Abstract
Oxytocin (OT) has gained considerable interest in recent years as a potential treatment for alcoholism and other substance use disorders. Evidence continues to mount that OT administered either centrally, peripherally or intranasally can decrease ethanol intake in both humans and animal models. The potential mechanisms for the ability of OT to decrease ethanol reward, and importantly, cue- and stress-induced ethanol relapse, are explored by reviewing the specific neuronal circuits involved in mediating these actions and their sensitivity to OT. In addition to dopamine neurons that project from ventral tegmental area (VTA) to nucleus accumbens (NAc) to signal positively reinforcing events, OT receptors (OxTR) are also expressed by dopamine neurons that project from VTA to brain regions that can convey aversive properties of a stimulus. Moreover, OxTR are expressed by non-dopaminergic neurons in the VTA, such as GABA and glutamate neurons, which can both modulate the activity of dopamine VTA neurons locally (in opposite directions) or can project to other brain regions, including the NAc, where it can alter either positive reinforcement or aversion caused by ethanol. The ability of OT to regulate limbic circuitry and the hypothalamic-pituitary-adrenal axis is discussed as a potential mechanism for the ability of OT to inhibit ethanol-induced negative reinforcement. Together, understanding the diversity and complexity of OT regulation of ethanol reward may contribute to more effective use of OT as pharmacotherapy for alcohol use disorder.
Keywords: Oxytocin, alcoholism, ventral tegmental area, dopamine, glutamate, GABA
Oxytocin (OT)1 has emerged in recent years as a potential treatment for alcoholism [see Lee and Weerts 2016]. OT decreased alcohol withdrawal symptoms, cravings and intake, both in humans [Pedersen et al., 2013; Mitchell et al., 2016; Hansson et al., 2018] and animals [Bowen 2011; Peters et al., 2012; MacFadyen et al., 2016; King et al., 2017; Stevenson et al., 2017a; Hansson et al., 2018; King & Becker, 2019; Tunstall et al., 2019]. Although there is face validity to the concept that a critical signal for social reward would effectively treat a substance use disorder that impairs social relationships [Vungkhanching et al., 2004; Caspers et al., 2005], the mechanisms for OT inhibition of ethanol craving and intake are not yet understood. In this review, we focus on potential mechanisms of OT regulation of ethanol reward and intake via an action at the OT receptor (OxTR), as well as evidence for dysregulation of these mechanisms by long-term alcohol exposure. Our goal is to evaluate the potential of OT treatment for alcohol use disorder (AUD) by providing a mechanistic framework for its actions on brain regions that mediate ethanol reward, including neurons in the ventral tegmental area (VTA) that project to nucleus accumbens (NAc) and other forebrain regions. Also included is OT regulation of brain regions that mediate stress and anxiety, since elevated levels of stress and anxiety contribute to increases in ethanol seeking and provide a mechanism for ethanol’s negative reinforcing actions. Evidence for ethanol-induced dysfunction of OT signaling is also summarized in order to support the validity of OT pharmacotherapy for AUD, and identify factors that may limit OT efficacy. This framework is intended to provide a starting point for understanding potential sites in the brain that may contribute to differences in the therapeutic usefulness of OT between patient subpopulations, including women, patients experiencing stress or social problems, and patients with different alcohol use patterns.
OT is a nonapeptide structurally similar to the hormone vasopressin [see Grinevich et al., 2015] that can also bind to vasopressin 1A receptors, albeit with 30–300-fold lower affinity than for OxTR [Mustoe et al., 2019]. Thus, OT actions at vasopressin receptors may also play a part in OT regulation of ethanol intake. The role of vasopressin receptors in ethanol-related behaviors has recently been reviewed [see Harper et al., 2018], therefore, this review will focus on OT actions mediated via OxTR.
Functionally, OT allows for animal species to successfully reproduce and thrive by inducing uterine contractions, lactation, and maternal attachments [see Pedersen et al., 1979; Pedersen et al., 1982] as well as strengthening of pair-bonding and social attachments [see Young and Wang, 2004]. Currently, intranasal (i.n.) OT is used to treat autism, depression, anxiety, psychosis, social dysfunction and other neuropsychiatric disorders [see Kirsch, 2015]. The presumed efficacy of OT in these disorders is based on an increase in social salience and a decrease in stress, both of which are relevant to addiction [see Lee & Weerts, 2016]. In addition to these two mechanisms contributing to the efficacy of OT to inhibit ethanol intake, the potential exists for a direct inhibition by OT of the neuronal substrates underlying the reinforcing properties of ethanol.
1.1. OT Efficacy in Substance Use Disorders
OT decreases drug seeking, withdrawal, and relapse of a number of abused substances including amphetamine-like stimulants, opioids, and cocaine [see McGregor and Bowen, 2012]. OT (40 IU i.n.) decreased stress-induced cravings in marijuana-dependent individuals [McRae-Clark et al., 2013] but not in opioid-dependent patients [Woolley et al., 2016]. OT (20 IU i.n.) decreased cue-induced cravings in daily cigarette smokers [Miller et al., 2016], but 40 IU i.n. OT did not alter stress-induced cigarette smoking [Van Hedger et al., 2018]. Thus, although OT may exert a common action on reward processes shared amongst substances of abuse, there are subtle differences in efficacy based on the drug and context of drug use. AUD likely provides further unique targets for OT regulation including the pharmacologic and caloric properties contributing to ethanol’s positive reinforcement, as well as anxiolysis and stress relief contributing to negative reinforcement by ethanol.
1.2. OT Efficacy in AUD
Clinical evidence for OT treatment of AUD has been promising though limited [see Lee and Weerts et al., 2016]. A small sample of alcohol-dependent subjects receiving 24 IU (i.n.) OT twice daily showed decreased alcohol craving, withdrawal-associated anxiety and need for lorazepam dosing, particularly in patients consuming greater than 16 standard drinks per day [Pedersen et al., 2013]. However, a second clinical trial using the same dosing regimen failed to reproduce OT’s ability to decrease oxazepam use [Melby et al 2019]. This failure was attributed to greater inter-individual variability in oxazepam use in the placebo group as well as a more heterogeneous sample population in terms of sex, age and a history of severe or complicated withdrawal [Melby et al 2019].
Along these lines, OT (40 IU i.n.) decreased cue-induced alcohol craving only in individuals with high attachment anxiety [Mitchell et al., 2016] thereby supporting the possibility that OT is more effective in specific populations of alcoholic patients. In fact, polymorphisms in the OT receptor (OxTR) gene were related to the degree of alcohol-related aggressive behavior [Johansson et al., 2012a and b] indicating that some populations of alcohol drinkers might possess altered postsynaptic responses to OT. As of yet, no studies have been conducted that test OT on ongoing alcohol drinking in humans (e.g. propensity to drink or degree of intoxication). In heavy social drinkers, 24 IU OT (i.n.) reduced neural reactivity to alcohol-related cues in a number of brain regions [Hansson et al., 2018] but whether this effect leads to a decrease in ethanol intake or also occurs in non-alcohol drinking subjects was not investigated. It is also unknown whether repeated OT treatment maintains efficacy without the development of tolerance, which is critical for its use as long-term treatment in alcoholic populations. These questions have been addressed in part by using animal models.
2. OT Inhibition of Ethanol Reinforcement using Animal Models
Using various rodent models of ethanol intake, considerable evidence of OT inhibition of ethanol intake has been reported in the past decade. OT (1 mg/kg i.p.) was first found to decrease free access ethanol intake in adult rats drinking 4.4% ethanol in a beer vehicle, with no effect on water consumption [Bowen et al., 2011]. In mice, OT (10 mg/kg, i.p.) decreased ethanol intake by 50% with no effect on water intake [Peters et al. 2012]. Thus, OT seems capable of selectively inhibiting ethanol intake without inhibiting fluid intake in general.
2.1. Selectivity of OT inhibition of ethanol intake
A major weakness of these initial studies is that they did not control for the high caloric density of ethanol. In addition to OT inhibition of fluid intake [Ryan et al., 2017], the inhibitory effects of OT on caloric intake are well documented [Maejima et al., 2015; Iwasaki et al., 2015]. Subsequent studies found doses of OT that decrease ethanol intake (0.3–1 mg/kg, i.p.) had little to no effect on calorically equal non-ethanol rewards [MacFadyen et al., 2016; King et al., 2017; Tunstall et al., 2019]. Additionally, doses of OT that inhibit ethanol consumption (0.3 – 1 mg/kg i.p.) did not cause sedation or locomotor impairment in male rats [MacFayden et al., 2016; Tunstall et al., 2019], male mice [King et al., 2017], or either male or female prairie voles [Stevenson 2017a]. Moreover, OT reductions in ethanol intake were offset by increases in water consumption [King et al., 2017] further indicating OT at these doses does not decrease fluid intake in general. Unfortunately, whether any of these actions were attributable to OT activation of vasopressin 1A receptors was not tested. A caveat of these studies is that although the purity of oxytocin (IU/mg powder) was not always specified in the methods sections of these animal studies, in general these doses were much higher per kg body weight compared to the doses used in the human studies described above (24–40 IU where 1 IU equals approximately 2 μg pure peptide),
2.2. OT inhibition of ethanol intake is centrally mediated
In the above studies, OT was given systemically but was assumed to act centrally by crossing the blood-brain barrier in small but physiologically relevant amounts [Neumann et al., 2013] or via a feed forward mechanism that increased central OT release [Striepens at al., 2013]. However, peripherally, OT may inhibit ethanol intake via an action on vagal afferents from the gut. Peripherally administered OT suppressed caloric intake via vagal activation of the nucleus tractus solitarius [Iwasaki et al., 2015]. Recent evidence indicates a gut-to-brain neural circuit links sensory neurons in the gut, via vagal neurons and the nucleus tractus solitarius, to activation of midbrain dopamine (DA) neurons such that optogenetic activation of the right vagal sensory ganglion is positively reinforcing [Han et al., 2018]. Ethanol activated vagal sensory afferents [Izbeki et al., 2002] and a subset of vagal sensory neurons that project from the proximal intestine to the nodose ganglia express OxTR [Bai et al., 2019]. Optogenetic activation of these OxTR-expressing sensory neurons inhibited caloric intake by over 50% [Bai et al., 2019], thus opening the possibility that OT regulates gut-to-brain sensations of ethanol intake via an action on the sensory vagus nerve or nodose ganglion. In this way, activation of OxTR in this gut-to-brain circuit might sate ethanol intake, similar to other peripheral receptors in the gut that inhibit ethanol intake [Godlewski et al., 2019].
Even so, varying the route of OT administration and employing OT analogs that do not cross the blood-brain barrier has implicated the importance of a central mechanism for OT inhibition of ethanol intake. Intracerebroventricular (i.c.v.) infusion of OT (0.5–30 μg) decreased ethanol preference and self-administration in male rats [Peters et al., 2016] and alcohol seeking behavior in alcohol-dependent male rats [Hansson et al., 2018] to the same extent as i.p., or i.n. routes of administration [Tunstall et al., 2019]. Inhibition of ethanol intake by OT (1 mg/kg, i.n.) was not altered by systemic administration (5 mg/kg i.p.) of the blood-brain-barrier-impermeable OxTR antagonist L-371,257 [Tunstall et al., 2019]. Along these lines, central administration of the blood-brain-barrier-impermeable OxTR agonist PF-06655075 (30 μg, i.c.v) inhibited ethanol intake to the same degree as 1 mg/kg OT given either i.p. or i.n. yet 1 mg/kg (s.c.) PF-06655075 did not alter ethanol intake [Tunstall et al., 2019]. Furthermore, PF-06655075 is an antagonist at vasopressin 1A receptors [Modi et al, 2018] thereby decreasing the likelihood that OT activation of central vasopressin 1A receptors was responsible for inhibition of ethanol intake [Tunstall et al., 2019]. In summary, although an action of OT on gut-to-brain signaling has the potential to decrease ethanol intake, the evidence supports a centrally-mediated effect of OT to decrease ethanol intake that is not confounded by OT-induced sedation, or inhibition of caloric or fluid intake.
2.3. OT inhibition of operant ethanol-seeking behavior
OT inhibition of ethanol intake can occur by either decreasing ethanol reward (i.e., decreased motivation to drink) or by increasing ethanol satiation (i.e., a smaller amount of ethanol is sufficiently rewarding). The use of different operant schedules of ethanol reinforcement can differentiate an effect of OT on motivation vs satiation of ethanol intake. Progressive ratio schedules of reinforcement revealed that 0.3 mg/kg, i.p. OT decreased breakpoint for ethanol but not sucrose reward [King et al., 2017]. Breakpoint, or the amount of work an individual is willing to perform for ethanol reward, is directly related to motivation for ethanol reinforcement [King et al., 2017]. Rats made dependent on ethanol (via exposure to ethanol vapor) exhibited both higher levels of operant responding for ethanol [Hansson et al., 2018; Tunstall et al., 2019] and higher breakpoints [Tunstall et al., 2019]. OT more potently (0.125–0.25 mg/kg, either i.p. or i.n.) inhibited both operant responding and breakpoints for ethanol in alcohol dependent rats compared to nondependent rats [Tunstall et al., 2019]. Thus, OT decreases the reward properties of ethanol especially when motivation for responding is increased by alcohol dependence.
Successful pharmacotherapy for AUD should decrease the rate of relapse in abstinent alcoholics especially during exposure to alcohol-related or stressful situations, both of which are major contributing factors to relapse [see Venniro et al., 2016]. The operant self-administration extinction-reinstatement model measures relapse of drug-seeking behaviors. After stable operant ethanol self-administration is established, the animal undergoes extinction training during which operant responses are no longer reinforced resulting in very low levels of responding (extinction). Relapse of ethanol-seeking behavior is prompted by stressful stimuli or by environmental cues that previously signaled drug delivery, such that operant responding increases even without ethanol delivery. Cue-induced reinstatement of alcohol seeking behavior was decreased by OT (10 nM i.c.v.) but only in alcohol-dependent rats [Hansson et al., 2018]. OT (0.5–1.0 mg/kg, i.p.) also decreased stress-induced ethanol relapse in rodent models [King & Becker, 2019]. Thus, OT has the potential to prevent relapse in abstinent alcoholics triggered by alcohol-related cues or stress.
3. OT Regulation of Mesolimbic Reward Circuitry
The OxTR is expressed in many brain areas that can conceivably regulate ethanol intake [see Grinevich 2015] including areas where there is no innervation by OT-containing neuronal fibers due to the ability of OT to act by volume transmission [see Ludwig and Leng, 2006]. Based on the evidence indicating OT inhibits motivation for ethanol reward, this review will focus on OT modulation of brain regions that mediate the positive and negative reinforcing actions of ethanol. OT neurons that originate in the paraventricular nucleus (PVN) and the supraoptic nucleus (SON) of the hypothalamus send long-range axonal projections to both the VTA and NAc [Sofroniew 1983; and see Grinevich 2015], and both areas possess OxTR [Elands et al., 1988; and see Grinevich 2015]. However, it is not known whether the OxTR is expressed by cell bodies in those regions or on axonal terminals originating from other brain regions. Additionally, the diversity of the neuronal phenotypes in VTA expressing OxTR, their projections to multiple brain regions, and the tendency for different firing patterns of these neurons to elicit unique behavioral phenotypes add to the complexity of OT regulation of ethanol reward.
3.1. VTA DA Neurons
OT inhibition of ethanol intake has been hypothesized to occur via direct stimulation of VTA DA neurons projecting to the NAc (Figure 1, Panel A) thus precluding the need for ethanol stimulation of those neurons. Certainly, DA neurons are a logical target for OT fibers considering that activation of VTA DA neurons that project to NAc has been proposed to signal the reward value of both natural (food, sex) and drug reinforcement [see Adinoff et al., 2004]. Optogenetic activation of OT fibers projecting to VTA DA neurons enhanced social reward, while silencing these fibers decreased social interactions [Hung et al., 2017]. A singular known OxTR exists both centrally and peripherally; a Class 1 G-coupled protein receptor that is generally coupled via Gq/11 and is excitatory in nature [see Gimpl & Fahrenholz, 2001]. Thus, OxTR activation is proposed to excite VTA DA neurons, thereby promoting positive reinforcement associated with natural rewards. However, in cell culture, OxTR can couple to Gi/o and both stimulate and inhibit inward rectifying potassium currents [Gravati et al., 2010]. Thus, although most reports find excitatory actions of OT at the cellular level (presumably via Gq), the potential for OT inhibition of neuronal signaling exists [Huber et al., 2005; Terenzi & Ingram 2005], and a solely excitatory action of OT on VTA DA neurons cannot be universally presumed.
Figure 1.
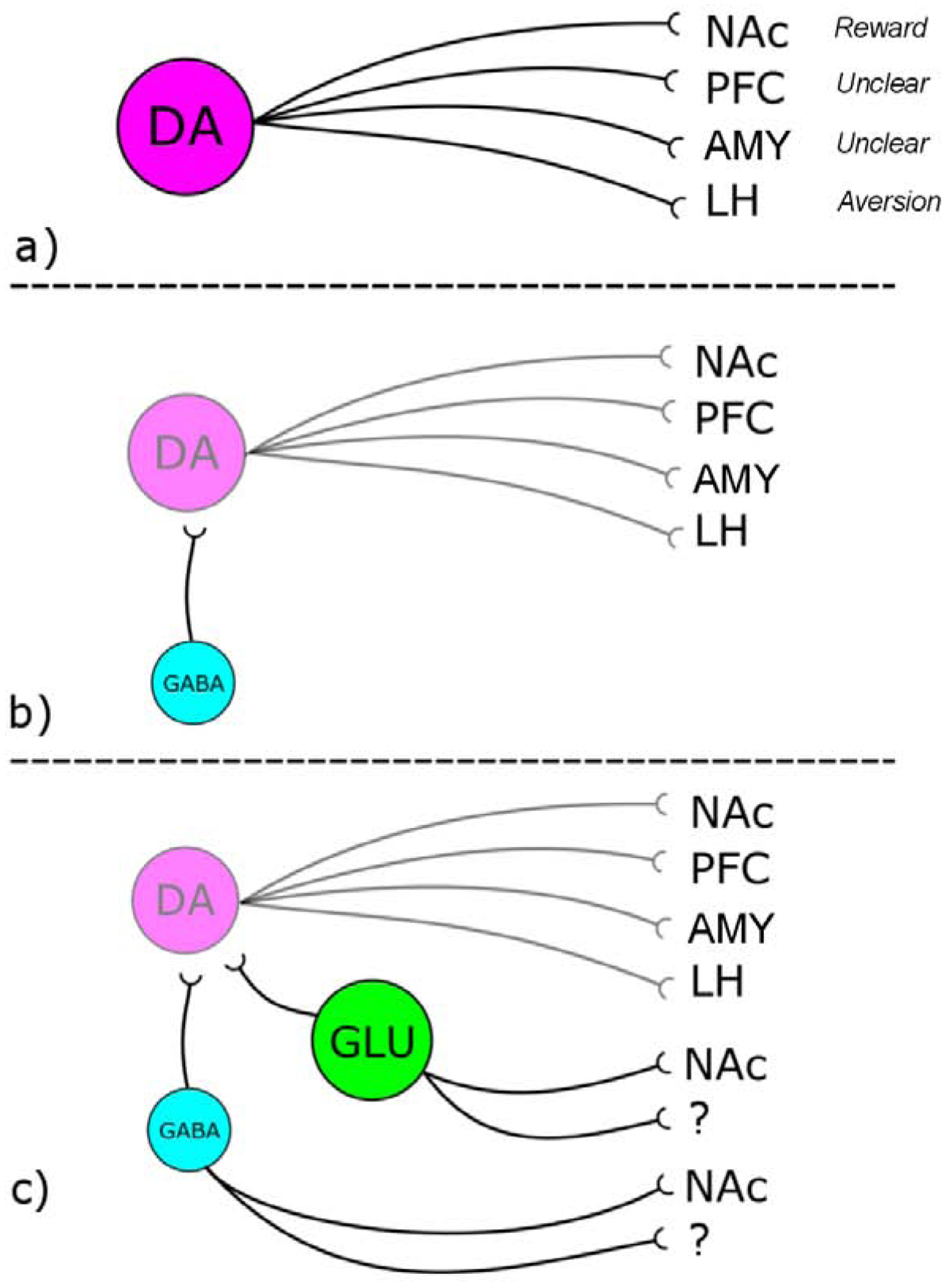
A schematic diagram of the neuronal phenotypes comprising the VTA and possible sites of OxTR action. Panel A: Current models of OxTR regulation of VTA neurotransmission assumes a direct action on DA neurons that originate in VTA and project to NAc to cause positive reinforcement. However projections of VTA DA neurons to other forebrain regions may mediate aversion. Panel B: OxTR are expressed by GABA interneurons in the VTA that inhibit firing of DA neurons thereby inhibiting positive reinforcement. Panel C: OxTR are expressed by GLU neurons that project locally, to NAc or to other regions. Additionally VTA GABA neurons also project to NAc and other forebrain regions. Abbreviations: prefrontal cortex (PFC), lateral habenula (LH), and amygdala (AMY).
In support of an excitatory action of OT on VTA DA neurons, ICV OT was itself rewarding both in operant [Donhoffner et al., 2016] and conditioned place preference assays [Liberzon et al., 1997; Kent et al., 2013]. OT microinjection into the VTA increased extracellular DA levels in the NAc [Melis et al., 2007] and bath-applied OT increased firing rates of VTA neurons, both in lateral VTA and in midline nuclei like the intrafasiclular nucleus [Tang et al., 2014; Xiao et al., 2017]. If OxTR activation directly stimulates DA signaling from the VTA to NAc to increase positive reinforcement (Figure 1, Panel A), then it is plausible this action of OT might preclude the changes in DA firing patterns that modulate craving for ethanol reward. Direct structural or physiological evidence of OxTR stimulation of VTA DA neurons would strengthen this argument.
The relatively recent availability of OxTR-cre mice has allowed for direct neuroanatomical evidence of OxTR expression by VTA DA neurons that project to NAc [Peris et al., 2017]. However, OxTR were also expressed by DA neurons that project to PFC, subregions of the amygdala (AMY) and the lateral habenula (LH). DA projections from VTA to PFC and LH were more sensitive to aversive stimuli [Root et al., 2014; Lammel et al., 2011 and see Lammel et al., 2014] and electrical stimulation of LH decreased voluntary ethanol intake in rats [Li et al 2016]. Thus, without knowing the relative strength of OxTR activation of NAc projecting pathways over LH projecting pathways, the effect of OT on ethanol reinforcement is unpredictable. For example, OxTR inhibition of ethanol intake might involve activation of VTA to LH pathways to increase the aversive properties of ethanol.
An alternative hypothesis is that OT actually interferes with the mechanism by which ethanol stimulates VTA DA neurons. Ethanol directly increased activation of VTA DA neurons [Brodie et al., 1990 and 1999] resulting in increased DA levels in NAc [Gonzales & Weiss, 1998; Doyon et al., 2005]. This action is mediated, at least in part, by ethanol inhibition of KCNK13, a two-pore potassium channel that maintains the negative resting membrane potential of VTA DA neurons, such that knockdown of this channel in the VTA decreased both ethanol-induced excitation of VTA neurons and binge ethanol intake [You et al., 2019]. Chronic ethanol treatment increased both ethanol-stimulated VTA DA activity [Brodie, 2002] and mRNA levels for KCNK13 [You et al., 2019] implying alterations of resting membrane potential provide a greater target for ethanol’s actions. OT inhibited potassium leak currents [Tirko et al., 2018; Singhal et al., 2018] which could interfere with this action of ethanol, thereby limiting stimulation of DA neurons and thus positive reinforcement. In support of this hypothesis, OT blocked ethanol-induced increases in DA release in NAc [Peters et al., 2016]. Thus, evidence exists for both OT stimulation of VTA DA neurons (which would reduce ethanol intake via a “satiety-type” mechanism) as well as OT blockade of ethanol-induced activation of VTA DA neurons (which would directly interfere with ethanol reward).
3.2. VTA GABA Neurons
The scenario is further complicated when OxTR regulation of non-DA neuronal phenotypes in VTA are considered. Although OT directly increased the firing rate of medially-located VTA DA neurons, at the same time OT decreased the firing rate of more lateral midbrain DA neurons [Xiao et al., 2017]. This latter effect occured via OT stimulation of inhibitory GABA interneurons that express OxTR such that the relative magnitude of these two mechanisms was biased towards excitation of VTA DA neurons yet inhibition of nigral DA neurons [Xiao et al., 2017]. Thus, OxTR regulation of DA neurons must also take into account the potential for functional expression of OxTR by GABA interneurons in the VTA (Figure 1 Panel B). VTA GABA neurons regulate both reward- and aversion-related behaviors via both local interneurons as well as GABAergic projections from the VTA to the NAc [see Creed et al., 2014]. Activity of local VTA GABA transmission was decreased by acute exposure to ethanol thereby contributing to ethanol-induced increases in VTA DA firing [Gallegos et al., 1999], but GABA signaling was increased by stress, aversive stimuli and drug withdrawal [Jhou et al., 2009]. OT may alter GABA neuronal activity and hence ethanol-induced changes in DA firing pattern to affect ethanol-seeking behavior. Thus, a more complicated scenario exists for OT regulation of ethanol-induced changes in VTA DA firing if local and projecting populations of VTA GABA neurons express OxTR (Figure 1 Panel B). The relative expression of OxTR by VTA GABA and DA phenotypes will determine whether OT ultimately decreases or increases DA output to NAc.
3.3. VTA GLU Neurons
In addition to DA projection neurons and GABA interneurons/projection neurons, the VTA also contains a population of GLU neurons [Hnasko et al., 2012; and see Morales & Root, 2014]. The neuronal phenotypes and axonal projections of VTA neurons differ according to anatomical location, with DA neurons more laterally and GLU and GABA neurons predominating in medial interfascilular and rostrolinear nuclei [see Ikemoto, 2007; Pupe & Wallen-Mackenzie, 2015]. GLU neurons are both interneurons and projection neurons targeting medial shell of NAc and other limbic structures [Dobi et al., 2010; Omelchenko et al., 2009; Yamaguchi et al., 2011; Taylor et al., 2014]. Neuroanatomical studies using OxTR-cre mice provided direct evidence of OxTR expression by both VTA DA and GLU neurons, however less than 10% of OxTR-expressing neurons in the VTA exhibited tyrosine hydroxylase staining in male mice [Peris et al., 2017]. Instead, about 50% of OxTR-expressing VTA neurons colocalized with vGlut2 mRNA, a marker for GLU, particularly in medial VTA [Peris et al., 2017]. Exogenous application of OT increased firing rate of VTA neurons both in lateral and medial regions but 76% of VTA neurons were OT-sensitive in interfascicular nucleus compared to only 28% in lateral areas [Tang et al., 2014]. OT (i.c.v.) increased firing rates of neurons in the medial NAc shell [Moaddab et al., 2015]. Together these findings indicate medial VTA neurons that project to medial NAc shell (and are more likely to be GLU neurons) exhibit significant OT sensitivity.
VTA GLU neurons play an important role in mediating both positive and negative hedonic value of a stimulus. VTA GLU neurons increased their firing rates in response to aversive airpuff, sucrose reward and in some cases, both, suggesting that VTA GLU neurons are heterogenous in function [Root et al 2018a]. In support of this heterogeneity, optogenetic activation of VTA GLU interneurons [Wang et al., 2015] as well as VTA GLU projection neurons [Yoo et al., 2016] was positively reinforcing under specific circumstances, while the same groups have found that activation of VTA GLU neurons was aversive [Qi et al., 2016; Yoo et al., 2016]. The duration of optogenetic activation was critical such that shorter stimulations were positively reinforcing but longer stimulations were aversive. During real time place preference experiments, mice avoided remaining in a compartment if it resulted in continuous activation of VTA GLU neurons and yet they persisted in entering that compartment [Qi et al., 2016; Yoo et al., 2016]. Similarly, mice operantly responded to halt continuous optogenetic activation of VTA GLU neurons [Qi et al., 2016] even though they learned to nose-poke for 1-sec stimulation [Yoo et al., 2016]. Taken together with the complexities of OxTR regulation of VTA projections to areas mediating aversion, it is likely that exogenously applied OT may have mixed effects on ethanol reward depending on the distribution of OxTR on specific neuronal phenotypes residing within discrete neural circuits. To make this even more complicated, this distribution is likely plastic and dependent on experience. Thus, if OxTR resides more abundantly on VTA GLU neurons vs DA neurons [Peris et al., 2017], it is possible OT may produce aversion rather than positive reinforcement depending on the extent to which OT excites/inhibits VTA GLU neurons and whether this GLU:DA ratio persists across gender, species and group housing conditions.
The consequences of OxTR regulation of GLU levels in NAc are relevant to alcohol seeking. Basal levels of extracellular GLU in NAc were increased after binge ethanol exposure which was related to increased motivation for ethanol self-administration [Li et al., 2010; Griffin et al., 2014; Pati et al., 2016]. Drugs, including OT, that alter the levels of GLU in NAc decreased reinstatement of ethanol self-administration [Das et al., 2015; Stennett et al., 2017; Weber et al., 2018]. Thus, OxTR regulation of GLU neurons that project to NAc from either VTA or other forebrain areas [Tan et al., 2019] provides a potential mechanism for OT inhibition of ethanol self-administration. However, possible aversive properties caused by OT overstimulation of VTA GLU neurons may complicate this scenario and impact ethanol reward in unpredictable ways.
In summary, OxTR regulation of VTA signaling of ethanol reward might include: 1) a direct action on VTA DA neurons that project to NAc, PFC, LH, or amygdala; 2) indirect effects on VTA DA neurons via GLU and GABA interneurons in the VTA; or, 3) direct actions on GLU and GABA VTA neurons that project to NAc or other regions (Figure 1, Panel C). It must be noted that co-release of these neurotransmitters is also evident: subpopulations of VTA neurons that project to NAc release both GLU and DA [Hnasko et al., 2010] while VTA projections to the LH release both GABA and GLU [Root et al., 2018b]. Additionally, for most of these neuronal phenotypes, it is not yet known whether OxTR are located on dendrites, soma or axon terminals, and whether this differs across VTA phenotypes or projection targets. Continued electrophysiological characterization of OT regulation of cellular function in VTA and target regions, such as that described above [Tang et al., 2014; Xiao et al., 2017] is required. Clearly, the OxTR-expressing VTA neuronal circuitry illustrated in Figure 1 includes only a small subset of the potential targets of OT throughout the brain that might regulate ethanol intake and reinforcement. Even so, it provides an example of the potential complexity of that regulation.
3.4. NAc Neurons
OT also regulates activity of medium spiny neurons in the NAc. OxTR density was greater in NAc compared to VTA [Dumais et al., 2013], where OxTR might be located on axonal projections from VTA and PFC, or on NAc medium spiny neurons or interneurons. ICV OT increased firing rate of medium spiny neurons in the medial shell of NAc but not interneurons [Moaddab et al., 2015] could have been due to a direct action on those neurons or mediated via activation of OxTR on GLU terminals that synapse onto medium spiny neurons. Direct infusion of OT into NAc inhibited drug seeking behavior [Ibragimov et al., 1987; Weber et al., 2018] and although a presynaptic action of OT on GLU afferents to NAc was proposed, a direct effect on NAc neurons was not eliminated [Weber et al., 2018]. Overexpression of OxTR by cell bodies in NAc reduced ethanol preference, ethanol intake and reinstatement of ethanol conditioned place preference [Bahi, 2015; Bahi et al., 2016]. However, D1- and D2-expressing GABAergic medium spiny neurons in NAc exhibit opposing actions on positive reinforcement and it is not clear which subpopulation of NAc neurons targeted by the lentivirus was responsible for inhibition of ethanol reward.
4. Other Potential Mechanisms for OT Regulation of Ethanol Intake
A number of other actions of OT might alter the physiological or behavioral effects of alcohol resulting in decreased ethanol intake. For example, OT (1 μg, i.c.v.) blocked ethanol-induced motor impairment while having minimal effects on righting reflex or open field activity when given alone [Bowen 2015]. However, one would expect that limiting ethanol impairment with OT would allow for higher ethanol intake not vice versa. OT (0.2–0.4 mg/kg, i.p.) administration prior to daily ethanol injection (2 or 4 g/kg) inhibited the formation of tolerance to the hypothermic, myorelaxant and locomotor impairing actions of ethanol [Szabo et al., 1985; Jodogne et al., 1991]. At these doses, OT did not alter wire-hanging or locomotor activity after a saline injection and did not affect impairment of these behaviors after the first ethanol injection. However, after the 5th ethanol injection, vehicle-injected mice exhibited much less impairment of motor behavior while OT-injected mice continued to be incapacitated [Jodogne et al., 1991]. By blocking development of tolerance, individuals should have less need to escalate ethanol consumption to achieve the same physiological response over repeated ethanol drinking sessions [see Sarnyai & Kovacs, 2013]. Whether OT can reverse tolerance after it has developed, (as opposed to blocking its development) has yet to be tested.
4.1. Anxiolytic and anti-stress effects of OT
The anxiolytic actions of OT [Windle et al., 1997; Frazier et al., 2013; László et al., 2016] may decrease the intensity of ethanol withdrawal thereby decreasing the potential for negative reinforcement by ethanol [see Koob, 2013]. OT injections decreased withdrawal symptoms in ethanol-dependent mice [Szabo et al., 1987] and blocked enhanced motivation for ethanol intake in alcohol-dependent rats [Tunstall et al., 2019]. Similarly, OT may help normalize ethanol-induced dysregulation of the stress response, thereby eliminating another factor that might contribute to the negative reinforcing properties of ethanol. Withdrawal following heavy drinking in humans decreased adrenocorticotropic hormone (ACTH) and increased plasma cortisol levels under basal conditions [Dai et al., 2002 and 2007; Gianoulakis et al., 2003] while decreasing both the ACTH and cortisol responses to stress [Dai et al., 2007]. Further, the extent of the blunted cortisol response to stress and alcohol-related cues predicted a greater risk of alcohol relapse in alcohol dependent patients [Junghanns et al., 2003 and 2005; Sinha et al., 2011]. In rats, basal corticosterone levels were increased by 7 days of ethanol vapor [Rivier et al., 1984] and the ACTH response to corticotropin-releasing hormone (CRH) or electric shocks was decreased [Rivier et al., 1990]. Repeated involuntary ethanol exposure for 5–7 weeks caused a long-lasting loss of inhibitory regulation of CRH-sensitive neurons in the central nucleus of the amygdala [Herman et al., 2016], persistently increased peak plasma corticosterone, and increased anxiety-like behaviors and ethanol intake [Somkuwar et al., 2017]. Ethanol-dependent rats exhibited fewer CRH neurons as well as decreased CRH mRNA in PVN which returned to normal after 2 months of withdrawal [Silva 2002b]. Thus, dysregulation of the HPA axis under both basal and stimulated conditions may ultimately contribute to the anxiogenic phenotype of abstinent alcoholics, inappropriate responses to external stressors, and greater negativereinforcing properties of further ethanol intake.
OT acts as a regulator of physiological and psychological stress [Smith et al., 2015] decreasing physiological alterations induced by stressors [Krause et al., 2011; Bülbül et al., 2011; Peters et al., 2014]. Both peripheral and central administration of OT, as well as chemogenetic activation of PVN OT neurons, decreased glucocorticoid release [Windle et al., 1997; Mantella et al., 2003; Ring et al., 2006; Blume et al., 2008; Grund et al., 2019]. Conversely, decreasing OT signaling with receptor antagonists [Mantella et al., 2003; Neumann et al., 2000a and b] or OT gene knockout [Amico et al., 2004; Mantella et al., 2003] increased glucocorticoid secretion. Thus, OT can potentially lower alcohol-induced elevations in basal cortisol levels and normalize the response of the HPA axis to stress. Accordingly, stress-induced reinstatement of ethanol intake was decreased by systemic injection of OT in both male and female rodents [Hansson et al., 2018; King and Becker, 2019]. The possibility that OT can also restore the blunted cortisol response to ethanol cues and stress in alcohol dependent patients should be further investigated.
Additionally, OT inhibited conditioned fear responses by activating local GABAergic inhibition of the central nucleus of the amygdala [Huber et al., 2005; Knobloch et al., 2012]. OT also blunted alcohol-related alterations in amygdalar GABAergic transmission which was proposed to be the mechanism for decreasing escalated ethanol intake in withdrawn animals [Tunstall et al., 2019]. A study of ethanol intake after social defeat stress [Anacker et al., 2014b] found dominant voles drank less ethanol than subordinate voles and the voles showing the most dominant behavior exhibited the highest numbers of PVN OT neurons with Fos immunoreactivity. These data indicate that dominant behavior increases OT signaling ultimately resulting in less ethanol intake. If OT decreases the psychological and physiological effects of stress, the need to drink may be reduced.
5. Regulation of OT signaling by long term ethanol intake
The ability of chronic ethanol exposure to disrupt OT signaling may contribute to a number of factors that may increase the risk of developing AUD such as enhanced stress responses or social deficits. If there is a deficit in OT production or release in alcoholics, then OT replacement therapy should exhibit significant efficacy. However, if at the same time OxTR signaling becomes dysfunctional after long term ethanol exposure, this could limit the efficacy of exogenous OT treatment for AUD.
5.1. Ethanol-induced impairment of OT signaling
Long-term alcoholics exhibited a loss of magnocellular OT neurons [Sivukhina et al., 2006]. Ethanol-dependent rats had fewer PVN OT neurons, however, OT mRNA levels were unchanged [Silva et al., 2002a]. Prairie voles given 7 weeks of either intermittent or continuous ethanol intake had fewer OT neurons in anterior PVN but not posterior PVN [Stevenson et al., 2017b]. As little as one week of voluntary ethanol drinking decreased the number of OT neurons in PVN of both male and female prairie voles with no change in vasopressin cells [Walcott & Ryabinin, 2017 and 2019]. Thus, both short term and long term repeated ethanol exposure decreases OT in the PVN providing a potential mechanism for OT as replacement therapy in alcoholics.
Postsynaptically, evidence for regulation of OxTR expression is mixed. OxTR mRNA was increased in ventral striatum of alcoholics [Hansson et al., 2018], but not in cortical and subcortical regions (including VTA, NAc and PFC) in post-mortem tissue from men with AUD [Lee et al., 2017]. In rodent models, OxTR mRNA was decreased in a number of brain regions including NAc during the first few days of alcohol withdrawal but both OxTR mRNA and immunoreactivity in PVN and SON was increased 3 weeks later [Hansson et al., 2018]. On the other hand, surface expression of OxTR in hypothalamus of adult male rats was decreased by intermittent ethanol treatment (4 g/kg via gavage every 48 hrs) during adolescence [Dannenhoffer et al., 2018] indicating that ethanol-induced downregulation of OxTR might be long lasting under certain circumstances. Thus, ethanol-induced changes in OxTR appear to occur in both a time-dependent and region-dependent fashion. Further elucidation of cell specific alterations in post-synaptic OxTR signaling during and after ethanol exposure is critical for understanding whether neuronal circuits important for regulating the reinforcing actions of ethanol will exhibit decreased sensitivity to OT, thereby limiting its clinical efficacy. At the same time, understanding how OxTR expression is altered by chronic ethanol exposure, particularly in specific subpopulations of VTA neurons, might shift the valence of OT from stimulation of DA neurons (and positive reinforcement) to inhibition via an increased action on GABA or GLU VTA neurons (and aversion). Such information would shed light on the pathophysiology underlying ethanol-induced social impairment.
5.2. Ethanol-induced Social Dysfunction
OT inhibition of addictive behaviors is proposed to involve reinforcement of social rewards that out-competes drug-induced rewards [see Sarnyai & Kovacs, 2013]. Isolated housing conditions increased the magnitude of social reward and at the same time, decreased drug reward [Yates et al., 2014]. Repeated exposure to amphetamine inhibited the formation of partner preference in female prairie voles, and central infusion of OT reversed that deficit [Young et al., 2014]. If repeated ethanol exposure decreases OT signaling, then social reward and its ability to inhibit drug seeking behavior may be diminished in subjects with AUD.
Ethanol-induced decreases in OT signaling may interfere with the normal behavioral and physiological reactions to social interaction (e.g., loss of OT-mediated positive reinforcement caused by social contacts) which may further contribute to negative affect and enhanced ethanol seeking. The negative affective state caused by ethanol-induced loss of OT signaling may be more subtle than the overt anxiogenesis caused by ethanol withdrawal. For example, OxTR gene knockout mice showed relatively little evidence of an anxiogenic phenotype but did exhibit deficits in maternal and social behaviors [Takayanagi et al., 2005; Lee et al., 2008; Horie et al., 2019]. In male prairie voles, ethanol hindered social bonding while females experienced an increase in partner preference [Anacker et al., 2014a; Walcott & Ryabinin, 2017]. Involuntary repeated ethanol exposure decreased social interaction but not social novelty in male DBA mice with no effect in C57 mice [Sidhu et al., 2018]. Daily binge ethanol intake decreased conditioned social preference in both male and female C57 mice without altering either social interactions or social novelty [Peris et al., 2019].
A fairly large body of research has been conducted on the effects of repeated ethanol intoxication during adolescence [see Spear 2018 for review] finding alterations in OT signaling to reward pathways [Dannenhoffer et al., 2018] with social deficits that may last into adulthood [Kim et al., 2019]. Intermittent ethanol exposure during adolescence decreased social interactions during adulthood in male rats and this deficit was reversed by treatment with the OT agonist WAY-267464 in a dose-related manner [Dannenhoffer et al., 2018]. OT treatment during adolescence had long term anxiolytic effects which paralleled a decrease in ethanol consumption behaviors [Bowen et al., 2011]. Thus, behavioral evidence supports a deficit in OT signaling caused by repeated ethanol exposure that can be persistent, especially if ethanol exposure occurs during adolescence. OT pharmacotherapy may be particularly effective in these patient populations.
6. Sex-related Differencesin OT Signaling
Individual differences in the efficacy of OT to treat AUD are likely, based on a number of factors. The mesolimbic reward circuitry may be differentially sensitive to OT across patient populations. The amount of OxTR in NAc varied by sex and estrus cyclicity [Dumais et al., 2013] indicating the likelihood of sex differences in OT inhibition of ethanol reward. There were sex differences in the actions of OT on caloric intake and locomotor activity in rats [Zhou et al., 2015], both of which could contribute to OT inhibition of ethanol intake. The reproductive status of females is also likely to contribute variability to OT efficacy. The size of OT neurons in SON was increased in pregnant rats and the number of fibers from OT neurons innervating the amygdala was increased in lactating rats, both compared to virgin females [Knobloch et al., 2012]. OxTR mRNA expression was elevated in pregnant rats compared to virgins [Young et al., 1997]. Virgin rats with intermittent ethanol access decreased daily intake upon becoming pregnant [Brancato et al., 2016]. After weaning, both ethanol intake and OxTR levels of the dam returned to normal.
As discussed in Section 3, the expression of OxTR by DA, GABA and GLU VTA neurons that project to multiple forebrain targets provide the basis for OT to exert biased modulation of VTA output. Variance in the number or location of OxTR expressed on these different VTA subpopulations can shift the bias from OT excitation to OT inhibition of mesolimbic output. Similarly, OT has the potential to exert both direct and indirect actions on MSNs in NAc. The nature or degree of these biases may differ depending on sex, genetic background, drug exposure, or social and familial conditions.
Certain populations may be more or less susceptible to ethanol-induced deficits in OT signaling. OT knockout increased anxiety in female but not male mice [Mantella et al., 2003]. In marmosets, manipulation of OxTR activity altered stress-induced changes in cortisol levels in a sex-dependent manner [Cavanaugh et al., 2016]. Ethanol impaired partner preference in prairie voles only if the male was intoxicated but not when both partners or only the female were impaired [Walcott & Ryabinin, 2017 and 2019]. Adolescent ethanol exposure decreased social interactions only in males [Dannenhoffer et al., 2018]. A greater ethanol-induced deficit in OT function underlying the formation of partner preference might predispose males to higher sensitivity to exogenous OT “replacement”. Similarly, greater HPA dysfunction in heavy alcohol drinking middle-aged males [Gianoulakis et al., 2003] might provide for greater OT normalization of HPA function. On the other hand, female alcoholics had more pronounced decreases in plasma ACTH levels after the age of 45 years old [Gianoulakis et al., 2003].
7. Summary
Relapse of alcohol use in abstinent alcoholics is a prime target for development of pharmacotherapies, including the major contributing factors to relapse: drug, environment, and stress cues that promote drug craving [Venniro et al., 2016]. OT decreases ethanol intake, cue-induced ethanol cravings and withdrawal-induced anxiety/stress. The similarity in the effects of intranasal OT and acute ethanol consumption on a number of emotional, cognitive and social behaviors in both animals and humans initially led to the proposal that OT may act as a replacement for ethanol [see Mitchell et al., 2015]. The findings presented above support that: 1) OT stimulation of DA reward circuitry may preclude craving for ethanol-induced stimulation of DA neurons; and, 2) OT-induced anxiolysis or stress relief precludes ethanol negative reinforcement. However, other evidence indicates a number of molecular actions of OT on specific neuronal populations that directly decrease ethanol’s reinforcing actions. In either case, variability in patient populations (e.g., sex, heavy drinking history) contribute to different relapse susceptibilities caused by ethanol exposure, anxiety, stress and environmental cues, which may be linked to alterations in OT signaling [see Bisagno & Cadet 2014]. Thus, a more complex scenario may exist. OT regulation of VTA neurons mediating ethanol reward likely depends on the phenotype and projection target of OxTR-expressing neuronal subpopulations, the excitatory/inhibitory nature of the OxTR-linked second messenger system, and co-release of GLU and/or GABA, either alone or with DA. A better understanding of the cellular location, density and activity of OxTR in specific VTA neuronal phenotypes and how they are affected by repeated ethanol exposure will prove insightful for understanding the potentially complicated effects of OT on ethanol reward. This complexity is likely related to the greater efficacy of OT in specific alcoholic patient populations e.g. those with high attachment anxiety [Mitchell et al., 2016] or heavy social drinking [Hansson et al., 2018]. Even more intriguing is evidence that voluntary abstinence by choosing social interaction over drug-taking behavior prevents drug seeking over the long term [Venniro et al., 2019]. Thus, the finely tuned regulation of neuronal activity caused by socially-induced increases in OT signaling may have greater efficacy than exogenous OT in decreasing alcohol relapse.
Although there are still gaps in the literature that might help to mitigate individual variability in OT efficacy for treating AUD, OT is proven to be an important regulator of a number of brain regions mediating the reinforcing properties of ethanol, including both DA and non-DA VTA neurons that project to numerous forebrain areas involved in ethanol reward. The ratio of direct and indirect effects of OxTR on VTA reward signaling is yet unknown, as is the exact nature of OxTR regulation of VTA neuronal subpopulations that project to regions mediating ethanol aversion. In addition to further studies to discern the nuanced mechanisms for OT regulation of reward circuitry, more clinical studies are needed to identify specific populations of alcoholics that demonstrate favorable therapeutic actions of OT. Once a better understanding of the factors contributing to OT sensitivity are better characterized, OT is likely to become an effective pharmacotherapy for treating AUD.
Highlights:
Oxytocin inhibits ethanol intake in human and animal models
Oxytocin receptors are expressed by multiple neuronal phenotypes in the ventral tegmental area
Oxytocin receptors can activate VTA circuitry mediating either reward or aversion
Long term alcohol intake can down-regulate oxytocin signaling
Evidence suggests high potential for individual variation in OTR regulation of VTA circuitry
Acknowledgements:
The authors wish to that Drs. Alex Service, Guillaume de Lartigue and Barry Setlow for proofreading, intellectual discussion and conceptual feedback, respectively.
Funding:
This work was supported by the National Institute of Health (grant number AA 026090).
Abbreviations:
- OT
oxytocin
- OxTR
oxytocin receptor
- DA
dopamine
- GLU
glutamate
- VTA
ventral tegmental area
- NAc
nucleus accumbens
- HPA
hypothalamic pituitary adrenal
- PVN
paraventricular nucleus
- ICV
intracerebroventricular
- SON
supraoptic nucleus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Abbreviations: ACTH = adrenal corticotropic hormone, CRH = corticotropin releasing hormone, DA = dopamine, GLU = glutamate, HPA = hypothalamic pituitary adrenal, ICV = intracerebroventricular, NAc = nucleus accumbens, OT = oxytocin, OxTR = oxytocin receptor, PVN = paraventricular nucleus, SON = supraoptic nucleus, VTA = ventral tegmental area
Literature Cited
- Adinoff B (2004) Neurobiologic processes in drug reward and addiction. Harv Rev Psychiatry. 12(6):305–320. doi: 10.1080/10673220490910844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amico JA, Mantella RC, Vollmer RR, Li X (2004) Anxiety and stress responses in female oxytocin deficient mice. J Neuroendocrinol. 16(4):319–24. doi: 10.1111/j.0953-8194.2004.01161.x [DOI] [PubMed] [Google Scholar]
- Anacker AMJ, Ahern TH, Hostetler CM, Dufou BD, Smith ML, Cocking DL. Ryabinin AE (2014a) Drinking alcohol has sex-dependent effects on pair bond formation in prairie voles. Proc Nat Acad Sci. 111(16):6052–6057. doi: 10.1073/pnas.1320879111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anacker AMJ, Smith ML, Ryabinin AE (2014b) Establishment of stable dominance interactions in prairie vole peers: relationships with alcohol drinking and activation of the paraventricular nucleus of the hypothalamus. Social Neurosci. 9(5):484–494. doi: 10.1080/17470919.2014.931885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahi A (2015) The oxytocin receptor impairs ethanol reward in mice. Physiol Beh. 139: 321–327. doi: 10.1016/j.physbeh.2014.11.046 [DOI] [PubMed] [Google Scholar]
- Bahi A, Al Mansouri S, Al Maamari E (2016) Nucleus accumbens lentiviral-mediated gain of function of the oxytocin receptor regulates anxiety- and ethanol-related behaviors in adult mice. Physiol Behav. 164(Pt A):249–58. doi: 10.1016/j.physbeh.2016.06.009 [DOI] [PubMed] [Google Scholar]
- Bai L, Mesgarzadeh S, Ramesh KS, Huey EL, Liu Y, Gray LA, Aitken TJ, Chen Y, Beutler LR, Ahn JS, Madisen L, Zeng H, Krasnow MA, Knight ZA (2019) Genetic identification of vagal sensory neurons that control feeding. Cell. 179(5):1129–1143.e23. doi: 10.1016/j.cell.2019.10.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisagno V, Cadet J (2014) Stress, sex, and addiction: potential roles of CRF, oxytocin and arginine vasopressin. Behav Pharmacol. 25(5–6):445–457. doi: 10.1097/FBP.0000000000000049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blume A, Bosch OJ, Miklos S, Torner L, Wales L, Waldherr M, Neumann ID (2008) Oxytocin reduces anxiety via ERK1/2 activation: local effect within the rat hypothalamic paraventricular nucleus. Eur J Neurosci. 27(8):1947–56. doi: 10.1111/j.1460-9568.2008.06184.x [DOI] [PubMed] [Google Scholar]
- Bowen MT, Carson DS, Spiro A, Arnold JC, McGregor IS (2011) Adolescent oxytocin exposure causes persistent reductions in anxiety and alcohol consumption and enhances sociability in rats. PLoS ONE. 6(11):e27237. doi: 10.1371/journal.pone.0027237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen MT, Peters ST, Absalom N, Chebib M, Neurmann ID, McGregor IS (2015) Oxytocin prevents ethanol actions at δ subunit-containing GABAA receptors and attenuates ethanol-induced motor impairment in rats. Proc Nat Acad Sci. 112(10):3104–3109. doi: 10.1073/pnas.1416900112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brancato A, Plescia F, Lavanco G, Cavallaro A, Cannizzaro C (2016) Continuous and intermittent alcohol free-choice from pre-gestational time to lactation: Focus on drinking trajectories and maternal behavior. Front Behav Neurosci. 10:31. doi: 10.3389/fnbeh.2016.00031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodie MS, Shefner SA, Dunwiddie TV (1990) Ethanol increases the firing rate of dopamine neurons of the rat ventral tegmental area in vitro. Brain Res. 1990 508(1):65–9. doi: 10.1016/0006-8993(90)91118-z [DOI] [PubMed] [Google Scholar]
- Brodie MS, Pesold C, Appel SB (1999) Ethanol directly excites dopaminergic ventral tegmental area reward neurons. Alc Clin Exp Res. 23:1848–52. doi: 10.1111/j.1530-0277.1999.tb04082.x [DOI] [PubMed] [Google Scholar]
- Brodie MS (2002) Increased ethanol excitation of dopaminergic neurons of the ventral tegmental area after chronic ethanol treatment. Alc Clin Exp Res. 26(11):1024–30. doi: 10.1097/01.ALC.0000021336.33310.6B [DOI] [PubMed] [Google Scholar]
- Bülbül M, Babygirija R, Cerjak D, Yoshimoto S, Ludwig K, Takahashi T (2011) Hypothalamic oxytocin attenuates CRF expression via GABA(A) receptors in rats. Brain Res. 1387:39–45. doi: 10.1016/j.brainres.2011.02.091 [DOI] [PubMed] [Google Scholar]
- Caspers KM, Cadoret RJ, Langbehn D, Yucuis R, Troutman B (2005) Contributions of attachment style and perceived social support to lifetime use of illicit substances. Addict Behav. 30(5):1007–11. doi: 10.1016/j.addbeh.2004.09.001 [DOI] [PubMed] [Google Scholar]
- Cavanaugh J, Carp SB, Rock CM, French JA (2016) Oxytocin modulates behavioral and physiological responses to a stressor in marmoset monkeys. Psychoneuroendocrin. 66:22–30. doi: 10.1016/j.psyneuen.2015.12.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creed MC, Ntamati NR, Tan KR (2014) VTA GABA neurons modulate specific learning behaviors through the control of dopamine and cholinergic systems. Front Behav Neurosci. 8:8. doi: 10.3389/fnbeh.2014.00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai X, Thavundayil J, Gianoulakis C (2002) Response of the hypothalamic-pituitary-adrenal axis to stress in the absence and presence of ethanol in subjects at high and low risk of alcoholism. Neuropsychopharmacology. 27(3):442–52. doi: 10.1016/S0893-133X(02)00308-1 [DOI] [PubMed] [Google Scholar]
- Dai X, Thavundayil J, Santella S, Gianoulakis C (2007) Response of the HPA-axis to alcohol and stress as a function of alcohol dependence and family history of alcoholism. Psychoneuroendocrinology 32 (3):293–305. doi: 10.1016/j.psyneuen.2007.01.004 [DOI] [PubMed] [Google Scholar]
- Dannenhoffer CA, Kim EU, Saalfield J, Werner DF, Varlinskaya EI, Spear LP (2018) Oxytocin and vasopressin modulation of social anxiety following adolescent intermittent ethanol exposure. Psychopharmacology (Berl). 235(10):3065–3077. doi: 10.1007/s00213-018-5003-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das SC, Yamamoto BK, Hristov AM, Sari Y (2015) Ceftriaxone attenuates ethanol drinking and restores extracellular glutamate concentration through normalization of GLT-1 in nucleus accumbens of male alcohol-preferring rats. Neuropharmacol. 97:67–74. doi: 10.1016/j.neuropharm.2015.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobi A, Margolis EB, Wang HL, Harvey BK, Morales M (2010) Glutamatergic and nonglutamatergic neurons of the ventral tegmental area establish local synaptic contacts with dopaminergic and nondopaminergic neurons. J Neurosci. 30(1):218–29. doi: 10.1523/JNEUROSCI.3884-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donhoffner ME, Goings SP, Atabaki K, Wood RI (2016) Intracerebroventricular oxytocin self-administration in female rats. J Neuroendocrinol. 28(10):10. doi: 10.1111/jne.12416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyon WM, Anders SK, Ramachandra VS, Czachowski CL, Gonzales RA (2005) Effect of operant self-administration of 10% ethanol plus 10% sucrose on dopamine and ethanol concentrations in the nucleus accumbens. J Neurochem. 93(6):1469–81. doi: 10.1111/j.1471-4159.2005.03137.x [DOI] [PubMed] [Google Scholar]
- Dumais KM, Bredewold R, Mayer TE, Veenema AH (2013) Sex differences in oxytocin receptor binding in forebrain regions: correlations with social interest in brain region- and sex- specific ways. Horm Behav. 64(4):693–701. doi: 10.1016/j.yhbeh.2013.08.012 [DOI] [PubMed] [Google Scholar]
- Elands J, Beetsma A, Barberis C, de Kloet ER (1988) Topography of the oxytocin receptor system in rat brain: an autoradiographical study with a selective radioiodinated oxytocin antagonist. J Chem Neuroanat. 1(6):293–302. [PubMed] [Google Scholar]
- Frazier CJ, Pati D, Hiller H, Nguyen D, Wang L, Smith JA, MacFadyen K, de Kloet AD, Krause EG (2013) Acute hypernatremia exerts an inhibitory oxytocinergic tone that is associated with anxiolytic mood in male rats. Endocrinology 154(7): 2457–2467. doi: 10.1210/en.2013-1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallegos RA, Lee RS, Criado JR, Henriksen SJ, Steffensen SC (1999) Adaptive responses of γ-aminobutyric acid neurons in the ventral tegmental area to chronic ethanol. J Pharmacol Exp Ther. 291(3):1045–1053 [PubMed] [Google Scholar]
- Gianoulakis C, Dai X, Brown T (2003) Effect of chronic alcohol consumption on the activity of the hypothalamic-pituitary-adrenal axis and pituitary beta-endorphin as a function of alcohol intake, age, and gender. Alc Clin Exp Res. 27(3):410–23. doi:0.1097/01.ALC.0000056614.96137.B8 [DOI] [PubMed] [Google Scholar]
- Gimpl G, Fahrenholz F (2001) The oxytocin receptor system: structure, function, and regulation. Physiol Rev. 8(2):629–83. doi: 10.1152/physrev.2001.81.2.629 [DOI] [PubMed] [Google Scholar]
- Godlewski G, Cinar R, Coffey NJ, Liu J, Jourdan T, Mukhopadhyay B, Chedester L, Liu Z, Osei-Hyiaman D, Iyer MR, Park JK, Smith RG, Iwakura H, Kunos G (2019) Targeting peripheral CB1 receptors reduces ethanol intake via a gut-rain axis. Cell Metab. 29(6):1320–1333.e8. doi: 10.1016/j.cmet.2019.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales RA, Weiss F (1998) Suppression of ethanol-reinforced behavior by naltrexone is associated with attenuation of the ethanol-induced increase in dialysate dopamine levels in the nucleus accumbens. J Neurosci. 18(24):10663–71. doi: 10.1523/JNEUROSCI.18-24-10663.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravati M, Busnelli M, Bulgheroni E, Reversi A, Spaiardi P, Parenti M, Toselli M, Chini B (2010) Dual modulation of inward rectifier potassium currents in olfactory neuronal cells by promiscuous G protein coupling of the oxytocin receptor. J Neurochem. 114(5):1424–35. doi: 10.1111/j.1471-4159.2010.06861.x [DOI] [PubMed] [Google Scholar]
- Griffin WC 3rd, Haun HL, Hazelbaker CL, Ramachandra VS, Becker HC (2014) Increased extracellular glutamate in the nucleus accumbens promotes excessive ethanol drinking in ethanol dependent mice. Neuropsychopharmacology. 39(3):707–717. doi: 10.1038/npp.2013.256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinevich V, Knobloch-Bollmann SH, Eliava M, Busnelli M, Chini B (2015) Assembling the puzzle: Pathways of oxytocin signaling in the brain. Biol Psych. 79(3):155–164. doi: 10.1016/j.biopsych.2015.04.013 [DOI] [PubMed] [Google Scholar]
- Grund T, Tang Y, Benusiglio D, Althammer F, Probst S, Oppenländer L, Neumann ID, Grinevich V (2019) Chemogenetic activation of oxytocin neurons: Temporal dynamics, hormonal release, and behavioral consequences. Psychoneuroendocrinology. 106:77–84. doi: 10.1016/j.psyneuen.2019.03.019. [DOI] [PubMed] [Google Scholar]
- Han W, Tellez LA, Perkins MH, Perez IO, Qu T, Ferreira J, Ferreira TL, Quinn D, Liu ZW, Gao XB, Kaelberer MM, Bohórquez DV, Shammah-Lagnado SJ, de Lartigue G, de Araujo IE (2018). A neural circuit for gut-induced reward. Cell. 175(3):665–678. doi: 10.1016/j.cell.2018.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson AC, Koopmann A, Uhrig S, Bühler S, Domi E, Kiessling E, Ciccocioppo R, Froemke RC, Grinevich V, Kiefer F, Sommer WH, Vollstädt-Klein S, Spanagel R (2018) Oxytocin reduces alcohol cue-reactivity in alcohol-dependent rats and humans. Neuropsychopharmacology. 43(6):1235–1246. doi: 10.1038/npp.2017.257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper KM, Knapp DJ, Criswell HE, Breese GR (2018) Vasopressin and alcohol: a multifaceted relationship. Psychopharmacology, 235(12): 3363–3379. doi: 10.1007/s00213-018-5099-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman MA, Contet C, Roberto M (2016) A functional switch in tonic GABA currents alters the output of central amygdala corticotropin releasing factor receptor-1 neurons following chronic ethanol exposure. J Neurosci. 36(42):10729–10741. doi: 10.1523/JNEUROSCI.1267-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hnasko TS, Chuhma N, Zhang H, Germaine YG, Sulzer D, Palmiter RD, Rayport S, Edwards RH (2010) Vesicular glutamate transport promotes dopamine storage and glutamate corelease in vivo. Neuron. 65(5):643–656. doi: 10.1016/j.neuron.2010.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hnasko TS, Hjelmstad GO, Fields HL, Edwards RH (2012) Ventral tegmental area glutamate neurons: electrophysiological properties and projections. J Neurosci. 32(43):15076–15085. doi: 10.1523/JNEUROSCI.3128-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie K, Inoue K, Suzuki S, Adachi S, Yada S, Hirayama T, Hidema S, Young LJ, Nishimori K (2019) Oxytocin receptor knockout prairie voles generated by CRISPR/Cas9 editing show reduced preference for social novelty and exaggerated repetitive behaviors. Horm Behav. 111:60–69. doi: 10.1016/j.yhbeh.2018.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber D, Veinante P, Stoop R (2005) Vasopressin and oxytocin excite distinct neuronal populations in the central amygdala. Science. 308(5719):245–248. doi: 10.1126/science.1105636 [DOI] [PubMed] [Google Scholar]
- Hung LW, Neuner S, Polepalli JS, Beier KT, Wright M, Walsh JJ, Lewis EM, Luo L, Deisseroth K, Dölen G, Malenka RC (2017) Gating of social reward by oxytocin in the ventral tegmental area. Science. 357(6358):1406–1411. doi: 10.1126/science.aan4994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibragimov R, Kovács GL, Szabó G, Telegdy G (1987) Microinjection of oxytocin into limbic-mesolimbic brain structures disrupts heroin self-administration behavior: a receptor-mediated event? Life Sci. 41(10):1265–71. doi: 10.1016/0024-3205(87)90205-0 [DOI] [PubMed] [Google Scholar]
- Ikemoto S (2007) Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res Rev. 56(1):27–78. doi: 10.1016/j.brainresrev.2007.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki Y, Maejima Y, Suyama S, Yoshida M, Arai T, Katsurada K, Kumari P, Nakabayashi H, Kakei M, Yada T (2015) Peripheral oxytocin activates vagal afferent neurons to suppress feeding in normal and leptin-resistant mice: a route for ameliorating hyperphagia and obesity. Am J Physiol Regul Integr Comp Physiol. 308(5):R360–9. doi: 10.1152/ajpregu.00344.2014. [DOI] [PubMed] [Google Scholar]
- Izbéki F, Wittmann T, Jancsó G, Csáti S, Lonovics J (2002) Inhibition of gastric emptying and small intestinal transit by ethanol is mediated by capsaicin-sensitive afferent nerves. Naunyn Schmiedebergs Arch Pharmacol. 365(1):17–21. doi: 10.1007/s00210-001-0491-0 [DOI] [PubMed] [Google Scholar]
- Jhou TC, Fields HL, Baxter MG, Saper CB, Holland PC (2009) The rostromedial tegmental nucleus (RMTg), a GABAergic afferent to midbrain dopamine neurons, encodes aversive stimuli and inhibits motor responses. Neuron. 61(5):786–800. doi: 10.1016/j.neuron.2009.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jodogne C, Tirelli E, Klingbiel P, Legros JJ (1991) Oxytocin attenuates tolerance not only to the hypothermic but also to the myorelaxant and akinesic effects of ethanol in mice. Pharmacol Biochem Behav. 40(2): 261–5. doi: 10.1016/0091-3057(91)90549-H [DOI] [PubMed] [Google Scholar]
- Johansson A, Bergman H, Corander J, Waldman ID, Karrani N, Salo B, Jern P, Algars M, Sandnabba K, Santtila P, Westberg L (2012a) Alcohol and aggressive behavior in men–moderating effects of oxytocin receptor gene (OXTR) polymorphisms. Genes Brain Beh. 11(2):214–221. doi: 10.1111/j.1601-183X.2011.00744.x [DOI] [PubMed] [Google Scholar]
- Johansson A, Westberg L, Sandnabba K, Jern P, Salo B, Santtila P (2012b) Associations between oxytocin receptor gene (OXTR) polymorphisms and self-reported aggressive behavior and anger: Interactions with alcohol consumption. Psychoneuroendocrinology. 37(9):1546–56. doi: 10.1016/j.psyneuen.2012.02.009 [DOI] [PubMed] [Google Scholar]
- Junghanns K, Backhaus J, Tietz U, Lange W, Bernzen J, Wetterling T, Rink L, Driessen M (2003) Impaired serum cortisol stress response is a predictor of early relapse. Alcohol Alcohol. 38(2):189–93. doi: 10.1093/alcalc/agg052 [DOI] [PubMed] [Google Scholar]
- Junghanns K, Tietz U, Dibbelt L, Kuether M, Jurth R, Ehrenthal D, Blank S, Backhaus J (2005) Attenuated salivary cortisol secretion under cue exposure is associated with early relapse. Alcohol Alcohol. 40(1):80–5. doi: 10.1093/alcalc/agh107 [DOI] [PubMed] [Google Scholar]
- Kent K, Arientyl V, Khachatryan MM, Wood RI (2013) Oxytocin induces a conditioned social preference in female mice. J Neuroendocrinology 24:803–810. doi: 10.1111/jne.12075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EU, Varlinskaya EI, Dannenhoffer CA, Spear LP (2019) Adolescent intermittent ethanol exposure: Effects on pubertal development, novelty seeking, and social interaction in adulthood. Alcohol. 75:19–29. doi: 10.1016/j.alcohol.2018.05.002 [DOI] [PubMed] [Google Scholar]
- King CE, Griffin WC, Luderman LN, Kate MM, McGinty JF, Becker HC (2017), Oxytocin reduces ethanol self-administration in mice. Alc Clin Exp Res. 41(5):955–964. doi: 10.1111/acer.13359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King CE, Becker HC (2019) Oxytocin attenuates stress-induced reinstatement of alcohol seeking behavior in male and female mice. Psychopharmacology. 236: 2613–2622. doi: 10.1007/s00213-019-05233-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch P (2015) Oxytocin in the socioemotional brain: implications for psychiatric disorders. Dialogues Clin Neurosci. 17(4):463–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knobloch HS, Charlet A, Hoffmann LC, Eliava M, Khrulev S, Cetin AH, Osten P, Schwarz MK, Seeburg PH, Stoop R, Grinevich V (2012) Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron. 73(3):553–66. doi: 10.1016/j.neuron.2011.11.030. [DOI] [PubMed] [Google Scholar]
- Koob GF (2013) Theoretical frameworks and mechanistic aspects of alcohol addiction: alcohol addiction as a reward deficit disorder. Curr Top Behav Neurosci. 13:3–30. doi: 10.1007/7854_2011_129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause EG, de Kloet AD, Flak JN, Smeltzer MD, Solomon MB, Evanson NK, Woods SC, Sakai RR, Herman JP (2011) Hydration state controls stress responsiveness and social behavior. J Neurosci. 31(14):5470–6. doi: 10.1523/JNEUROSCI.6078-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Ion DI, Roeper J, Malenka RC (2011) Projection-specific modulation of dopamine neuron synapses by aversive and rewarding stimuli. Neuron. 70(5):855–862. doi: 10.1016/j.neuron.2011.03.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Lim BK, Malenka RC (2014) Reward and aversion in a heterogeneous midbrain dopamine system. Neuropharmacology. 76 Pt B(00):351–359. doi: 10.1016/j.neuropharm.2013.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- László K, Kovács A, Zagoracz O, Ollmann T, Péczely L, Kertes E, Lacy DL, Lénárd L (2016) Positive reinforcing effect of oxytocin microinjection in the rat central nucleus of the amygdala. Behav Brain Res 296:279–285. doi: 10.1016/j.bbr.2015.09.021 [DOI] [PubMed] [Google Scholar]
- Lee HJ, Caldwell HK, Macbeth AH, Tolu SG, Young WS 3rd (2008) A conditional knockout mouse line of the oxytocin receptor. Endocrinology. 149(7):3256–3263. doi: 10.1210/en.2007-1710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MR, Weerts EM (2016) Oxytocin for the treatment of drug and alcohol use disorders. Behav Pharmacol. 27(8):640–648. doi: 10.1097/FBP.0000000000000258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MR, Schwandt ML, Sankar V, Suchankova P, Sun H, Leggio L (2017) Effect of alcohol use disorder on oxytocin peptide and receptor mRNA expression in human brain: A post-mortem case-control study. Psychoneuroendocrinology. 85:14–19. doi: 10.1016/j.psyneuen.2017.07.481. [DOI] [PubMed] [Google Scholar]
- Li J, Zuo W, Fu R, Xie G, Kaur A, Bekker A, Ye JH (2016) High frequency electrical stimulation of lateral habenula reduces voluntary ethanol consumption in Rrats. Int J Neuropsychopharmacol. 19(10):pyw050. doi: 10.1093/ijnp/pyw050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Zharikova A, Vaughan CH, Bastian J, Zandy S, Esperon L, Axman E, Rowland NE, Peris J (2010) Intermittent high-dose ethanol exposures increase motivation for operant ethanol self-administration: possible neurochemical mechanism. Brain Res. 1310:142–53. doi: 10.1016/j.brainres.2009.11.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberzon I1, Trujillo KA, Akil H, Young EA (1997) Motivational properties of oxytocin in the conditioned place preference paradigm. Neuropsychopharmacology. 17(6):353–9. doi: 10.1016/S0893-133X(97)00070-5 [DOI] [PubMed] [Google Scholar]
- Ludwig M, Leng G (2006) Dendritic peptide release and peptide-dependent behaviours. Nature Rev Neurosci. 7(2):126–136. doi: 10.1038/nrn1845 [DOI] [PubMed] [Google Scholar]
- MacFadyen K, Loveless R, DeLucca B, Wardley K, Deogan S, Thomas C, Peris J (2016) Peripheral oxytocin administration reduces ethanol consumption in rats. Pharmacol Biochem Behav. 140: 27–32. doi: 10.1016/j.pbb.2015.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maejima Y, Rita RS, Santoso P, Aoyama M, Hiraoka Y, Nishimori K, Gantulga D, Shimomura K, Yada T (2015) Nasal oxytocin administration reduces food intake without affecting locomotor activity and glycemia with c-Fos induction in limited brain areas. Neuroendocrinology. 101(1):35–44. doi: 10.1159/000371636. [DOI] [PubMed] [Google Scholar]
- Mantella Vollmer RR, Li X, Amico JA (2003) Female oxytocin-deficient mice display enhanced anxiety-related behavior. Endocrinology. 144(6):2291–6. doi: 10.1210/en.2002-0197 [DOI] [PubMed] [Google Scholar]
- cGregor IS, Bowen MT (2012) Breaking the loop: oxytocin as a potential treatment for drug addiction. Horm Behav. 61(3):331–9. doi: 10.1016/j.yhbeh.2011.12.001 [DOI] [PubMed] [Google Scholar]
- McRae-Clark AL (2013) Effect of oxytocin on craving and stress response in marijuana-dependent individuals: A pilot study. Psychopharmacology. 228(4): 623–631. doi: 10.1007/s00213-013-3062-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melby K, Gråwe RW, Aamo TO, Salvesen Ø, Spigset O (2019) Effect of intranasal oxytocin on alcohol withdrawal syndrome: A randomized placebo-controlled double-blind clinical trial. Drug Alcohol Depend. 197:95–101. doi: 10.1016/j.drugalcdep.2019.01.003 [DOI] [PubMed] [Google Scholar]
- Melis MR, Melis T, Cocco C, Succu S, Sanna F, Pillolla G, Boi A, Ferri GL, Argiolas A (2007) Oxytocin injected into the ventral tegmental area induces penile erection and increases extracellular dopamine in the nucleus accumbens and paraventricular nucleus of the hypothalamus of male rats. Eur J Neurosci. 26(4):1026–35. doi: 10.1111/j.1460-9568.2007.05721.x [DOI] [PubMed] [Google Scholar]
- Miller MA, Bershad A, King AC, Lee R, de Wit H (2016) Intranasal oxytocin dampens cue-elicited cigarette craving in daily smokers: a pilot study. Behav Pharmacol. 27(8):697–703. doi: 10.1097/FBP.0000000000000260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell IJ, Gillespie SM, Abu-Akel A (2015) Similar effects of intranasal oxytocin administration and acute alcohol consumption on socio-cognitions, emotions and behaviour: Implications for the mechanisms of action. Neurosci Biobehav Rev. 55:98–106. doi: 10.1016/j.neubiorev.2015.04.018 [DOI] [PubMed] [Google Scholar]
- Mitchell JM, Arcuni PA, Weinstein D, Woolley JD (2016) Intranasal oxytocin selectively modulates social perception, craving, and approach behavior in subjects with alcohol use disorder. J Addict Med. 10(3):182–9. doi: 10.1097/ADM.0000000000000213 [DOI] [PubMed] [Google Scholar]
- Moaddab M, Hyland B, Brown CH (2015) Oxytocin excites nucleus accumbens shell neurons in vivo. Mol Cell Neurosci. 68:323–30. doi: 10.1016/j.mcn.2015.08.013 [DOI] [PubMed] [Google Scholar]
- Modi ME, Majchrzak MJ, Fonseca KR, Doran A, Osgood S, Vanase-Frawley M, Feyfant E, McInnes H, Darvari R, Buhl DL, Kablaoui NM (2016) Peripheral administration of a long-acting peptide oxytocin receptor agonist inhibits fear-induced freezing. J Pharmacol Exp Ther. 358(2):164–172. doi: 10.1124/jpet.116.232702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales M, Root DH (2014) Glutamate neurons within the midbrain dopamine regions. Neuroscience. 282:60–68. doi: 10.1016/j.neuroscience.2014.05.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustoe A, Schulte NA, Taylor JH, French JA, Toews ML (2019) Leu8 and Pro8 oxytocin agonism differs across human, macaque, and marmoset vasopressin 1a receptors. Sci Rep. 9(1):15480. doi: 10.1038/s41598-019-52024-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann ID, Torner L, Wigger A (2000a) Brain oxytocin: differential inhibition of neuroendocrine stress responses and anxiety-related behaviour in virgin, pregnant and lactating rats. Neuroscience. 95(2):567–75. doi: 10.1016/S0306-4522(99)00433-9 [DOI] [PubMed] [Google Scholar]
- Neumann ID, Wigger A, Torner L, Holsboer F, Landgraf R (2000b) Brain oxytocin inhibits basal and stress-induced activity of the hypothalamo-pituitary-adrenal axis in male and female rats: partial action within the paraventricular nucleus. J Neuroendocrinol. 12(3):235–243. doi: 10.1046/j.1365-2826.2000.00442.x [DOI] [PubMed] [Google Scholar]
- Neumann ID, Maloumby R, Beiderbeck DI, Lukas M, Landgraf R (2013) Increased brain and plasma oxytocin after nasal and peripheral administration in rats and mice. Psychoneuroendocrinology. 38:1985–1993. doi: 10.1016/j.psyneuen.2013.03.003 [DOI] [PubMed] [Google Scholar]
- Omelchenko N, Sesack SR (2009) Ultrastructural analysis of local collaterals of rat ventral tegmental area neurons: GABA phenotype and synapses onto dopamine and GABA cells. Synapse. 63(10):895–906. doi: 10.1002/syn.20668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pati D, Kelly K, Stennett B, Frazier CJ, Knackstedt LA (2016) Alcohol consumption increases basal extracellular glutamate in the nucleus accumbens core of Sprague-Dawley rats without increasing spontaneous glutamate release. Eur J Neurosci. 44(2):1896–1905. doi: 10.1111/ejn.13284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen CA, Prange AJ (1979) Induction of maternal behavior in virgin rats after intracerebroventricular administration of oxytocin. Proc Nat Acad Sci. 76(12): 6661–6665. doi: 10.1073/pnas.76.12.6661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen CA, Ascher JA, Monroe YL, Prange AJ Jr. (1982) Oxytocin induces maternal behavior in virgin female rats. Science. 216(4546): 648–50. doi: 10.1126/science.7071605 [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Smedley KL, Leserman J, Jarskog LF, Rau SW, Kampov-Polevoi A, Casey RL, Fender T, Garbutt J (2013) Intranasal oxytocin blocks alcohol withdrawal in human subjects. Alc Clin Exp Res. 37(3):484–489. doi: 10.1111/j.1530-0277.2012.01958.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peris J, MacFadyen K, Smith JA, de Kloet AD, Wang L, Krause EG (2017) Oxytocin receptors are expressed on dopamine and glutamate neurons in the mouse ventral tegmental area that project to nucleus accumbens and other mesolimbic targets. J Comp Neurol. 525(5):1094–1108. doi: 10.1002/cne.24116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peris J, Montgomery D, Totten K, Weatherington A, Ortiz Y, Scott KA, Tan Y, Krause EG (2019) Drinking-in-the-dark for two months decreases conditioned social preference in mice. Alcoholism: Clin. Exp. Res 43:211A. doi: 10.111/acer.14056 [DOI] [Google Scholar]
- Peters S, Bowen MT, Bohrer K, McGregor IS, Neumann ID (2016) Oxytocin inhibits ethanol consumption and ethanol-induced dopamine release in the nucleus accumbens. Addiction Biol. 22(3):702–711. doi: 10.1111/adb.1236 [DOI] [PubMed] [Google Scholar]
- Peters S, Slattery DA, Flor PJ, Neumann ID, Reber SO (2012) Differential effects of baclofen and oxytocin on the increased ethanol consumption following chronic psychosocial stress in mice. Addiction Biol. 18(1):66–77. doi: 10.1111/adb.12001 [DOI] [PubMed] [Google Scholar]
- Peters S, Slattery DA, Uschold-Schmidt N, Reber SO, Neumann ID (2014) Dose-dependent effects of chronic central infusion of oxytocin on anxiety, oxytocin receptor binding and stress-related parameters in mice. Psychoneuroendocrinology. 42:225–36. doi: 10.1016/j.psyneuen.2014.01.021 [DOI] [PubMed] [Google Scholar]
- Pupe S, Wallén-Mackenzie Å (2015) Cre-driven optogenetics in the heterogeneous genetic panorama of the VTA. Trends Neurosci. 38(6):375–86. doi: 10.1016/j.tins.2015.04.005 [DOI] [PubMed] [Google Scholar]
- Qi J, Zhang S, Wang HL, Barker DJ, Miranda-Barrientos J, Morales M (2016) VTA glutamatergic inputs to nucleus accumbens drive aversion by acting on GABAergic interneurons. Nat Neurosci. 19:725–733. doi: 10.1038/nn.4281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ring RH, Malberg JE, Potestio L, Ping J, Boikess S, Luo B, Schechter LE, Rizzo S, Rahman Z, Rosenzweig-Lipson S (2006) Anxiolytic-like activity of oxytocin in male mice: behavioral and autonomic evidence, therapeutic implications. Psychopharmacol. 185(2):218–225. doi: 10.1007/s00213-005-0293-z [DOI] [PubMed] [Google Scholar]
- Rivier C, Bruhn T, Vale W (1984) Effect of ethanol on the hypothalamic-pituitary-adrenal axis in the rat: role of corticotropin-releasing factor (CRF). J Pharmacol Exp Ther. 229(1):127–31. [PubMed] [Google Scholar]
- Rivier C, Imaki T, Vale W (1990) Prolonged exposure to alcohol: effect on CRF mRNA levels, and CRF- and stress-induced ACTH secretion in the rat. Brain Res. 520(1–2):1–5. doi: 10.1016/0006-8993(90)91685-a [DOI] [PubMed] [Google Scholar]
- Root DH, Mejias-Aponte CA, Qi J, Morales M (2014) Role of glutamatergic projections from ventral tegmental area to lateral habenula in aversive conditioning. J Neurosci. 34(42):13906–13910. doi: 10.1523/JNEUROSCI.2029-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root DH, Estrin DJ, Morales M (2018a) Aversion or salience signaling by ventral tegmental area glutamate neurons. iScience. 2:51–62. doi: 10.1016/j.isci.2018.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root DH, Zhang S, Barker DJ, Miranda-Barrientos J, Liu B, Wang HL, Morales M (2018b) Selective Brain Distribution and Distinctive Synaptic Architecture of Dual Glutamatergic-GABAergic Neurons. Cell Rep. 23(12):3465–3479. doi: 10.1016/j.celrep.2018.05.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan PJ, Ross SI, Campos CA, Derkach VA, Palmiter RD (2017) Oxytocin-receptor-expressing neurons in the parabrachial nucleus regulate fluid intake. Nat Neurosci. 20(12):1722–1733. doi: 10.1038/s41593-017-0014-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarnyai Z, Kovács GL (2013) Oxytocin in learning and addiction: From early discoveries to the present. Pharmacol Biochem Behav. 119:3–9. doi: 10.1016/j.pbb.2013.11.019 [DOI] [PubMed] [Google Scholar]
- Sidhu H, Kreifeldt M, Contet C (2018) Affective disturbances during withdrawal from chronic intermittent ethanol inhalation in C57BL/6J and DBA/2J male mice. Alcohol Clin Exp Res. 42(7):1281–1290. doi: 10.1111/acer.13760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva SM, Madeira MD, Ruela C, Paula-Barbosa MM (2002a) Prolonged alcohol intake leads to irreversible loss of vasopressin and oxytocin neurons in the paraventricular nucleus of the hypothalamus. Brain Res. 925(1):76–88. doi: 10.1016/S0006-8993(01)03261-9 [DOI] [PubMed] [Google Scholar]
- Silva SM, Paula-Barbosa MM, Madeira MD (2002b) Prolonged alcohol intake leads to reversible depression of corticotropin-releasing hormone and vasopressin immunoreactivity and mRNA levels in the parvocellular neurons of the paraventricular nucleus. Brain Res. 954(1):82–93. doi: 10.1016/S0006-8993(01)03261-9 [DOI] [PubMed] [Google Scholar]
- Singhal SM Harden SW, Krause EG, Frazier CJ (2018) Oxytocin enhances excitatory and inhibitory synaptic transmission in a layer specific manner in the rat medial prefrontal cortex. Society for Neuroscience Abstracts 44: 287.16. [Google Scholar]
- Sinha R, Fox HC, Hong KI, Hansen J, Tuit K, Kreek MJ (2011) Effects of adrenal sensitivity, stress- and cue-induced craving, and anxiety on subsequent alcohol relapse and treatment outcomes. Arch Gen Psychiatry. 68(9):942–52. doi: 10.1001/archgenpsychiatry.2011.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivukhina EV, Dolzhikov AA, Morozov E, Jirikowski GF, Grinevich V (2006) Effects of chronic alcoholic disease on magnocellular and parvocellular hypothalamic neurons in men. Horm Metab Res. 38(6):382–390. doi: 10.1055/s-2006-944522 [DOI] [PubMed] [Google Scholar]
- Smith JA, Pati D, Wang L, de Kloet AD, Frazier CJ, Krause EG (2015) Hydration and beyond: neuropeptides as mediators of hydromineral balance, anxiety and stress-responsiveness. Front Syst Neurosci. 9:46. doi: 10.3389/fnsys.2015.00046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew MV (1983) Vasopressin and oxytocin in the mammalian brain and spinal cord. Trends in Neurosciences. 6: P467–472. doi: 10.1016/0166-2236(83)90221-7 [DOI] [Google Scholar]
- Somkuwar SS, Vendruscolo LF, Fannon MJ, Schmeichel BE, Nguyen TB, Guevara J, Sidhu H, Contet C, Zorrilla EP, Mandyam CD (2017) Abstinence from prolonged ethanol exposure affects plasma corticosterone, glucocorticoid receptor signaling and stress-related behaviors. Psychoneuroendocrinology. 84:17–31. doi: 10.1016/j.psyneuen.2017.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP (2018). Effects of adolescent alcohol consumption on the brain and behaviour. Nat Rev Neurosci. 19:197–214. doi: 10.1136/bmjopen-2018-023629 [DOI] [PubMed] [Google Scholar]
- Stennett BA, Frankowski JC, Peris J, Knackstedt LA (2017) Ceftriaxone reduces alcohol intake in outbred rats while upregulating xCT in the nucleus accumbens core. Pharmacol Biochem Behav. 159:18–23. doi: 10.1016/j.pbb.2017.07.001 [DOI] [PubMed] [Google Scholar]
- Stevenson JR, Wenner SM, Freestone DM, Romaine CC, Parian MC, Christian SM, Bohidar AE, Ndem JR, Vogel IR, O’Kane CM (2017a) Oxytocin reduces alcohol consumption in prairie voles. Physiol Behav. 179:411–421. doi: 10.1016/j.physbeh.2017.07.021 [DOI] [PubMed] [Google Scholar]
- Stevenson JR, Young KA, Bohidar AE, Francomacaro LM, Fasold TR, Buirkle JM, Ndem JR, Christian SC (2017b). Alcohol consumption decreases oxytocin neurons in the anterior paraventricular nucleus of the hypothalamus in prairie voles. Alcohol Clin Exp Res. 41:1444–1451. doi: 10.1111/acer.13430 [DOI] [PubMed] [Google Scholar]
- Striepens N, Kendrick KM, Hanking V, Landgraf R, Wüllner U, Maier W, Hurlemann R (2013) Elevated cerebrospinal fluid and blood concentrations of oxytocin following its intranasal administration in humans. Sci Rep. 3:3440. doi: 10.1038/srep03440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabó G, Kovács GL, Székeli S, Telegdy G (1985) The effects of neurohypophyseal hormones on tolerance to the hypothermic effect of ethanol. Alcohol. 2(4):567–74. doi: 10.1016/0741-8329(85)90082-5 [DOI] [PubMed] [Google Scholar]
- Szabó G, Kovács GL, Telegdy G (1987) Effects of neurohypophyseal peptide hormones on alcohol dependence and withdrawal. Alcohol Alcohol. 22(1):71–74. doi: 10.1093/oxfordjournals.alcalc.a044668 [DOI] [PubMed] [Google Scholar]
- Takayanagi Y, Yoshida M, Bielsky IF, Ross HE, Kawamata M, Onaka T, Yanagisawa T, Kimura T, Matzuk MM, Young LJ, Nishimori K (2005) Pervasive social deficits, but normal parturition, in oxytocin receptor-deficient mice. Proc Natl Acad Sci USA. 102(44):16096–101. doi: 10.1073/pnas.0505312102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Y, Singhal SM, Harden SW, Cahill KM, Nguyen DM, Colon-Perez LM, Sahagian TJ, Thinschmidt JS, de Kloet AD, Febo M, Frazier CJ, Krause EG (2019) Oxytocin receptors are expressed by glutamatergic prefrontal cortical neurons that selectively modulate social recognition. J Neurosci. 39(17):3249–3263. doi: 10.1523/JNEUROSCI.2944-18.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Chen Z, Tao H, Li C, Zhang X, Tang A, Liu Y (2014) Oxytocin activation of neurons in ventral tegmental area and interfascicular nucleus of mouse midbrain. Neuropharmacology. 77:277–84. doi: 10.1016/j.neuropharm.2013.10.004 [DOI] [PubMed] [Google Scholar]
- Taylor SR, Badurek S, Dileone RJ, Nashmi R, Minichiello L, Picciotto MR (2014) GABAergic and glutamatergic efferents of the mouse ventral tegmental area. J Comp Neurol. 522(14):3308–34. doi: 10.1002/cne.23603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terenzi MG, Ingram CD (2005) Oxytocin-induced excitation of neurones in the rat central and medial amygdaloid nuclei. Neuroscience. 134(1):345–54. doi: 10.1016/j.neuroscience.2005.04.004 [DOI] [PubMed] [Google Scholar]
- Tirko NN, Eyring KW, Carcea I, Mitre M, Chao MV, Froemke RC, Tsien RW (2018) Oxytocin Transforms Firing Mode of CA2 Hippocampal Neurons. Neuron 100(3):593–608.e3. doi: 10.1016/j.neuron.2018.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunstall BJ, Kirson D, Zallar LJ, McConnell SA, Vendruscolo JCM, Ho CP, Oleata CS, Khom S, Manning M, Lee MR, Leggio L, Koob GF, Roberto M, Vendruscolo LF (2019) Oxytocin blocks enhanced motivation for alcohol in alcohol dependence and blocks alcohol effects on GABAergic transmission in the central amygdala. PLoS Biol. 17:e2006421. doi: 10.1371/journal.pbio.2006421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hedger K, Bershad AK, Lee R, de Wit H (2018) Effects of intranasal oxytocin on stress-induced cigarette craving in daily smokers. Nicotine Tob Res. doi: 10.1093/ntr/nty159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venniro M, Caprioli D, Shaham Y (2016) Animal models of drug relapse and craving: From drug priming-induced reinstatement to incubation of craving after voluntary abstinence. Prog Brain Res. 224:25–52. doi: 10.1016/bs.pbr.2015.08.004 [DOI] [PubMed] [Google Scholar]
- Venniro M, Caprioli D2, Shaham Y (2019) Novel models of drug relapse and craving after voluntary abstinence. Neuropsychopharmacology. 44(1):234–235. doi: 10.1038/s41386-018-0196-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vungkhanching M1, Sher KJ, Jackson KM, Parra GR (2004) Relation of attachment style to family history of alcoholism and alcohol use disorders in early adulthood. Drug Alcohol Depend. 75(1):47–53. doi: 10.1016/j.drugalcdep.2004.01.013 [DOI] [PubMed] [Google Scholar]
- Walcott AT, Ryabinin AE (2017) Alcohol’s effects on pair-bond maintenance in male prairie voles. Front Psychiatry. 8:226. doi: 10.3389/fpsyt.2017.00226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walcott AT, Ryabinin AE (2019) Effects of alcohol consumption on pair bond maintenance and potential neural substrates in female prairie voles. Alcohol Alcohol. pii: agz041. doi: 10.1093/alcalc/agz041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HL, Qi J, Zhang S, Wang H, Morales M (2015) Rewarding effects of optical stimulation of ventral tegmental area glutamatergic neurons. J Neurosci. 35(48):15948–54. doi: 10.1523/JNEUROSCI.3428-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber RA, Logan CN, Leong KC, Peris J, Knackstedt L, Reichel CM (2018) Regionally specific effects of oxytocin on reinstatement of cocaine seeking in male and female rats. Int J Neuropsychopharmacol. 21(7):677–686. doi: 10.1093/ijnp/pyy025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windle RJ, Shanks N, Lightman SL, Ingram CD (1997) Central oxytocin administration reduces stress-induced corticosterone release and anxiety behavior in rats. Endocrinology. 138(7):2829–34. doi: 10.1210/endo.138.7.5255 [DOI] [PubMed] [Google Scholar]
- Woolley JD, Arcuni PA, Stauffer CS, Fulford D, Carson DS, Batki S, Vinogradov S (2016) The effects of intranasal oxytocin in opioid-dependent individuals and healthy control subjects: a pilot study. Psychopharmacology (Berl). 233(13):2571–80. doi: 10.1007/s00213-016-4308-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L, Priest MF, Nasenbeny J, Lu T, Kozorovitskiy Y (2017) Biased oxytocinergic modulation of midbrain dopamine systems. Neuron. 95(2):368–384. doi: 10.1016/j.neuron.2017.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Wang HL, Li X, Ng TH, Morales M (2011) Mesocorticolimbic glutamatergic pathway. J Neurosci. 31(23):8476–90. doi: 10.1523/JNEUROSCI.1598-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates JR, Beckmann JS, Meyer AC, Bardo MT (2013) Concurrent choice for social interaction and amphetamine using conditioned place preference in rats: effects of age and housing condition. Drug Alc.Dep 129(3):240–246. doi: 10.1016/j.drugalcdep.2013.02.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo JH, Zell V, Gutierrez-Reed N (2016) Ventral tegmental area glutamate neurons co-release GABA and promote positive reinforcement. Nat Commun. 7:13697. doi: 10.1038/ncomms13697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- You C, Savarese A, Vandegrift BJ, He D, Pandey SC, Lasek AW, Brodie MS (2019) Ethanol acts on KCNK13 potassium channels in the ventral tegmental area to increase firing rate and modulate binge-like drinking. Neuropharmacology. 144:29–36. doi: 10.1016/j.neuropharm.2018.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young KA, Liu Y, Gobrogge KL, Wang H, Wang Z (2014) Oxytocin reverses amphetamine-induced deficits in social bonding: Evidence for an interaction with nucleus accumbens dopamine. J Neurosci. 34(25):8499–8506. doi: 10.1523/JNEUROSCI.4275-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LJ, Muns S, Wang Z, Insel TR (1997) Changes in oxytocin receptor mRNA in rat brain during pregnancy and the effects of estrogen and interleukin-6. J Neuroendocrinol. 9(11):859–65. doi: 10.1046/j.1365-2826.1997.00654.x [DOI] [PubMed] [Google Scholar]
- Young LJ, Wang Z (2004) The neurobiology of pair bonding. Nat. Neurosci 7(10):1048–1054. doi: 10.1038/nn1327 [DOI] [PubMed] [Google Scholar]
- Zhou L, Ghee SM, See RE, Reichel CM (2015) Oxytocin differentially affects sucrose taking and seeking in male and female rats. Behav Brain Res. 283:184–190. doi: 10.1016/j.bbr.2015.01.050 [DOI] [PMC free article] [PubMed] [Google Scholar]


