Abstract
The novel coronavirus (Sars-CoV-2) pandemic has spread rapidly, from December to the end of March, to 185 countries, and there have been over 3,000,000 cases identified and over 200,000 deaths. For a proportion of hospitalized patients, death can occur within a few days, mainly for adult respiratory distress syndrome or multi-organ dysfunction syndrome. In these patients, clinical signs and symptoms, as well as laboratory abnormalities, suggest a cytokine storm syndrome in response to the viral infection. No current targeted treatment is yet available for COVID-19, an unknown disease up to 2 months ago, which challenges doctors and researchers to find new drugs or reallocate other treatments for these patients. Since the beginning of the COVID-19 outbreak, a growing body of information on diagnostic and therapeutic strategies has emerged, mainly based on preliminary experience on retrospective studies or small case series. Antivirals, antimalarials, corticosteroids, biotechnological and small molecules, convalescent plasma and anticoagulants are among the drugs proposed for the treatment or in tested for COVID-19. Given the complexity of this new condition, a multidisciplinary management seems to be the best approach. Sharing and integrating knowledge between specialists, to evaluate the correct timing and setting of every treatment, could greatly benefit our patients. We reviewed the literature, combining it with our experiences and our specialist knowledge, to propose a management algorithm, correlating the clinical features with laboratory and imaging findings to establish the right timing for each treatment.
|
Key Points • Critically ill COVID-19 patients show signs of cytokine storm syndrome. • No current targeted therapy is available, but a lot of drugs are in tested. • A multidisciplinary approach is crucial to manage COVID-19. • Choosing the correct timing of treatment is of pivotal importance to avoid the most severe complications. |
Keywords: Algorithm, COVID-19, Management, Sars-CoV-2
Introduction
Since December 2019, the novel coronavirus (Sars-CoV-2) pandemic spread rapidly, from the Hubei province in China to 185 countries causing over 3,000,000 cases. More than 200,000 deaths have been attributed to the coronavirus disease (named COVID-19), and these numbers are growing steadily [1].
As an emerging acute respiratory infectious disease, COVID-19 primarily spreads through the airway’s tract, by droplets, respiratory secretions, and direct contact. Spread by aerosol (airborne transmission) is suspected to be another important route of transmission but unestablished now [2]. Some patients with SARS-CoV-2 infection have viral RNA or live infectious virus present in faeces, which suggests that another possible route might be faecal-oral transmission [3]. Based on current epidemiological data, the incubation period ranges from 1 to 14 days, with an estimated median incubation period of 5.1 days, and the transmission can also occur during the pre-symptomatic stage. Moreover, also asymptomatic cases, which represent a considerable percentage of the infections, are likely to contribute to virus circulation [4]. Elderly patients, especially with other comorbidities, such as hypertension, cardiovascular diseases and diabetes, and subjects with primary or secondary immunodeficiencies have the highest mortality rate [5]. Although children tend to experience only mild symptoms, previously healthy young adults have also succumbed to COVID-19 [6, 7]. Although most patients have mild symptoms and good prognosis after infection, some patients develop severe forms and die within few days, mainly for adult respiratory distress syndrome (ARDS) and/or for multi-organ dysfunction syndrome (MODS). In these patients, clinical signs and symptoms, as well as laboratory abnormalities, suggest a cytokine storm syndrome (CSS) triggered by the viral infection [8].
Since the beginning of the COVID-19 outbreak, a growing body of information on diagnostic and therapeutic strategies has emerged, mainly based on preliminary experience on retrospective studies or small case series.
Together with supportive intensive care and antivirals, the use of immunomodulatory agents and/or convalescent plasma transfusion has been proposed, and some of them are currently under investigation by clinical trials.
In this context, understanding all the different phases of the disease is crucial, integrating diagnostic and therapeutic armamentarium to develop appropriate strategies with a multidisciplinary approach.
This manuscript aims at reviewing the current available literature on the main diagnostic and pharmacologic approaches, in order to develop a management and therapeutic algorithm, which provides a practical guide to healthcare workers involved in the management of COVID-19 patients.
Methods
A multidisciplinary group comprising three rheumatologists, one clinical immunologist, two experts in infectious diseases and 4 anaesthesiologists was set up, based on their expertise in (a) the management of immunosuppressants/immunomodulatory agents, (b) the management of patients infected by COVID-19 in non-intensive care units and (c) management of patients infected by COVID-19 in intensive care units.
This manuscript was based on the results of a comprehensive search in PubMed matching the key search terms “Sars-CoV-2” and “COVID-19”. We searched PubMed for English-language studies published from January 2020 to April 2, 2020. We also manually searched the references of selected articles. Full texts and abstracts of published and pre-published articles were reviewed by 2 independent components of the multidisciplinary group (GF and FPM). The resulting manuscript was distributed by email to experts, who approved and critically revised the final version of this document. Finally, a management algorithm was proposed, based on the review of the literature and the experience of the participants of this multidisciplinary group.
Clinical, laboratory and imaging findings
Common clinical manifestations include fever, cough, fatigue, sputum production, shortness of breath, sore throat, headache, arthro-myalgia, anosmia, dysgeusia, pleuritic pain, conjunctivitis, diarrhoea and vomiting [9, 10]. Most patients, especially in the early asymptomatic or pauci-symptomatic phase, have a normal routine blood test with only mild lymphocytopenia. As the disease progresses, laboratory tests may show a reduction in the lymphocyte count; an in increase ferritin, D-dimer and lactate dehydrogenase (LDH); and coagulation abnormalities (mainly prolonged PT and aPTT). These values tend to increase as the disease progresses with an increase of the C-reactive protein (CRP) and interleukin (IL)-6. Not infrequently a progressive increase in transaminases, triglycerides, NT-proBNP and troponin, creatinine and blood urea has been observed. Platelets and neutrophils may also decrease, while procalcitonin is generally negative in COVID-19 patients [11–13].
Blood gas analyses vary from normal in the early and mild phase to mild hypoxia with or without hypocapnia in mild to moderate stage, while severe hypoxia with hypercapnia predominates in the late stage. Thoracic imaging is of great value in the diagnosis and monitoring of the therapeutic efficacy of C0VID-19. High-resolution CT (HRCT) is the gold-standard imaging modality. In HRCT scans, COVID-19 at the early stage often presents with multifocal patchy shadows or ground-glass opacities located in the lung periphery, subpleural area and both lower lobes. Later, interlobular septal thickening and intralobular interstitial thickening (“crazy paving”) are being observed in some ground-glass opacities. A small number of cases may show solitary, local lesions or nodular/patchy lesion distributed consistent with bronchi with peripheral ground-glass opacities changes. Progression of the disease mostly occurs within 7–10 days, with enlarged and increased density of the lesions and consolidated lesions with air bronchogram sign. Critical cases may show further expanded consolidation, with the whole lung density showing increased opacity (“white lung”) [9–12]. Although less sensitive than HRCT, chest radiography is typically the first-line imaging modality used for patients with suspected COVID-19, also for ease of decontamination (especially with portable radiography units). However, chest radiograph may be normal in early or mild disease. The most frequent findings are airspace opacities, described as consolidation or ground-glass opacity. The distribution is most often bilateral, peripheral and lower zone predominant [13]. Lung ultrasound (LUS) may be useful in diagnosis and follow-up of COVID-19 pulmonary involvement. The following patterns have been described in COVID-19 patients: (i) localized or diffused B-lines (thickened subpleural interlobular septa), (ii) irregular and thickened pleural line with scattered discontinuities, (iii) subpleural consolidations and (iv) alveolar consolidations (tissue-like with air bronchograms) [14]. LUS is widely available; it allows rapid examination without moving the patient, thus reducing the risk of infection and patient destabilization, especially in critically ill patients. Although its specificity is not yet known, LUS can also be used as the first imaging step, indicating patients who need HRCT, and as a first screening measure for the asymptomatic or pauci-symptomatic patients (suspected or confirmed), who might present signs of early lung involvement [15].
Management
Antiviral agents
At present, several drugs approved for other indications as well as a few new compounds are under investigation in randomized clinical trials (RCT) across the globe (https://clinicaltrials.gov/), but none has definitely proven efficacy for treatment of COVID-19. Retrospective data from SARS epidemic suggested that an early antiviral treatment with lopinavir/ritonavir (LPV/RTV) can potentially reduce the incidence of severe and critical cases [16]. Recently, a clinical trial in hospitalized adults with severe COVID-19 in China did not show reduction in time to clinical improvement and mortality with LPV/RTV compared with standard of care; however, some evidence suggest that LPV/RTV initiation within 12 days after symptom onset is associated with shorter time to clinical improvement might be assumed [17]. Additional study is needed to evaluate possible clinical benefits of early use of LPV/RPV in COVID-19. Remdesivir, a broad-spectrum investigational antiviral, previously tested for SARS, MERS, and Ebola, has in vitro activity against SARS-CoV-2 [18]. The drug has been used in hundreds of COVID-19 patients in the USA and Europe under compassionate use, and anecdotal evidence of benefit have been reported, but no hard data [19]. Some RCT are ongoing and will provide data on the efficacy of remdesivir in the treatment of COVID-19. Currently, the manufacturer (Gilead) is transitioning from individual compassionate use requests to an expanded access program for emergency access to the drug for severely ill patients with confirmed COVID-19. Clinical trials on other antivirals like arbidol, oseltamivir, favipiravir, interferon beta-1A, darunavir and cobicistat are ongoing, but data are not yet available (NCT04255017, NCT04261270, NCT04310228, NCT04315948, NCT04252274).
Antimalarial and other agents with potential antiviral and immunomodulating activity
Antiviral activity of antimalarial drug chloroquine (CQ) and hydroxychloroquine (HCQ) has been recently tested [18, 20–27]. Studies show that chloroquine can inhibit pH-dependent steps of the viral replication, with a potent effect on SARS-CoV-2 infection and spread [24]. Moreover, it works as a novel class of autophagy inhibitor [25], which may interfere with viral infection and replication.
Chloroquine has been used at an oral dose of 500 mg twice daily, in several clinical trials in China during this outbreak: while peer reviewed data from the trials are currently unavailable, it was announced that promising early results have been demonstrated [26]. As for HCQ, preliminary data from a randomized clinical trial revealed that after 5 days of treatment (200 mg/bid), the symptoms of patients with COVID-19 were significantly relieved, with a shorten time of recovery for cough and fever and an enhanced improvement from pneumonia [27]. Gautret et al. recently published their preliminary data on the impact of oral hydroxychloroquine 200 mg every 8 h on viral clearance in patients with COVID-19 [28]. Although the study included only a small non-randomized cohort, promising results were obtained and in combination with azithromycin seems to be enhanced.
The antiviral activity of the antimalarials and the potential synergy with the other drugs would indicate their use mainly in the early or mild phase of the disease, starting from the clinical onset or the early radiological signs (LUS or HRCT). Furthermore, chloroquine and hydroxychloroquine have well-known immunomodulatory effects, suppressing the production/release of TNF-α and IL-6, thus modulating the host hyperimmune response. Optimal dosage and duration of treatment remain unknown and still under investigation.
Finally, ivermectin, a broad spectrum anti-parasitic agent, demonstrated antiviral action against the SARS-CoV-2 clinical isolate in vitro, but further investigation is warranted to understand its possible benefits in humans [29].
Convalescent plasma
A significant reduction of viral load and mortality has been shown in studies using convalescent plasma for the treatment of severe acute viral respiratory infections, like SARS, MERS, Ebola, H1N1 and H5N1 [30–34] with a significantly better outcome obtained with earlier transfusion [35]. High titre–specific antibodies should be able to bind to SARS-CoV-2 and neutralize the viral particles, blocking the viral access of uninfected cells, and activate potent effector mechanisms [36]. Recent data show that the administration of convalescent plasma containing neutralizing antibody is followed by improvement in the patients’ clinical status [37]. With the growing number of convalescents, it could become a treatment of choice in the moderate phase up to rescue therapy.
Anticoagulants
Disseminated intravascular coagulation (DIC) is frequently reported also in the mild and early stages of the disease [38], and it is strongly associated with a significantly higher mortality (71.4% of non-survivors—0.6% of survivors) [39]. Although the dysregulation of the haemostatic system is well documented in sepsis, SARS-CoV-2 is probably more prone to induce DIC, also thanks to the hyperimmune host response [40]. According to our experience, heparin is recommended at the initial dose of 50 UI/Kg or 25 UI/Kg in patients with bleeding or platelet count < 50xl 09/L with aPPT being 40–60 s as a target of anticoagulation maintenance dosage.
Immunomodulatory drugs, corticosteroids and rescue therapies
A subgroup of COVID-19 patients may develop secondary hemophagocytic lymphohistiocytosis (sHLH). This is a hyper-inflammatory syndrome characterized by a fulminant cytokine storm/macrophage activation syndrome (CSS/MAS), which rapidly led to multi-organ failure [8, 41]. It is well-known that sHLH is frequently triggered by viral infection [42, 43]. Unfortunately, in these conditions, the therapeutic window is narrow because the irreversible damage is premature and permanent, and the mortality for the most severe cases reaches up to 60% of patients [44]. Clinical features of this syndrome are unremitting fever, hyperferritinemia, cytopenia and elevation of inflammatory markers like C-reactive protein and IL-6 [8, 41]. Based on this notion, treatments with biotechnological immunomodulators or small molecules have been proposed since the beginning of the COVID-19 outbreak.
Immunomodulatory drugs
Tocilizumab, a recombinant humanized anti-human IL-6 receptor monoclonal antibody, is firstly approved for the treatment of rheumatoid arthritis and giant cell arteritis and more recently for the treatment of the CSS secondary to chimeric antigen receptors (CAR)-T [45]. A recent retrospective study on 21 COVID-19 patients in antiviral treatment for a week with persistent fever and worsening of CT images and hypoxia suggested a potential therapeutic role of tocilizumab. Indeed, after treatment, in addition to the improvement of body temperature, the respiratory function, imaging and lymphopenia improved in most of the patients with normalization of inflammatory markers without significant adverse event [46]. Based on this observation, a multicentre, single-arm, open-label, phase 2 study (NCT04317092) is currently ongoing. Sarilumab, a human IgG1 monoclonal antibody that binds specifically to both soluble and membrane-bound IL-6Rs, and siltuximab, an anti-IL-6 chimeric monoclonal antibody, are currently being tested in COVID-19 patients (NCT04327388, NCT04324073, NCT04329650, NCT04322188, NCT04330638). Anakinra, a recombinant human IL-1 receptor antagonist, currently used in several autoinflammatory diseases, has been also tested in conditions associated with a hyper-inflammation, like sepsis and sHLH/MAS [47, 48]. Anakinra can be administered subcutaneously or intravenously, in a wide range of dosage and with an excellent safety profile and is currently being in tested (NCT04324021). Janus kinases inhibitors baricitinib and ruxolitinib are treatments currently approved for RA and myelofibrosis/polycythaemia vera, respectively. These small molecules were found capable of reducing or interrupting the passage of the virus into target cells and to inhibit the JAK1- and JAK2-mediated cytokine release [49–51]. They are currently being tested (NCT04320277, NCT04321993), but no data are available.
At the best of our knowledge, fever, persistent and progressive lymphopenia and elevation of serum CRP and IL-6 associated with hypoxia and ground glass establish the correct timing of treatment, mainly if also associated with haemostasis alteration. Platelet to lymphocyte ratio may also be a novel indicator of CSS in COVID-19 [52].
Corticosteroids and rescue therapies
Although the use of corticosteroids in Sars-COV-2 viral pneumonia is not clearly recommended [53], in case of signs of an exaggerated immune response or in patients with symptoms of myocardial involvement, the use of moderate dosage of corticosteroids for a short time (0.5–1 mg/kg/day of prednisone equivalent for 5–7 days) is suggested [54, 55].
Plasma exchange, intravenous immunoglobulin (IVIG), etoposide and emapalumab are currently used in refractory or relapsing MAS/HLH and can be considered as a rescue therapy when other therapies have failed [56–58]. An interventional clinical trial evaluating the safety and efficacy of anakinra and emapalumab in COVID-19 has been recently registered (NCT04324021).
Conclusions
The high number of patients, who contracted the virus and developed the disease in a relative short period, has compromised the health system worldwide that responded to the pandemic by reorganizing hospitals and engaging doctors from different specializations to face this health emergency. No current targeted treatment is yet available for COVID-19, an unknown disease until 2 months ago, which challenges doctors and researchers to find new drugs or reallocate other treatments for these patients.
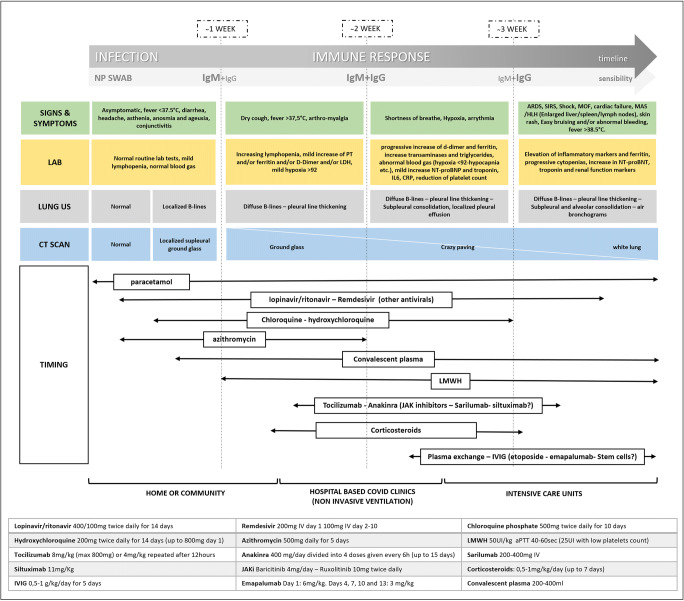
The lesson we learned is that there are distinct phases of COVID-related disease that are dominated by infection, invasion and viral replication first and then by the host immune response, up to hyper-inflammation (CSS). The overload of work that healthcare professionals must face with, often in risky conditions as seen in China, Europe and now also overseas, is combined with the lack of standardized protocols or algorithms for the management and treatment of these patients. It now seems evident that a part of the COVID-19 patients suddenly worsens, frequently a week after symptom onset, especially in patients with an exaggerated immune response is triggered. The experience, both from literature data and clinical practice, confirms as we could expect, the sooner the treatment, the better the outcomes. Given the complexity of this new condition, a multidisciplinary management seems to be the best approach. Sharing and integrating knowledge between specialists, to evaluate the correct timing and setting of every treatment, could greatly benefit our patients. Based on this, we reviewed the literature, combining it with our experiences and our specialist knowledge, to establish a management algorithm. We tried to correlate the clinical features with laboratory and imaging findings to establish the right timing for each treatment. We harmonized all this information in a visual algorithm (Fig. 1), to make it easy to read and update based on new literature results.
Fig. 1.
Following the timeline of the disease and correlating with the clinical, laboratory and imaging data, supported also by the first anatomopathological reports, we can distinguish two main phases: (a) the first in which the viral shedding and replication with direct cell damage predominate and (b) the second in which an exaggerated host immune response with cytokine storm triggered the inflammatory cascade. Based on this concept, we correlated clinical, laboratory and imaging data in order to use the more appropriate drug. By further dividing the patients according to the clinical picture and correlating with the diagnostic tests, the most severe forms of the disease are predominated by the host hyperimmune response and the consequent damage. However, this response begins with the earlier stages, and therefore, the timing of the treatments is critical to optimize the outcome. Finally, the dosages of the drugs are reported
It is crucial to identify patients early, especially those with negative laboratory prognostic factors (cytopenia, high CRP and D-dimer, hyperferritinemia) and early signs of pulmonary involvement in HRCT as well as in LUS, to start treatment and monitor them closely. Since this approach is easily available also outside the hospitals, the new challenge will be to also extend it in a community-based setting.
Authors’ contribution
GF and FPM: research, selection and review of literature, group coordinators. ET, GMA, AA, PHM, AR, EG, SM and TJI: critical revision of the text and final approval. These authors contribute equally to the work.
Compliance with ethical standards
Disclosures
None.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis; published online Feb 19. 10.1016/S1473-3099(20)30120-1 [DOI] [PMC free article] [PubMed]
- 2.Han Q, Lin Q, Ni Z, You L (2020) Uncertainties about the transmission routes of 2019 novel coronavirus. Infuenza Other Respir Viruses. 10.1111/irv.12735 [DOI] [PMC free article] [PubMed]
- 3.Hindson J. COVID-19: faecal-oral transmission? Nat Rev Gastroenterol Hepatol. 2020;17:259. doi: 10.1038/s41575-020-0295-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bai Y, Yao L, Wei T et al (2020) Presumed asymptomatic carrier transmission of COVID-19 [published online ahead of print, 2020. JAMA.:e202565 [DOI] [PMC free article] [PubMed]
- 5.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J et al (2020) Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel coronavirus-infected pneumonia in Wuhan, China. JAMA [DOI] [PMC free article] [PubMed]
- 6.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS, China Medical Treatment Expert Group for Covid-19 Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lei C, Huigo L, Wei L, Jing L, Kui L, Jin S et al (2020) Analysis of clinical features of 29 patients with 2019 novel coronavirus pneumonia. Chin J Tuberc Respir Dis
- 8.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kui L, Fang YY, Deng Y, Liu W, Wang MF, Ma JP, et al. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J. 2020;133:1025–1031. doi: 10.1097/CM9.0000000000000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J', Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pan F, Ye T, Sun P, Gui S, Liang B, Li L, Zheng D, Wang J, Hesketh RL, Yang L, Zheng C (2020) Time course of lung changes on chest CT during recovery from 2019 novel coronavirus (COVID-19) pneumonia. Radiology. 10.1148/radiol.2020200370 [DOI] [PMC free article] [PubMed]
- 13.Wong HYF, Lam HYS, Fong AH, Leung ST, Chin TW, Lo CSY, Lui MM, Lee JCY, Chiu KW, Chung T, Lee EYP, Wan EYF, Hung FNI, Lam TPW, Kuo M, Ng MY (2019) Frequency and distribution of chest radiographic findings in COVID-19 positive patients. Radiology. 10.1148/radiol.2020201160
- 14.Peng Q-Y, Wang X-T, Zhang L-N (2020) Findings of lung ultrasonography of novel corona virus pneumonia during the 2019–2020 epidemic. Intensive Care Medic. 10.1007/s00134-020-05996-6 [DOI] [PMC free article] [PubMed]
- 15.Soldati G, Smargiassi A, Inchingolo R, Buonsenso D, Perrone T, Briganti DF, Perlini S, Torri E, Mariani A, Mossolani EE, Tursi F, Mento F, Demi L (2020) Is there a role for lung ultrasound during the COVID-19 pandemic? J Ultrasound Med. 10.1002/jum.15284 [DOI] [PMC free article] [PubMed]
- 16.Chan C, Lai S, Chu C, Tsui C, Tam M, Wong M et al Treatment of severe acute respiratory syndrome with lopinavir/ritonavir: a multicentre retrospective matched cohort study. Hong Kong Med J 9 [PubMed]
- 17.Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, Ruan L, Song B, Cai Y, Wei M, Li X, Xia J, Chen N, Xiang J, Yu T, Bai T, Xie X, Zhang L, Li C, Yuan Y, Chen H, Li H, Huang H, Tu S, Gong F, Liu Y, Wei Y, Dong C, Zhou F, Gu X, Xu J, Liu Z, Zhang Y, Li H, Shang L, Wang K, Li K, Zhou X, Dong X, Qu Z, Lu S, Hu X, Ruan S, Luo S, Wu J, Peng L, Cheng F, Pan L, Zou J, Jia C, Wang J, Liu X, Wang S, Wu X, Ge Q, He J, Zhan H, Qiu F, Guo L, Huang C, Jaki T, Hayden FG, Horby PW, Zhang D, Wang C. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M et al (2020) Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 10.1038/s41422-020-0282-0 [DOI] [PMC free article] [PubMed]
- 19.Kupferschmidt K, Cohen J. Race to find COVID-19 treatments accelerates. Science. 2020;367(6485):1412–1413. doi: 10.1126/science.367.6485.1412. [DOI] [PubMed] [Google Scholar]
- 20.Chih-Cheng L, Tzu-Ping S, Wen-Chien K, Hung-Jen T, Po-Ren H. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int J Antimicrob Agents. 2020;55(3):105924. doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.(2020) Multicenter collaboration group of Department of Science and Technology of Guangdong Province and Health Commission of Guangdong Province for chloroquine in the treatment of novel coronavirus pneumonia. [Expert consensus on chloroquine phosphate for the treatment of novel coronavirus pneumonia]. Zhonghua Jie He He Hu Xi Za Zhi 43:E019 [DOI] [PubMed]
- 22.Nicastri E, Petrosillo N, Bartoli TA, et al. National Institute for the Infectious Diseases “L. Spallanzani”, IRCCS. Recommendations for COVID-19 clinical management. Infect Dis Rep. 2020;12(1):8543. doi: 10.4081/idr.2020.8543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yao X, Ye F, Zhang M et al (2020) In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin Infect Dis [DOI] [PMC free article] [PubMed]
- 24.Vincent MJ, Bergeron E, Benjannet S, Erickson BR, Rollin PE, Ksiazek TG, Seidah NG, Nichol ST. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005;2:69. doi: 10.1186/1743-422X-2-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Golden EB, Cho HY, Hofman FM, Louie SG, Schonthal AH, Chen TC. Quinoline-based antimalarial drugs: a novel class of autophagy inhibitors. Neurosurg Focus. 2015;38(3):E12. doi: 10.3171/2014.12.FOCUS14748. [DOI] [PubMed] [Google Scholar]
- 26.Gao J, Tian Z, Yang X (2020) Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. 10.5582/bst.2020.01047 [DOI] [PubMed]
- 27.Chen Z Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial. MedRxiv preprint. 10.1101/2020.03.22.20040
- 28.Gautret et al. (2020) Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents (In Press 17 March 2020). 10.1016/j.ijantimicag.2020.105949 [DOI] [PMC free article] [PubMed] [Retracted]
- 29.Caly L, Druce JD, Catton MG, Jans DA, Wagstaff KM The FDA approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antivir Res. 10.1016/j.antiviral.2020.104787 [DOI] [PMC free article] [PubMed]
- 30.Kraft CS, Hewlett AL, Koepsell S, et al. Nebraska biocontainment unit and the emory serious communicable diseases unit. The use of TKM-100802 and convalescent plasma in 2 patients with Ebola virus disease in the United States. Clin Infect Dis. 2015;61(4):496–502. doi: 10.1093/cid/civ334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Griensven J, Edwards T, de Lamballerie X, et al. Ebola-Tx Consortium. Evaluation of convalescent plasma for Ebola virus disease in Guinea. N Engl J Med. 2016;374(1):33–42. doi: 10.1056/NEJMoa1511812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou B, Zhong N, Guan Y. Treatment with convalescent plasma for influenza A (H5N1) infection. N Engl J Med. 2007;357(14):1450–1451. doi: 10.1056/NEJMc070359. [DOI] [PubMed] [Google Scholar]
- 33.Hung IF, To KK, Lee CK, et al. Convalescent plasma treatment reduced mortality in patients with severe pandemic influenza A (H1N1) 2009 virus infection. Clin Infect Dis. 2011;52(4):447–456. doi: 10.1093/cid/ciq106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burnouf T, Radosevich M. Treatment of severe acute respiratory syndrome with convalescent plasma. Hong Kong Med J. 2003;9(4):309. [PubMed] [Google Scholar]
- 35.Cunningham AC, Goh HP, Koh D. Treatment of COVID-19: old tricks for new challenges. Crit Care. 2020;24(1):91. doi: 10.1186/s13054-020-2818-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen L, Xiong J, Bao L, Shi Y. Convalescent plasma as a potential therapy for COVID-19. Lancet Infect Dis. 2020;20:398–400. doi: 10.1016/s1473-3099(20)30141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shen C, Wang Z, Zhao F, Yang Y, Li J, Yuan J, Wang F, Li D, Yang M, Xing L, Wei J, Xiao H, Yang Y, Qu J, Qing L, Chen L, Xu Z, Peng L, Li Y, Zheng H, Chen F, Huang K, Jiang Y, Liu D, Zhang Z, Liu Y, Liu L. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020;323:1582. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lillicrap D. Disseminated intravascular coagulation in patients with 2019-nCoV pneumonia. J Thromb Haemost. 2020;18:786–787. doi: 10.1111/jth.14781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin L, Lu L, Cao W, Li T. Hypothesis for potential pathogenesis of SARS-CoV-2 infection-a review of immune changes in patients with viral pneumonia. Emerg Microbes Infect. 2020;9(1):727–732. doi: 10.1080/22221751.2020.1746199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ruan Q, Yang K, Wang W, Jiang L, Song J (2020; published online March 3.) Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 10.1007/s00134-020-05991-x [DOI] [PMC free article] [PubMed]
- 42.Ramos-Casals M, Brito-Zeron P, Lopez-Guillermo A, Khamashta MA, Bosch X. Adult haemophagocytic syndrome. Lancet. 2014;383:1503–1516. doi: 10.1016/S0140-6736(13)61048-X. [DOI] [PubMed] [Google Scholar]
- 43.Karakike E, Giamarellos-Bourboulis EJ. Macrophage activation-like syndrome: a distinct entity leading to early death in sepsis. Front Immunol. 2019;10:55. doi: 10.3389/fimmu.2019.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang X, Yu Y, Xu J et al (2020; Published Online February 21.) Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 10.1016/S2213-2600(20)30079-5 [DOI] [PMC free article] [PubMed]
- 45.Brudno JN, Kochenderfer JN. Recent advances in CAR T-cell toxicity: mechanisms, manifestations and management. Blood Rev. 2019;34:45–55. doi: 10.1016/j.blre.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu X, Han M, Li T et al Effective treatment of severe COVID-19 patients with tocilizumab. chinaXiv 202003.00026v1 [DOI] [PMC free article] [PubMed]
- 47.Shakoory B, Carcillo JA, Chatham WW, Amdur RL, Zhao H, Dinarello CA, Cron RQ, Opal SM. Interleukin-1 receptor blockade is associated with reduced mortality in sepsis patients with features of macrophage activation syndrome: reanalysis of a prior phase iii trial. Crit Care Med. 2016;44:275–281. doi: 10.1097/CCM.0000000000001402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eloseily EM, Weiser P, Crayne CB, Haines H, Mannion ML, Stoll ML et al (2019) Benefit of anakinra in treating pediatric secondary hemophagocytic lymphohistiocytosis. Arthritis Rheumatol [DOI] [PubMed]
- 49.Stebbing J, Phelan A, Griffin I, Tucker C, Oechsle O, Smith D, Richardson P (2020) COVID-19: combining antiviral and anti-inflammatory treatments. Lancet Infect Dis. 10.1016/S1473-3099(20)30132-8 [DOI] [PMC free article] [PubMed]
- 50.Richardson P, Griffin I, Tucker C, Smith D, Oechsle O, Phelan A, Stebbing J. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet. 2020;395(10223):e30–e31. doi: 10.1016/S0140-6736(20)30304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goldsmith SR, Saif Ur Rehman S, Shirai CL, Vij K, DiPersio JF. Resolution of secondary hemophagocytic lymphohistiocytosis after treatment with the JAK1/2 inhibitor ruxolitinib. Blood Adv. 2019;3(23):4131–4135. doi: 10.1182/bloodadvances.2019000898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Qu R, Ling Y, Zhang Y-h-z et al (2020) Platelet-to-lymphocyte ratio is associated with prognosis in patients with coronavirus disease-19. J Med Virol:1–9. 10.1002/jmv.25767 [DOI] [PMC free article] [PubMed]
- 53.Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395:473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shang L, Zhao J, Hu Y, Du R, Cao B. On the use of corticosteroids for 2019-nCoV pneumonia. Lancet. 2020;395(10225):683–684. doi: 10.1016/S0140-6736(20)30361-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Inciardi RM, Lupi L, Zaccone G et al (2020) Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19). [published online ahead of print]., JAMA Cardiol. 10.1001/jamacardio.2020.1096 [DOI] [PMC free article] [PubMed]
- 56.Simon DW, Aneja R, Carcillo JA, Halstead ES. Plasma exchange, methylprednisolone, IV immune globulin, and now anakinra support continued PICU equipoise in management of hyperferritinemia-associated sepsis/multiple organ dysfunction syndrome/macrophage activation syndrome/secondary hemophagocytic lymphohistiocytosis syndrome*. Pediatr Crit Care Med. 2014;15(5):486–488. doi: 10.1097/PCC.0000000000000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bergsten E, Horne A, Aricó M, Astigarraga I, Egeler RM, Filipovich AH, Ishii E, Janka G, Ladisch S, Lehmberg K, McClain KL, Minkov M, Montgomery S, Nanduri V, Rosso D, Henter JI. Confirmed efficacy of etoposide and dexamethasone in HLH treatment: long-term results of the cooperative HLH-2004 study. Blood. 2017;130(25):2728–2738. doi: 10.1182/blood-2017-06-788349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vallurupalli M, Berliner N. Emapalumab for the treatment of relapsed/refractory hemophagocytic lymphohistiocytosis. Blood. 2019;134(21):1783–1786. doi: 10.1182/blood.2019002289. [DOI] [PMC free article] [PubMed] [Google Scholar]