Abstract
Objective
To evaluate the recurrence and functional outcomes in a primarily hereditary cohort of patients undergoing partial adrenalectomy for pheochromocytoma.
Methods
A retrospective review from a prospectively managed database of patients undergoing partial adrenalectomy from 1995–2018 at the National Cancer Institute was performed. Local recurrence was defined as imaging evidence of a recurrent or de novo lesion on the operative side. Steroid dependency was defined as requiring daily steroid replacement at time of last follow-up.
Results
124 partial adrenalectomies, removing 162 tumors, were performed in 107 patients. Most patients had a known hereditary predisposition to develop bilateral, multifocal and recurrent pheochromocytoma. Median tumor size was 2cm (IQR 1.5–2.8). Median follow-up was 60 months (IQR 13–131). Local recurrence occurred in 17 patients (15.8%) and were managed with active surveillance or surgery. A single patient (1/106, 0.9%) developed metastatic spread of pheochromocytoma approximately 14 years after his first of two partial adrenalectomies and remains alive under active surveillance. Median time to recurrence was 71 months (IQR 26–127) with 10 patients (9.3%) requiring daily steroid replacement at time of last follow-up.
Conclusions
Partial adrenalectomy offers excellent oncologic and functional outcomes, sparing most patients from lifelong steroid replacement therapy. Recurrences can be easily managed with repeat surgery or active surveillance via functional work-up and imaging. Partial adrenalectomy remains the recommended surgical management for patients pre-disposed to development of bilateral, multifocal and recurrent pheochromocytoma.
Keywords: Pheochromocytoma, Partial Adrenalectomy, Steroid-Dependency
Introduction
Pheochromocytoma is a rare, neuroendocrine tumor of the adrenal medulla with an annual incidence of approximately 2–8 per million While early studies suggested that 10% of these tumors were hereditary and 10% were bilateral,1 more recent studies have called the “rule of 10s” into question. Germline alterations in at least 15 genes are implicated in pheochromocytoma and are found in upwards of 40% of cases2. Even among patients without clinical evidence of a hereditable pheochromocytoma syndrome, 25% are found to be positive for an associated germline mutation.3
In patients with a hereditary predisposition for developing pheochromocytoma, the risk for development of bilateral or multifocal tumors is high.4 In one early series of patients with von Hippel-Lindau (VHL), 47% were ultimately found to have bilateral pheochromocytoma, with many cases presenting in a metachronous fashion.5 Historically, total adrenalectomy is the treatment of choice for pheochromocytoma. In patients at risk for bilateral tumors, bilateral total adrenalectomy necessitates the need for life-long steroid dependence, along with its many associated complications. 6 To help minimize risk of steroid dependence, partial adrenalectomy(PA) has been employed to spare normal cortical tissue.7–9 At our institution, we started performing PA in 1995, motivated by a large population of patients at increased lifetime risk of development of adrenal pheochromocytoma. Our default surgical approach is partial adrenalectomy for suspected pheochromocytoma, unless there is anatomic or intraoperative technical issue that prevents our ability to spare normal adrenal tissue. While previous studies have demonstrated the safety and feasibility of this technique, longer term outcomes have been lacking. A meta-analysis based mostly on small retrospective series published in 2015 by Nagaraja et al noted that recurrence (overall 8%) and steroid dependent rates (12–39%) are relatively low in patients undergoing partial adrenalectomy for numerous different types of adrenal lesions.10 We sought to update functional and oncologic outcomes in our primarily hereditary cohort of patients undergoing PA for pheochromocytoma.11
Subjects/patients and Methods
A prospectively managed database of patients treated at the Urologic Oncology Branch of the National Cancer Institute on an IRB-approved protocol was queried for all consecutive patients undergoing PA for pathologically confirmed pheochromocytoma between 1995–2018. Patient demographics, hereditary syndrome, surgical indication, perioperative characteristics, recurrence and steroid replacement data were collected. Local recurrence was defined as a radiographic evidence of a recurrent or de novo lesion on the operative side. Steroid dependence was defined as requiring daily steroid replacement at the time of last follow-up. Univariate logistic regression was used to determine predictors steroid dependency as this is an important clinical endpoint. Statistical analysis was performed using STATA, Version 14 (StataCORP, Tx, USA)
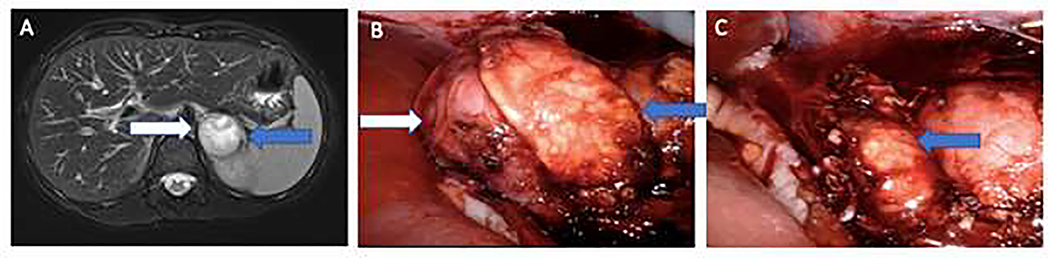
Our operative technique has been previously described.12 Briefly, all patients had pre-operative blockade with phenoxybenzamine and metyrosine.13 A variety of approaches were used at the discretion of the primary surgeon, with changing practice patterns over the study period. For open approaches, both transperitoneal approaches via midline and subcostal incisions were performed, as well as retroperitoneal approaches via a flank incision per surgeon preference. In both laparoscopic and robotic cases, partial adrenalectomy was performed via a transperitoneal approach. In all cases, dissection of the adrenal gland was minimized to maintain collateral blood flow. Intraoperative ultrasound was used when available to help identify the tumor. A plane is then developed between the pseudocapsule of the tumor and normal adrenal and the lesion is removed, occasionally with a small rim of normal cortex. While adrenal vein sparing was performed when feasible, in this cohort, the majority were divided. Patients were followed with cross-sectional at 3 months post operatively and every 1 – 3 years thereafter with concomitant annual serum catecholamine and subjective symptom screening. Representative pre-operative imaging and intra-operative imaging included in Figure 2.
2.
T2-weighted MRI demonstrated 4 cm right pheochromocytoma (white arrow) and normal adrenal limb (blue arrow). B) Intra-operative view of pheochromocytoma and normal adrenal. C). Post-resection view of normal remnant adrenal gland.
Results
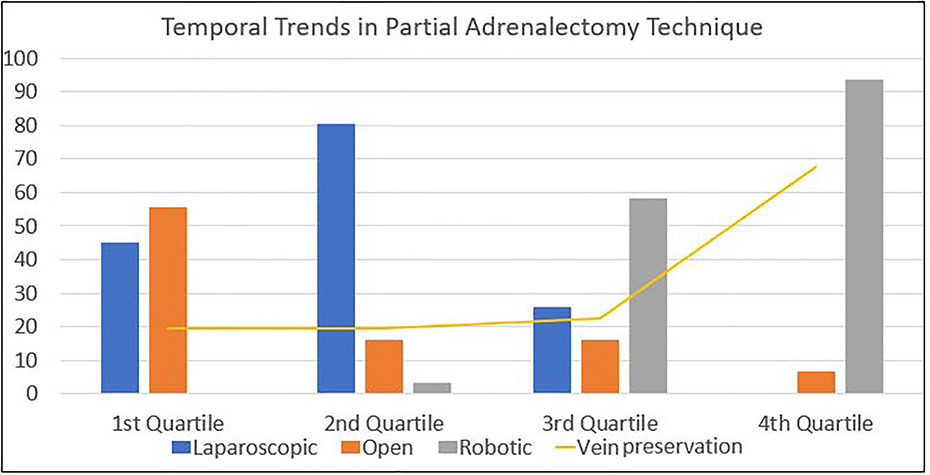
We identified 124 partial adrenalectomies performed in 107 patients between 1995 and 2018, with a total of 162 tumors removed. Most partial adrenalectomies (95–76.6%) were performed minimally invasively with 47 (37.9%) pure laparoscopic cases and 48(38.7%) robotic cases; with 29 procedures completed via an open approach. Thirty-three partial adrenalectomies were performed in combination with an additional surgical procedure, with the majority (22, 67%%) undergoing concurrent ipsilateral partial nephrectomy. Four patients (12%) underwent concurrent pancreatic resection, 3 patients (9%) underwent concurrent retroperitoneal lymph node dissection, 2 (6%) underwent concurrent contralateral total adrenalectomy, 1 (3%) underwent concurrent laparoscopic radiofrequency ablation of a renal mass, and 1 (3%) underwent concurrent pelvic mass excision. Temporal trends in procedure type at our institution are highlighted in Figure 1. The robotic approach increased over the study period along with the utilization of adrenal vein preservation. Median patient age at time of surgery was 31 (IQR 21–42). Median follow-up from time of surgery was 60 months (IQR 13–131). Most patients (99, 92.5%) had a germline alteration, with VHL being the most common (83, 77.5%). The demographics of the study cohort are detailed in Table 1.
1.
Temporal trends demonstrating increased utilization of minimally-invasive approaches to partial adrenalectomy over time.
Table 1.
Demographic, Operative and Clinical Details
| n (%) | |||
|---|---|---|---|
| Partial Adrenalectomies | 24 | ||
| Patients | 107 | ||
| Median Age | 31(IQR 21–42) | ||
| Sex | |||
| Male | 61 (57) | ||
| Female | 46 (43) | ||
| Laterality | |||
| Right | 60 (48.4) | ||
| Left | 48 (38.7) | ||
| Bilateral | 16 (12.9) | ||
| Operative Technique | |||
| Open | 29(23.4) | ||
| Laparoscopic | 47(37.9) | ||
| Robotic | 48 (38.7) | ||
| Concurrent Surgery | |||
| Yes | 34 (27.4) | ||
| No | 90(72.6) | ||
| Genetic Basis | |||
| VHL | 83(77.5) | ||
| MEN 2a | 14 (13.1) | ||
| Sporadic | 8(7.5) | ||
| TS/NF1 | 2 (1.9) | ||
| Total Tumors Removed | 162 | ||
| Median Tumor Size (cm) | 2 (IQR 1.5–2.7) | ||
| Vein Preservation | |||
| Yes | 39(38.6) | ||
| No | 62 (61.4%) |
Seventeen patients (16%) developed a recurrent lesion at a median time of 71 months (IQR 26–171) after partial adrenalectomy. Recurrences were managed with active surveillance if asymptomatic or surgical resection if patients had significantly elevated catecholamines (2x the upper limit of normal) or were symptomatic suggestive of a catecholamine producing tumor. In this series, five went on to have repeat surgery at a median time of 9 months (IQR 5–40) and twelve patients are still on active surveillance at last follow up (median total follow-up 46 months (IQR 18–91)). The majority of patients (3/5) whom had surgery for recurrence underwent repeat minimally invasive resection, while the remaining 2 underwent repeat open exploratory laparotomy. Of note, these repeat procedures were performed via the same approach of the original partial adrenalectomy. There were no positive margins seen on final pathologic analysis in this series.
Long term steroid replacement was required in 10(9.3%) patients. While the median age of these patients were higher (38.5 vs 30 years), it did not reach our defined level of statistical significance(p=0.07). Most patients (7, 70%) requiring steroid replacement at last follow-up had either a history of or underwent concurrent contralateral total adrenalectomy at the time of partial adrenalectomy. An additional patient had a completion ipsilateral total adrenalectomy for recurrence approximately 7 years after the initial partial adrenalectomy. Eight of these patients are still alive at time of last follow-up. One patient died after suffering a cardiac arrest while admitted for inpatient treatment of pneumonia and the other died of metastatic renal cell carcinoma. Additional details on patients requiring steroid replacement are included in Supplementary Table 1. Results of a univariate logistic regression analysis are included in Table 2. Variables analyzed included gender, tumor size, total number of tumors removed, laterality, simultaneous bilateral partial adrenalectomy and solitary adrenal gland. The only significant predictor found was the presence of a solitary adrenal gland (OR 13.7, p<0.001).
Table 2.
Logistic Regression Predictors of Steroid Dependence
| Odds Ratio (95% CI) | P value | |
|---|---|---|
| Male Gender | 1.36 (0.3–5.74) | 0.7 |
| Tumor Size (cm) | 1.20 (0.65–2.20) | 0.6 |
| Number Tumors removed | 1.30 (.55–3.07) | 0.5 |
| Left side | 1.33 (032–5.64) | 0.7 |
| Simultaneous Bilateral Partial Adrenalectomy | 1.75 (0.34–9.09 | 0.5 |
| Solitary Adrenal | 13.7 (3.36–56.4) | < 0.001 |
A single patient in this cohort developed metastatic pheochromocytoma. He underwent his first partial adrenalectomy at age 14, followed by a bilateral partial adrenalectomy four years later. Fourteen years after his first partial adrenalectomy, he developed a lingual lobe lesion and underwent a wedge resection that was shown to be consistent with pheochromocytoma. Six years following his wedge resection, a new thoracic spine bone lesion was seen and was shown to be an additional metastatic pheochromocytoma site. Despite this new lesion, he remains asymptomatic without elevated catecholamines and systemic therapy has not been initiated at this time, now 22 years after his initial adrenal surgery. He is followed closely with every 6-month cross sectional imaging and catecholamine testing.
Comments
Pheochromocytoma is a disease in which a large proportion of patients have a germline predisposition, meaning many more patients are at risk for the development of bilateral, multifocal and/or recurrent pheochromocytoma than previously recognized. Because of our institutional interest in hereditary cancer syndromes, our early adoption of partial adrenalectomy has enabled us to obtain long-term outcomes of patients treated with this procedure
Reported risk factors of pheochromocytoma recurrence have been reported and have included age at diagnosis, laterality, and familial predisposition. In one of the largest analyses of pheochromocytoma and paragangliomas, Amar and colleagues noted that right sided tumors, younger age at initial diagnosis, familial predisposition and extra-adrenal tumors were all associated with increased chance of tumor recurrence.14 Pamporaki et al showed in their series comparing pediatric and adult pheochromocytoma and paragangliomas that children had a 2.1x higher risk of a recurrent lesions. This may be associated with a higher prevalence of hereditary syndromes seen in children presented with pheochromocytoma and paraganglioma as compared to adults in the same study (80.4% of children vs 52.6% of adults)15 Length of total follow-up in our series was shown to be much longer in those patients who had a recurrence when compared to those who did not (51 months vs 144 months, p=0.0031), providing evidence that long term surveillance is important in this group of patients.
A single patient in this series developed metastatic pheochromocytoma, leading further evidence to its rarity. Generally, metastatic pheochromocytoma has been associated with a relatively good prognosis and survival. In one single center study, median age at diagnosis was 39 with overall and disease specific survivals of 24.6 and 33.7 years.16 A recent meta-analysis reported a similar conclusion with 5 and 10 year mortality rates of 37% and 29%, respectively. 17 Our series provides additional evidence that the metastatic potential of pheochromocytoma is low and can likely be managed with close imaging follow-up surveillance. This is important information as treatment options for this rare malignancy are limited and would be without curative intent, with various treatment associated toxicities reported.18
It is unclear what effect adrenal vein preservation has on the risk of development of steroid dependence. While some suggest preserving the main renal vein is important,19 others suggest division of the vein is safe as long as the remaining retroperitoneal vein plexus is left undisturbed.20 Brauckhoff and colleagues noted that preservation of the vein is not essential, while maintaining approximately 15%−30% of the gland volume can preserve adequate function. 21 For our cohort, in those with available data on adrenal vein status, 62/101 veins were ligated (61.4%) while the remainder (39/101; 38.6%) were preserved. As shown in Figure 1, adrenal vein preservation increased over the study period. While this study was not designed to determine the factors associated with changing surgical technique, we feel the increased adrenal vein preservation is likely related to both the increased magnification afforded by the minimally invasive/robotic approaches in addition to the increasing comfort level with performing these procedures over time. There was no difference in steroid dependence based on the operative management of the vein with ligation being performed in 4 (57.1%) of the patients who were steroid dependent vs 3(42.9%) who had the vein preserved (p=1.00), but with low numbers it may be difficult to see a difference. On univariate analysis, the only clinical parameter predictive of steroid dependence was the presence of a solitary adrenal gland. The majority of patients on steroids had had previous contralateral total adrenalectomy prior. If a partial adrenalectomy was feasible and performed on the other side, it is likely that steroid replacement would have been avoided. Given our increasing comfort level with robotic laparoscopic partial adrenalectomy, our goal is vein preservation when feasible and to minimize excessive dissection of the remaining normal gland to preserve as much of the normal venous plexus that is possible. Use of adjuncts, such as indocyanine green, may be helpful to help identify the vasculature and subjectively note the perfusion of the remnant adrenal gland following resection, however, there is suggestion that pheochromocytomas may be isoflourescent to the remainder of the adrenal gland limiting its utility to assist with identification and resection of these lesions. 22 Additionally, our preference for most adrenal lesions, if clinically appropriate, is to perform adrenal sparing surgery.
Previous outcomes of partial adrenalectomy have been published. In one of the largest series reported, primarily composed of MEN2 patients, oncologic and steroid dependent outcomes were reported for patients undergoing partial adrenalectomy.23 A total of 625 patients were included with 324 (52%) patients undergoing an adrenal sparing approach. Overall, recurrences were seen in 13% of patients undergoing adrenal sparing surgery at a median time of 8 years (IQR 4–17). All patients with recurrence in this series underwent repeat surgery, with no comment whether patients were symptomatic. The rate of steroid dependency in those with primary partial adrenalectomies was 23.5%. Rates from other small published series for recurrence range from 3.4–12% and steroid dependent rates range from 0–22%. 9,24–26 While the recurrence rate in our series is slightly higher, we took the most conservative definition of local recurrence based on several factors. First, we based recurrence on a composite of imaging findings, symptoms and/or catecholamine elevation. Indeed, in our series, most recurrences were based on imaging alone (ie. asymptomatic), likely representing early lesions and these patients are followed with active surveillance. If the definition were narrowed to symptomatic recurrences only, as some series do, our recurrence rate would be 4.6%. Additionally, there appear to be biological differences with pheochromocytoma recurrences in patients with VHL compared to patients without VHL which portend a higher overall recurrence rate. In the large series from Neumann et al, ipsilateral recurrences occurred in a larger number of patients with identified VHL mutations when compared to all patients undergoing cortical sparing surgery ( 16% vs 6%)23 Finally, patients with hereditary predisposition to pheochromocytoma are at risk for de novo tumors in the residual adrenal tissue. We made no distinction between de novo tumors and recurrence from residual or missed tumors during resection. Given that the vast majority of patients had no evidence of residual tumor(s) on first follow up imaging, many “recurrences” are likely de novo.
With the increased utilization of laparoscopic surgery, minimally invasive adrenalectomy became the gold standard after this technique was described in the 1990s. 27 As robotics have become widely adopted in surgery, robotic approaches to adrenal masses have been increasingly utilized.28,29 Perceived benefits to the use of a robotic approach when compared to standard laparoscopy include increased magnification, stereoscopic vision and greater range of motion via the endowrist.30 Robotic approaches have been associated with decreased blood loss, shorter length of stay and fewer complications when compared to standard laparoscopy, however no formal randomized controlled trials have been performed for total or partial adrenalectomy. A robotic approach was used more frequently later in our series as shown in Figure 1 given our increasing comfort with this technology.
Limitations of the analysis include those inherent to single-institution, retrospective reviews. Additionally, while steroid dependent rates are low in our series, patients did not undergo a standardized assessment of adrenal function (such as with a routine post-operative adrenocorticotrophic hormone stim test) and steroid initiation was per the judgement of the treating physician based on history and patient symptoms. Furthermore, because of the long-nature of the study period, practice patterns evolved over time and were not standardized. Strengths include the relatively large number of patients and procedures included, which to our knowledge is one of the largest ever reported.
Conclusions
Partial adrenalectomy for pheochromocytoma is an oncologically sound treatment strategy that spares most patients from requiring life-long steroid replacement. Given the late recurrences seen (one patient had a recurrence over 14 years following his initial procedure), long term surveillance should be performed in patients undergoing partial adrenalectomy for pheochromocytoma. While recurrences are seen, they are usually only detected by imaging and can be managed, at least initially, with active surveillance. If repeat surgery is pursued, an adrenal sparing approach, if feasible, should be performed.
Supplementary Material
Acknowledgements
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tischler AS. Pheochromocytoma and Extra-adrenal Paraganglioma: Updates. Archives of Pathology & Laboratory Medicine. 2008;132(8):1272–1284. [DOI] [PubMed] [Google Scholar]
- 2.Fishbein L, Leshchiner I, Walter V, et al. Comprehensive Molecular Characterization of Pheochromocytoma and Paraganglioma. Cancer Cell. 2017;31(2):181–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neumann HPH, Bausch B, McWhinney SR, et al. Germ-Line Mutations in Nonsyndromic Pheochromocytoma. New England Journal of Medicine. 2002;346(19):1459–1466. [DOI] [PubMed] [Google Scholar]
- 4.Jenny W, Peter S, Oliver G. Genetics and clinical characteristics of hereditary pheochromocytomas and paragangliomas. Endocrine-Related Cancer. 2011;18(6):R253–R276. [DOI] [PubMed] [Google Scholar]
- 5.Walther MM, Reiter R, Keiser HR, et al. Clincial and Genetic Characterization of Pheocrhomocytoma in Von Hippel-Lindau Familys: Comparison with Sporadic Pheochromocytoma Gives Insited into Natorual History of Pheochromocytoma The Journal of Urology. 1999;162(3, Part 1):659–664. [DOI] [PubMed] [Google Scholar]
- 6.Charmandari E, Nicolaides NC, Chrousos GP. Adrenal insufficiency. The Lancet. 2014;383(9935):2152–2167. [DOI] [PubMed] [Google Scholar]
- 7.Walther MM, Keiser HR, Choyke PL, Rayford W, Lyne JC, Linehan WM. Management of Hereditary Pheochromocytoma in Von Hippel-Lindau Kindreds with Partial Adrenalectomy. The Journal of Urology. 1999;161(2):395–398. [PubMed] [Google Scholar]
- 8.Madala A, Daugherty M, Bratslavsky G. Partial Adrenalectomy—Why Should it be Considered? Urology Practice. 2015;2(6):359–366. [DOI] [PubMed] [Google Scholar]
- 9.Grubbs EG, Rich TA, Ng C, et al. Long-Term Outcomes of Surgical Treatment for Hereditary Pheochromocytoma. Journal of the American College of Surgeons. 2013;216(2):280–289. [DOI] [PubMed] [Google Scholar]
- 10.Nagaraja V, Eslick GD, Edirimanne S. Recurrence and functional outcomes of partial adrenalectomy: a systematic review and meta-analysis. International journal of surgery (London, England). 2015;16(Pt A):7–13. [DOI] [PubMed] [Google Scholar]
- 11.Benhammou JN, Boris RS, Pacak K, Pinto PA, Linehan WM, Bratslavsky G. Functional and oncologic outcomes of partial adrenalectomy for pheochromocytoma in patients with von Hippel-Lindau syndrome after at least 5 years of followup. The Journal of urology. 2010;184(5):1855–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Asher KP, Gupta GN, Boris RS, Pinto PA, Linehan WM, Bratslavsky G. Robot-assisted laparoscopic partial adrenalectomy for pheochromocytoma: the National Cancer Institute technique. European urology. 2011;60(1):118–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perry RR, Keiser HR, Norton JA, et al. Surgical management of pheochromocytoma with the use of metyrosine. Annals of surgery. 1990;212(5):621–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amar L, Serváis A, Gimenez-Roqueplo AP, Zinzindohoue F, Chatellier G, Plouin PF. Year of diagnosis, features at presentation, and risk of recurrence in patients with pheochromocytoma or secreting paraganglioma. The Journal of clinical endocrinology and metabolism. 2005;90(4):2110–2116. [DOI] [PubMed] [Google Scholar]
- 15.Pamporaki C, Eisenhofer G, Fliedner S, et al. Characteristics of Pediatric vs Adult Pheochromocytomas and Paragangliomas. The Journal of Clinical Endocrinology & Metabolism. 2017;102(4):1122–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamidi O, Young JWF, Iñiguez-Ariza NM, et al. Malignant Pheochromocytoma and Paraganglioma: 272 Patients Over 55 Years. The Journal of Clinical Endocrinology & Metabolism. 2017;102(9):3296–3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamidi O, Young WF, Gruber L, et al. Outcomes of patients with metastatic phaeochromocytoma and paraganglioma: A systematic review and meta-analysis. Clinical Endocrinology. 2017;87(5):440–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jimenez C Treatment for Patients With Malignant Pheochromocytomas and Paragangliomas: A Perspective From the Hallmarks of Cancer. Frontiers in endocrinology. 2018;9:277–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roukounakis N, Dimas S, Kafetzis I, et al. Is preservation of the adrenal vein mandatory in laparoscopic adrenal-sparing surgery? JSLS : Journal of the Society of Laparoendoscopic Surgeons. 2007;11(2):215–218. [PMC free article] [PubMed] [Google Scholar]
- 20.Ikeda Y, Takami H, Niimi M, Kan S, Sasaki Y, Takayama J. Laparoscopic partial or cortical-sparing adrenalectomy by dividing the adrenal central vein. Surgical Endoscopy. 2001;15(7):747–750. [DOI] [PubMed] [Google Scholar]
- 21.Brauckhoff M, Gimm O, Thanh PN, et al. Critical size of residual adrenal tissue and recovery from impaired early postoperative adrenocortical function after subtotal bilateral adrenalectomy. Surgery. 2003;134(6):1020–1027. [DOI] [PubMed] [Google Scholar]
- 22.Tuncel A, Balci M, Aykanat C, Aslan Y, Berker D, Guzel O. Laparoscopic partial adrenalectomy using near-infrared imaging: the initial experience. Minimally invasive therapy & allied technologies : MITAT : official journal of the Society for Minimally Invasive Therapy. 2019:1–7. [DOI] [PubMed] [Google Scholar]
- 23.Neumann HPH, Tsoy U, Bancos I, et al. Comparison of Pheochromocytoma-Specific Morbidity and Mortality Among Adults With Bilateral Pheochromocytomas Undergoing Total Adrenalectomy vs Cortical-Sparing Adrenalectomy. JAMA Network Open. 2019;2(8):e198898–e198898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neumann HP, Bender BU, Reincke M, Eggstein S, Laubenberger J, Kirste G. Adrenal-sparing surgery for phaeochromocytoma. The British journal of surgery. 1999;86(1):94–97. [DOI] [PubMed] [Google Scholar]
- 25.Walz MK, Alesina PF, Wenger FA, et al. Laparoscopic and Retroperitoneoscopic Treatment of Pheochromocytomas and Retroperitoneal Paragangliomas: Results of 161 Tumors in 126 Patients. 2006;30(5):899–908. [DOI] [PubMed] [Google Scholar]
- 26.Walz MK, Iova LD, Deimel J, et al. Minimally Invasive Surgery (MIS) in Children and Adolescents with Pheochromocytomas and Retroperitoneal Paragangliomas: Experiences in 42 Patients. World journal of surgery. 2018;42(4):1024–1030. [DOI] [PubMed] [Google Scholar]
- 27.Gagner M, Lacroix A, Bolte E. Laparoscopic adrenalectomy in Cushing’s syndrome and pheochromocytoma. The New England journal of medicine. 1992;327(14):1033. [DOI] [PubMed] [Google Scholar]
- 28.Boris RS, Gupta G, Linehan WM, Pinto PA, Bratslavsky G. Robot-assisted Laparoscopic Partial Adrenalectomy: Initial Experience. Urology. 2011;77(4):775–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ball MW, Hemal AK, Allaf ME. International Consultation on Urological Diseases and European Association of Urology International Consultation on Minimally Invasive Surgery in Urology: laparoscopic and robotic adrenalectomy. BJU Int. 2017;119(1):13–21. [DOI] [PubMed] [Google Scholar]
- 30.Hyams ES, Stifelman MD. The role of robotics for adrenal pathology. Current Opinion in Urology. 2009;19(1):89–96. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.