Abstract
Objectives:
The Women’s Health Initiative (WHI) randomized trial identified age differences in the benefit-risk profile of estrogen alone (ET) use. However, WHI trial impact on disease-associated medical expenditures attributable to subsequent decreased ET utilization has not been measured. Therefore, the objective of this analysis was to quantify the age-specific disease- associated medical expenditures attributable to reduced ET utilization after the WHI HT trials.
Methods:
Population-level disease counts and associated expenditures between 2003 and 2015 were compared between an observed ET-user population versus a hypothetical ET-user population assuming absence of the WHI HT trials, constructed by extrapolating ET utilization rates from 1996–2002 assuming pre-WHI HT rates would have continued without publication of the WHI HT trial data (2002–2004). Analyses were stratified by age (50–59, 60–69 and 70–79 years). Input data were extracted from Medical Expenditure Panel Survey (MEPS) and the literature. The primary outcomes were: ET utilization, chronic diseases (breast cancer, stroke, CHD, colorectal cancer, pulmonary embolism and hip fracture) and disease-associated direct medical expenditures.
Results:
Over 13 years, the decline in ET utilization was associated with $4.1 billion expenditure for excess chronic diseases (37,549 excess events) among women in their 50s, compared to savings of $1.5 billion and $4.4 billion for diseases averted by lower ET utilization among women in their 60s (13,495 fewer events) and 70s (40,792 fewer events), respectively.
Conclusion:
The decline in ET utilization had differential disease and expenditure consequences by age groups in the US. These results are limited by the lack of inclusion of vasomotor symptom benefit and costs of alternative medications for these symptoms in the analysis.
Keywords: Estrogen, risk and benefits of estrogen, hormone therapy, economic impact, Women’s Health Initiative
INTRODUCTION
Findings from the Women’s Health Initiative (WHI) clinical trials led to major changes in hormone therapy (HT) use after demonstration that estrogen alone (ET), in women with prior hysterectomy, had significant effects on several chronic conditions, collectively referred to as the global index, including breast cancer, coronary heart disease (CHD), stroke, pulmonary embolism, colorectal cancers, and hip fracture (1–3).
While ET had been commonly prescribed for women with prior hysterectomy for climacteric symptom management (4), concerns regarding ET influence on other clinical conditions had generated debate regarding its risks and benefits. These concerns were heightened by initial reports from the WHI randomized trial that, in the overall cohort aged 50–79 years, during the intervention period, while ET decreased hip fractures and led to a borderline reduction in breast cancer risk, stroke incidence was significantly increased and CHD influence was neutral (1). However, LaCroix et al (2011) reported that, with longer-term follow-up, risks became attenuated and the decreased risk of breast cancer persisted (3). In another long-term follow-up analysis, Manson et al (2013) reported differential risk profiles of ET use across the age categories, with the balance of risks and benefits of ET more favorable among women in their 50s compared to older age groups (2).
Despite emerging evidence supportive of ET use in younger postmenopausal women with climacteric symptoms and no contraindications, ET utilization continues to reflect the substantial reduction seen following initial reports (5) of the WHI hormone therapy trials beginning in 2002 (6–9). Although Roth et al (2014) reported a corresponding net economic return of $37.1 billion ($140 per dollar invested in the trial) for the WHI estrogen plus progestin (E+P) trial, a trial conducted in parallel to the ET trial, corresponding data on the economic consequences of the ET trial have not been previously published (10).
Therefore, our primary study objective was to evaluate the health and economic consequences of declines in ET therapy after publication of the WHI hormone therapy trial results in 2002–2004. We hypothesized that, compared to the hypothetical non-WHI ET scenario, declines in ET utilization would benefit older women but adversely affect younger women aged less than 60 years old. This evaluation of the economic impacts of changes in ET use after 2004 will provide clinicians, patients, and other stakeholders with a more complete picture of the payoffs associated with the landmark WHI trials.
METHODS
Data source
We used the Household Component (HC) of the Medical Expenditure Panel Survey (MEPS) data to calculate estrogen usage and medical expenditures for global index diseases between 1996 and 2015 (11). The HC data are based on questionnaires from individual household members and their medical providers and include the Population Characteristics (demographic characteristics, e.g., age, sex, race, ethnicity, marital status, etc.), Medical Condition (MC) and Prescribed Medicines (PM) event files (12, 13). Expenditures on events of prescription medicines, hospital inpatient stays, emergency room visits, outpatient visits, office-based medical provider visits, and home health care are sourced from out-of-pocket payments and payments by private insurance, Medicaid, Medicare, and other sources. Therefore, these data are more generalizable to the US population than any single data source. Each record of the expenditure has a condition index indicating the medical condition with which the event is associated and can be linked to the medical condition file with the condition index and the unique personal ID. Additional description of the MEPS files are in the Supplement. The Population Characteristics, MC and PM files were linked by unique survey participant ID numbers in all files.
Study sample
Our analysis focused on women aged between 50 and 79 years old who had undergone hysterectomy and used estrogens between 1996 and 2015. Hysterectomy status was determined from participants’ responses to the following question included in the 2000–2015 MEPS survey questionnaires: “Have (has) you (person) had a hysterectomy?”. All outcome measures were stratified by age groups as reported by the WHI HT trials: 50–59, 60–69 and 70–79 years old.
Estimation of estrogen Usage
In this study, estrogen users are those reporting estrogen use without use of progestin or any sex hormone combination products within a given survey year. As hysterectomy status was not included in earlier MEPS surveys, we imputed counts of estrogen users by hysterectomy for the period of 1996 – 1999 using data from the 2000 and 2001 cycles because the overall prevalence of estrogen usage declined dramatically in late 2002 after publication of the WHI estrogen plus progestin trial results (13). The annual point-prevalence of estrogen usage was calculated by dividing the weighted population size of estrogen users and the weighted size of hysterectomy population within each calendar year of the MEPS survey. These point-prevalences were also calculated for pre-specified age groups by dividing the weighted counts of users by the weighted counts of women in each age group.
Estrogen users in non-WHI scenario:
We assumed that the prevalence of estrogen usage would have followed a linear trend from 1996 throughout 2015 if the results of WHI were never published. We therefore extrapolated the linear trend of the 1996–2002 estrogen usage prevalence into the period of 2003–2015 to obtain the hypothetical non-WHI prevalent estrogen user population for years 2003–2015. Using the weighted population sizes of eligible women (aged 50–79) from the MEPS population files, we obtained the hypothetical non-WHI population sizes of estrogen users and non-users annually by each age group between 2003 and 2015.
Disease risk estimates
Measures of selected disease included in the global index disease risks were extracted from published data on the WHI ET trial (2). These risks were calculated from the probability of WHI participants experiencing an estrogen-related disease from end-point analysis in both the ET in-trial and the post-trial phases. Manson et. al. (2013) previously reported differential chronic disease risk by the following age groups, 50–59 (hazard ratios [HR], 0.82), 60–69 (HR, 1.03) and 70–79 (HR, 1.10) years old, P-trend = 0.01 (2). Therefore, all analyses were stratified by age groups to capture age-dependent effects of estrogen therapy on women’s health. Disease risk input data is provided in Supplement: e-Table 1.
Expenditures for Diseases
The literature suggests different methods for calculating the medical expenditure for diseases which can result in a wide range of figures depending on the method being used (14). We adopted the primary diagnosis approach, which is a simple method of allocating spending from medical encounter to the condition (“primary diagnosis”) that resulted in the encounter (15). We calculated the annual average medical expenditure for each chronic condition among women using MEPS data (2003–2015) on medical expenditures for each disease condition associated with services from the following settings: hospital inpatient stays, emergency room visits, outpatient visits, office-based medical provider visits, and home health care. We calculated the individual-level annual mean expenditures for each chronic condition and the global index of monitored clinical events in the 2018 US dollars based on the 1983-based Consumer Price Index (CPI) to allow for cross-year expenditure aggregations. The unit expenditure for the global index was calculated as the arithmetic average of the six chronic conditions that make up the global disease index. The unit expenditures for individual diseases and the global disease index are provided in Supplement: e-Table 2.
Statistical Analysis
Base-case analysis
The primary goal of the analysis was to measure the difference in counts of disease attributable to ET and the associated direct medical expenditures between the observed and hypothetical non-WHI scenarios between 2003 and 2015. Although the WHI ET trial data were not officially published until 2004, publication of the WHI E+P trial resulted in substantial declines in ET utilization across board as previously reported. We assumed that, in the absence of WHI, the post-WHI ET trends (2003–2015) in ET utilization would have followed the same trend as the observed pre-WHI ET period (1996 – 2002). Therefore, we framed our analysis around comparing excess diseases (both individual diseases and the global index) that would have occurred if the WHI ET trial results were not published (hypothetical non-WHI ET scenario) versus the observed WHI ET scenario in the post-WHI ET period. All primary analysis were stratified by prespecified age categories.
The total hysterectomy population level counts of diseases in both the real and hypothetical scenarios were obtained by applying the long-term disease risks from the WHI ET trial (2) to observed ET users and non-users based on extrapolations. Disease counts in both the real and hypothetical scenarios were calculated by multiplying the probability for each disease occurring among ET users and non-users, based on the WHI ET data. The disease counts attributable to the WHI ET trial was then calculated by subtracting the absolute cumulative counts of excess diseases in the hypothetical non-WHI ET scenario from those estimated in the real WHI scenario over a 13-year period between 2003 and 2015.
Next, the calculated differences in absolute excess disease counts were multiplied by the annual medical expenditures associated with treatment of each disease to obtain disease-specific medical expenditure differences between the real and hypothetical WHI ET scenarios. The difference in calculated medical expenditures between the real and non WHI ET scenarios, therefore, are interpreted as the cumulative increment in annual medical costs due to the changes of estrogen usage over the 2003–2015 period.
Sensitivity analysis
Four major sets of sensitivity analyses were conducted to characterize the uncertainty around results of the base-case analysis. First, although ET is recommended among only women who have undergone hysterectomy, some women with intact uterus also use ET alone as opposed to ET plus progestin. Therefore, we replicated all base-case analysis among the overall ET user population with/without hysterectomy. Second, we replicated the base-case analysis using medical expenditure data from WHI participants with Medicare fee-for-service benefits (16). This expenditure data (1998 – 2012) comes directly from WHI participants based on healthcare spending Medicare Carrier, Hospice, Home Health and Durable Medical Equipment claims (16). Third, because the first WHI HT trial, Estrogen+Progestin (E+P), was published in July of 2002, we repeated the analysis by comparing a pre-WHI period from 1996–2001 with a post-WHI period of 2003–2015. Fourth, we repeated the analysis by including average annual expenditure on estrogen prescriptions among estrogen users, so that the net impact of WHI on expenditures includes potential savings in medication costs.
Uncertainty analysis
We calculated alternative non-WHI hypothetical estrogen usage trends based on the 95% CI of the slope of the pre-WHI estrogen usage trend. Based on the lower confidence interval (LCI) and upper confidence interval (UCI) of the non-WHI estrogen usage, we calculated and reported corresponding disease and expenditure consequences, to reflect potential measurement errors from unobserved factors that may have affected estrogen usage in the pre-WHI period.
Additionally, to assess the uncertainty around the reported disease risks that were applied in the base-case analysis, we also replicated the analysis by using the lower and upper confidence interval values of each disease risk estimate reported by Manson et. al. (2013) as point estimates of disease risks to create two sets of analysis; one based on the LCI value and another set on the UCI values.
RESULTS
Estrogen Usage
Figures 1a, 1b and 1c provide a graphical representation of trends of the annual point-prevalence of ET usage for women who had undergone hysterectomy, stratified by age groups, from 1996 to 2015. The decline in prevalence of estrogen use after 2002 was abrupt and steep across all three age groups. The average of annual point-prevalence of estrogen use pre-WHI (1996 – 2002) vs. post-WHI (2003 – 2015) were 58% vs. 29%, 47% vs. 20% and 29% vs. 18% for the 50 – 59, 60 – 69 and 70 – 79 year groups, respectively. Similar trends were observed in the overall population of women (Supplement: e-Figures 1a, 1b and 1c).
Figure 1:
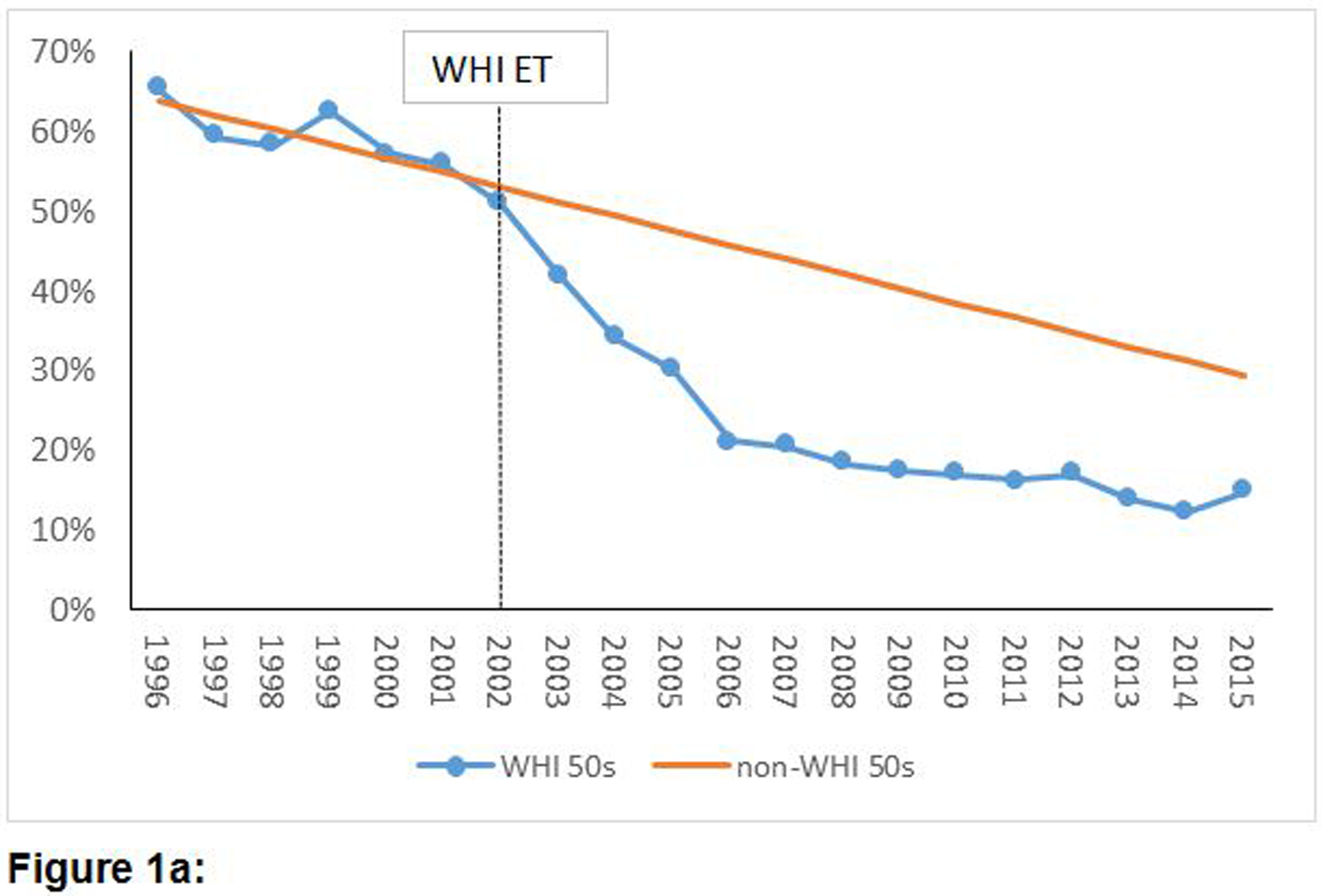
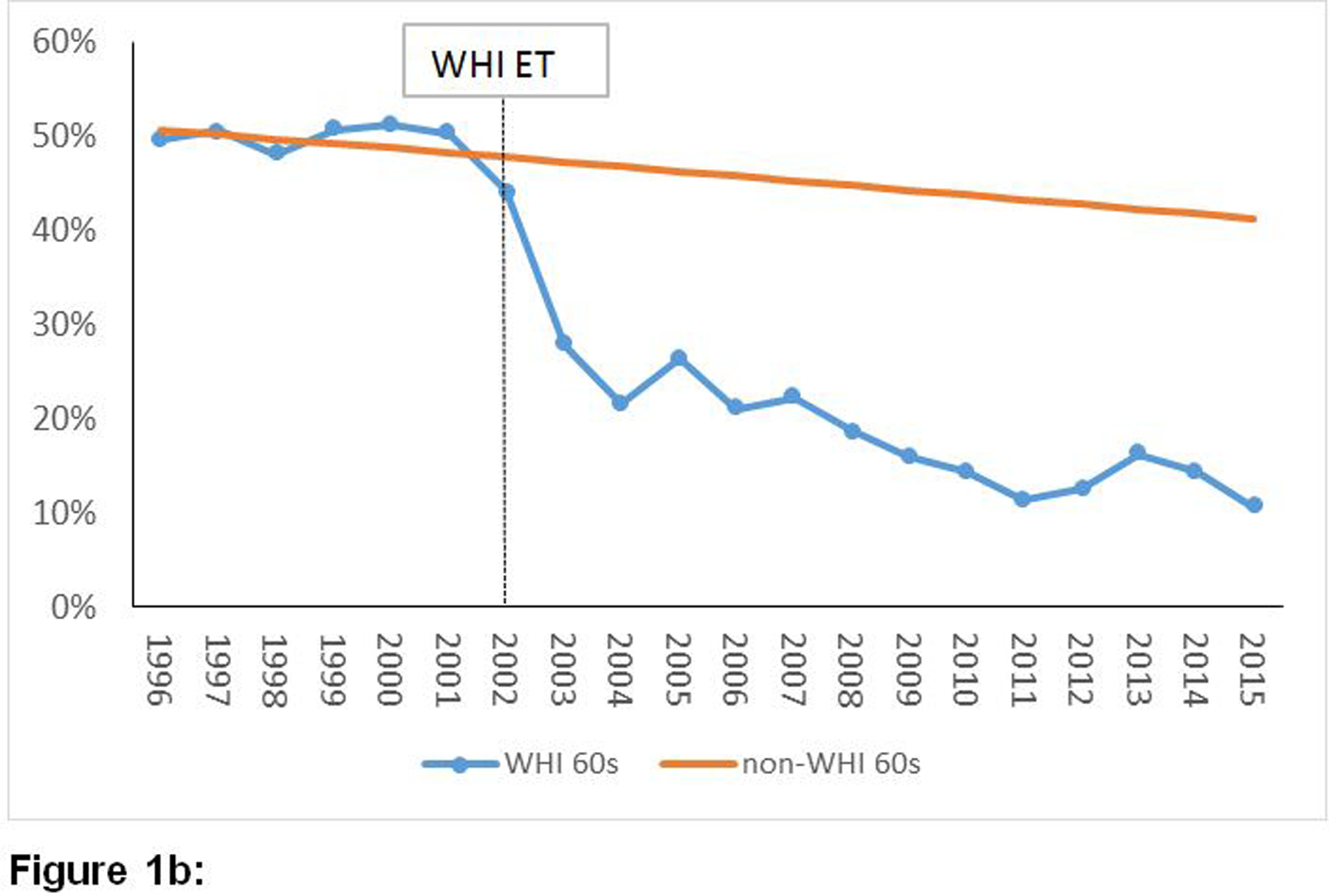
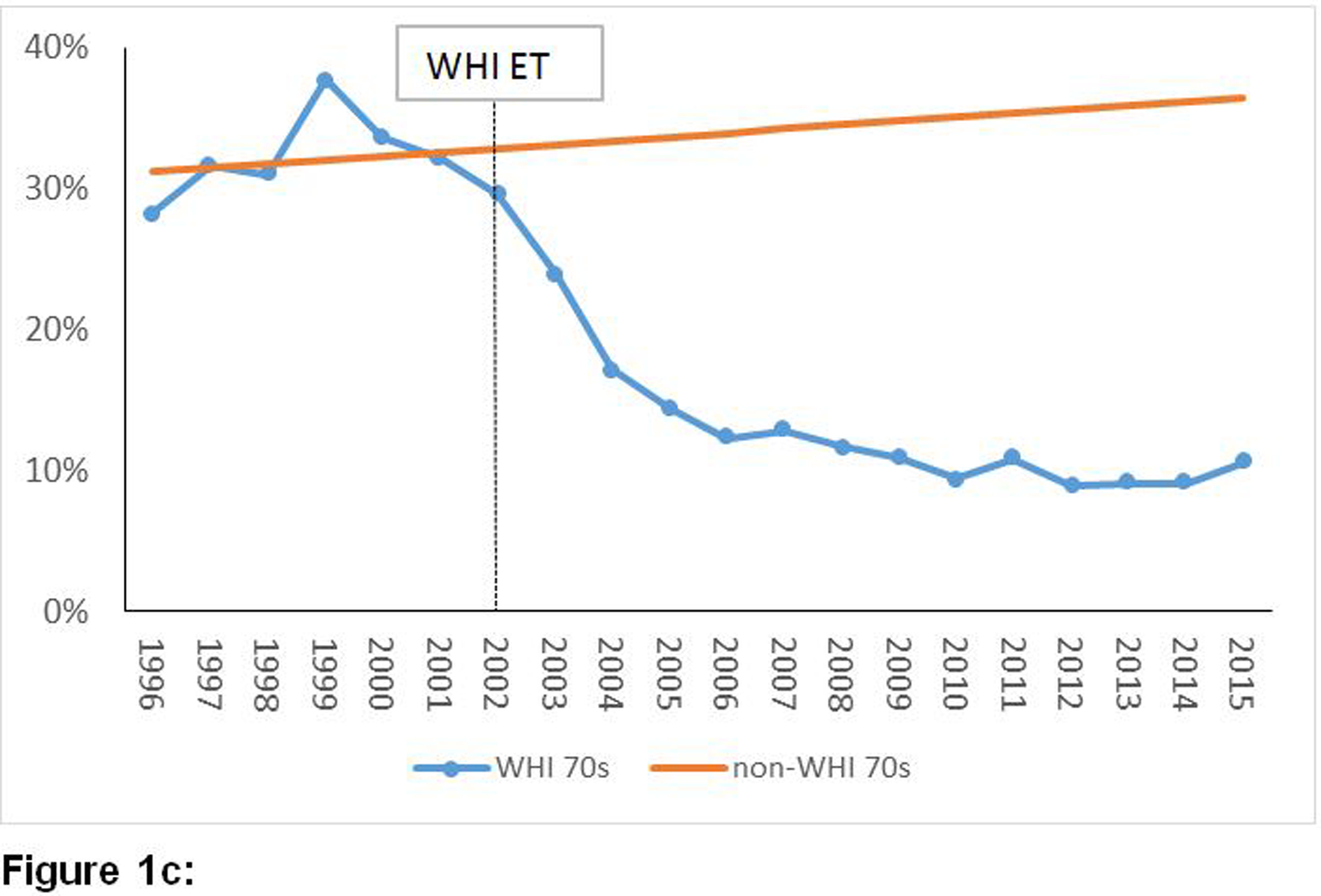
Temporal trends of prevalence of estrogen-alone utilization before and after publication of the WHI ET results among women with a history of hysterectomy by age groups.
Figure 1a: Temporal trends of prevalence of estrogen-alone utilization before and after publication of the WHI ET results among women with a history of hysterectomy aged 50–59 years old
Figure 1b: Temporal trends of prevalence of estrogen-alone utilization before and after publication of the WHI ET results among women with a history of hysterectomy aged 60–69 years old
Figure 1c: Temporal trends of prevalence of estrogen-alone utilization before and after publication of the WHI ET results among women with a history of hysterectomy aged 70–79 years old
Attributable diseases of ET after the WHI ET trial
In Table 1, we present the anticipated absolute differences in counts of disease rates between women in the real vs. hypothetical non-WHI (stable ET use, assuming WHI had not been conducted) scenarios. A positive difference in the absolute counts of diseases corresponds to the counts of excess diseases that would have occurred under the real WHI scenario compared to the hypothetical non-WHI scenario. Conversely, a negative difference corresponds to the counts of diseases that would have been averted in the real vs. a hypothetical non-WHI scenario. Overall, the reductions in ET utilization did not favor younger women. Compared to the non-WHI ET scenario, there were excess numbers of global index cases (n=37,549) that occurred in the real WHI ET scenario attributable to lower ET utilizations among younger women in their 50s. On the other hand, 23,495 and 40,792 global index cases were averted among women in their 60s and 70s, respectively, as a consequence of reduced ET utilization after the WHI HT trials were published. The excess diseases attributable to ET for each component of the global disease index are also presented in Table 1. The temporal trends of these differences among women with history of hysterectomy are presented in Supplement: e-Figures 2a, 2b and 2c. Similar figures are presented for individual diseases, Supplement: e-Figures 3a–3r.
Table 1:
Differences in medical expenditures for treating disease conditions between real and hypothetical non-WHI scenarios over a 13-year cumulative period (2003–2015): Only Women with Hysterectomy
| Disease conditions stratified by age groups (years) | Counts of disease attributable to ET (rates in ET group minus rate in placebo) (95%LCL,95%UCL) | aCumulative medical expenditure differences between real and hypothetical non-WHI scenarios ($millions) | |
|---|---|---|---|
| Annual (95%LCL,95%UCL) | 13-Years (95%LCL,95%UCL) | ||
| Breast Cancer | |||
| 50–59 | −10109 (−4404, −15814) | −61 (−26, −95) | −792 (−345, −1239) |
| 60–69 | −15426 (−7520, −23329) | −93 (−45, −141) | −1209 (−589, −1828) |
| 70–79 | −6181 (−2250, −10110) | −37 (−13, −61) | −484 (−176, −792) |
| Coronary heart disease (CHD) | |||
| 50–59 | −15887 (−6922, −24849) | −70 (−30, −110) | −912 (−397, −1426) |
| 60–69 | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) |
| 70–79 | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) |
| Stroke | |||
| 50–59 | −2888 (−1259, −4516) | −17 (−7, −28,) | −216 (−94, −338) |
| 60–69 | 17355 (8461, 26245) | 100 (49, 151) | 1300 (634, 1966) |
| 70–79 | 8651 (3151, 14153) | 50 (18, 82) | 648 (236, 1060) |
| Pulmonary Embolism | |||
| 50–59 | 1446 (630, 2262) | 6 (3, 10) | 80 (35, 125) |
| 60–69 | 11569 (5643, 17499) | 49 (24, 74) | 640 (312, 968) |
| 70–79 | −6179 (−2248, −10107) | −26 (−9, −43) | −342 (−124, −559) |
| Colorectal Cancer | |||
| 50–59 | −4333 (−1888, −6778) | −59 (−26, −92) | −767 (−334, −1200) |
| 60–69 | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) |
| 70–79 | 13599 (4953, 22247) | 185 (67, 303) | 2407 (877, 3938) |
| Hip Fracture | |||
| 50–59 | −1447 (−629, −2260) | −23 (−10, −36) | −297 (−129, −464) |
| 60–69 | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) |
| 70–79 | −8653 (−3151, −14155) | −137 (−50, −224) | −1779 (−648, −2910) |
| Global Index | |||
| 50–59 | −37549 (−16359, −58739) | −312 (−136, −488) | −4059 (−1768, −6349) |
| 60–69 | 13495 (6577, 20410) | 112 (55, 170) | 1459 (711, 2206) |
| 70–79 | 40792 (14853, 66732) | 339 (124, 555) | 4409 (1606, 7213) |
The unit expenditure per disease based on MEPS (2003–2015) data are: $6027.91, breast cancer; $4415.65, CHD; $5761.94, stroke; $4257.01, PE; $13618.06, colorectal cancer; $15812.83, hip fracture; $8315.01, global index. The 95% CIs are obtained from repeating the analysis using the 95% CIs of the non-WHI estrogen usage trend.
The annual expenditures accrued during each year of the observation period, 2003–2015, were summed up to generate 13-year cumulative expenditures associated with treating chronic condition. A positive difference in the medical expenditures corresponds to excess cost of treatment of excess diseases. Conversely, negative differences correspond to monetary savings in medical expenditures for diseases averted.
Economic consequence of WHI ET
The direct medical expenditures accrued or saved after the WHI ET trial are presented in Table 1. These numbers are interpreted as the annual expenditure for treating a patient given the patient has the disease (it is not the average expenditure for the patient throughout her life cycle). As expected, the medical expenditure consequences of the WHI ET trial followed the patterns observed for the trends of chronic conditions attributable to ET use (Table 1). Lower ET utilization, as a result of the WHI ET trial, had an adverse consequence for young women but not older women, for whom a favorable consequence was observed. Based on the overall medical expenditure for the global disease index, nearly $312 million dollars annually (cumulative $4.1 billion over 13 years) was spent on treating excess diseases that occurred among women in their 50s because of lower ET utilization. Conversely, about $112 million annually ($1.5 billion over 13 years) and $339 million annually ($4.4 billion over 13 years) were saved by not having to treat excess diseases due to ET because of lower ET utilization among women in their 60s and 70s respectively. The pattern of net savings on medical expenditures did not change when alternative non-WHI hypothetical ET utilization trends were calculated based on the 95% CIs of the slopes of pre-WHI estrogen utilization trends. Excess expenditures for women in their 50s can be up to $6.3 billion; savings for older women can be up to $2.2 billion (for women in 60s) and $7.2 billion (for women in 70s). Age group differences in the global index were largely accounted for by differences in CHD, stroke and colorectal cancer. We also repeated the base-case analysis combining (pooling) women of all ages between 50 and 79, Table 2. The estimated savings on global index expenditure was $1.7 billion; the magnitude of the savings was very close to the summation of results for women in the different age groups.
Table 2:
Differences in medical expenditures for treating disease conditions between real and hypothetical non-WHI scenarios over a 13-year cumulative period (2003–2015): Women of All Ages between 50–79
| Disease conditions stratified by age groups (years) | Counts of disease attributable to ET (rates in ET group minus rate in placebo) (95%LCL,95%UCL) | aCumulative medical expenditure differences between real and hypothetical non-WHI scenarios ($millions) | |
|---|---|---|---|
| Annual (95%LCL,95%UCL) | 13-Years (95%LCL,95%UCL) | ||
| Women with Hysterectomy | |||
| Breast Cancer | −13820 (−5206, −22435) | −83(−31, −135) | −1079 (−403, −1755) |
| Coronary heart disease (CHD) | 9214 (3470, 14957) | 125 (47, 204) | 1625 (611, 2652) |
| Stroke | −32248 (−12146, −52349) | −142 (−54, −231) | −1846 (−702, −3003) |
| Pulmonary Embolism | 4607 (1735, 7478) | 38 (14, 62) | 494 (182, 806) |
| Colorectal Cancer | −9214 (−3470, −14957) | −146 (−55, −237) | −1898 (−715, −3081) |
| Hip Fracture | 9214 (3470, 14957) | 39 (15, 64) | 507 (195, 832) |
| Global Index | 23034 (8676, 37392) | 133 (50, 215) | 1729 (650, 2795) |
| All Women | |||
| Breast Cancer | −18314 (−2767, −34609) | −110 (−17, −209) | −1430 (−221, −2717) |
| Coronary heart disease (CHD) | 12209 (1845, 23073) | 166 (25, 314) | 2158 (325, 4082) |
| Stroke | −42732 (−6457, −80755) | −189 (−29, −357) | −2457 (−377, −4641) |
| Pulmonary Embolism | 6104 (922, 11536) | 51 (8, 96) | 663 (104, 1248) |
| Colorectal Cancer | −12209 (−1845, −23073) | −193 (−29, −365) | −2509 (−377, −4745) |
| Hip Fracture | 12209 (1845, 23073) | 52 (8, 98) | 676 (104, 1274) |
| Global Index | 30523 (4612, 57682) | 176 (27, 332) | 2288 (351, 4316) |
The unit expenditure per disease based on MEPS (2003–2015) data are: $6027.91, breast cancer; $4415.65, CHD; $5761.94, stroke; $4257.01, PE; $13618.06, colorectal cancer; $15812.83, hip fracture; $8315.01, global index. The 95% CIs are obtained from repeating the analysis using the 95% CIs of the non-WHI estrogen usage trend.
The annual expenditures accrued during each year of the observation period, 2003–2015, were summed up to generate 13-year cumulative expenditures associated with treating chronic condition. A positive difference in the medical expenditures corresponds to excess cost of treatment of excess diseases. Conversely, negative differences correspond to monetary savings in medical expenditures for diseases averted.
Results from sensitivity and uncertainty analysis
The data observed from the base-case analysis were largely replicated among all estrogen-alone users, with/without hysterectomy (Table 3). The medical expenditures were slightly larger in this larger population because of greater counts of excess disease that occurred or were averted in this larger group of ET users. When savings on reduced estrogen prescriptions (the annual average was $317.97 for each estrogen user), the savings became $5.6 billion for women in their 50s, $9.6 billion for women in their 60s, and $9.6 billion for women in their 70s over the 13-year period. The magnitude of savings on estrogen prescriptions substantially increased the overall medical expenditure savings, reflecting the fact that it represents observed estrogen usage decline versus expenditures on probability-adjusted disease occurrence rates. The magnitude of medical expenditures associated with treating diseases as a result of lower ET utilization was smaller (Supplement: e-Table 3) when we used only estimates on medical expenditure data derived from WHI participants with Medicare benefits (Supplement: e-Table 2). Larger differences in medical expenditures were observed when comparisons were made using a 1996–2001 pre-WHI versus a 2003–2015 post-WHI period (Supplement: e-Table 4). Supplement: e-Table 5a and 5b are replicates of the base-case analysis based on the LCI and UCI values of the disease risk estimates due to ET.
Table 3:
Differences in medical expenditures for treating disease conditions between real and hypothetical non-WHI scenarios over a 13-year cumulative period (2003–2015): with or without hysterectomya.
| Disease conditions stratified by age groups (years) | Counts of disease attributable to ET (rates in ET group minus rate in placebo) (95%LCL,95%UCL) | bCumulative medical expenditure differences between real and hypothetical non-WHI scenarios ($millions) | |
|---|---|---|---|
| Annual (95%LCL,95%UCL) | 13-Years (95%LCL,95%UCL) | ||
| Breast Cancer | |||
| 50–59 | −10842(3294, −26722) | −65(20,−161) | −850(258,−2094) |
| 60–69 | −24165(−9243,−39092) | −146(−56,−236) | −1894(−724,−3063) |
| 70–79 | −7667(−3545,−11788) | −46(−21,−71) | −601(−278,−924) |
| Coronary heart disease (CHD) | |||
| 50–59 | −17036(5175,−41993) | −75(23,−185) | −978(297,−2410) |
| 60–69 | 0(0,0) | 0(0,0) | 0(0,0) |
| 70–79 | 0(0,0) | 0(0,0) | 0(0,0) |
| Stroke | |||
| 50–59 | −3098(941,−7634) | −18(5,−44) | −232(70,−572) |
| 60–69 | 27189(10399,43980) | 157(60,253) | 2037(779,3294) |
| 70–7 | 10734(4965,16508) | 62(29, 95) | 804(372,1237) |
| Pulmonary Embolism | |||
| 50–59 | 1547(−471,3814) | 7(−2,16) | 86(−26,211) |
| 60–69 | 18125(6935,29317) | 77(30,125) | 1003(384,1622) |
| 70–79 | −7666(−3544,−11791) | −33(−15,−50) | −424(−196,−652) |
| Colorectal Cancer | |||
| 50–59 | −4647(1412,−11454) | −63(19,−156) | −823(250,−2028) |
| 60–69 | 0(0,0) | 0(0,0) | 0(0,0) |
| 70–79 | 16868(7802,25936) | 230(106,353) | 2986(1381,4592) |
| Hip Fracture | |||
| 50–59 | −1547(469,−3816) | −24(7,−60) | −318(96,−784) |
| 60–69 | 0(0,0) | 0(0,0) | 0(0,0) |
| 70–79 | −10735(−4963,−16504) | −170(−78,−261) | −2207(−1020,−3393) |
| Global Index | |||
| 50–59 | −40274(12225,−99261) | −335(102,−825) | −4353(1321,−10730) |
| 60–69 | 21148(8090,34208) | 176(67,284) | 2286(874,3698) |
| 70–79 | 50604(23397,77810) | 421(195,647) | 5470(2529,8411) |
All women includes women with or without documented hysterectomy in MEPS.
The unit expenditure per disease based on MEPS (2003–2015) data are: $6027.91, breast cancer; $4415.65, CHD; $5761.94, stroke; $4257.01, PE; $13618.06, colorectal cancer; $15812.83, hip fracture; $8315.01, global index. The unit expenditure for the global index was calculated as the arithmetic average of the 6 comprising diseases. The 95% CIs are obtained from repeating the analysis using the 95% CIs of the non-WHI estrogen usage trend.
The annual expenditures accrued during each year of the observation period, 2003–2015, were summed up to generate 13-year cumulative expenditures associated with treating chronic condition. A positive difference in the medical expenditures corresponds to excess cost of treatment of excess diseases. Conversely, negative differences correspond to monetary savings in medical expenditures for diseases averted.
DISCUSSION
In this analysis, we evaluated the chronic conditions attributable to ET and the associated direct medical expenditures for treating these conditions after publication of the WHI HT trial results. Because the annual prevalence rates of ET utilization declined sharply after 2002 as a result of the WHI HT trial publications (9, 17–21), we attributed the excess chronic conditions that occurred or were averted and the associated treatment expenditures among ET users to the WHI HT trials. Reduced ET utilization trends, overall, had adverse consequences among younger postmenopausal women (50–59 years old). In contrast, lower ET utilization had favorable consequences among older postmenopausal women, especially those aged 70–79 years old. The impact on women in their 60s was relatively modest. These patterns and trends in estrogen-alone treatment and population disease burdens translated into nearly $4.1 billion excess cost for treating excess chronic disease events due to lower ET utilization among younger women in their 50s with prior hysterectomy over a 13-year cumulative period (2003 – 2015). Conversely, $4.4 billion in direct medical expenditure was saved among their older counterparts aged 70–79 because of excess chronic conditions averted from reduced ET utilization during the same time horizon. A relatively smaller amount of $1.5 billion was saved among the 60–69 year group from 2003 – 2015.
The observed age-disease specific differential economic impact of the WHI ET trial is consistent with published data on the population attributable risks of ET. Manson et. al. (2013) previously conducted a comprehensive and integrated analysis of the long-term outcomes of the WHI ET trial and reported that ET was associated with long-term reduced global index of monitored clinical events among women aged 50–59 years old whereas older women, 70–79 years old, were at increased risk; a very modest increased risk was observed for the 60–69 year group, with significant interactions by age over the cumulative 13 years of follow up (2). The biological basis for the relative benefits of estrogen-alone treatment among younger women and the adverse effects in older women has been extensively discussed (22–24).
Implications of the results
In spite of evidence of a balanced to favorable benefit:risk profile for ET among younger postmenopausal women, ET utilization declined in this subgroup at similar rates to those observed for older age groups. We were not able to ascertain reasons for declining utilization trends in younger women in this analysis. However, it is plausible that these younger women and their clinicians were deciding against ET because the earlier WHI estrogen plus progestin trial had significant adverse effects on several chronic conditions across age groups of postmenopausal women (2, 5).
A previous analysis of the 10-year health and economic impacts of the WHI (E+P) trial suggested the trial resulted in $9.0B in reduced disease-related direct medical expenditure in the U.S. from 2003 to 2012 after accounting for potential increases in fractures and colorectal cancer cases. Compared to this study, shifts in E+P use were expected to have a larger effect on incidence of diseases of interest (e.g. 2.6-fold more cases of breast cancer avoided) and associated direct medical expenditure (e.g. 13.6-fold more savings). Collectively, disease expenditure impacts stemming from reductions in HT use following reports from the WHI ET and E+P trials are approximately $10B. While these two analyses of WHI clinical trial impacts analyzed different time horizons and used different methods, they similarly reinforce a conclusion that the WHI hormone therapy clinical trials yielded considerable societal value in the United States.
LIMITATIONS
Our analysis has limitations. We could not distinguish consequences of the WHI HT trials between prevalent and new initiators of estrogen-alone therapy because the sample sizes of new initiators was too low. Nevertheless, the proportion of all women considered as new initiators (based on no prior use in the past two years) declined in a similar trend as that for prevalent users. Further, previous data showed no differential effects of estrogen-alone therapy between prevalent and new initiators (10). Our analysis assumed that estrogen-alone therapy would have the same risk as those observed in the WHI ET trial. However, potential differences in ET adherence in real world settings as well as formulation, dose, route of administration, and duration of use were not accounted for in our analysis.
Time since menopause and vasomotor symptoms were not addressed in our analysis because this information was not available in the MEPS data. Therefore, our study findings, especially in the participants 60 years of age or older, are limited to estrogen alone use as was given in the trial, namely, with estrogen alone use initiated in the vast majority, a decade or more from menopause. We provide limited or no evidence regarding initiation of estrogen alone use in the peri-menopausal and early postmenopausal situation with continuation of use through the sixth or seventh decade.
The estimated disease treatment expenditures did not factor in mortality. However, over 13 years, it was only the younger women who had a meaningful difference in mortality by ET-alone treatment group (mortality reduction with ET vs placebo) and the other age groups had generally neutral results. Thus, the expenditures for the younger women on ET may have been overestimated (and their savings underestimated) – and the other age groups probably saw minimal impact on cost estimates related to mortality. Because women are choosing alternative non-hormonal medications to treat menopausal symptoms and given that we do not have information on treatment indications or the cost of these alternative treatments, we factored the cost of ET into sensitivity analyses but not our main analyses (25–31). Factoring in ET cost, without that of alternative treatments, may produce imbalanced expenditures.
STRENGTHS
Our analysis has several strengths. We applied a simplified economic evaluation technique to generate data on the health and economic consequences of the WHI ET trial (32, 33). This approach is objective and uses randomized trial data for medication-disease associations and is not restricted to any particular viewpoint or perspective, unlike traditional economic evaluation approaches. The cost consequence analysis technique is a recommended economic evaluation for interventions that have multiple potential effects and are centered on patient-oriented outcomes such as the WHI ET trial (32, 33). Our analysis provides an assessment of the health and economic consequences of the WHI ET trial by directly estimating temporal trends in ET utilization and attributable disease estimates and medical expenditure costs over a 13-year period. By showing that the health and economic consequences of the WHI ET results differed by age, the clinical community will be better able to personalize care and optimize the benefit:risk ratio of ET use by considering the age of patients. We applied simplified models to extrapolate a hypothetical ET user population and further applied direct disease risk estimates for the cumulative long-term effects of ET use to quantify the population-attributable disease and cost estimates.
CONCLUSION
These data add to the existing body of literature that encourages personalized decision-making, in this case to better balance the risk:benefit ratio of estrogen-alone therapy based on the patient’s age and/or time since menopause. Our data show that the WHI ET results were extrapolated across postmenopausal women of all ages and the subsequent decline in ET utilization had adverse effects in younger women but favorable effects in older women. Taking age into account in clinical decision making about ET use will allow for improved care for women of all ages.
Supplementary Material
ACKNOWLEDGEMENT:
The authors thank the WHI participants, clinical sites, investigators, and staff for their dedicated efforts. The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts HHSN268201600018C, HHSN268201600001C, HHSN268201600002C, HHSN268201600003C, and HHSN268201600004C.
Sources of funding: This study was partially funded by the National Institutes of Health, U.S. Department of Health and Human Services through contracts HHSN268201600018C, HHSN268201600001C, HHSN268201600002C, HHSN268201600003C, and HHSN268201600004C.
Footnotes
Conflict of interest/financial disclosure: Dr. Chlebowski has received funding from Pfizer. Dr. Roth has received funding from Bayer. The other authors have no relevant disclosures.
References
- 1.Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291(14):1701–12. [DOI] [PubMed] [Google Scholar]
- 2.Manson JE, Chlebowski RT, Stefanick ML, Aragaki AK, Rossouw JE, Prentice RL, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310(13):1353–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.LaCroix AZ, Chlebowski RT, Manson JE, Aragaki AK, Johnson KC, Martin L, et al. Health outcomes after stopping conjugated equine estrogens among postmenopausal women with prior hysterectomy: a randomized controlled trial. JAMA. 2011;305(13):1305–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The NHTPSAP. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24(7):728–53. [DOI] [PubMed] [Google Scholar]
- 5.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–33. [DOI] [PubMed] [Google Scholar]
- 6.Crawford SL, Crandall CJ, Derby CA, El Khoudary SR, Waetjen LE, Fischer M, et al. Menopausal hormone therapy trends before versus after 2002: impact of the Women’s Health Initiative Study Results. Menopause. 2018;26(6):588–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsai SA, Stefanick ML, Stafford RS. Trends in menopausal hormone therapy use of US office-based physicians, 2000–2009. Menopause. 2011;18(4):385–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wysowski DK, Governale LA. Use of menopausal hormones in the United States, 1992 through June, 2003. Pharmacoepidemiol Drug Saf. 2005;14(3):171–6. [DOI] [PubMed] [Google Scholar]
- 9.Hersh AL, Stefanick ML, Stafford RS. National use of postmenopausal hormone therapy: annual trends and response to recent evidence. JAMA. 2004;291(1):47–53. [DOI] [PubMed] [Google Scholar]
- 10.Roth JA, Etzioni R, Waters TM, Pettinger M, Rossouw JE, Anderson GL, et al. Economic return from the Women’s Health Initiative estrogen plus progestin clinical trial: a modeling study. Ann Intern Med. 2014;160(9):594–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Services UDoHH. MEPS HC-096 2005 Medical Conditions In: Quality AfHRa, ed. Rockville MD; 2007. [Google Scholar]
- 12.Services UDoHH. MEPS HC-183 Panel 19 Longitudinal Data File In: Quality AfHRa, ed. Rockville MD; 2017. [Google Scholar]
- 13.;Pageshttps://meps.ahrq.gov/mepsweb/about_meps/survey_back.jsp.
- 14.Anne E Hall TH. CALCULATING DISEASE‐BASED MEDICAL CARE EXPENDITURE INDEXES FOR MEDICARE BENEFICIARIES: A COMPARISON OF METHOD AND DATA CHOICES In: Analysis BoE, ed. 1441 L St. NW, Washington, DC; 2014. [Google Scholar]
- 15.Aizcorbe A, Bradley R, Greenaway‐McGrevy R, Herauf B, Kane R, Liebman E, Pack S, and Rozental L. Alternative Price Indexes for Medical Care: Evidence from the MEPS Survey. Bureau of Economic Analysis; 2011. [Google Scholar]
- 16.Shreibati JB, Manson JE, Margolis KL, Chlebowski RT, Stefanick ML, Hlatky MA. Impact of hormone therapy on Medicare spending in the Women’s Health Initiative randomized clinical trials. Am Heart J. 2018;198:108–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guay MP, Dragomir A, Pilon D, Moride Y, Perreault S. Changes in pattern of use, clinical characteristics and persistence rate of hormone replacement therapy among postmenopausal women after the WHI publication. Pharmacoepidemiol Drug Saf. 2007;16(1):17–27. [DOI] [PubMed] [Google Scholar]
- 18.Hillman JJ, Zuckerman IH, Lee E. The impact of the Women’s Health Initiative on hormone replacement therapy in a Medicaid program. J Womens Health (Larchmt). 2004;13(9):986–92. [DOI] [PubMed] [Google Scholar]
- 19.Hing E, Brett KM. Changes in U.S. prescribing patterns of menopausal hormone therapy, 2001–2003. Obstet Gynecol. 2006;108(1):33–40. [DOI] [PubMed] [Google Scholar]
- 20.Majumdar SR, Almasi EA, Stafford RS. Promotion and prescribing of hormone therapy after report of harm by the Women’s Health Initiative. JAMA. 2004;292(16):1983–8. [DOI] [PubMed] [Google Scholar]
- 21.Crawford SL, Crandall CJ, Derby CA, El Khoudary SR, Waetjen LE, Fischer M, et al. Menopausal hormone therapy trends before versus after 2002: impact of the Women’s Health Initiative Study Results. Menopause. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mikkola TS, Clarkson TB. Estrogen replacement therapy, atherosclerosis, and vascular function. Cardiovasc Res. 2002;53(3):605–19. [DOI] [PubMed] [Google Scholar]
- 23.North American Menopause S. Estrogen and progestogen use in postmenopausal women: 2010 position statement of The North American Menopause Society. Menopause. 2010;17(2):242–55. [DOI] [PubMed] [Google Scholar]
- 24.Writing Group on behalf of Workshop Consensus G. Aging, menopause, cardiovascular disease and HRT. International Menopause Society Consensus Statement. Climacteric. 2009;12(5):368–77. [DOI] [PubMed] [Google Scholar]
- 25.Drewe J, Bucher KA, Zahner C. A systematic review of non-hormonal treatments of vasomotor symptoms in climacteric and cancer patients. Springerplus. 2015;4:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johns C, Seav SM, Dominick SA, Gorman JR, Li H, Natarajan L, et al. Informing hot flash treatment decisions for breast cancer survivors: a systematic review of randomized trials comparing active interventions. Breast Cancer Res Treat. 2016;156(3):415–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peng W, Adams J, Hickman L, Sibbritt DW. Longitudinal analysis of associations between women’s consultations with complementary and alternative medicine practitioners/use of self-prescribed complementary and alternative medicine and menopause-related symptoms, 2007–2010. Menopause. 2016;23(1):74–80. [DOI] [PubMed] [Google Scholar]
- 28.Bair YA, Gold EB, Zhang G, Rasor N, Utts J, Upchurch DM, et al. Use of complementary and alternative medicine during the menopause transition: longitudinal results from the Study of Women’s Health Across the Nation. Menopause. 2008;15(1):32–43. [DOI] [PubMed] [Google Scholar]
- 29.Wathen CN. Health information seeking in context: how women make decisions regarding hormone replacement therapy. J Health Commun. 2006;11(5):477–93. [DOI] [PubMed] [Google Scholar]
- 30.Ma J, Drieling R, Stafford RS. US women desire greater professional guidance on hormone and alternative therapies for menopause symptom management. Menopause. 2006;13(3):506–16. [DOI] [PubMed] [Google Scholar]
- 31.Kaunitz AM, Manson JE. Management of Menopausal Symptoms. Obstet Gynecol. 2015;126(4):859–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brazier J. Measuring and valuing mental health for use in economic evaluation. J Health Serv Res Policy. 2008;13 Suppl 3:70–5. [DOI] [PubMed] [Google Scholar]
- 33.Drummond M, Sculpher M, Torrance G, O’Brien B and Stoddart G. Methods for the Economic Evaluation of Health Care Programmes. Oxford, UK: Oxford University Press; 2005. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


