Abstract
Background:
REM-sleep behavior disorder (RBD) is a common finding among patients with synucleinopathies. We aimed to determine the degree of autonomic dysfunction in patients presenting with idiopathic RBD (iRBD), and the predictive value of autonomic dysfunction for phenoconversion to a defined neurodegenerative disease.
Methods:
We searched our electronic medical record for patients diagnosed with iRBD who also underwent standardized autonomic function testing within 6 months of iRBD diagnosis, and who had clinical follow-up of at least 3 years following iRBD diagnosis. The composite autonomic severity score (CASS) was derived and compared between phenoconverters and non-converters using chi-square and Wilcoxon rank-sum tests.
Results:
We identified 18 patients who fulfilled in- and exclusion criteria. Average age at autonomic testing was 67 ± 6.6 years. Twelve (67%) patients phenoconverted during the follow-up period; 6 developed PD, the other 6 DLB. Fifteen (83%) patients had at least mild autonomic dysfunction. There were no significant differences between overall converters and non-converters in total CASS or CASS subscores. However, iRBD patients who developed DLB had significantly higher total and cardiovagal CASS scores compared with those who developed PD (p <0.05), and a trend for higher adrenergic CASS scores compared to those who developed PD and those who did not phenoconvert.
Discussion:
Autonomic dysfunction was seen in 83% of iRBD patients, and more severe baseline cardiovagal autonomic dysfunction in iRBD wasassociated with phenoconversion to DLB but not PD. Prospective studies are needed to confirm the value of autonomic testing for predicting phenoconversion and disease phenotype in iRBD.
Keywords: REM sleep behavior disorder (RBD), Autonomic, Phenoconversion, Parkinson’s disease, Dementia with Lewy bodies
Introduction
Rapid eye movement sleep behavior disorder (RBD) is strongly associated with synucleinopathy neurodegeneration, particularly Parkinson’s disease (PD), dementia with Lewy bodies (DLB) pure autonomic failure (PAF) and multiple system atrophy (MSA) [20]. Patients with idiopathic RBD, which is RBD in the absence of a clinically overt neurodegenerative disease, phenoconvert to DLB, PD or MSA at a rate of approximately 6% per year [2, 16]. The relationship between RBD and synucleinopathy has led to a search for biomarkers of risk for phenoconversion, and there is evidence that objective hyposmia, nonspecific motor impairment, autonomic dysfunction, and REM sleep without atonia represent markers of increased risk for accelerated development of PD or DLB [8, 13, 16]. Autonomic dysfunction, particularly orthostatic hypotension, constipation and erectile dysfunction, is common in RBD patients and may present up to 20 years prior to the development of clinical signs of PD [4]. Despite the well-known association between autonomic dysfunction and RBD, few quantitative studies of autonomic function in iRBD have been reported.
Mild sudomotor dysfunction has been reported in 29–41% of RBD patients, and was found to be more common in patients with PD and RBD compared to iRBD alone [8, 17]. Another small study reported that iRBD patients had more symptoms of orthostatic intolerance and overall autonomic symptoms than controls, but less than PD patients, and an overall degree of autonomic dysfunction in iRBD that was between that of controls and PD patients [5]. Cardiovagal and adrenergic function have shown a similar degree of impairment in PD and iRBD patients, with 70–80% of iRBD patients having mild-moderate impairment in these domains [8, 17]. Taken together, current data suggests that iRBD patients do exhibit mild-moderate autonomic dysfunction. However, prior studies have focused on the similarities in autonomic impairment between iRBD and PD patients to show further evidence for the synucleinopathy link, but no previous studies to our knowledge have analyzed predictive capability of autonomic testing for phenoconversion. We therefore aimed to report quantitative cardiovagal, adrenergic and sudomotor function in patients at time of iRBD diagnosis, and to determine if type or severity of autonomic dysfunction at iRBD diagnosis is predictive of future synucleinopathy development or ultimate phenotype.
Methods
A text-based search of our electronic medical record was performed for a diagnosis of REM sleep behavior disorder. We required patients to have undergone both polysomnography (PSG) and autonomic testing between 1998–2008 at Mayo Clinic Rochester as identified by CPT codes. This search yielded 378 unique patients. The date range was chosen to allow for sufficient duration of follow-up after autonomic testing to identify phenoconverters. Detailed clinical and autonomic data were collected for patients identified in this search who did not meet exclusion criteria for this study. RBD diagnosis was based on clinical interview with a board-certified sleep specialist and review of video-PSG with qualitative review for the presence of REM sleep without atonia. Reason for sleep evaluation was RBD in 15 (83%) and OSA in 3 (17%). Reason for autonomic testing included: Orthostatic intolerance in 11 (61%), screening for autonomic dysfunction in 5 (3 with constipation/urinary dysfunction, 2 without clear autonomic symptoms) (28%), evaluate asymptomatic orthostatic blood pressure drop on bedside testing (1 patient) and syncope (1 patient). Patients were excluded if they met any of the following criteria (Figure 1):
No diagnosis of idiopathic RBD or RBD at time of autonomic testing based on international classification of sleep disorders-1 or 2 criteria (depending on year of diagnosis)
RBD associated with synucleinopathy (PD, DLB, MSA, PAF) at time of autonomic testing or diagnosed based on autonomic testing results
Diagnosis of autoimmune autonomic disorder (such as acetylcholine receptor ganglionic antibody neuropathy) since the results of autonomic testing would not be reflective of true idiopathic RBD
>6 months between iRBD diagnosis and autonomic testing to limit risk of evolving phenoconversion prior to autonomic testing that would impact results
Less than 3 years of in-person clinical follow up after autonomic testing
Figure 1:
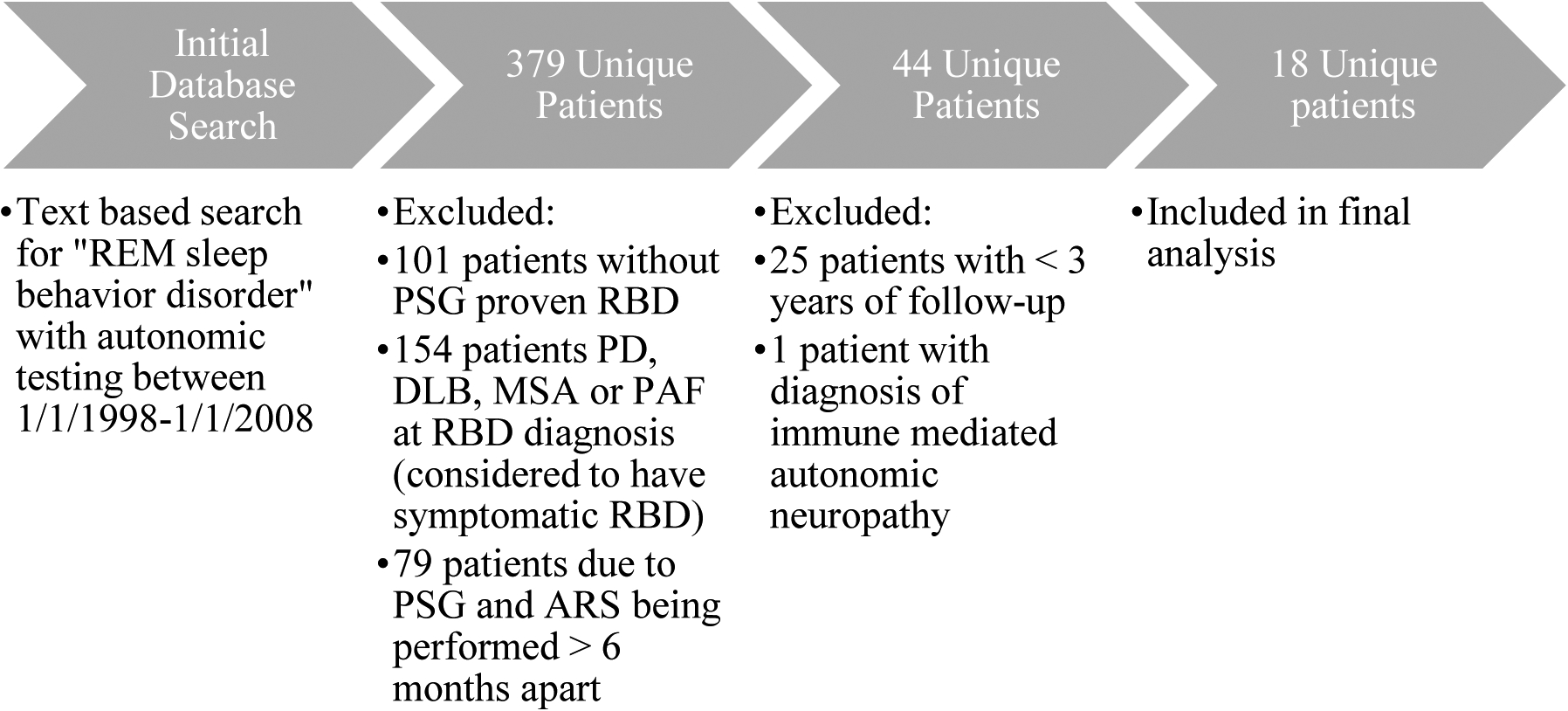
Study Flowchart
REM=rapid eye movement sleep; PSG=polysomnography; ARS=autonomic reflex screen; RBD=REM sleep behavior disorder; DLB=dementia with Lewy bodies; MSA=multiple system atrophy; PD=Parkinson’s disease
Categorization of converters to MSA, PD, or DLB was based on established clinical diagnostic criteria [6, 7, 14, 18]. The Mayo Clinic IRB approved this study.
Clinical Data
Autonomic variables
Autonomic testing is highly standardized and composed of (1) the autonomic reflex screen (ARS), which assesses postganglionic sudomotor, cardiovagal, and cardiovascular adrenergic function; and (2) the thermoregulatory sweat test (TST), which quantitatively assesses preganglionic and postganglionic sudomotor pathways over the entire anterior body surface, although not necessarily all patients underwent TST. Patients were routinely instructed to hold medications that may interfere with autonomic function at least 48 hours prior to autonomic function testing.
Autonomic reflex screen
Postganglionic sympathetic sudomotor function was assessed using a quantitative sudomotor axon reflex test (QSART) utilizing acetylcholine iontophoresis at 4 standardized sites [11]. Cardiovagal function was assessed using heart rate responses to deep breathing and the Valsalva maneuver while cardiovascular adrenergic function was evaluated by assessing BP responses to the Valsalva maneuver and passive head-up tilt [9]. In order to quantify and compare sudomotor, cardiovagal, and cardiovascular adrenergic function independent of age and sex, a Composite Autonomic Severity Score (CASS) was derived. The CASS score is a validated instrument to quantify the overall severity and distribution of autonomic failure utilizing findings on the ARS yielding a score of 0–3 points for sudomotor, 0–3 points for cardiovagal, and 0–4 points for cardiovascular adrenergic impairment, resulting in a total score ranging from 0 (no impairment) to 10 (severe autonomic failure) [12]. Variables were extracted from autonomic testing at the time closest to the original diagnosis of iRBD (within 6 months in all cases by inclusion critiera). Chart review was performed to extract Total CASS and CASS subscores derived by a single investigator blinded to phenoconversion status (W.S.).
Thermoregulatory sweat test
The TST provides a quantitative evaluation of thermoregulatory sweat function. It was performed under standardized conditions in a cabinet with a hot and humid environment using indicator powder that changes color when exposed to sweat which allows quantification of percent anhidrosis of the anterior body [10].
Statistical Analysis
Clinical, demographic, and ARS data are presented as means, standard-deviations, and frequencies. Quantitative variables were analyzed using Wilcoxon rank-sum tests while chi-square tests were used to analyze categorical variables between groups with JMP statistical software (JMP, Version 13. SAS Institute Inc., Cary, NC). Time to phenoconversion or censor (date of last in-person follow-up or 9/1/2019) was determined using Kaplan-Meier analysis and Cox-Proportional Hazards adjusting for age and sex to evaluate association between autonomic data and rate of phenoconversion. Significance was set at an alpha <0.05.
Results
Eighteen patients (15 males) were included in this study. Average age at RBD diagnosis was 67.1 ± 6.7 years. Demographic, clinical and autonomic data for the entire cohort is shown in Table 1. Fifteen (83%) patients had total CASS scores ≥2, indicating at least mild autonomic dysfunction at the time of iRBD diagnosis. TST was abnormal in all 6 patients who had TST testing, but the findings were generally modest with four patients having findings of distal sweat loss, one patient having a multifocal pattern, and one patient having a mixed (including distal) pattern of anhidrosis. Average overall CASS score was 2.8 ± 1.8 for the cohort with average sudomotor, cardiovagal and adrenergic subscores of 1.3 ± 1.1, 0.5 ± 0.8, and 1.1 ± 1.1, respectively. Twelve (67%) patients had evidence of sudomotor dysfunction. Five patients (33%) had cardiovagal dysfunction and 61% had adrenergic dysfunction. Twelve patients (67%) phenoconverted, of which 6 developed PD and 6 DLB (4 pathologically confirmed). None of the patients developed MSA or PAF (as all patients diagnosed with PAF or MSA were diagnosed immediately following autonomic testing based on interpretation of the results). Eight (44) of patients were taking antidepressant medications at or prior to RBD diagnosis, although there was no difference in antidepressant use between converters and non-converters. Duration of follow-up was not significantly different between those remaining iRBD vs phenoconverters (8.8 ± 4.8 vs 5.4 ± 4.2 years, p = 0.2) and duration of RBD symptom onset to autonomic testing was not different between iRBD and phenoconverters (4.5 ± 2.9 vs 4.6 ± 4.2 years, p = 0.5). Patients who developed DLB had longer duration of autonomic symptoms than those who developed PD (p <0.05). Severity of autonomic dysfunction as measured by CASS score did not predict later phenoconversion, however, comparing iRBD patients who developed DLB versus PD, iRBD patients with higher total CASS scores and cardiovagal CASS subscores at diagnosis were more likely to develop DLB than PD (p<0.05). There were otherwise no significant differences between those patients who phenoconverted and those who remained with iRBD (Table 2 and Table 3). For the overall cohort, five years after iRBD diagnosis, 40% of patients had phenoconverted, which increased to 47% at 7 years and 55% at 10 years from iRBD diagnosis with 67% of patients having converted 11 year after diagnosis. Eight patients died during the follow-up period. Total CASS or CASS subscale was not associated with phenconversion status by Cox-Proportional Hazards.
Table 1:
Demographics, Clinical and Autonomic Variables of Idiopathic RBD Patients Undergoing Autonomic Testing
| Sex (M/F) | 15/3 |
| Age at RBD Symptom Onset (years) | 62.1 ± 8.3 |
| Age at RBD Diagnosis (years) | 67.1 ± 6.7 |
| Age at 1st Autonomic Symptoms (years) | 65.3 ± 5.6 |
| Age at Autonomic Testing | 67.3 ± 6.6 |
| Duration RBD Symptoms to Dx (years) | 4.6 ± 3.7 |
| Duration Autonomic Symptoms to testing (years) | 2.2 ± 2.6 |
| # Phenoconverters | 12 (6 PD, 6 DLB) |
| Duration Autonomic Testing to Phenoconversion Dx (years) | 5.4 ± 4.2 |
| Total CASS Score(mean and SD) | 2.8 ± 1.8 |
| Sudomotor CASS Score (mean and SD) | 1.3 ± 1.1 |
| Cardiovagal CASS Score(mean and SD) | 0.5 ± 0.8 |
| Adrenergic CASS Score (mean and SD) | 1.1 ± 1.1 |
| % Anhidrosis* | 11.0 ± 11.4 |
n=6 patients
CASS=Composite autonomic severity score; RBD=REM sleep behavior disorder, PD=Parkinson’s disease; DLB=Dementia with Lewy bodies
Table 2:
Comparison of Demographic and Autonomic Variables Between Phenoconverters and Non-Phenoconverters
| iRBD | Phenoconverters | p <0.05 | |
|---|---|---|---|
| Sex (M/F) | 4/2 | 11/1 | |
| Age at RBD Symptom Onset (yrs) | 57.7 + 8.5 | 64.5 + 7.6 | |
| Age at RBD Diagnosis (yrs) | 62.7 + 8.6 | 69.3 + 4.3 | |
| Duration RBD Symptoms to RBD Dx (yrs) | 4.5 + 2.9 | 4.6 + 4.2 | |
| Duration RBD Symptoms to Last Follow up or Phenoconversion (yrs) | 13.7 + 3.5 | 9.7 + 6.1 | |
| Age at 1st Autonomic Symptoms (yrs) | 62.8 + 4.5 | 66.5 + 5.9 | |
| Age at Autonomic Testing (yrs) | 62.7 + 8.6 | 68.8 + 3.1 | |
| Duration Autonomic Symptoms to ARS (yrs) | 2.1 + 1.7 | 2.2 + 3.0 | |
| Duration Autonomic Testing to Phenoconversion Dx (years) | NA | 5.4 + 4.2 | |
| Total CASS Score | 2.8 + 1.7 | 2.8 + 1.9 | |
| Sudomotor CASS Score | 1.8 + 1.2 | 1.0 + 1.0 | |
| Cardiovagal CASS Score | 0.3 + 0.8 | 0.7 + 0.9 | |
| Adrenergic CASS Score | 0.7 + 0.8 | 1.3 + 1.2 | |
| % Anhidrosis* | 5.5 + 3.5 | 13,7 + 13.5 |
n=6 patients
CASS=Composite autonomic severity score; RBD=REM sleep behavior disorder, PD=Parkinson’s disease; DLB=Dementia with Lewy bodies, Dx=diagnosis, ARS=autonomic reflex screen
Table 3:
Comparison of Demographic and Autonomic Variables Between Phenoconversion Subgroups and Non-Phenoconverters
| iRBDA | DLBB | PDC | p <0.05 | |
|---|---|---|---|---|
| Sex (M/F) | 4/2 | 6/0 | 5/1 | |
| Age at RBD Symptom Onset (yrs) | 57.7 + 8.5 | 60.2 + 7.8 | 68.0 + 5.8 | |
| Age at RBD Diagnosis (yrs) | 62.7 + 8.6 | 68.3 + 3.4 | 70.3 + 5.1 | |
| Duration RBD Symptoms to RBD Dx (yrs) | 4.5 + 2.9 | 7.4 + 4.6 | 2.3 + 2.0 | |
| Age at 1st Autonomic Symptoms (yrs) | 62.8 + 4.5 | 65.3 + 6.9 | 68.3 + 4.5 | |
| Age at Autonomic Testing (yrs) | 62.7 + 8.6 | 68.8 + 3.1 | 70.3 + 5.1 | |
| Duration Autonomic Symptoms to ARS (yrs) | 2.1 + 1.7 | 3.8 + 3.4 | 0.4 + 0.5 | B>C |
| Duration Autonomic Testing to Phenoconversion Dx (years) | NA | 5.3 + 3.4 | 5.5 + 5.2 | |
| Total CASS Score | 2.8 + 1.7 | 4.0 + 2.0 | 1.7 + 1.0 | B>C |
| Sudomotor CASS Score | 1.8 + 1.2 | 1.0 + 1.3 | 1.0 + 0.8 | |
| Cardiovagal CASS Score | 0.3 + 0.8 | 1.2 + 0.8 | 0 + 0 | B>C |
| Adrenergic CASS Score | 0.7 + 0.8 | 2.0 + 1.3 | 0.7 + 0.8 | |
| % Anhidrosis* | 5.5 + 3.5 | 7.0 + 0 | 15.9 + 15.6 |
n=1 DLB, 2 iRBD, 3 PD patients
CASS=Composite autonomic severity score; RBD=REM sleep behavior disorder, PD=Parkinson’s disease; DLB=Dementia with Lewy bodies, Dx=diagnosis, ARS=autonomic reflex screen
Discussion
Our study confirms a high frequency of autonomic dysfunction (over 80%) in patients with idiopathic RBD at the time of diagnosis. Our findings suggest generally modest impairment, but impairment across sudomotor, cardiovagal, and cardiovascular adrenergic domains of a degree consistent with previous reports and consistent with the concept of RBD as prodromal synucleinopathy [1, 3, 8, 17]. Importantly, more severe autonomic dysfunction at iRBD diagnosis, driven primarily by cardiovagal and to a lesser degree adrenergic impairment, seems to differentiate patients who ultimately develop DLB rather than PD. This is consistent with previous studies comparing CASS scores between DLB and PD patients showing more severe autonomic failure in DLB compared to PD, and another study using iodine-123-labeled metaiodobenzylguanidine (123I-MIBG) myocardial scintigraphy showing significantly greater cardiac sympathetic denervation in patients with early stage DLB compared to PD [21, 22]. Mean CASS scores differed between patients who developed DLB and those who developed PD, but were similar in patients with persisting iRBD compared to phenoconverters overall. It is possible that patients who remained iRBD in our study will ultimately phenoconvert to a motor synucleinopathy, but this assumption requires continued surveillance.
We found that patients who developed DLB compared with PD had longer duration of autonomic symptoms. It is possible that the longer duration of autonomic symptoms may explain more severe autonomic dysfunction, alternatively, it may be that patients destined to develop DLB have earlier onset of autonomic symptoms than those who develop PD. Since there was no difference in the duration of follow-up between persisting iRBD and phenoconverters in our study, more severe autonomic failure does not appear to be associated with increased risk of near-term phenoconversion. However, we considered patients with severe autonomic dysfunction leading to a diagnosis of MSA or PAF to be “phenoconverted,” so our cohort may have been biased toward a milder degree of autonomic dysfunction. There were not significant differences in sudomotor function between converters and non-converters. Only 6 patients underwent TST in our cohort, so the predictive value of TST could not be assessed reliably, but all were abnormal with a predominant pattern of limited, distal sweat loss, similar to what has been reported in DLB and PD patients.[22] One patient who converted to PD had a mixed, patchy, and more widespread pattern of anhidrosis. As we required patients to remain idiopathic RBD for at least 6 months following autonomic testing, all MSA patients included in the initial search met our exclusion criteria as they were diagnosed immediately following autonomic testing, classifying them as symptomatic and not idiopathic RBD (n=95). It may be that as is the case for conversion from PAF to MSA, the time to conversion from developing iRBD to MSA tends to be short and may be the reason for the apparent lack of MSA conversion in this study [19]. The lack of patients converting from iRBD to PAF may relate to the relative rarity of PAF and the relatively small number of patients studied or due to the fact that the presence of PAF based on autonomic testing results lead to exclusion (as we consider PAF to be evidence of a symptomatic synucleinopathy).
We found that approximately 40% of iRBD patients developed parkinsonism or cognitive impairment within 5 years after RBD diagnosis, consistent with previous studies [15]. We did not find that total CASS scores or CASS subscores were associated with phenoconversion. This may be related to small sample size, the heterogeneity of our patient group, and the retrospective nature of our study.
Our study has several limitations as a retrospective study subject to referral and sampling biases. Additionally, our cohort may overestimate the prevalence and severity of autonomic dysfunction in RBD, given that most patients were referred for autonomic function testing for presumed autonomic symptoms. Three patients remaining iRBD were lost to follow-up 10 years prior to medical record review for this study, so it is possible that these patients had also phenoconverted during the time they were lost to follow-up. Only 4 patients who phenoconverted had pathologic confirmation of their diagnosis (all had DLB), possibly leading to patient misclassification. We did not exclude patients who had a history of diabetes, which could have influenced our results although only two patients had diabetes so it is unlikely this had a significant impact. Further, given the retrospective nature of our study, evaluation of motor and/or cognitive impairment were not uniformly assessed at each visit (ie. UPDRS, Short Test of Mental Status) potentially limiting earlier identification of evolving phenoconversion. Finally, we did not detect MSA or PAF phenoconverters in this study presumably for reasons further discussed above, so the predictive value of autonomic testing for those entities at the stage of idiopathic RBD remains unknown. Future prospective autonomic function studies of idiopathic RBD patients are planned to better answer this question.
In conclusion, we found a high frequency of overall limited autonomic dysfunction in patients with idiopathic RBD, but did not find the degree of autonomic dysfunction at that stage to predict rate of phenoconversion to a neurodegenerative synucleinopathy. However, iRBD patients who eventually differentiated toward a prodromal DLB trajectory had significantly more cardiovagal dysfunction and trended toward greater adrenergic dysfunction when compared with those who developed PD. Future prospective studies are needed to confirm and further elaborate on these findings, but our data suggests that autonomic testing at time of iRBD diagnosis deserves further attention in larger studies to confirm its ability discriminate iRBD patients predestined to develop DLB from those who develop PD early in the disease course.
Funding Sources:
This work was supported in part by National Institutes of Health (R01 NS092625).
Footnotes
Financial Disclosure/Conflict of Interest:
The authors report no relevant disclosures to the work presented in the manuscript.
References
- 1.Low Al-Qassabi A, Pelletier A, Fereshtehnejad SM, Postuma RB (2018) Autonomic Sweat Responses in REM Sleep Behavior Disorder and Parkinsonism. J Parkinsons Dis 8:463–468 [DOI] [PubMed] [Google Scholar]
- 2.Dauvilliers Y, Schenck CH, Postuma RB, Iranzo A, Luppi PH, Plazzi G, Montplaisir J, Boeve B (2018) REM sleep behaviour disorder. Nat Rev Dis Primers 4:19. [DOI] [PubMed] [Google Scholar]
- 3.Doppler K, Jentschke HM, Schulmeyer L, Vadasz D, Janzen A, Luster M, Hoffken H, Mayer G, Brumberg J, Booij J, Musacchio T, Klebe S, Sittig-Wiegand E, Volkmann J, Sommer C, Oertel WH (2017) Dermal phospho-alpha-synuclein deposits confirm REM sleep behaviour disorder as prodromal Parkinson’s disease. Acta Neuropathol 133:535–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferini-Strambi L, Oertel W, Dauvilliers Y, Postuma RB, Marelli S, Iranzo A, Arnulf I, Hogl B, Manni R, Miyamoto T, Fantini ML, Puligheddu M, Jennum P, Sonka K, Santamaria J, Zucconi M, Rancoita PM, Leu-Semenescu S, Frauscher B, Terzaghi M, Miyamoto M, Unger M, Stiasny-Kolster K, Desautels A, Wolfson C, Pelletier A, Montplaisir J (2014) Autonomic symptoms in idiopathic REM behavior disorder: a multicentre case-control study. J Neurol 261:1112–1118 [DOI] [PubMed] [Google Scholar]
- 5.Frauscher B, Nomura T, Duerr S, Ehrmann L, Gschliesser V, Wenning GK, Wolf E, Inoue Y, Hogl B, Poewe W (2012) Investigation of autonomic function in idiopathic REM sleep behavior disorder. J Neurol 259:1056–1061 [DOI] [PubMed] [Google Scholar]
- 6.Gilman S, Wenning GK, Low PA, Brooks DJ, Mathias CJ, Trojanowski JQ, Wood NW, Colosimo C, Durr A, Fowler CJ, Kaufmann H, Klockgether T, Lees A, Poewe W, Quinn N, Revesz T, Robertson D, Sandroni P, Seppi K, Vidailhet M (2008) Second consensus statement on the diagnosis of multiple system atrophy. Neurology 71:670–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hughes AJ, Daniel SE, Kilford L, Lees AJ (1992) Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. Mov Disord 30:1843–1848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee H, Cho YW, Kim HA (2015) The Severity and Pattern of Autonomic Dysfunction in Idiopathic Rapid Eye Movement Sleep Behavior Disorder. J Clin Neurophysiol 10:14–27 [DOI] [PubMed] [Google Scholar]
- 9.Low PA, Benarroch EE, Sletten DM (1993) Autonomic nervous system function. J Clin Neurophysiol 10:14–27 [DOI] [PubMed] [Google Scholar]
- 10.Low PA, Benarroch EE, Sletten DM (2008) Laboratory evaluation of autonomic failure. Lippincott Williams & Wilkins, Philadelphia [Google Scholar]
- 11.Low PA, Caskey PE, Tuck RR, Fealey RD, Dyck PJ (1983) Quantitative sudomotor axon reflex test in normal and neuropathic subjects. Ann Neurol 14:573–580 [DOI] [PubMed] [Google Scholar]
- 12.Low PA, Denq JC, Opfer-Gehrking TL, Dyck PJ, O’Brien PC, Slezak JM (1997) Effect of age and gender on sudomotor and cardiovagal function and blood pressure response to tilt in normal subjects. Muscle Nerve 20:1561–1568 [DOI] [PubMed] [Google Scholar]
- 13.McCarter SJ, Sandness DJ, McCarter AR, Feemster JC, Teigen LN, Timm PC, Boeve BF, Silber MH, St Louis EK (2019) REM sleep muscle activity in idiopathic REM sleep behavior disorder predicts phenoconversion. Neurology 93:e1171–e1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McKeith IG, Dickson DW, Lowe J, Emre M, O’Brien JT, Feldman H, Cummings J, Duda JE, Lippa C, Perry EK, Aarsland D, Arai H, Ballard CG, Boeve B, Burn DJ, Costa D, Del Ser T, Dubois B, Galasko D, Gauthier S, Goetz CG, Gomez-Tortosa E, Halliday G, Hansen LA, Hardy J, Iwatsubo T, Kalaria RN, Kaufer D, Kenny RA, Korczyn A, Kosaka K, Lee VM, Lees A, Litvan I, Londos E, Lopez OL, Minoshima S, Mizuno Y, Molina JA, Mukaetova-Ladinska EB, Pasquier F, Perry RH, Schulz JB, Trojanowski JQ, Yamada M, Consortium on DLB (2005) Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology 65:1863–1872 [DOI] [PubMed] [Google Scholar]
- 15.Postuma RB, Gagnon JF, Bertrand JA, Genier Marchand D, Montplaisir JY (2015) Parkinson risk in idiopathic REM sleep behavior disorder: preparing for neuroprotective trials. Neurology 84:1104–1113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Postuma RB, Iranzo A, Hu M, Hogl B, Boeve BF, Manni R, Oertel WH, Arnulf I, FeriniStrambi L, Puligheddu M, Antelmi E, Cochen De Cock V, Arnaldi D, Mollenhauer B, Videnovic A, Sonka K, Jung KY, Kunz D, Dauvilliers Y, Provini F, Lewis SJ, Buskova J, Pavlova M, Heidbreder A, Montplaisir JY, Santamaria J, Barber TR, Stefani A, St Louis EK, Terzaghi M, Janzen A, Leu-Semenescu S, Plazzi G, Nobili F, Sixel-Doering F, Dusek P, Bes F, Cortelli P, Ehgoetz Martens K, Gagnon JF, Gaig C, Zucconi M, Trenkwalder C, Gan-Or Z, Lo C, Rolinski M, Mahlknecht P, Holzknecht E, Boeve AR, Teigen LN, Toscano G, Mayer G, Morbelli S, Dawson B, Pelletier A (2019) Risk and predictors of dementia and parkinsonism in idiopathic REM sleep behaviour disorder: a multicentre study. Brain 142:744–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rocchi C, Placidi F, Liguori C, Del Bianco C, Lauretti B, Diomedi M, Pisani A, Mercuri NB, Izzi F (2018) Daytime autonomic activity in idiopathic rapid eye movement sleep behavior disorder: a preliminary study. Sleep Med 52:163–167 [DOI] [PubMed] [Google Scholar]
- 18.Schatz IJ, Bannister R, Freeman RL, Goetz CG, Jankovic J, Kaufmann HC, Koller WC, Low PA, Mathias CJ, Polinsky RJ, Quinn NP, Robertson D, Streeten DHP (1996) Consensus statement on the definition of orthostatic hypotension, pure autonomic failure, and multiple system atrophy. Neurology 46:1470–14708628505 [Google Scholar]
- 19.Singer W, Berini SE, Sandroni P, Fealey RD, Coon EA, Suarez MD, Benarroch EE, Low PA (2017) Pure autonomic failure: Predictors of conversion to clinical CNS involvement. Neurology 88:1129–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.St Louis EK, Boeve AR, Boeve BF (2017) REM Sleep Behavior Disorder in Parkinson’s Disease and Other Synucleinopathies. Mov Disord 32:645–658 [DOI] [PubMed] [Google Scholar]
- 21.Suzuki M, Kurita A, Hashimoto M, Fukumitsu N, Abo M, Ito Y, Urashima M, Inoue K (2006) Impaired myocardial 123I-metaiodobenzylguanidine uptake in Lewy body disease: comparison between dementia with Lewy bodies and Parkinson’s disease. J Neurol Sci 240:15–19 [DOI] [PubMed] [Google Scholar]
- 22.Thaisetthawatkul P, Boeve BF, Benarroch EE, Sandroni P, Ferman TJ, Petersen R, Low PA (2004) Autonomic dysfunction in dementia with Lewy bodies. Neurology 62:1804–1809 [DOI] [PubMed] [Google Scholar]


