Abstract
Heart rate variability (HRV) and End Tidal CO2 (ETCO2) in relation to treatment response have not been studied in Latino populations or in comorbid asthma and panic disorder (PD). An extension of previously published research, the current study explored psychophysiological variables as possible mediators of treatment response. Latino treatment completers (N = 32) in the Bronx with asthma-PD received either Cognitive-Behavioral Psychophysiological Therapy (CBPT) or Music Relaxation Therapy (MRT). CBPT included HRV-biofeedback (HRVB); in-the-moment heart rate data to help an individual learn to influence his/her own heart rate. The sample was primarily female (93.8%) and Puerto Rican (81.25%). Treatment groups did not differ on demographics, except for less education in CBPT. The Panic Disorder Severity Scale (PDSS) and Asthma Control Questionnaire (ACQ) assessed changes in symptoms. HRV and ETCO2 were measured at four of eight therapy sessions. Baseline ETCO2 and changes in HRV from first to last of psychophysiology sessions were investigated as mediators of change on ACQ and PDSS. Mixed model analyses indicated in the CPBT group, changes in both asthma control and PD severity were not mediated by changes in HRV. In the CBPT and MRT groups combined, changes in PD severity were not mediated by baseline ETCO2. These findings may be due to the brevity of HRVB in CBPT, multiple treatment components, ETCO2 not directly targeted, and/or unique physiological pathways in Latinos with asthma-PD.
Keywords: HRV, ETCO2, asthma, panic, Latinos, cognitive behavior therapy (CBT)
Introduction
Asthma and panic disorder (PD) are characterized by similar symptoms and psychophysiological markers. For example, both disorders are associated with low Heart Rate Variability (HRV), the variation in the time interval between heartbeats due to breathing (Garakani et al. 2009; Friedman and Thayer 1998; Klein et al. 1995; Yeragani et al. 1993; Garrard et al. 1992; Kazuma et al. 1997). Low end tidal carbon dioxide (ETCO2), or the maximal concentration of carbon dioxide at the end of an exhaled breath, is another shared feature. Comorbidity rates are high (Hasler et al. 2005; Nascimento et al. 2002; Goodwin et al. 2003) and patients with comorbid asthma and PD report higher levels of subjective distress as compared with those with asthma alone (Boudreau et al. 2015). Individuals of Puerto Rican, Cuban, and Dominican backgrounds are two- to five-times more likely to report lifetime asthma than individuals of Mexican descent (National Heart Lung and Blood Institute 2013). Puerto Rican background was associated with particularly high prevalence rate of lifetime asthma of 35.8% (National Heart Lung and Blood Institute 2013). There is currently a lack of research on psychophysiological factors in Latino populations and ethnic subgroups.
End Tidal Carbon Dioxide (ETCO2).
Patients with PD have shown increased sensitivity to CO2 (Papp et al. 1997; Gorman et al. 1988; Maddock and Carter 1991; Dager et al. 1995). This unique respiratory dysfunction may necessitate modifications to treatment interventions. Additionally, lower baseline levels of ETCO2 have predicted less response to psychotherapy treatments in a non-asthmatic, mixed-anxiety disorders sample of participants (Davies and Craske 2014). While PD symptomatology can be addressed through multiple treatment approaches, targeting physiological factors has been shown to impact underlying physiology most effectively (Meuret et al. 2010; Meuret et al. 2001; Meuret et al. 2009).
ETCO2 also plays an important role in the physiology of asthma: low levels of CO2 (hypocapnia) may contribute to airway obstruction (Van den Elshout et al. 1991). Treatments targeting ETCO2 have been successful in reducing symptoms and improving reported asthma control (Meuret 2007), as well as increasing ETCO2 levels, reducing airway obstruction, and reducing asthma symptoms over time (Ritz et al. 2014).
Heart Rate Variability (HRV).
Increased HRV may predict cardiovascular health and decreased risk of mortality (Kleiger et al. 1987; Dekker et al. 2000; La Rovere et al. 2003; Tsuji et al. 1996). It may also contribute to physical and mental health through relationships with cognitive performance and brain functioning (Thayer et al. 2009). HRV may be reduced in those with PD (Yeragani et al. 1993; Klein et al. 1995; Friedman and Thayer 1998; Garakani et al. 2009) as well as adults (Garrard et al. 1992) and children (Kazuma et al. 1997) with asthma. HRVB was effective as a complementary treatment for asthma resulting in improvements in pulmonary function and reduced need for steroid medications. It appears that HRV may be influenced through a range of clinical approaches (Chuang et al. 2010; Raglio et al. 2010; Garakani et al. 2009; Lehrer et al. 2006; Lehrer et al. 2004). In contrast, ETCO2 appears to require specific targeting CO2 levels in order to be normalized.
The current study builds on an RCT designed to treat Latinos with comorbid asthma-PD. HRV and ETCO2 were examined using data comparing two psychological treatment approaches (Feldman et al. 2016). The experimental intervention of Cognitive-Behavioral Psychophysiological Therapy (CBPT) included 4 sessions of HRV Biofeedback (HRVB). Music Relaxation Therapy (MRT) was an active comparison treatment and included paced breathing at participants’ average resting respiration rate as a control for the HRVB component of CBPT. In a previous publication, we reported that both treatment groups improved significantly on measures of asthma and PD, including: PD severity, asthma control, Beck Depression Inventory, and anxiety measures (Anxiety Sensitivity Index, Agoraphobia Cognitions Questionnaire, Body Sensations Questionnaire) (Feldman et al. 2016). CBPT had significant improvements in self-reported asthma medication adherence to daily inhaled corticosteroids compared with MRT (Feldman et al. 2016). We also found that psychological factors mediated outcomes: decreases in PD severity in the CBPT group were mediated by anxiety sensitivity, while decreases in the MRT group were mediated by depression (Feldman et al. 2016).
Psychophysiological measures indicated no changes in ETCO2 on between-group and within-group analyses. HRV variables of LF and LF/HF ratio strongly increased within each session, with accompanying decreases in respiration from baseline task to HRV biofeedback, providing evidence of treatment fidelity that HRV biofeedback was successful (Feldman et al. 2016). These findings together led to our interest in exploring possible mechanisms of change. Psychophysiological data collected during this study have not been previously examined in mediational analyses, thus we investigated whether asthma and PD outcomes might additionally be mediated by psychophysiological variables. We hypothesized that in the CBPT treatment, increases in LF and LF/HF would mediate decreases in PDSS and ACQ scores. We also hypothesized that higher levels of ETCO2 at baseline would mediate greater decreases in PDSS score in both groups.
Methods
This was a mixed design study and participants were randomly assigned (through stratified computer generated sequence) to one of two groups: MRT or CBPT. Treatments were available in English and Spanish. The Institutional Review Boards at the Albert Einstein College of Medicine and Rutgers University approved all study protocols. CONSORT guidelines were followed and the study was registered at ClinicalTrials.gov (NCT01583296). Please see the primary publication for extensive description of procedures (Feldman et al. 2016).
Participants.
Latino adults age 18 and older were recruited through hospital-based outpatient clinics from 2010 to 2012. Inclusion criteria were: (i) DSM-IV diagnosis of PD, with or without Agoraphobia, (ii) asthma diagnosis, (iii) fluency in English or Spanish, (iv) during the two months prior to and while in the active treatment intervention, participants agreed to avoid changes in prescription levels of psychotropic medications. Exclusion criteria included: bipolar disorder, psychosis, cognitive impairment, or organic brain syndrome; current alcohol or substance use/dependence; less than six months participation in alternative psychotherapy for anxiety or PD.
In each of the CBPT and MRT groups, 24 randomized participants attended at least the first therapy session and 16 were treatment completers at both the post-treatment and three-month follow-up time points (see Figure 1 for additional details). The sample was primarily female (93.8%) and Puerto Rican (81.25%), with poorly controlled asthma (85.4%). Diagnosis of PD with agoraphobia was current for 83.4% of participants and 37.5% met criteria for current Major Depressive Disorder.
Fig. 1.
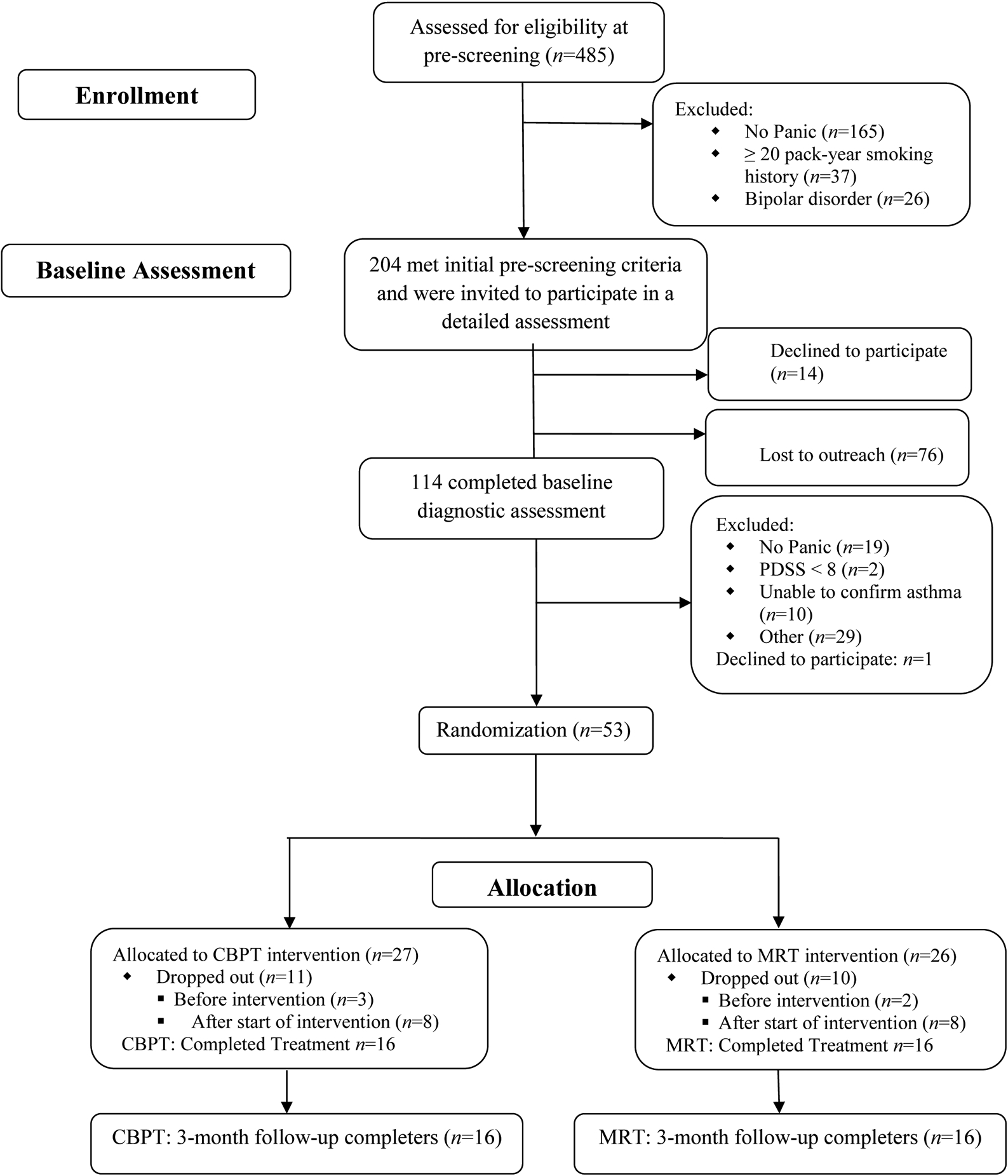
Participant Flowchart. Reproduced with permission from Feldman et al. (2016).
Panic Disorder Diagnosis.
PD was assessed using DSM-IV criteria with the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I), Research Version, Patient Edition (First et al. 1995). Administrations of the SCID-I were recorded and reviewed by licensed clinical psychologists who established diagnosis by consensus.
Asthma Diagnosis.
Asthma diagnosis was determined by a board-certified pulmonary physician based on data from baseline assessment and electronic medical chart review in concordance with NHLBI Guidelines (National Heart Lung and Blood Institute 1997). If needed, participants completed a bronchodilator test during the baseline session to confirm asthma diagnosis.
Psychological and Physiological Measures
Asthma Control Questionnaire (ACQ).
Change in asthma control was measured with the ACQ, as assessed by blinded research assistants. This 7-item questionnaire measures the adequacy of asthma control and change in asthma control over the past week with each item being measured on a 7-point scale. Responses are averaged, with 0 indicating good control and 6 indicating extremely poor control. The ACQ has good measurement properties and is valid, reproducible, internally consistent, and sensitive to changes within a participant (Juniper et al. 1999).
Panic Disorder Severity Scale (PDSS).
The PDSS is based on clinician rating of overall PD severity and was used to measure change in PD severity over time. The PDSS has a possible range of 0 to 28 and a cut-off score of 8 to identify current PD. PDSS ratings were determined by blinded clinical psychology graduate students trained in the use of this instrument and supervisors. The PDSS has good measurement properties and is reliable, internally consistent and valid (Shear et al. 2001).
End Tidal Carbon Dioxide (ETCO2).
ETCO2 was measured with a capnometer (Better Breathing, Boulder CO), which sampled exhaled gas via a nasal cannula.
Heart Rate Variability (HRV).
HRV data were edited using Kubios software (Version 2.1, University of Eastern Finland, MATLAB). Analyses of inter-beat-intervals included low frequency (LF) band (0.04–0.15 Hz), high frequency (HF) band (0.15–0.4 Hz), and as a ratio of low frequency to high frequency (LF/HF).
Physiologicial Recording Procedures.
For both groups, the entire treatment included 8 weekly sessions lasting from 60–90 minutes, with ETCO2 and HRV data collected during therapy sessions 3, 4, 5, and 7. Data were recorded on a six-channel I-330-C2+ physiograph device (J&J Engineering, Poulsbo, WA). Biofeedback software CapnoPolygraph 5 was used during the sessions. Both groups breathed at various frequencies for two minute intervals, accomplished by following a pacer. The first 5 minutes consisted of a plain vanilla task to establish baseline levels. The next two 5 minute tasks consisted of breathing at resonance or designated frequency, dependent upon group assignment. The CBPT group practiced these second and third tasks both with and without pacers. The fourth and final recording was another 5 minute plain vanilla task. Previously established procedures for HRV biofeedback were utilized (Lehrer et al. 2000). In order to avoid the MRT group breathing at the resonance frequency, therapists were trained to ensure that the frequency range remained between 9 and 18 breaths per minute. This range was also intended to restrict participants from breathing at higher rates, which often result in dyspnea (i.e. shortness of breath) for patients with PD. Participants in both groups were asked to practice at home twice daily for 20 minutes each session.
Statistical Analyses
Analyses were conducted using Statistical Analysis System version 9.4 (SAS 9.4) and R software (R Foundation for Statistical Computing). Analyses were conducted on an intent-to-treat basis and included all participants that were randomized to one of the two treatment groups (n = 24/group) and completed at least through the third therapy session, which was the first to include psychophysiology measurements. Missing data were assumed to be missing at random (Ibrahim and Molenberghs 2009; Feldman et al. 2016). Age, gender, and height were included as covariates for HRV analyses (LF and LF/HF ratio). The natural logarithm was used to transform HRV (LF and LF/HF) variables and ACQ data. No covariates were included for analyses of ETCO2. Threshold for statistical significance was defined by p-value <0.05. Preliminary analyses included t-tests to evaluate group differences at baseline on the following variables: demographics (age, weight, race), LF, LF/HF, ETCO2, and respiration rate.
Mixed model analyses evaluated longitudinal mediational models to determine whether changes in PDSS or ACQ scores over time were mediated by LF or LF/HF ratio and their longitudinal changes. HRV data included the third therapy appointment (first of the psychophysiological recordings) and the seventh therapy appointment (fourth and last of the psychophysiological recordings). Data for PDSS and ACQ were included from the baseline and post-treatment timepoints. We hypothesized that in the CBPT treatment, increases in LF and LF/HF would mediate decreases in PDSS and ACQ scores from baseline to post-treatment. Mixed model analyses also evaluated whether changes in PDSS scores over time (from baseline to post-treatment timepoints) were mediated by baseline levels of ETCO2 in both CBPT and MRT. We hypothesized that higher baseline levels of ETCO2 would mediate decreases in PDSS score in both groups from baseline to post-treatment. PDSS data were z-scored to facilitate path coefficient interpretation. The following three models were fitted: Model 1: Yij= u1i+ b1+ c’ timeij +e1i, Model 2: Mij= u2i+ b2+ a time ij+e2i, Model 3: Yij= u3i+ b3+ c timeij + b Mij+ e3i. Statistical hypotheses of H0: ab=0 vs. H1: ab≠0, at α = 5% were utilized to test the mediation models (model 2 and model 3).
Results
Analyses were conducted to obtain baseline characteristics of the participants who were primarily women of Puerto Rican ethnicity. Comparison of between group demographic characteristics did not reveal any significant group differences at baseline. Comparisons of the 21 participants who dropped out with the 32 participants who completed the study revealed no differences on demographics, asthma control, depression, or PD and asthma severity. Disconnected cell phones with no alternative contact information was the primary reason for participant drop out. Thus, data missing from participant drop outs are likely missing at random across the two groups.
In the CBPT group only, the mediated pathways from time to PDSS or ACQ through LF or LF/HF ratio were examined for each combination of variables (e.g. PDSS with LF as mediator, PDSS with LF/HF, ACQ with LF, and ACQ with LF/HF). Mixed model analyses revealed that HRV measures did not mediate improvements in PD (PDSS) or asthma control (ACQ) (Table 1). Figures 2a–2d illustrate the non-significant regression coefficients.
Table 1.
Model Coefficients for CBPT Group Mediational Analyses
| Model | a | b | c’ | Indirect Effect | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mediator | Outcome | Coeff. | SE | p | Coeff. | SE | p | Coeff. | SE | p | Estimate | 95% CI |
| LF | PDSS | −0.619 | 0.271 | 0.062 | −0.372 | 0.188 | 0.104 | 0.097 | 0.214 | 0.658 | 0.230 | [−0.012, 0.608] |
| LF/HF | PDSS | −0.887 | 0.569 | 0.170 | −0.204 | 0.145 | 0.218 | 0.097 | 0.214 | 0.658 | 0.181 | [−0.100, 0.646] |
| LF | ACQ | −0.619 | 0.271 | 0.062 | 0.039 | 0.227 | 0.870 | −0.533 | 0.281 | 0.079 | −0.024 | [−0.354, 0.287] |
| LF/HF | ACQ | −0.887 | 0.569 | 0.170 | −0.136 | 0.162 | 0.439 | −0.533 | 0.281 | 0.079 | 0.121 | [−0.187, 0.573] |
Fig. 2.
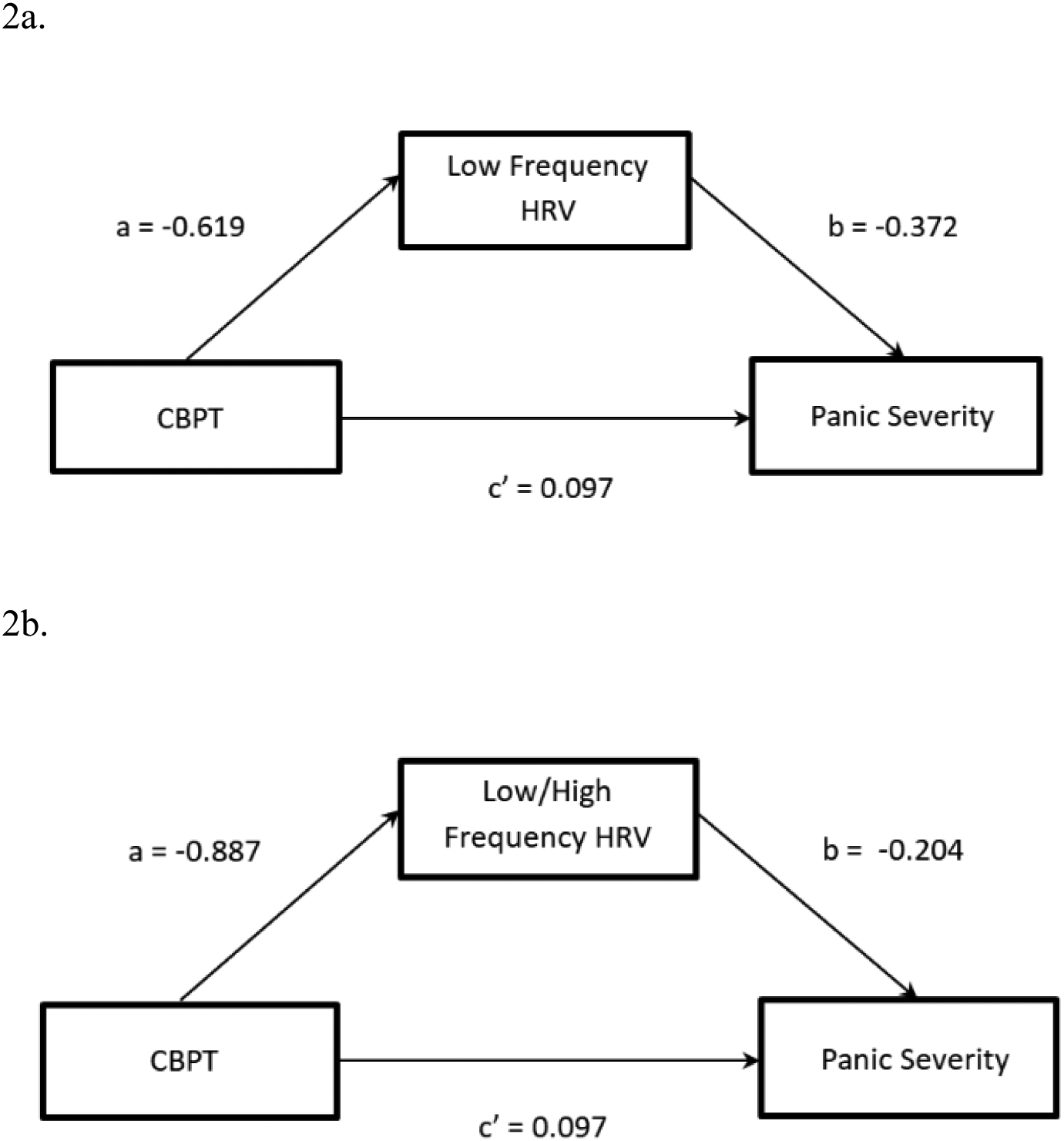
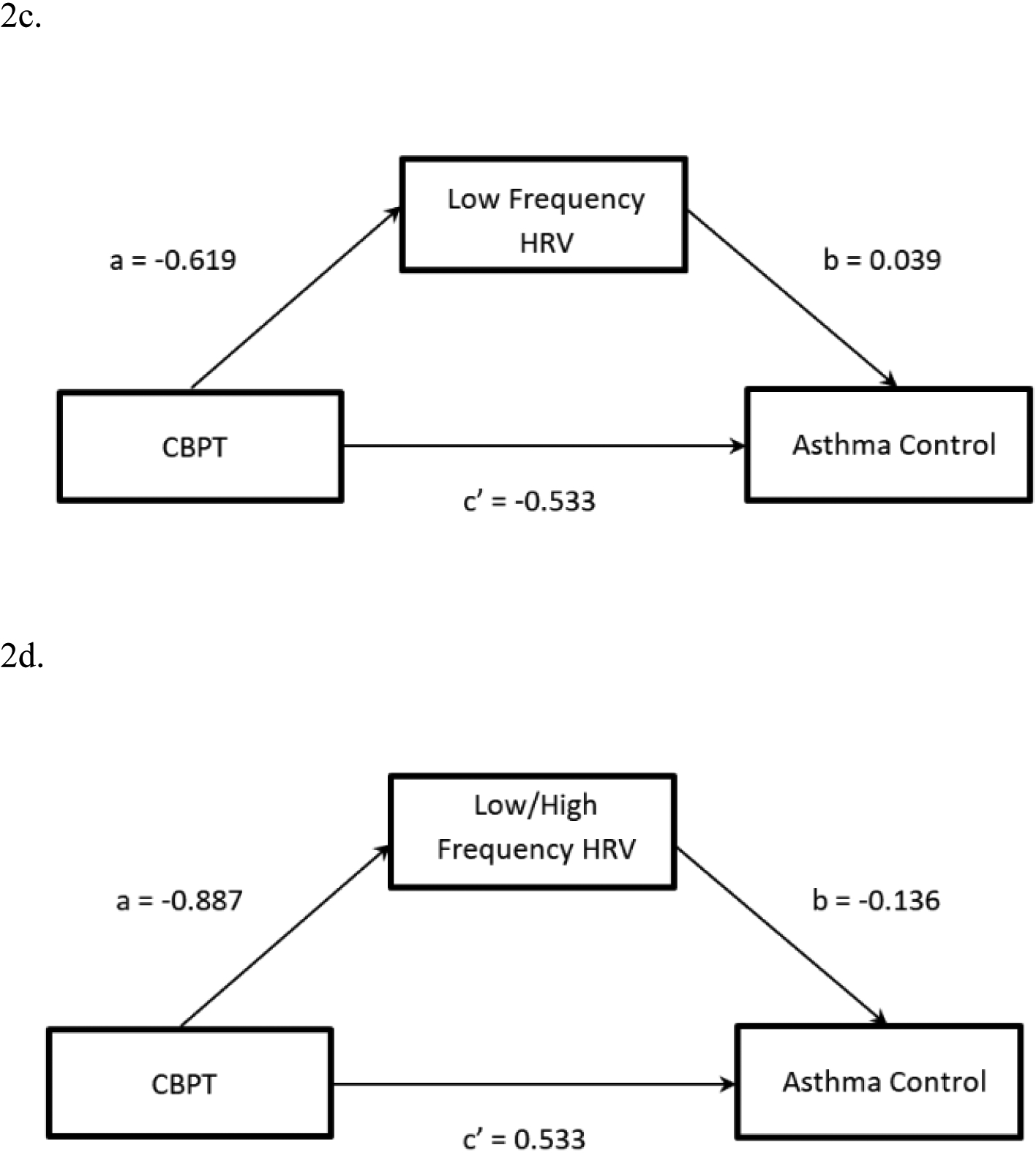
Model 2a. Mediated pathway for CBPT and PDSS through low frequency (LF) heart rate variability (HRV). Model 2b. Mediated pathway for CBPT and PDSS through low/high frequency (LF/HF) heart rate variability (HRV). Model 2c. Mediated pathway for CBPT and asthma control questionnaire (ACQ) through LF HRV. Model 2d. Mediated pathway for CBPT and ACQ through LF/HF ratio.
Mixed model analyses revealed that changes in PD severity were not mediated by baseline ETCO2 (Table 2). Figure 3 shows that ETCO2 did not mediate changes in PDSS for the CBPT and MRT groups combined.
Table 2.
Model Coefficients for combined CBPT and MRT Group Mediational Analyses
| Model | Predictors of Mediator | Predictors of Outcome | Predictors of the outcome in mediated model | |||||
|---|---|---|---|---|---|---|---|---|
| Mediator | Outcome | Time (a1) | Time2 (a2) | Time (c1) | Time2 (c2) | Time (c1’) | Time2 (c2’) | Mediator (b) |
| ETCO2 | PDSS | −4.405 | 0.983 | 0.305 | −0.102 | 0.407 | −0.131 | −0.008 |
Fig. 3.
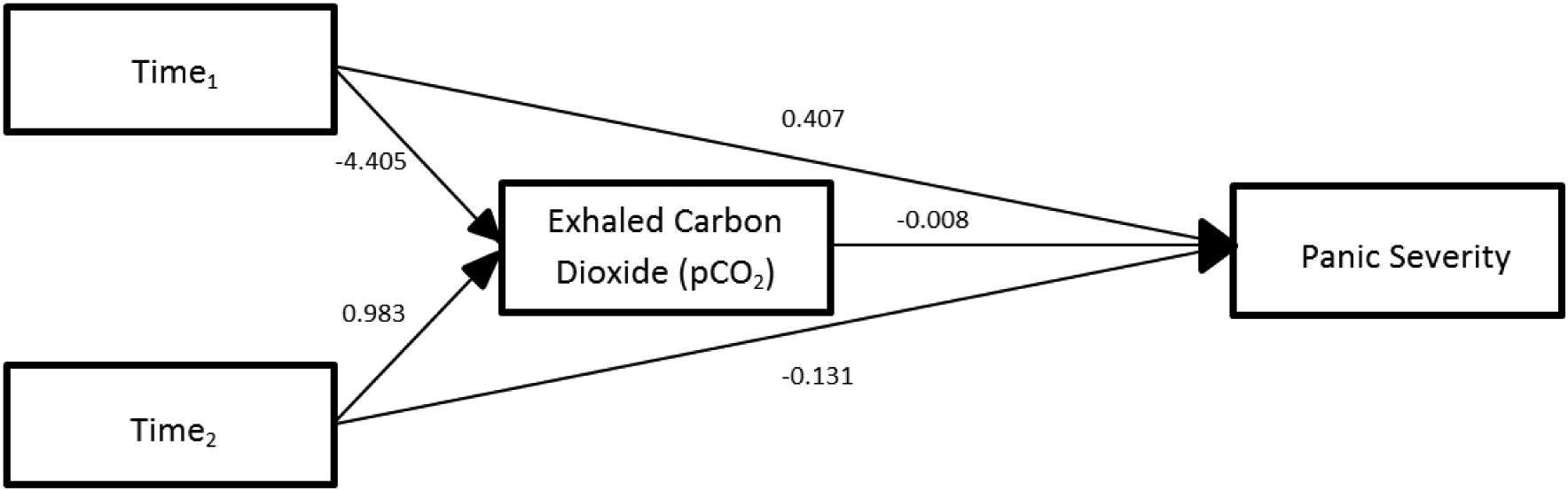
Model 3. Mediated pathway for both CBPT and MRT through end tidal carbon dioxide (ETCO2).
Discussion
While both CBPT and MRT groups showed decreases in PD severity and increases in asthma control from baseline to post-treatment (Feldman et al. 2016), in the CBPT group these changes were not mediated by changes in HRV (LF and LF/HF ratio) across time. Similarly, baseline levels of ETCO2 did not mediate decreases in PD severity from baseline to post-treatment in both CBPT and MRT groups combined. While it appears that HRV and ETCO2 are important markers and treatment targets of asthma and PD when not comorbid, such findings were not replicated in the current study of Latinos with both conditions.
In the current study, heart rate variability was specifically targeted through HRVB in the CBPT group. The conclusion that HRV was not a primary mechanism of change may be premature, however, as the HRVB was but one aspect of a multi-component treatment. The integration of other therapeutic elements may have minimized the influence of HRV or ETCO2 as a causal factor of change on asthma and PD outcomes. In contrast with HRV, ETCO2 was not a specific target of treatment, which may at least partially explain why it was not a mediator of clinical change outcomes. Previous research found that targeting this variable was necessary to influence ETCO2 levels over time (Ritz et al. 2014; Meuret et al. 2010).
Future interventions may benefit from utilizing treatment protocols that include only biofeedback procedures. Comparison of a treatment targeting ETCO2 with one targeting HRV in a sample of individuals with comorbid asthma and PD may be particularly interesting. As HRVB was not the primary therapeutic intervention, the biofeedback sessions were brief and limited to four sessions. In individuals with comorbid asthma-PD the physiological disturbance may be increased as compared to asthma or PD alone and require a higher treatment dose. Depression has been linked to decreased HRV (Stapelberg et al. 2012), reduced HF HRV (Musselman et al. 1998), and may contribute to disruption of related autonomic processes. In both CBPT and MRT groups, 37.5% of participants were depressed at baseline and, thus depression may have impacted psychophysiological processes. However, previous research also suggests depressive symptoms may be positively influenced by HRVB treatments. For example, in two small preliminary studies, HRVB was associated with significant reductions in depressive symptoms at follow-up (Siepmann et al. 2008) or in as few as 4 sessions (Karavidas et al. 2007). These studies included predominantly Caucasian samples, which is a potentially meaningful difference from the sample used in the current study. Future studies should incorporate more frequent sessions of longer duration to examine the optimal dose of biofeedback and include participants of more diverse ethnic and racial backgrounds.
This study had several limitations including a sample that was small and predominantly female. There was also a high drop-out rate of 40%, however, participant attrition was equivalent between the treatment groups. The sample was quite homogeneous, limiting generalizability of the current findings. An additional limitation was the number of time points available for inclusion in the psychophysiological analyses. It is possible that mediating effects of HRV and ETCO2 would be more readily detected with additional data points. It may be possible that therapeutic effects were not caused by chronic changes in HRV, but in acute changes that may have utility if implemented when experiencing panic symptoms.
Asthma-PD comorbidity rates are high and patients often experience increased emotional and physical distress due to their combined symptoms. Understanding the mechanisms for promoting physiological health may help improve available therapies and outcomes. Further research is needed to examine alternative mediating influences of symptom decreases in Latinos with asthma-PD. The current research takes an initial step in understanding the impact of HRV and ETCO2 on treatment response in Latinos. Race may be an important factor in psychophysiological functioning: differences in HRV have been observed across age, sex, and race, with significant differences between HRV in Caucasian and African Americans (Liao et al. 1995; Choi et al. 2006). Prior research has established ETCO2 as a mediator of anxiety disorder symptoms (Davies and Craske 2014; Meuret et al. 2010), but this was not replicated in the current sample of Latinos with comorbid asthma-PD. Continued study of psychophysiological phenomenon in diverse samples may help clarify the mechanisms of treatment response across ethnic/racial categories.
Acknowledgments
Funding for this study was provided by the National Institute of Mental Health: 1R34MH087679, PI: Jonathan M. Feldman, Ph.D. Spanish translation of measures was funded by the Einstein-Montefiore ICTR 5UL-1TR00107-02. Special thanks to Dr. Melissa D. McKee, Ms. Claudia Lechuga, and the New York City Research and Improvement Networking Group for their assistance with recruitment of participants. Special thanks also for the cooperation from the New York City Health and Hospitals Corporation, Jacobi Medical Center, and Montefiore Medical Center.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
This study has been approved by the appropriate ethics committee and all procedures were performed in accordance with the ethical standards of the institution. All procedures performed were also in accordance with the ethical standards enumerated in the 1964 Declaration of Helsinki, as well as its later amendments or comparable ethical standards. All participants gave their informed consent prior to their inclusion in the current research.
Conflict of Interest: The authors declare that they have no conflict of interest. The authors do not have a financial relationship with the funding organizations. The authors have full control of all primary data and agree to allow the journal to review their data, if requested.
References
- Boudreau M, Lavoie KL, Cartier A, Trutshnigg B, Morizio A, Lemière C, et al. (2015). Do asthma patients with panic disorder really have worse asthma? A comparison of physiological and psychological responses to a methacholine challenge. Respiratory Medicine, 109(10), 1250–1256. [DOI] [PubMed] [Google Scholar]
- Choi J-B, Hong S, Nelesen R, Bardwell WA, Natarajan L, Schubert C, et al. (2006). Age and ethnicity differences in short-term heart-rate variability. Psychosomatic Medicine, 68(3), 421–426. [DOI] [PubMed] [Google Scholar]
- Chuang C-Y, Han W-R, Li P-C, & Young S-T (2010). Effects of music therapy on subjective sensations and heart rate variability in treated cancer survivors: a pilot study. Complementary Therapies in Medicine, 18(5), 224–226. [DOI] [PubMed] [Google Scholar]
- Dager SR, Strauss WL, Marro KI, Richards TL, Metzger GD, & Artru AA (1995). Proton magnetic resonance spectroscopy investigation of hyperventilation in subjects with panic disorder and comparison subjects. American Journal of Psychiatry, 152(5), 666–672. [DOI] [PubMed] [Google Scholar]
- Davies CD, & Craske MG (2014). Low baseline pCO2 predicts poorer outcome from behavioral treatment: evidence from a mixed anxiety disorders sample. Psychiatry Research, 219(2), 311–315, doi: 10.1016/j.psychres.2014.06.003. [DOI] [PubMed] [Google Scholar]
- Dekker JM, Crow RS, Folsom AR, Hannan PJ, Liao D, Swenne CA, et al. (2000). Low heart rate variability in a 2-minute rhythm strip predicts risk of coronary heart disease and mortality from several causes The ARIC Study. Circulation, 102(11), 1239–1244. [DOI] [PubMed] [Google Scholar]
- Feldman JM, Matte L, Interian A, Lehrer PM, Lu S-E, Scheckner B, et al. (2016). Psychological treatment of comorbid asthma and panic disorder in Latino adults: Results from a randomized controlled trial. Behaviour Research and Therapy, 87, 142–154, doi: 10.1016/j.brat.2016.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, & Williams JB (1995). Structured clinical interview for DSM-IV axis I disorders-patient edition (SCID-I/P, Version 2.0) New York: Biometrics Research Department, New York State Psychiatric Institute, 722. [Google Scholar]
- Friedman BH, & Thayer JF (1998). Autonomic balance revisited: panic anxiety and heart rate variability. Journal of Psychosomatic Research, 44(1), 133–151. [DOI] [PubMed] [Google Scholar]
- Garakani A, Martinez JM, Aaronson CJ, Voustianiouk A, Kaufmann H, & Gorman JM (2009). Effect of medication and psychotherapy on heart rate variability in panic disorder. Depression and Anxiety, 26(3), 251–258. [DOI] [PubMed] [Google Scholar]
- Garrard CS, Seidler A, McKibben A, McAlpine LE, & Gordon D (1992). Spectral analysis of heart rate variability in bronchial asthma. Clinical Autonomic Research, 2(2), 105–111. [DOI] [PubMed] [Google Scholar]
- Goodwin RD, Pine DS, & Hoven CW (2003). Asthma and panic attacks among youth in the community. Journal of Asthma, 40(2), 139–145. [DOI] [PubMed] [Google Scholar]
- Gorman JM, Fyer MR, Goetz R, Askanazi J, Liebowitz MR, Fyer AJ, et al. (1988). Ventilatory physiology of patients with panic disorder. Archives of General Psychiatry, 45(1), 31–39. [DOI] [PubMed] [Google Scholar]
- Hasler G, Gergen PJ, Kleinbaum DG, Ajdacic V, Gamma A, Eich D, et al. (2005). Asthma and panic in young adults: a 20-year prospective community study. American Journal of Respiratory and Critical Care Medicine, 171(11), 1224–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim JG, & Molenberghs G (2009). Missing data methods in longitudinal studies: a review. Test, 18(1), 1–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juniper EF, byrne PM, Guyatt G. h., Ferrie P. j., & King D. r. (1999). Development and validation of a questionnaire to measure asthma control. European Respiratory Journal, 14(4), 902–907, doi: 10.1034/j.1399-3003.1999.14d29.x. [DOI] [PubMed] [Google Scholar]
- Karavidas MK, Lehrer PM, Vaschillo E, Vaschillo B, Marin H, Buyske S, et al. (2007). Preliminary results of an open label study of heart rate variability biofeedback for the treatment of major depression. Applied Psychophysiology and Biofeedback, 32(1), 19–30. [DOI] [PubMed] [Google Scholar]
- Kazuma N, Otsuka K, Matsuoka I, & Murata M (1997). Heart rate variability during 24 hours in asthmatic children. Chronobiology International, 14(6), 597–606. [DOI] [PubMed] [Google Scholar]
- Kleiger RE, Miller JP, Bigger JT, & Moss AJ (1987). Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. The American journal of cardiology, 59(4), 256–262. [DOI] [PubMed] [Google Scholar]
- Klein E, Cnaani E, Harel T, Braun S, & Ben-Haim SA (1995). Altered heart rate variability in panic disorder patients. Biological Psychiatry, 37(1), 18–24. [DOI] [PubMed] [Google Scholar]
- La Rovere MT, Pinna GD, Maestri R, Mortara A, Capomolla S, Febo O, et al. (2003). Short-term heart rate variability strongly predicts sudden cardiac death in chronic heart failure patients. Circulation, 107(4), 565–570. [DOI] [PubMed] [Google Scholar]
- Lehrer P, Vaschillo E, Lu S-E, Eckberg D, Vaschillo B, Scardella A, et al. (2006). Heart rate variability biofeedback: effects of age on heart rate variability, baroreflex gain, and asthma. Chest Journal, 129(2), 278–284. [DOI] [PubMed] [Google Scholar]
- Lehrer PM, Vaschillo E, & Vaschillo B (2000). Resonant frequency biofeedback training to increase cardiac variability: Rationale and manual for training. Applied Psychophysiology and Biofeedback, 25(3), 177–191. [DOI] [PubMed] [Google Scholar]
- Lehrer PM, Vaschillo E, Vaschillo B, Lu S-E, Scardella A, Siddique M, et al. (2004). Biofeedback treatment for asthma. Chest Journal, 126(2), 352–361. [DOI] [PubMed] [Google Scholar]
- Liao D, Barnes RW, Chambless LE, Simpson RJ, Sorlie P, Heiss G, et al. (1995). Age, race, and sex differences in autonomic cardiac function measured by spectral analysis of heart rate variability—the ARIC study. The American journal of cardiology, 76(12), 906–912. [DOI] [PubMed] [Google Scholar]
- Maddock RJ, & Carter CS (1991). Hyperventilation-induced panic attacks in panic disorder with agoraphobia. Biological Psychiatry, 29(9), 843–854, doi: 10.1016/0006-3223(91)90051-M. [DOI] [PubMed] [Google Scholar]
- Meuret AE (2007). Targeting pCO(2) in asthma: pilot evaluation of a capnometry-assisted breathing training. Applied Psychophysiology and Biofeedback, 32(2), 99–109, doi: 10.1007/s10484-007-9036-8. [DOI] [PubMed] [Google Scholar]
- Meuret AE, Rosenfield D, Hofmann SG, Suvak MK, & Roth WT (2009). Changes in respiration mediate changes in fear of bodily sensations in panic disorder. Journal of Psychiatric Research, 43(6), 634–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuret AE, Rosenfield D, Seidel A, Bhaskara L, & Hofmann SG (2010). Respiratory and cognitive mediators of treatment change in panic disorder: evidence for intervention specificity. Journal of Consulting and Clinical Psychology, 78(5), 691–704, doi: 10.1037/a0019552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuret AE, Wilhelm FH, & Roth WT (2001). Respiratory biofeedback-assisted therapy in panic disorder. Behavior Modification, 25(4), 584–605. [DOI] [PubMed] [Google Scholar]
- Musselman DL, Evans DL, & Nemeroff CB (1998). The relationship of depression to cardiovascular disease: epidemiology, biology, and treatment. Archives of General Psychiatry, 55(7), 580–592. [DOI] [PubMed] [Google Scholar]
- Nascimento I, Nardi AE, Valença AM, Lopes FL, Mezzasalma MA, Nascentes R, et al. (2002). Psychiatric disorders in asthmatic outpatients. Psychiatry Research, 110(1), 73–80. [DOI] [PubMed] [Google Scholar]
- National Heart Lung and Blood Institute (1997). Expert panel report 2: guidelines for the diagnosis and management of asthma. DIANE Publishing. [Google Scholar]
- National Heart Lung and Blood Institute (2013). Hispanic Community Health Study: Study of Latinos Data Book. NIH Publication No. 13–7951
- Papp LA, Martinez JM, Klein DF, Coplan JD, Norman RG, Cole R, et al. (1997). Respiratory psychophysiology of panic disorder: three respiratory challenges in 98 subjects. American Journal of Psychiatry, 154(11), 1557–1565. [DOI] [PubMed] [Google Scholar]
- Raglio A, Oasi O, Gianotti M, Manzoni V, Bolis S, C Ubezio M, et al. (2010). Effects of music therapy on psychological symptoms and heart rate variability in patients with dementia. A pilot study. Current aging science, 3(3), 242–246. [DOI] [PubMed] [Google Scholar]
- Ritz T, Rosenfield D, Steele AM, Millard MW, & Meuret AE (2014). Controlling asthma by training of Capnometry-Assisted Hypoventilation (CATCH) vs slow breathing: a randomized controlled trial. Chest Journal, 146(5), 1237–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shear MK, Rucci P, Williams J, Frank E, Grochocinski V, Vander Bilt J, et al. (2001). Reliability and validity of the Panic Disorder Severity Scale: replication and extension. Journal of Psychiatric Research, 35(5), 293–296, doi: 10.1016/S0022-3956(01)00028-0. [DOI] [PubMed] [Google Scholar]
- Siepmann M, Aykac V, Unterdörfer J, Petrowski K, & Mueck-Weymann M (2008). A pilot study on the effects of heart rate variability biofeedback in patients with depression and in healthy subjects. Applied Psychophysiology and Biofeedback, 33(4), 195–201. [DOI] [PubMed] [Google Scholar]
- Stapelberg NJ, Hamilton-Craig I, Neumann DL, Shum DH, & McConnell H (2012). Mind and heart: heart rate variability in major depressive disorder and coronary heart disease-a review and recommendations. Australian and New Zealand Journal of Psychiatry, 46(10), 946–957. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Hansen AL, Saus-Rose E, & Johnsen BH (2009). Heart rate variability, prefrontal neural function, and cognitive performance: the neurovisceral integration perspective on self-regulation, adaptation, and health. Annals of Behavioral Medicine, 37(2), 141–153. [DOI] [PubMed] [Google Scholar]
- Tsuji H, Larson MG, Venditti FJ, Manders ES, Evans JC, Feldman CL, et al. (1996). Impact of reduced heart rate variability on risk for cardiac events The Framingham Heart Study. Circulation, 94(11), 2850–2855. [DOI] [PubMed] [Google Scholar]
- Van den Elshout F, Van Herwaarden C, & Folgering H (1991). Effects of hypercapnia and hypocapnia on respiratory resistance in normal and asthmatic subjects. Thorax, 46(1), 28–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeragani VK, Pohl R, Berger R, Balon R, Ramesh C, Glitz D, et al. (1993). Decreased heart rate variability in panic disorder patients: a study of power-spectral analysis of heart rate. Psychiatry Research, 46(1), 89–103. [DOI] [PubMed] [Google Scholar]


