.8 - 18.8) |
| Moderate/Heavy | 6.1 ( 4.3 - 8.6) | 6.2 ( 4.5 - 8.4) | 14.8 (11.6 - 18.7) |
Abbreviations: ANA = antinuclear antibodies; CI = confidence interval.
The weighted estimate of ANA prevalence was derived from a logistic regression model that adjusted for the survey design variables (strata, clusters, and sampling weights) and a categorical covariate for the characteristic of interest but not for other covariates. The estimated numbers of persons with ANA in the U.S. (with CI) in millions are: 22 (CI=19-25) for Period 1, 27 (CI=23-30) for Period 2, and 41 (CI=34-48) for Period 3.
BMI was categorized as underweight/healthy, overweight, or obese using standard cut points of <25, 25 to <30, or ≥30 kg/m2 for persons ≥20 years old and by applying 2000 CDC growth chart percentiles of <85, 85 to <95, or ≥95 for persons 12-19 years old. Smoking exposure was based on current measured cotinine levels and classified as none (<0.05 ng/ml), second-hand (0.05 to 15 ng/ml), or active (>15 ng/ml). Alcohol consumption (available for ages ≥20 years) was based on the number of drinks in the past year and classified as none (<12 total), light (1-3 per week), or moderate/heavy (>3 per week).
ANA correlates.
Weighted but unadjusted analyses supported several known associations, including higher ANA prevalence in females and older adults (Table 2). Among non-Hispanics, blacks had a higher prevalence than whites in 1988-1991, but that difference was attenuated in 2011-2012 consequent to the steeper increasing time trend in whites. Also, ANA prevalence was higher in non-smokers than active smokers, and in non-drinkers versus moderate/heavy drinkers.
Covariate-adjusted models confirmed several ANA correlates (Table 3). All three periods showed an ANA association with sex (P<0.0001) and age (P≤0.002), whereas evidence of an ANA association with other characteristics was either lacking or varied across periods. The odds of having ANA were 2-3 times higher for females than males, with OR=2.53 (CI=1.90-3.36) in 1988-1991, OR=2.97 (CI=2.30-3.84) in 1999-2004, and OR=1.94 (CI=1.57-2.40) in 2011-2012. Similarly, the period-specific ANA odds ratios for older adults relative to adolescents were OR=3.63 (CI=2.02-6.55), OR=1.80 (CI=1.23-2.63), and OR=1.71 (CI=1.21-2.42), respectively. Relative to non-Hispanic whites, the odds of having ANA were higher for non-Hispanic blacks (OR=1.75; CI=1.33-2.31) and Mexican-Americans (OR=1.87; CI=1.40-2.50) in 1988-1991, but racial/ethnic differences diminished in 1999-2004 and 2011-2012. Compared with being underweight/healthy, the period-specific ANA associations with being overweight or obese transitioned from inverse to positive across the three periods, though all CIs included the null value of 1.0. The ANA associations for active smokers versus nonsmokers were inverse in all three periods, but most CIs included 1.0. Compared with no alcohol use, moderate/heavy drinking was inversely associated with ANA in 1988-1991 (OR=0.56; CI=0.34-0.92) and 1999-2004 (OR=0.64; CI=0.44-0.94), but not in 2011-2012, as support for an overall ANA association with alcohol use lessened over time.
Table 3.
Covariate-adjusted estimates of ANA associations with selected characteristics by time period. a
| ANA Prevalence Odds Ratio (95% CI) for Characteristic Category | ||||
|---|---|---|---|---|
| Characteristic b | Period 1: 1988-1991 | Period 2: 1999-2004 | Period 3: 2011-2012 | |
| Sex | ||||
| Male | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | |
| Female | 2.53 (1.90 - 3.36) | 2.97 (2.30 - 3.84) | 1.94 (1.57 - 2.40) | |
| P: | < 0.0001 | < 0.0001 | < 0.0001 | |
| Age (years) | ||||
| Adolescent (12-19) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | |
| Younger Adult (20-49) | 2.27 (1.43 - 3.62) | 0.97 (0.66 - 1.42) | 1.00 (0.73 - 1.38) | |
| Older Adult ( ≥ 50 ) | 3.63 (2.02 - 6.55) | 1.80 (1.23 - 2.63) | 1.71 (1.21 - 2.42) | |
| P: | 0.0007 | 0.002 | 0.0009 | |
| Race/Ethnicity | ||||
| Non-Hispanic White | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | |
| Non-Hispanic Black | 1.75 (1.33 - 2.31) | 1.47 (1.13 - 1.92) | 1.20 (0.95 - 1.51) | |
| Mexican-American | 1.87 (1.40 - 2.50) | 1.34 (1.01 - 1.76) | 0.87 (0.63 - 1.19) | |
| Other | 1.39 (0.62 - 3.13) | 0.90 (0.60 - 1.35) | 0.90 (0.70 - 1.15) | |
| P: | 0.0007 | 0.03 | 0.18 | |
| Body Mass Index (BMI) | ||||
| Underweight/Healthy | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | |
| Overweight | 0.74 (0.54 - 1.02) | 0.83 (0.62 - 1.11) | 1.20 (0.96 - 1.49) | |
| Obese | 0.90 (0.65 - 1.25) | 1.00 (0.79 - 1.27) | 1.13 (0.88 - 1.46) | |
| P: | 0.19 | 0.39 | 0.10 | |
| Smoking Exposure | ||||
| None | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | |
| Second-Hand | 0.68 (0.44 - 1.05) | 1.14 (0.87 - 1.50) | 1.02 (0.79 - 1.32) | |
| Active | 0.56 (0.31 - 1.01) | 0.70 (0.50 - 0.97) | 0.83 (0.55 - 1.23) | |
| P: | 0.13 | 0.07 | 0.54 | |
| Alcohol Consumption | ||||
| None | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | |
| Light | 1.07 (0.70 - 1.63) | 0.96 (0.66 - 1.40) | 0.90 (0.65 - 1.25) | |
| Moderate/Heavy | 0.56 (0.34 - 0.92) | 0.64 (0.44 - 0.94) | 0.91 (0.62 - 1.34) | |
| P: | 0.03 | 0.06 | 0.80 | |
Abbreviations: ANA = antinuclear antibodies; CI = confidence interval.
The ANA association with each characteristic category was assessed by estimating a period-specific odds ratio under a logistic regression model that adjusted for the survey design variables (strata, clusters, and sampling weights) and categorical covariates for sex, age, race/ethnicity, and the characteristic of interest. The P value for assessing a characteristic’s overall association with ANA in a given time period was based on an F-test from a statistical contrast.
BMI was categorized as underweight/healthy, overweight, or obese using standard cut points of <25, 25 to <30, or ≥30 kg/m2 for persons ≥20 years old and by applying 2000 CDC growth chart percentiles of <85, 85 to <95, or ≥95 for persons 12-19 years old. Smoking exposure was based on current measured cotinine levels and classified as none (<0.05 ng/ml), second-hand (0.05 to 15 ng/ml), or active (>15 ng/ml). Alcohol consumption (available for ages ≥20 years) was based on the number of drinks in the past year and classified as none (<12 total), light (1-3 per week), or moderate/heavy (>3 per week).
ANA time trends.
There was strong evidence that ANA prevalence increased over time, primarily from the second to the third period (Table 4). Adjusted for covariates, estimated ORs for the second and third time periods relative to the first were 1.02 (CI=0.85-1.24) and 1.47 (CI=1.22-1.78), respectively, reflecting an overall ANA time trend (P<0.0001). In stratified analyses, the ANA time trend was seen in both males (P<0.0001) and females (P=0.008). Within age subgroups, the time trend was clearly apparent in adolescents (P<0.0001), with ORs that steadily increased across all periods (from 1.00 to 2.02 to 2.88). Although we observed no time trend in adults 20-49 years old, ANA prevalence increased over time in adults aged 50 or older (P=0.001). ANA time trends were also apparent in other subgroups (Table 4), notably non-Hispanic whites, overweight participants, second-hand smokers, and moderate/heavy drinkers. Further adjustment for BMI, smoking, or alcohol (in addition to sex, age, and race/ethnicity) had little impact on the ANA time trends.
Table 4.
Covariate-adjusted assessments of ANA time trends in selected characteristic-based subgroups. a
| Number of Participants ANA+ / Total |
ANA Prevalence Odds Ratio (95% CI) for Time Period | Trend P |
|||
|---|---|---|---|---|---|
| Characteristic b | Period 1: 1988-1991 | Period 2: 1999-2004 | Period 3: 2011-2012 | ||
| Overall | 1,976 / 14,211 | 1.00 (reference) | 1.02 (0.85 - 1.24) | 1.47 (1.22 - 1.78) | <0.0001 |
| Sex | |||||
| Male | 650 / 6,983 | 1.00 (reference) | 0.91 (0.67 - 1.23) | 1.73 (1.31 - 2.30) | <0.0001 |
| Female | 1,326 / 7,228 | 1.00 (reference) | 1.07 (0.85 - 1.36) | 1.35 (1.08 - 1.69) | 0.008 |
| Age (years) | |||||
| Adolescent (12-19) | 261 / 2,709 | 1.00 (reference) | 2.02 (1.16 - 3.53) | 2.88 (1.64 - 5.04) | <0.0001 |
| Younger Adult (20-49) | 711 / 6,203 | 1.00 (reference) | 0.86 (0.65 - 1.14) | 1.26 (0.96 - 1.66) | 0.10 |
| Older Adult ( ≥ 50 ) | 1,004 / 5,299 | 1.00 (reference) | 1.07 (0.79 - 1.44) | 1.51 (1.17 - 1.95) | 0.001 |
| Race/Ethnicity | |||||
| Non-Hispanic White | 769 / 5,844 | 1.00 (reference) | 1.09 (0.85 - 1.38) | 1.66 (1.30 - 2.12) | <0.0001 |
| Non-Hispanic Black | 536 / 3,376 | 1.00 (reference) | 0.94 (0.74 - 1.19) | 1.16 (0.91 - 1.48) | 0.21 |
| Mexican-American | 421 / 3,142 | 1.00 (reference) | 0.83 (0.62 - 1.11) | 0.85 (0.62 - 1.17) | 0.41 |
| Other | 250 / 1,849 | 1.00 (reference) | 0.75 (0.34 - 1.62) | 1.15 (0.57 - 2.31) | 0.44 |
| Body Mass Index (BMI) | |||||
| Underweight/Healthy | 780 / 5,800 | 1.00 (reference) | 1.04 (0.81 - 1.33) | 1.26 (1.00 - 1.59) | 0.06 |
| Overweight | 581 / 4,306 | 1.00 (reference) | 1.03 (0.72 - 1.47) | 1.88 (1.33 - 2.65) | 0.0001 |
| Obese | 595 / 4,000 | 1.00 (reference) | 1.04 (0.76 - 1.43) | 1.43 (0.99 - 2.08) | 0.04 |
| Smoking Exposure | |||||
| None | 515 / 2,974 | 1.00 (reference) | 0.73 (0.45 - 1.18) | 1.02 (0.62 - 1.67) | 0.19 |
| Second-Hand | 1,023 / 7,672 | 1.00 (reference) | 1.25 (0.94 - 1.65) | 1.65 (1.29 - 2.12) | 0.0002 |
| Active | 396 / 3,332 | 1.00 (reference) | 0.83 (0.55 - 1.25) | 1.42 (0.99 - 2.04) | 0.07 |
| Alcohol Consumption | |||||
| None | 690 / 3,957 | 1.00 (reference) | 0.96 (0.67 - 1.37) | 1.37 (0.96 - 1.96) | 0.14 |
| Light | 490 / 3,408 | 1.00 (reference) | 0.86 (0.61 - 1.21) | 1.26 (0.92 - 1.73) | 0.08 |
| Moderate/Heavy | 252 / 2,461 | 1.00 (reference) | 1.04 (0.63 - 1.69) | 2.41 (1.55 - 3.75) | <0.0001 |
Abbreviations: ANA = antinuclear antibodies; ANA+ = positive for ANA; CI = confidence interval.
The ANA time trend assessments were based on two logistic regression models that adjusted for the survey design variables (strata, clusters, and sampling weights) and categorical covariates for sex, age, and race/ethnicity. One model added a categorical covariate for time period and estimated the ANA prevalence odds ratio for each period, relative to the first. The other model added a quantitative covariate for the number of years between period midpoints, relative to the first, and produced a P value from a χ2-test to assess an ANA time trend.
BMI was categorized as underweight/healthy, overweight, or obese using standard cut points of <25, 25 to <30, or ≥30 kg/m2 for persons ≥20 years old and by applying 2000 CDC growth chart percentiles of <85, 85 to <95, or ≥95 for persons 12-19 years old. Smoking exposure was based on current measured cotinine levels and classified as none (<0.05 ng/ml), second-hand (0.05 to 15 ng/ml), or active (>15 ng/ml). Alcohol consumption (available for ages ≥20 years) was based on the number of drinks in the past year and classified as none (<12 total), light (1-3 per week), or moderate/heavy (>3 per week).
Supplemental analyses.
We performed supplemental analyses to assess possible ANA correlates and time trends within additional subgroups, such as those based on finer age groups, sex/age combinations, smoking history, PIR, and education (Supplementary Tables 1-4). Though there was little indication of an overall ANA association with smoking history, PIR, or education, we found strong evidence of increasing ANA time trends in the higher income (P<0.0001) and higher education (P=0.0007) subgroups.
To further explore changes in ANA over time, we considered trends in ANA staining intensities, titers, and patterns in ANA-positive participants. None of these factors was informative, though there was weak evidence suggesting that “mitotic” patterns increased over time (Supplementary Table 5).
We also investigated changes over time in the prevalence of thyroid disease, and its association with ANA. The overall prevalence of self-reported, doctor-diagnosed thyroid disease increased across the three periods (trend P<0.0001), as well as in various sex-by-age subgroups (Supplementary Table 6). In each period, ANA rates were higher among those with thyroid disease (21-24%) compared to those without (12-16%).
DISCUSSION
Most autoimmune diseases are persistent conditions, with unknown etiologies and diverse pathologies. They impact as many as one in 20 individuals in the adult U.S. population, with substantial personal and societal costs. Recent studies suggest the incidence of some autoimmune diseases may be increasing (4-6). However, true temporal trends are difficult to determine due to the lack of national registries and changes in the assessment and diagnosis of specific diseases (16). We hypothesized that the prevalence of ANA, an objective and common biomarker of autoimmunity, may also have increased over time.
The NHANES databases and serum repositories provided a unique opportunity to assess this hypothesis in nationally-representative samples of the U.S. population ≥12 years old across three time periods (1988-1991, 1999-2004, and 2011-2012). As expected, a considerable proportion of the population had ANA. Our novel and robust findings suggest that ANA prevalence increased substantially in the U.S. over the 25-year timeframe examined, rising from 11.0% in 1988-1991 to 11.5% in 1999-2004 to 15.9% in 2011-2012, which corresponds to 22, 27, and 41 million affected persons, respectively. We adjusted for sex, age, and race/ethnicity and found positive ANA time trends overall and in certain subgroups. Further adjustment for key health characteristics, some of which have shifted in recent years (e.g., obesity, smoking, and drinking), had little impact.
Growing evidence suggests that autoantibodies precede the onset of symptomatic autoimmune disease by many years (17, 18); thus, ANA may be an intermediate marker on the pathway to disease or may signal increased susceptibility to autoimmune diseases through related causal pathways. ANA have also been associated with other factors, including chemical exposures, infections, medications, and parity (9, 11-13), some of which are likely changing in frequency in the U.S. population. Like ANA, autoimmune thyroid disease is more common in women and increases with age (19). Additionally, an elevated prevalence of ANA has been seen in patients with thyroid disease (20). In exploratory analyses of the same samples of NHANES data, we observed both an increasing prevalence of self-reported thyroid disease and an association between thyroid disease and ANA. Because trends in ANA could be a marker of increasing susceptibility to developing autoimmune diseases, the concurrent time trends in thyroid disease and ANA exemplify the potential clinical relevance of our broader findings.
Our previous research identified several ANA correlates (8). The present study confirmed that ANA prevalence increased with age and was relatively high in females and non-Hispanic blacks. Obesity and overweight have increased dramatically in the population, and, though statistical support was weak, our results suggest a possibly shifting association between ANA prevalence and overweight: from inverse associations in the first two periods to a positive association in the third period (when ANA prevalence also increased the most). As higher BMI has been associated with risk of systemic autoimmune diseases, such as systemic lupus erythematosus and rheumatoid arthritis (21, 22), further study is needed to understand the relationship of ANA with BMI. While smoking is a risk factor for some autoimmune diseases, it appears protective for others (23). Current smoking was weakly associated with lower ANA. Rates of smoking have decreased in the population, but inclusion of smoking in our models had little impact on the observed ANA time trends. The data also suggested a possible inverse association between ANA and alcohol consumption in the first two time periods. These findings are in part consistent with growing evidence, including that from two recent prospective cohorts, of a possible protective role of moderate alcohol consumption on the risk of developing lupus (24, 25). Thus, further investigation is needed to understand and expand on these issues.
Our study had several strengths. The ANA subsamples were large, spanned 25 years, and were representative of the U.S. population. Also, all ANA assays were performed in the same laboratory and used the same methods. In addition, our analyses accounted for sociodemographic factors and various health behaviors as potential trend modifiers.
Our findings, however, should be interpreted in the context of certain limitations: associations were based on cross-sectional data rather than repeated measures; some variables were self-reported, including the limited questionnaire data on autoimmune diseases; ANA were not assessed in children <12 years old; and NHANES excludes institutionalized participants, such as the elderly in residential care. Although some of the serum samples were three decades old, there were no gross differences in appearance or behavior of the samples to suggest degradation, and antibodies are known to be stable over time in frozen storage (26). Moreover, the observed time trends were not apparent in all subgroups, as might be expected if the age of the specimens was influencing the measured levels of ANA.
Recently, Pisetsky and colleagues (27) reconfirmed that different ANA assay kits can give different results. They were interested in assessing variation in ANA assays, and thus used three ANA kits, an ANA ELISA, and a bead-based multiplex assay, whereas we purposely used a single assay (performed in one laboratory) to provide as much consistency as possible in our evaluation of ANA changes over time. We used the NOVA View system due to familiarity, previous good experiences, and the need to improve efficiency for the large number of samples in our study by using a semi-automated system; thus, we were restricted to the ANA assay that accompanied the system and we knew that this assay can detect some autoantibodies that others cannot (e.g., autoantibodies to cytoplasmic rods and rings). Using another assay could have led to systematically higher or lower ANA prevalence estimates, but we focused on trends across time periods. Even if period-specific estimates shift upward or downward with one assay versus another, presumably the same trends would be seen across time.
The reported P values were not adjusted for multiple comparisons and some apparent trends and associations could be due to chance. Nevertheless, our main finding that ANA increased over time is consistent, with P<0.0001 for the trend overall and in many subgroups, so these P values would remain noteworthy even after making conservative Bonferroni-type corrections that multiply by the number of comparisons.
The standard HEp-2 assay for ANA detects a heterogeneous group of autoantibodies and is a commonly used diagnostic tool in a clinical context (15). However, relatively little is known about the natural history of ANA, absent an autoimmune disease. Given that memory B-cells typically persist once tolerance to self-antigens is broken, currently-detected ANA may reflect both past and recent exposures. Our cross-sectional data cannot determine the timing of ANA development relative to aging and other factors, such as smoking; however, observed differences across demographic subgroups or covariates suggest research opportunities to better understand the determinants of autoimmunity and autoimmune diseases. The ANA staining pattern is an important consideration for understanding the relevance of ANA in symptomatic and healthy populations. One common staining pattern, dense-fine-speckled (DFS), has been associated with anti-DFS70 autoantibodies and may be more common in healthy individuals than those with autoimmune diseases (28, 29); however, neither this nor other ANA patterns appeared to explain the increasing ANA time trends seen in our study. The autoantigens recognized by the mitotic staining pattern, which showed weak evidence of increasing over time, are poorly understood and have uncertain clinical implications (15, 30).
Although ANA prevalence increased across the three periods in many subgroups, the rate and timing of this rise were not always the same, especially with respect to age. Reasons for the generation of ANA at different times across the lifespan may vary. For example, the occurrence of ANA in older adults may be related to immunosenescence (31) or to exposures that increase with age, such as medications. Notably, while ANA prevalence was highest in adults ≥70 years old, it varied little over time in this age group (21-24%; Supplementary Table 2). In contrast, ANA prevalence in adolescents aged 12-19 years increased dramatically from 5% to 9% to 13% across the three periods. While investigations of ANA in healthy children are limited (32, 33), potential explanations for an increase in ANA prevalence include changes in perinatal or early-life exposures, such as childhood infections or other types of exposures during developmentally sensitive periods, possibly leading to dysregulated immunity. The rising ANA time trend observed in this age group may be particularly concerning if ANA are harbingers of increased susceptibility to future autoimmune diseases.
In conclusion, the overall prevalence of ANA in the U.S. increased from 1988 to 2012, with a larger increase in recent years. Both sex and age were consistently strong ANA correlates, while ANA associations with race/ethnicity, BMI, smoking exposure, and alcohol consumption varied over time. The positive ANA time trends were most pronounced in adolescents, males, and non-Hispanic whites. Additional studies to complement our exploratory investigation, particularly of the aforementioned sociodemographic groups, should be the focus of future research to determine the driving forces underlying these ANA increases and to inform the development of possible preventative measures.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Drs. Charles Dillon, Helen Meier, and Paivi Salo for helpful comments, to Justin Nicholas and Rodrigo Mora for technical laboratory assistance, to Dr. Geraldine McQuillan for administrative and regulatory help, to Wayne Pereanu for technical editing, and to members of the NHANES Autoimmunity Study Group (including Drs. Linda Birnbaum, Richard Cohn, Dori Germolec, Minoru Satoh, Nigel Walker, and Irene Whitt) for initiating the studies that motivated much of this research. We thank Dr. Michael Mahler and Inova Diagnostics for providing the use of the NOVA View automated fluorescence microscope system for data collection.
This research was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Environmental Health Sciences under projects Z01 ES025041 and Z01 ES101074, and under contract HHSN273201600011C to Social & Scientific Systems. The interpretation and conclusions contained herein are those of the authors and do not necessarily represent positions of any of the authors’ affiliations.
There was no financial support nor other benefits from commercial sources for the work reported on in the manuscript, and there are no other financial interests of the authors, which could create a potential conflict of interest or the appearance of a conflict of interest with regard to the work.
REFERENCES
- 1.Davidson A, Diamond B. Autoimmune diseases. N Engl J Med. 2001;345(5):340–50. [DOI] [PubMed] [Google Scholar]
- 2.Wang L, Wang FS, Gershwin ME. Human autoimmune diseases: a comprehensive update. J Intern Med. 2015;278(4):369–95. [DOI] [PubMed] [Google Scholar]
- 3.Bach JF. The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med. 2002;347(12):911–20. [DOI] [PubMed] [Google Scholar]
- 4.Lerner A, Jeremias P, Matthias T. The World Incidence and Prevalence of Autoimmune Diseases is Increasing. International J of Celiac Disease. 2015;3(4):151–5. [Google Scholar]
- 5.Fatoye F, Gebrye T, Svenson LW. Real-world incidence and prevalence of systemic lupus erythematosus in Alberta, Canada. Rheumatol Int. 2018;38(9):1721–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mayer-Davis EJ, Lawrence JM, Dabelea D, Divers J, Isom S, Dolan L, et al. Incidence Trends of Type 1 and Type 2 Diabetes among Youths, 2002-2012. N Engl J Med. 2017;376(15):1419–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmidt CW. Questions persist: environmental factors in autoimmune disease. Environ Health Perspect. 2011;119(6):A249–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Satoh M, Chan EK, Ho LA, Rose KM, Parks CG, Cohn RD, et al. Prevalence and sociodemographic correlates of antinuclear antibodies in the United States. Arthritis Rheum. 2012;64(7):2319–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parks CG, Miller FW, Satoh M, Chan EK, Andrushchenko Z, Birnbaum LS, et al. Reproductive and hormonal risk factors for antinuclear antibodies (ANA) in a representative sample of U.S. women. Cancer Epidemiol Biomarkers Prev. 2014;23(11):2492–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liao KP, Kurreeman F, Li G, Duclos G, Murphy S, Guzman R, et al. Associations of autoantibodies, autoimmune risk alleles, and clinical diagnoses from the electronic medical records in rheumatoid arthritis cases and non-rheumatoid arthritis controls. Arthritis Rheum. 2013;65(3):571–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang C, Gershwin ME. Drugs and autoimmunity--a contemporary review and mechanistic approach. J Autoimmun. 2010;34(3):J266–J75. [DOI] [PubMed] [Google Scholar]
- 12.Miller FW, Alfredsson L, Costenbader KH, Kamen DL, Nelson LM, Norris JM, et al. Epidemiology of environmental exposures and human autoimmune diseases: findings from a National Institute of Environmental Health Sciences Expert Panel Workshop. J Autoimmun. 2012;39(4):259–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dinse GE, Jusko TA, Whitt IZ, Co CA, Parks CG, Satoh M, et al. Associations between selected xenobiotics and antinuclear antibodies in the National Health and Nutrition Examination Survey, 1999-2004. Environ Health Perspect. 2016;124(4):426–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson CL, Paulose-Ram R, Ogden CL, Carroll MD, Kruszon-Moran D, Dohrmann SM, et al. National Health and Nutrition Examination Survey: analytic guidelines, 1999-2010. Vital Health Stat 2 2013(161):1–24. [PubMed] [Google Scholar]
- 15.Damoiseaux J, Andrade LEC, Carballo OG, Conrad K, Francescantonio PLC, Fritzler MJ, et al. Clinical relevance of HEp-2 indirect immunofluorescent patterns: the International Consensus on ANA patterns (ICAP) perspective. Ann Rheum Dis. 2019;78(7):879–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ungprasert P, Sagar V, Crowson CS, Amin S, Makol A, Ernste FC, et al. Incidence of systemic lupus erythematosus in a population-based cohort using revised 1997 American College of Rheumatology and the 2012 Systemic Lupus International Collaborating Clinics classification criteria. Lupus. 2017;26(3):240–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arbuckle MR, McClain MT, Rubertone MV, Scofield RH, Dennis GJ, James JA, et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med. 2003;349(16):1526–33. [DOI] [PubMed] [Google Scholar]
- 18.Lingampalli N, Sokolove J, Lahey LJ, Edison JD, Gilliland WR, Holers VM, et al. Combination of anti-citrullinated protein antibodies and rheumatoid factor is associated with increased systemic inflammatory mediators and more rapid progression from preclinical to clinical rheumatoid arthritis. Clin Immunol. 2018;195:119–26. [DOI] [PubMed] [Google Scholar]
- 19.Stathatos N, Daniels GH. Autoimmune thyroid disease. Curr Opin Rheumatol. 2012;24(1):70–5. [DOI] [PubMed] [Google Scholar]
- 20.Tektonidou MG, Anapliotou M, Vlachoyiannopoulos P, Moutsopoulos HM. Presence of systemic autoimmune disorders in patients with autoimmune thyroid diseases. Ann Rheum Dis. 2004;63(9):1159–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tedeschi SK, Barbhaiya M, Malspeis S, Lu B, Sparks JA, Karlson EW, et al. Obesity and the risk of systemic lupus erythematosus among women in the Nurses’ Health Studies. Semin Arthritis Rheum. 2017;47(3):376–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tedeschi SK, Cui J, Arkema EV, Robinson WH, Sokolove J, Lingampalli N, et al. Elevated BMI and antibodies to citrullinated proteins interact to increase rheumatoid arthritis risk and shorten time to diagnosis: A nested case-control study of women in the Nurses’ Health Studies. Semin Arthritis Rheum. 2017;46(6):692–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perricone C, Versini M, Ben-Ami D, Gertel S, Watad A, Segel MJ, et al. Smoke and autoimmunity: The fire behind the disease. Autoimmun Rev. 2016;15(4):354–74. [DOI] [PubMed] [Google Scholar]
- 24.Cozier YC, Barbhaiya M, Castro-Webb N, Conte C, Tedeschi SK, Leatherwood C, et al. Relationship of cigarette smoking and alcohol consumption to incidence of systemic lupus erythematosus in a prospective cohort study of black women. Arthritis Care Res (Hoboken). 2019;71(5):671–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barbhaiya M, Lu B, Sparks JA, Malspeis S, Chang SC, Karlson EW, et al. Influence of alcohol consumption on the risk of systemic lupus erythematosus among women in the Nurses’ Health Study cohorts. Arthritis Care Res (Hoboken). 2017;69(3):384–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Argentieri MC, Pilla D, Vanzati A, Lonardi S, Facchetti F, Doglioni C, et al. Antibodies are forever: a study using 12-26-year-old expired antibodies. Histopathology. 2013;63(6):869–76. [DOI] [PubMed] [Google Scholar]
- 27.Pisetsky DS, Spencer DM, Lipsky PE, Rovin BH. Assay variation in the detection of antinuclear antibodies in the sera of patients with established SLE. Ann Rheum Dis. 2018;77(6):911–3. [DOI] [PubMed] [Google Scholar]
- 28.Mariz HA, Sato EI, Barbosa SH, Rodrigues SH, Dellavance A, Andrade LE. Pattern on the antinuclear antibody-HEp-2 test is a critical parameter for discriminating antinuclear antibody-positive healthy individuals and patients with autoimmune rheumatic diseases. Arthritis Rheum. 2011;63(1):191–200. [DOI] [PubMed] [Google Scholar]
- 29.Mahler M, Andrade LE, Casiano CA, Malyavantham K, Fritzler MJ. Implications for redefining the dense fine speckled and related indirect immunofluorescence patterns. Expert Rev Clin Immunol. 2019;15(5):447–8. [DOI] [PubMed] [Google Scholar]
- 30.Chan EK, Damoiseaux J, Carballo OG, Conrad K, de Melo Cruvinel W, Francescantonio PL, et al. Report of the first international consensus on standardized nomenclature of antinuclear antibody HEp-2 cell patterns 2014-2015. Front Immunol. 2015;6:412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weyand CM, Goronzy JJ. Aging of the immune system. Mechanisms and therapeutic targets. Ann Am Thorac Soc. 2016;13 Suppl 5:S422–s8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sperotto F, Cuffaro G, Brachi S, Seguso M, Zulian F. Prevalence of antinuclear antibodies in schoolchildren during puberty and possible relationship with musculoskeletal pain: a longitudinal study. J Rheumatol. 2014;41(7):1405–8. [DOI] [PubMed] [Google Scholar]
- 33.Hilario MO, Len CA, Roja SC, Terreri MT, Almeida G, Andrade LE. Frequency of antinuclear antibodies in healthy children and adolescents. Clin Pediatr (Phila). 2004;43(7):637–42. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.