Abstract
Objective
To determine the association between daily water intake and 24-hour urine volume among adolescents with nephrolithiasis in order to estimate a “fluid prescription”, the additional water intake needed to increase urine volume to a target goal.
Methods
We conducted a secondary analysis of an ecological momentary assessment study that prospectively measured daily water intake of 25 adolescents with nephrolithiasis over 7 days. We identified 24-hour urine volumes obtained for clinical care within 12 months of water intake assessment. A linear regression model was fit to estimate the magnitude of the association between daily water intake and 24-hour urine volume, adjusting for age, sex, race, and daily temperature.
Results
Twenty-two participants completed fifty-seven 24-hour urine collections within 12 months of the study period. Median daily water intake was 1.4 L (IQR 0.67–1.94). Median 24-hour urine volume was 2.01 L (IQR 1.20–2.73). A 1 L increase in daily water intake was associated with a 710 mL increase in 24-hour urine output (95% CI 0.55–0.87). Using the model output, the equation was generated to estimate the additional fluid intake needed (fluid prescription; FP) to produce the desired increase in urine output (dUOP): FP=dUOP/0.71.
Conclusions
The fluid prescription equation (FP = dUOP)/0.71), which reflects the relationship between water intake and urine volume, could be used to help adolescents with nephrolithiasis achieve urine output goals to decrease stone recurrence.
Keywords: Nephrolithiasis, urine volume, pediatrics, fluid intake
Introduction
Kidney stone disease (nephrolithiasis) results in an annual healthcare cost of $10 billion per year in the United States.1 The prevalence of nephrolithiasis in the United States has increased 70% since the 1990s.3 Between 1997 and 2007, the incidence of kidney stones among adolescents doubled.2 Approximately 50% of patients who develop kidney stones in childhood will have a symptomatic recurrence within three years.4
A key intervention in reducing kidney stone recurrence is increasing fluid intake.5–8 High fluid intake is associated with a decreased risk of incident and recurrent kidney stones, and the American Urological Association currently recommends increasing fluid intake to achieve a urine output of at least 2.5 liters per day for adults.7–11 Although guidelines do not specify the type of fluid intake, high intake of water is considered to be the ideal fluid for children as sugary beverages increase urine calcium and are associated with an increased risk of forming incident stones.11,13–15 Additionally, alcoholic and caffeinated beverages are not sensible for consumption by children.
There have been no studies examining the association between water intake volume and changes in 24-hour urine volume in children with nephrolithiasis. Clinicians often recommend drinking enough water to achieve a 24-hour urine output of 2–2.5 liters.7 However, these recommendations are inherently difficult to operationalize due to the uncertainty of how much additional water intake is needed to achieve the goal urine output. Our goal was to develop an equation that estimates the relationship between daily water intake and 24-hour urine volume in order to inform personalized water intake recommendations for adolescent kidney stone formers.
Materials and Methods
Study Design and Population
This study is a secondary analysis of data collected as part of the Barriers to Water Intake study conducted at The Children’s Hospital of Philadelphia (CHOP).17 Briefly, 25 patients aged 12 to 18 years with a history of at least one stone of any composition except stones formed by infection (e.g. struvite and carbapatite) were enrolled between June 2016 and April 2017. In this study, participants’ daily water intake was measured using HidrateSpark, which is a Bluetooth-enabled “smart” water bottle that records water intake and has been shown to be accurate to within 3% of actual water intake.12 To reflect water intake behaviors over the year, enrollment was staggered over the year (i.e. no more than 7 participants were enrolled within a 3-month season). The study was designed to take place during weekdays and weekend days so as to reflect natural variation in activities and location that could impact water intake. As part of clinical care, participants were provided with a personalized daily water intake goal per National Academy of Medicine recommendations for adequate daily water intake.5 These recommendations, which consider water from food and other beverages, were used as the daily water intake goal. These recommendations are used in our Kidney Stone Center because specific 24-hour urine volume guidelines do not exist for pediatric stone formers, but maintaining higher than adequate fluid intake will produce sufficient urine volume to reduce kidney stone risk. We excluded patients who did not speak English, could not operate a mobile device, had a condition causing increased fluid loss (e.g. chronic diarrhea), or had changes in dietary intake (e.g. bariatric surgery). This study was approved by CHOP’s institutional review board (IRB 14–011343).
Outcome
The primary outcome was 24-hour urine volume (L), which was measured by 24-hour urine collections analyzed by Litholink as part of standard clinical care. For each participant, we considered all 24-hour urine volumes 12 months before and 12 months after the study period during which participants completed the Barriers to Water Intake study (i.e. measured daily water intake). Twenty four hour urine volume results were retrospectively abstracted from chart review specifically for this secondary analysis. No additional interventions were made for this analysis. Participants from the initial study who did not have a 24-hour urine volume collection recorded within this time frame were excluded from this analysis.
Primary exposure
Water intake data was collected prospectively for a single 7-day period as part of the initial Barriers to Water Intake study. Daily water intake volumes were collected over 7 consecutive days for each participant. We utilized HidrateSpark smart water bottles connected to participants’ personal mobile devices to capture real-time water intake.17 Participants were instructed to drink all water from the water bottle during the duration of the study. Participants recorded water not consumed from the bottle using a manual add feature on the HidrateSpark mobile app that recorded volume with 1 mL precision. Although the volumes of any other consumed beverage were not recorded, 27% of participants indicated they consumed beverages other than water during the course of the study.
Covariates
The ecological momentary assessment app (LifeData) provided GPS coordinates for participants. We used these locations to determine the local daily wet bulb temperature using National Weather Service data for each day of the study period during which water intake was measured.6 Wet bulb temperature measures the complex thermodynamic relationship between temperature, surface pressure, and humidity, and represents the combined effect of temperature and humidity on human physiology We also obtained self-reported age, sex, race, BMI and season from the prospectively collected data in the Barriers to Water Intake study.
Statistical Analysis
A linear regression model with robust standard errors and a random intercept was fit to estimate the association between median daily water intake and 24-hour urine volume for each participant. This model accounted for clustering of 24-hour urine volumes at the participant level. The model was adjusted for age, sex, race, season, BMI and daily median wet-bulb temperature. The mean daily wet bulb temperature was included in the model to adjust for possible insensible water losses due to temperature and changes in water intake dependent on temperature. In a sensitivity analysis, we excluded 24-hour urine volumes of collections with creatinine excretion values in the upper and lower 5th percentiles. A two-tailed P-value <0.05 was the threshold for significance. Analyses were performed in Stata v15.1 (College Station, TX).
Results
Twenty-two of the 25 participants included in the Barriers study had 24-hour urine volume collections within the specified time period (Table 1). Fifty-seven 24-hour urine collections were completed within 12 months of the water intake measurement and were included in the analysis. Median daily water intake was 1.4 L (IQR 0.67–1.94 L). Median 24-hour urine volume was 2.01 L (IQR 1.20–2.73 L).
Table 1:
Participant Characteristics
| Median Daily Water Intake (IQR) | 1.4 L (0.67–1.94) |
| Median 24-hour Urine Volume (IQR) | 2.01 L (1.20–2.73) |
| Median Daily Wet Bulb Temperature (IQR) | 49°F (37–68) |
| Median Age (IQR) | 16 (15–17) |
| Median Body Mass Index (IQR) | 22.1 (20.1–25.2) |
| Season of Study Completion (%) | |
| Winter | 8 (36%) |
| Spring | 2 (9%) |
| Summer | 5 (23%) |
| Fall | 7 (32%) |
| Sex (%) | |
| Male | 9 (41%) |
| Female | 13 (59%) |
| Race (%) | |
| Black | 2 (9%) |
| White | 20 (91%) |
A 1 L increase in median daily water intake was associated with a 710 mL increase in 24-hour urine output (95% CI 550 – 870 mL, p<0.001). Using the model output, the following equation was generated that estimates the additional fluid intake needed (fluid prescription; FP) to produce the desired increase in urine output (dUOP):
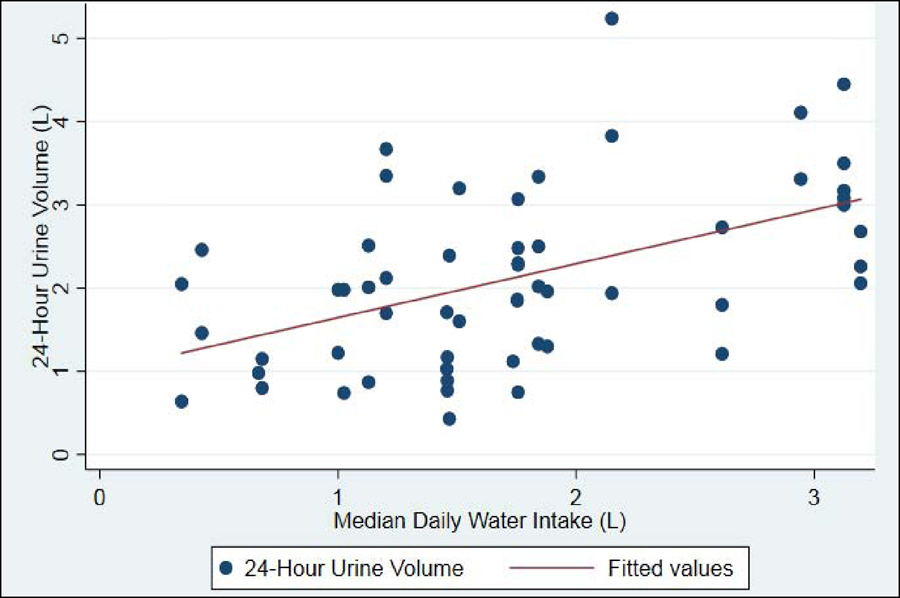
Applied to a hypothetical patient who needs to increase their 24-hour urine volume by 550 mL to reach a goal of 2.5L, the recommended increase in daily water intake would be 775 mL (Table 2 and Figure).
Table 2:
Association between Daily Water Intake and 24-hour Urine Volume, Adjusted for Covariates
| Variable | Coefficient | Standard Error | 95% Confidence Interval | P | |
|---|---|---|---|---|---|
| Median Daily Water Intake(L) | 0.712 | 0.077 | 0.553 | 0.871 | 0.000 |
| Median Daily Wet Bulb Temp(°F) | 0.028 | 0.006 | 0.015 | 0.042 | 0.000 |
| Female sex | −0.081 | 0.199 | −0.495 | 0.334 | 0.690 |
| Age | 0.021 | 0.056 | −0.095 | 0.137 | 0.709 |
| Black Race (white referent) | −0.571 | 0.221 | −1.031 | −0.111 | 0.017 |
| Body Mass Index | −0.026 | 0.016 | −0.059 | 0.007 | 0.115 |
| Season | 0.167 | 0.101 | −0.043 | 0.376 | 0.113 |
Figure 1-.
Relationship between Daily Water Intake and 24-hour Urine Volume
Mean wet bulb temperature during the participants’ study period and Black race had a modest yet statistically significant association with 24-hour urine output (Table 2). Specifically, a 1 °F increase in mean wet bulb temperature was associated with a 28 mL higher 24-hour urine output (95% CI 15–42 mL, p<0.0001). Black race was associated with a 571 mL lower 24-hour urine output (95% CI −1031-−111mL, p<0.017). Other measured values included age, sex, BMI, and season of study completion were not significantly associated with changes in urine output (Table 2).
In the sensitivity analysis excluding participants with creatinine concentration values in the upper and lower 5th percentiles (n=54 urine volumes), a 1L increase in median daily water intake was associated with a 680 mL increase in 24-hour urine output (95% CI 490–870 mL, p<0.000).
Discussion
The objective for all stone formers is to drink a sufficient amount of fluid to produce enough urine to decrease kidney stone formation. In a randomized trial of adults with nephrolithiasis, fluid intake sufficient to produce a urine output of 2.5L/day decreased stone recurrence by 55% and increased the time to recurrence by more than 1 year.10,21 However, patients with kidney stones do not routinely measure 24-hour urine volume except for the one to two times a year that 24-hour urine analyses are performed. This secondary analysis of a prospective cohort study of 22 adolescents with kidney stone disease provides useable information that could help patients reduce kidney stone recurrence by estimating the volume of additional daily fluid intake needed to achieve urine output goals (FP = dUOP/0.71). The wide availability of smart water bottles such as those used in this study would allow patients to operationalize this fluid prescription by measuring this additional water volume during the course of their everyday lives.
This study differs from previous studies of urine volume among patients with kidney stones, which have largely focused on the inability to maintain high water intake and associated low urine volume.17–23 Rather, we estimated the relationship between daily water intake and 24-hour urine volume in order to generate knowledge that helps patients achieve urine output goals to prevent stone recurrence. This approach may fill gaps in recommendations for water intake by creating a more concrete plan to attain age-and sex-specific water intake goals. This study also provides insight into the variables that may impact 24-hour urine volume in adolescent patients, including ambient temperature which affects insensible water loss due to perspiration and respiration.
Using wet bulb temperature helped mitigate confounding by the combination of ambient temperature and humidity, which affect fluid intake and insensible losses. In particular, adults lose approximately 700 mL of water per day to insensible losses. This loss is comprised of approximately 350 mL through the skin and an additional approximately 350 mL from normal respiration. An additional 100 mL may be lost in sweat, although this amount is dependent on ambient temperature and activity.25 Infants and small children are believed to lose more water to insensible losses than adults due to a greater skin surface area per body weight. For children, it is estimated that 45 mL of water are lost per 100 kcal of energy expended. A child weighing 20 kilograms requires roughly 1500 kcals of energy per day. This expenditure leads to approximately 675 mL of insensible water loss, similar to expected water loss of an adult.26 While this study did not measure insensible water losses or body surface area, the difference between a 1 liter increase in water intake and a 710 mL increase in urine output is consistent with insensible water loss.
Differences in insensible water losses could also explain the association between Black race and lower urine volume observed in this study. Our study showed Black participants had significantly lower urine output than white participants, though only two participants identified as Black which limits the inferences that could be drawn from this finding. While there is a demonstrated difference in sweat production and kidney stone risk in black individuals and white individuals, the differences seen in our study may also be due in part to differences in the indiviudals not accounted for in our model.27
Age was not associated with a statistically significant change in 24-hour urine output among this population of adolescents between 12 and 18 years old (IQR 15–17). Despite the potential for older children to drink more water due to having higher water intake goals, only 20% of participants in the intial study met their water intake goal for 4 or more days.17 The few individuals who achieved these goals may have resulted in a lack of association with age and urine output in this secondary analysis. Nevertheless, the resulting fluid prescription equation accounts for age differences in the relationship between water intake and urine output.
Sex (identication as female vs male referent) was also not associated with a statistically significant change in 24-hour urine output. There were 9 male participants and 13 female participants considered in this analysis. Similarly to age, water intake guidelines vary based on sex. According to the National Academy of Medicine guidelines, females 14–18 years old need to drink 1 L less per day than their male counterparts (2.3 L/d vs 3.3 L/d)5. Despite these differences in goals, there was no significant association between sex and urine output. This is likely due to water intake being multifactorial, rather than based simply on the goals set out in this study. Additionally, since most participants in the initial study did not attain their goals, the potential impact of higher goals for males is likely diminished. BMI was also not associated with changes in 24-hour urine volume. The IQR of BMIs in our cohort was 20.1–25.2 kg/m2. A majority of our particpants were within the BMI range for normal weight. Therefore, it was anticipated that BMI would have little effect on urine output, as many of the participants had similar BMIs, though this does diminish generalizability to underweight and obese adolescents.
This study also demonstrates the utility of using Bluetooth or other smart devices to monitor water intake outside the clinic. In the initial Barriers to Water Intake study, 82% of participants indicated the smart water bottle was easy to use and helped them drink more water while 86% indicated the were more likely to drink to avoid a recurrent kidney stone.17 The bottle also gave the study team the ability to monitor each “sip” or drinking event remotely. This allowed for the study team to determine that participants were not simply filling up and dumping out their water bottles to artificially inflate water intake scores. The low goal attainment by study participants also supports the belief that participants did not dump water for goal attainment.
Our results contextualize and operationalize the desired increase in urine output as a function of additional fluid intake. While these results apply to adolescents with kidney stones, they would likely also apply to adults with kidney stones. Adolescents have similar expected insensible water loss to adults, though little is known about differences in water intake habits between adolescents and adults.25 In addition, the association between water intake and 24-hour urine volume may be relevant to other clinical situations in which fluid intake and urine volume is important, including reducing the risk of chronic kidney disease progression28 and urinary tract infection.29,30 These results may also provide guidance in fluid management in the post-operative period, particularly for patients after discharge from the hospital when urine output is not routinely measured.
There are several limitations to this study. Median daily water intake was 1.7 L while the median 24-hour urine volume measured 12 months before and after the study period was 2.1 L. There are several possibilities explaining the discrepancy between median water intake and median 24-hour urine volume. First, roughly 20% of daily fluid intake comes from food, which was not tracked during this study.16,24 Second, we did not collect volumes of alternative beverage consumption, such as soda or juice. In the original Barriers study, 27% of participants reported drinking an alternative beverage and alternative beverage consumption was not associated with less water intake.17 Third, although a Hawthorne effect was not observed in the 7-day observation period of the Barriers to Water Intake study, it is possible that the amount of water consumed during the study period was greater than “normal” days.17 It is also possible that 24-hour urine collections, which are moments in time, reflect greater than typical fluid intake. However, the purpose of this current study was to estimate the relationship between the change in water intake and the change in urine volume. In this context, the average water intake and average 24-hour urine volumes are less important than the magnitude of the association between increases in water intake and urine volume. These results therefore provide insight into the additional daily water intake needed to increase 24-hour urine volume among typical kidney stone formers. Fourth, we included 24-hour urine volumes from 12 months before and after water intake was measured. Ideally, 24-hour urine output and water intake would be prospectively collected to decrease the potential for changes in drinking habits and subsequent urine output. However, participants received the same water intake recommendations throughout the period during which both urine volumes and water intake were measured (i.e. the National Academy of Medicine guidelines used for goals in the study were also the recommendations given to patients as part of routine care in the clinic). Fifth, we also did not collect data on the activity level of participants, including participation of athletics, or on body surface area or BMI percentile. This could limit the generalizability of the findings to patients who are not exceptionally active or at the extremes of body size as increased insensible losses of water due to sweat or a large body surface are may decrease urine output and change the amount of water intake needed to meet a daily goal. Finally, the results of this study are limited to adolescents with kidney stones. Future prospective studies are required to determine if prescription-style recommendations for increases in water intake increase 24-hour urine output and decrease stone recurrence. The ongoing Prevention of Urinary Stones with Hydration (PUSH) randomized clinical trial is testing the efficacy of fluid prescriptions, financial incentives, and coaching to maintain high fluid intake to decrease kidney stone recurrence (https://clinicaltrials.gov/ct2/show/NCT03244189). Both intervention and control arm participants receive the HidrateSpark bottle, which reflects that the bottle is a means of measurement more than an intervention itself.
Conclusions
The fluid prescription equation (FP = dUOP)/0.71), which reflects the relationship between water intake and urine volume, could be used to help adolescents with kidney stones achieve urine output goals to decrease stone recurrence. Future research examining the utility of these recommendations as part of kidney stone prevention strategies is needed.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Litwin M, Saigal C. Economic impact of urologic disease In: National Institute of Diabetes and Digestive and Kidney Diseases NIoH, US Department of Health and Human Services, ed. Urologic Diseases in America. NIH Publication; 12-78652012:486. [Google Scholar]
- 2.Tasian GE, Ross ME, Song L, et al. Annual Incidence of Nephrolithiasis among Children and Adults in South Carolina from 1997 to 2012. Clinical journal of the American Society of Nephrology : CJASN. 2016;11(3):488–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scales CD Jr., Smith AC, Hanley JM, Saigal CS, Urologic Diseases in America P. Prevalence of kidney stones in the United States. Eur Urol 2012;62(1):160–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tasian GE, Kabarriti AE, Kalmus A, Furth SL. Kidney Stone Recurrence among Children and Adolescents. J Urol 2017;197(1):246–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pearle MS, Goldfarb DS, Assimos DG, et al. AUA GuidelinesMedical Management of Kidney Stones: AUA Guideline JURO Vol 192: Elsevier Ltd; 2014:316–324. [DOI] [PubMed] [Google Scholar]
- 6.Ross Michelle E., et al. “Assessment of the Combination of Temperature and Relative Humidity on Kidney Stone Presentations.” Environmental Research, vol. 162, 2018, pp. 97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borghi L, Meschi T, Amato F, Briganti A, Novarini A, Giannini A. Urinary volume, water and recurrences in idiopathic calcium nephrolithiasis: a 5-year randomized prospective study. J Urol 1996;155(3):839–843. [PubMed] [Google Scholar]
- 8.Lotan Y, Buendia Jimenez I, Lenoir-Wijnkoop I, et al. Increased water intake as a prevention strategy for recurrent urolithiasis: major impact of compliance on cost-effectiveness. J Urol 2013;189(3):935–939. [DOI] [PubMed] [Google Scholar]
- 9.Curhan GC, Willett WC, Rimm EB, Stampfer MJ. A prospective study of dietary calcium and other nutrients and the risk of symptomatic kidney stones. N Engl J Med 1993;328(12):833–838. [DOI] [PubMed] [Google Scholar]
- 10.Qaseem A, Dallas P, Forciea MA, Starkey M, Denberg TD, Physicians CGCotACo. Dietary and pharmacologic management to prevent recurrent nephrolithiasis in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med Vol 1612014:659–667. [DOI] [PubMed] [Google Scholar]
- 11.Curhan GC, Willett WC, Speizer FE, Stampfer MJ. Beverage use and risk for kidney stones in women. Ann Intern Med 1998;128(7):534–540. [DOI] [PubMed] [Google Scholar]
- 12.Borofsky MS, Dauw CA, York N, Terry C, & Lingeman JE (2018). Accuracy of daily fluid intake measurements using a “smart” water bottle. Urolithiasis, 46(4), 343–348. [DOI] [PubMed] [Google Scholar]
- 13.Ferraro PM, Taylor EN, Gambaro G, Curhan GC. Soda and Other Beverages and the Risk of Kidney Stones. Clin J Am Soc Nephrol 2013. August; 8(8):1389–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nguyen NU, Dumoulin G, Henriet MT, Regnard J. Increase in urinary calcium and oxalate after fructose infusion. Hormone and metabolic research = Hormon-und Stoffwechselforschung = Hormones et metabolisme. 1995;27(3):155–158. [DOI] [PubMed] [Google Scholar]
- 15.Taylor EN, Curhan GC. Fructose consumption and the risk of kidney stones. Kidney International. 2008;73(2):207–212. [DOI] [PubMed] [Google Scholar]
- 16.Kant AK, Graubard BI. Contributors of water intake in US children and adolescents: associations with dietary and meal characteristics--National Health and Nutrition Examination Survey 2005–2006. The American journal of clinical nutrition. 2010;92(4):887–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tasian GE, Ross M, Song L, Audrain-McGovern J, Wiebe D, Warner SG, & Furth SL (2018). Ecological Momentary Assessment of Factors Associated with Water Intake Among Adolescents with Kidney Stone Disease. The Journal of Urology. 2019; 201(3): 606–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parks JH, Goldfischer ER, Coe FL. Changes in urine volume accomplished by physicians treating nephrolithiasis. J Urol 2003;169(3):863–866. [DOI] [PubMed] [Google Scholar]
- 19.DeFoor WR, Jackson E, Minevich E, et al. The risk of recurrent urolithiasis in children is dependent on urinary calcium and citrate. Urology 2010;76(1):242–245. [DOI] [PubMed] [Google Scholar]
- 20.Atan L, Andreoni C, Ortiz V, et al. High kidney stone risk in men working in steel industry at hot temperatures. Urology 2005;65(5):858–861. [DOI] [PubMed] [Google Scholar]
- 21.DeFoor W, Minevich E, Jackson E, et al. Urinary metabolic evaluations in solitary and recurrent stone forming children. J Urol 2008;179(6):2369–2372. [DOI] [PubMed] [Google Scholar]
- 22.DeFoor WR, Jackson E, Minevich E, et al. The Risk of Recurrent Urolithiasis in Children Is Dependent on Urinary Calcium and Citrate. URL 2010;76(1):242–245. [DOI] [PubMed] [Google Scholar]
- 23.McCauley LR, Dyer AJ, Stern K, Hicks T, Nguyen MM. Factors influencing fluid intake behavior among kidney stone formers. J Urol 2012;187(4):1282–1286. [DOI] [PubMed] [Google Scholar]
- 24.Guelinckx I, Tavoularis G, König J, Morin C, Gharbi H, Gandy J. Contribution of Water from Food and Fluids to Total Water Intake: Analysis of a French and UK Population Surveys. Nutrients 2016;8(10):630 Published 2016 Oct 14. doi: 10.3390/nu8100630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hall J (2016). Fluid Intake And Output Are Balanced During Steady-State Conditions. Guyton and Hall Textbook of Medical Physiology. Retrieved July 17, 2019 from https://www.r2library.com/Resource/Title/1455770051/ch0025s0467
- 26.Holliday MA, & Segar WE (1957). The maintenance need for water in parenteral fluid therapy. Pediatrics, 19(5), 823–832. [PubMed] [Google Scholar]
- 27.Marino FE, Lambert MI, & Noakes TD (2004). Superior performance of African runners in warm humid but not in cool environmental conditions. Journal of Applied Physiology, 96(1), 124–130. [DOI] [PubMed] [Google Scholar]
- 28.Clark WF, Sontrop JM, Huang S, et al. Effect of Coaching to Increase Water Intake on Kidney Function Decline in Adults With Chronic Kidney Disease: The CKD WIT Randomized Clinical Trial. JAMA 2018;319(18):1870–1879. doi: 10.1001/jama.2018.4930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hooton TM, Vecchio M, Iroz A, Tack I, Dornic Q, Seksek I, & Lotan Y (2018). Effect of increased daily water intake in premenopausal women with recurrent urinary tract infections: a randomized clinical trial. JAMA internal medicine, 178(11), 1509–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Popkin BM, D’Anci KE, & Rosenberg IH (2010). Water, hydration, and health. Nutrition reviews, 68(8), 439–58. [DOI] [PMC free article] [PubMed] [Google Scholar]