Abstract
Neuroblastoma (NB) is a malignant embryonal tumor of the sympathetic nervous system that is most commonly diagnosed in the abdomen, often presenting with signs and symptoms of metastatic spread. Three decades ago, high-risk NB metastatic to bone and bone marrow in children was not curable. Today, with multimodality treatment, 50% of these patients will survive, but most suffer from debilitating treatment-related complications. Novel targeted therapies to improve cure rates while minimizing toxicities are urgently needed. Recent molecular discoveries in oncology have spawned the development of an impressive array of targeted therapies for adult cancers, yet the paucity of recurrent somatic mutations or activated oncogenes in pediatric cancers poses a major challenge to the evolving paradigm of personalized medicine. Although low tumor mutational burden is a major hurdle for immune checkpoint inhibitors, an immature or impaired immune system and inhibitory tumor microenvironment can further complicate the prospects for successful immunotherapy. In this regard, despite the poor immunogenic properties of NB, the success of antibody-based immunotherapy and radioimmunotherapy directed at single targets (eg, GD2 and B7-H3) is both encouraging and surprising, given that most solid tumor antibodies that use Fc-dependent mechanisms or radioimmunotargeting have largely failed. Here, we summarize the current information on the immunologic properties of this tumor, its potential immunotherapeutic targets, and novel antibody-based strategies on the horizon.
INTRODUCTION
Most metastatic solid tumors are not curable with chemotherapies alone. Immunotherapy, a modality that achieves durable and sometimes complete tumor regression in metastatic melanoma, renal cell cancer, or chemotherapy-resistant non–small-cell lung cancers (NSCLCs), is emerging as a viable alternative or adjuvant to current standards of care. However, major hurdles persist. Intensive chemotherapy and its sequelae severely compromise both innate and adaptive immunities in patients. With low tumor mutation burdens (TMBs) and the downregulation or absence of surface HLA expression in some cancers (eg, neuroblastoma [NB]), classic T-cell immunity, which relies on tumor-derived peptides presented on the HLA molecule, is no longer functional. Although low TMB is a major hurdle for immune checkpoint inhibitors (ICIs), additional roadblocks such as an immature or impaired immune system (eg, from chemotherapy), the paucity of tumor-infiltrating lymphocytes, and immune suppression by tumor microenvironment (TME) combine to derail the antitumor immune response. As of 2019, there are 33 US Food and Drug Administration (FDA)–approved antibodies or conjugates for human cancer, 2 vaccines (sipuleucel-T [Provenge; Dendreon, Seal Beach, CA] and talimogene laherparepvec), and 2 cell therapies (axicabtagene ciloleucel [Yescarta; Kite Pharma, Santa Monica, CA] and tisagenlecleucel [Kymriah; Novartis, Basel, Switzerland]). This review will provide a focused update on antibody-based immunotherapy for high-risk metastatic NB, which has achieved the most success among pediatric solid tumors, with an emphasis on the immunologic properties of this tumor and its potential immunotherapeutic targets for novel antibody formats1 and their clinical applications.
Treatment of high-risk NB currently includes induction chemotherapy, surgical resection, radiotherapy, high-dose chemotherapy with autologous hematopoietic stem-cell transplantation, the differentiating agent isotretinoin, and immunotherapy with anti-GD2 monoclonal antibodies (mAbs; dinutuximab [ch14.18] or 3F8) plus cytokines, achieving long-term overall survival of > 50%.2,3 In addition, compartmental radioimmunotherapy (RIT) with iodine-131 [131I]-8H9 has contributed to major survival improvements in patients with CNS relapsed NB.4 Active immunity elicited by a bivalent anti-GD2 and anti-GD3 vaccine trial also improved survival rates for patients with NB with a history of prior relapse.5 However, major challenges remain in optimizing anti-GD2 immunotherapy and expanding therapeutic targets for NB immunotherapy. A better understanding of the limitations and opportunities of antibody-based immunotherapy is critical in shaping the new treatment perspective. Classic T-cell cytotherapy,6 oncolytic viral therapy,7 dendritic cell vaccines,8 and chimeric antigen receptor (CAR) T cells9 will not be discussed; readers are referred to reviews that address these topics in depth.
IMMUNOLOGIC PROPERTIES OF NB
Clinically, a subset of NB undergoes spontaneous regression or maturation, whereas others will rapidly progress despite intensive multimodal treatment. Although low-risk NBs show whole chromosome gains without segmental aberrations or gene amplifications, high-risk metastatic NBs frequently show segmental aberrations and MYCN amplification.10 Within these clinical and genetic heterogeneities, 2 distinct immunologic profiles emerge. Among low-risk subtypes, NB has the characteristics of hot tumor, where spontaneous regression or maturation is not uncommon (eg, among locoregional disease and stage 4S NB). Most stage 4S tumors express normal levels of HLA class I antigen and have strong CD3+ T-cell infiltration,11 suggesting recognition of NB cells by T cells.12,13 In addition, patients with low-risk NB can manifest the opsoclonus-myoclonus-ataxia syndrome associated with the presence of antineuronal antibodies. Cerebellar gray matter volume and visual and motor cortex thickness can be significantly reduced,14 and neurofilament light chain in CSF is markedly increased, consistent with neuronal damage.15 These ganglioneuroblastomas or differentiating NBs are characterized by the presence of diffuse immune cell infiltrates and tumor-associated lymphoid follicles (containing CD20+ B cells), suggesting an active immune reaction against NB.16
In contrast, high-risk metastatic NBs have the characteristics of cold tumors, armed with immune evasion mechanisms (Fig 1). First, these tumors are embedded in an immunosuppressive TME, typically infiltrated by CD163+ tumor-associated macrophages (TAMs) that paralyze T-cell responses.17,18 The TAM promotes T-cell apoptosis via Fas-Fas ligand (FasL) interactions, while activating myeloid-derived suppressor cells (MDSCs) and regulatory T cells, suppressing active immune response.19-21 Second, by downregulating HLA class I antigens and NKG2D ligands, activating immunoreceptor expressed by natural killer (NK) cells, NBs make themselves nearly invisible to classic T cells or NK cells.11,22 Third, NB cells express high levels of gangliosides and sialic acid–containing sugars and proteins, which are immunosuppressive when they shed into TME.23,24 Fourth, lymphocytes in the NB-infiltrated bone marrow (stage 4 metastatic NB) express programmed cell death 1 (PD-1) receptor, whereas HLA class I–positive NB cell lines constitutively express programmed death ligand 1 (PD-L1); interferon-γ (IFN-γ) could also induce PD-L1 expression in NB tumors. This PD-1/PD-L1 pathway is thought to mediate immune resistance mechanisms in metastatic NB.25,26
FIG 1.
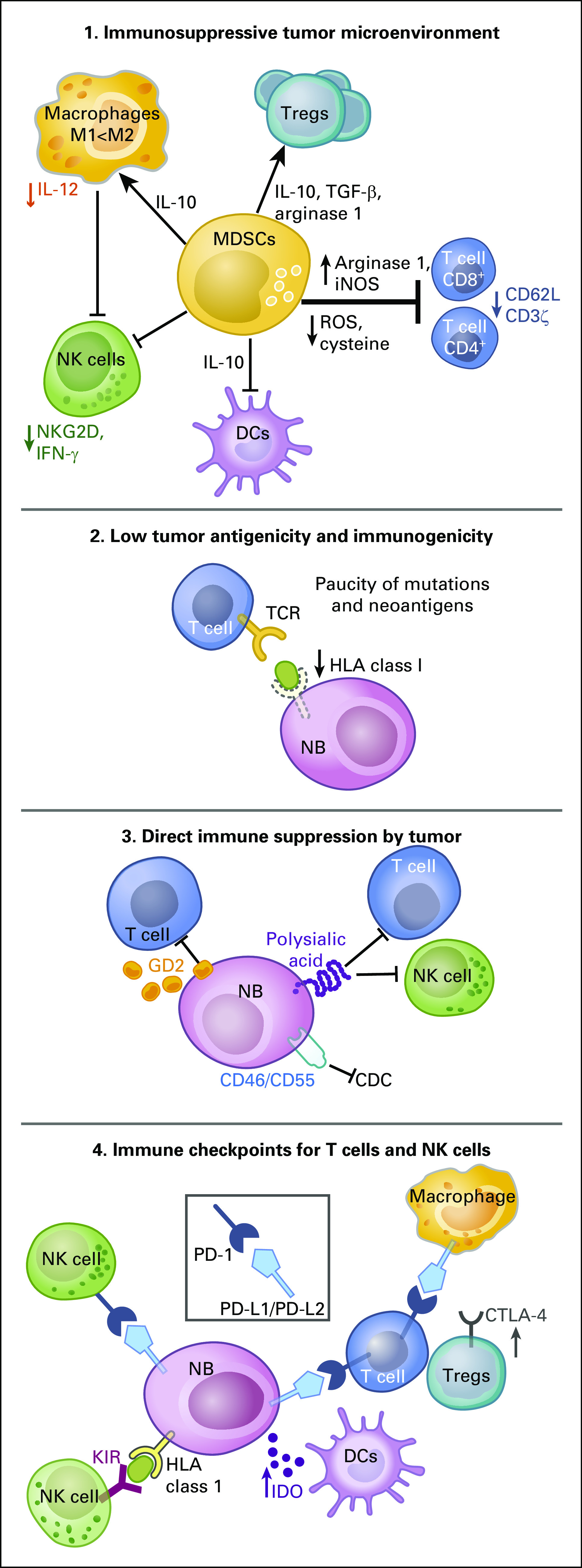
Mechanisms of immune evasion of neuroblastoma (NB). NBs may evade the immune destruction mediated by cytotoxic T cells (CTLs) and natural killer (NK) cells through multiple mechanisms, including the following: (1) immunosuppressive tumor microenvironment mediated by myeloid-derived suppressor cells (MDSCs)147; (2) rarity of somatic mutations or neoantigens recognizable by classic T-cell receptors (TCRs) and downregulation of HLA class I molecules and antigen processing and presenting pathways; (3) expression of immunosuppressive tumor antigens such as gangliosides and sialic acids and membrane complement inhibitors; and (4) upregulation of multiple immune checkpoint inhibitors on immune effector cells and NB tumor cells. DCs, dendritic cells; IFN, interferon; IL, interleukin; iNOS, inducible nitric oxide synthase; ROS, reactive oxygen species; TGF-β, transforming growth factor-β; Treg, regulatory T cells.
IMMUNOTHERAPEUTIC TARGETS FOR NB
Disialoganglioside GD2
Among the immune surface targets for NB (Appendix Tables A1 and A2, online only), disialoganglioside GD2 is one of the most often studied clinically. It belongs to a unique class of carbohydrate antigens expressed at high density on all primary or metastatic tumors regardless of stage, with proximity to the cell membrane and homogenous distribution within and across NBs, as well as rare antigen loss, which are all properties highly desirable for cancer immunotherapy; they ranked 12th among National Cancer Institute (NCI) cancer antigens.27,28 As an oncofetal antigen, GD2 is expressed during fetal development, and after birth, its expression is restricted to the CNS, predominantly on neurons, as well as peripheral nerves and skin melanocytes.29 Although monosialogangliosides, such as GM1 or GM3, function as negative regulators of receptor tyrosine kinases (RTK) signaling, disialoganglioside GD2 activates RTK-mediated signal transduction, leading to the activation of c-Met, engaging the MEK/ERK and PI3K/Akt pathways, and resulting in increased cancer cell proliferation and migration.30-32 Changes in ganglioside and glycan profiles occur in pathologic conditions and are observed in a variety of embryonal cancers (eg, NB, brain tumor, retinoblastoma, Ewing sarcoma, rhabdomyosarcoma), bone tumors (eg, osteosarcoma), soft tissue sarcomas (eg, leiomyosarcoma, liposarcoma, fibrosarcoma), and neural crest–derived tumors (eg, small-cell lung cancer, melanoma).27 Anti-GD2 immunoglobulin G (IgG) mAbs and anti-GD2 radioimmunoconjugates have shown successes in preclinical and clinical studies.27,33 T-cell–based approaches targeting GD2 are also actively pursued using both bispecific antibodies (BsAbs)34 and CAR T-cells.9,35
B7-H3
B7-H3 (CD276), a type I transmembrane glycoprotein molecule, is ubiquitously transcribed in normal human tissues, but its protein expression is restricted by a tight post-transcriptional control. In some tumors, B7-H3 is highly overexpressed by microRNA-29, IFN-γ stimulation, and immunoglobulin-like transcript 4 (ILT-4) signaling, enabling immunotherapies targeting B7-H3 to circumvent on-target off-tumor toxicity.36-39 This protein is homogeneously expressed in both primary and metastatic NBs40 and many pediatric and adult solid cancers, including primary and metastatic brain cancers. It is correlated with worse prognosis and increased potential for metastasis, and this protein ranked 66th among NCI cancer antigens.28 The mAb 8H9 (omburtamab) is specific for 4Ig-B7-H3, the long and principal form of B7-H3. Although most normal tissues were negative for 8H9 staining, liver tissue showed positive, and moderate uptake of 8H9 in the liver was observed in patient imaging studies using IgG1 131I-8H9 (ClinicalTrials.gov identifier: NCT00582608). To increase the therapeutic index (TI) and to avoid liver uptake of intravenous 8H9 and subsequent liver toxicity, compartmental radioimmunotherapy (RIT) was given among patients with CNS metastasis, and radioimmunoconjugates using omburtamab have shown the most success so far.4 Intrathecal (through an Ommaya reservoir) 131I- or 124I-conjugated omburtamab has increased the cure rate for patients with CNS involvement.4 A phase I clinical trial of intraperitoneal 131I-8H9 for patients with desmoplastic small round cell tumors and other solid tumors involving the peritoneum is ongoing (ClinicalTrials.gov identifier: NCT01099644).41 Another B7-H3–targeting antibody, enoblituzumab, notable for its nonreactivity with liver,42 is currently in phase I trials for diverse solid tumors including refractory tumors and pediatric cancers. Furthermore, a clinical trial of a T-cell–engaging BsAb built on the dual-affinity retargeting (DART) platform (MGD009) is underway in patients with B7-H3–positive advanced solid tumors (ClinicalTrials.gov identifier: NCT02628535). The prevalence of B7-H3 overexpression across NB, lung, breast, brain, kidney, and prostate cancers, and dendritic cells makes B7-H3 a particularly intriguing tumor target or a checkpoint ligand.43
ALK
Aberrant anaplastic lymphoma kinase (ALK) expression is found in anaplastic large-cell lymphoma (ALCL), NSCLC, rhabdomyosarcoma,44 and NB.45 ALK is ranked 33rd among the NCI cancer antigens,28 and the majority of NBs (22 of 24 NBs) and half of 29 cell lines of neural origin were found to express ALK transcripts and ALK protein.45 Mutations in ALK have been implicated in 9% of NBs, and it is adversely prognostic, especially in the presence of MYCN amplification.46,47 ALK mutations hyperactivate the RAS-MAPK signaling pathways in NB, promoting cancer formation. Immunodominant peptide epitopes of ALK for both class I and II major histocompatibility complex (MHC) and circulating ALK-specific T cells have been identified in patients with ALCL, providing the basis for peptide vaccine immunotherapy for ALK-driven tumors.48,49 Prediction of T-Cell Epitopes for Cancer Therapy (ProTECT) analyses have identified 2 neoepitopes created by the R1275Q mutation in the ALK protein that could complex with HLA-B*15:01 to drive cytotoxic T-cell response.50 IgGs targeting the ALK ectodomain have also shown activity against NB tumors in preclinical models irrespective of ALK mutation, and the combination of crizotinib with anti-ALK mAb induced cell surface accumulation of ALK, resulting in enhanced apoptosis of NB cells.51 In addition, an antibody-drug conjugate directly targeting ALK receptor, CDX-0125-TEI, exhibited efficient ALK antigen binding and internalization, showing cytotoxicity against both ALK-wild and ALK-mutant patient-derived xenografts (PDXs).52 ALK could be a viable immunotherapeutic target, with relevance for NB and other ALK-positive cancers, irrespective of ALK mutation.
ANTIBODY-BASED IMMUNOTHERAPY FOR NB
IgG mAbs
Hybridoma technology first introduced by Köhler and Milstein53 has generated numerous mAbs targeting human malignancies and immune cells, leading to major breakthroughs in cancer therapy in the past 3 decades. Anti-GD2 mAbs can induce direct cell death54; Fcγ receptor (FcγR)–mediated antibody-dependent cell-mediated cytotoxicity (ADCC) by NK cells,55,56 neutrophils,57 and macrophages58; and complement-mediated cytotoxicity (CMC)59,60 (Fig 2). Through complement breakdown products (eg, C3bi) deposited on NB, complement-dependent cell-mediated cytotoxicity (CDCC) or phagocytosis (CDCP) could potentially become relevant.
FIG 2.
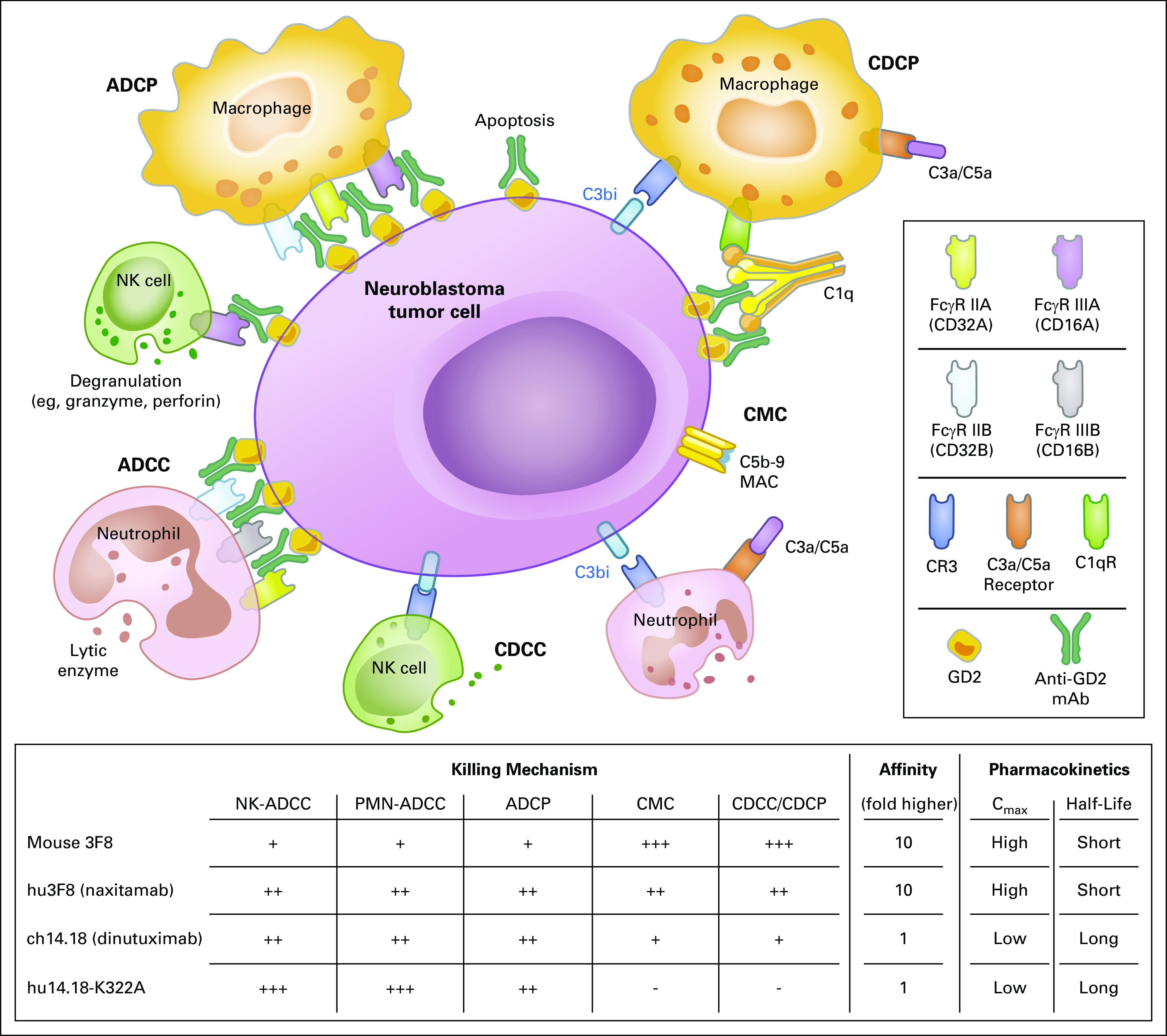
Mechanisms of action of anti-GD2 monoclonal antibodies. Anti-GD2 monoclonal antibodies (mAbs) mediate active immune response against disialoganglioside (GD2)–positive tumor cells. Anti-GD2 mAbs bind to cell surface GD2 and induce immune reactions including direct tumor cell apoptosis. Recruitment and signaling of type I receptors (FcγR I-III and their isoforms) through antigen-antibody complexes trigger antibody-dependent cellular cytotoxicity (ADCC) and antibody-dependent cellular phagocytosis (ADCP). Alternatively, activation of complement pathway leads to tumor cell killing by the following 2 distinct processes: first, direct tumor cell lysis through complement-mediated cytotoxicity (CMC) by assembly of membrane attack complex (MAC; C5b-C9); and second, complement receptors (CRs) on effector cells recognize opsonins, such as C3b, and trigger complement-dependent cellular cytotoxicity (CDCC) and complement-dependent cellular phagocytosis (CDCP). These various immune responses by anti-GD2 mAbs can be modified further through Fc engineering by mutation and/or glycomodification to reduce immunogenicity or toxicity and increase the antitumor effect of engaging immune effector cells. Cmax, maximum concentration; NK, natural killer; PMN, polymorphonuclear leukocyte.
Two anti-GD2 mouse IgG3 antibody families have been the most studied (ie, 3F8 and 14.18). Early on, 14.18 was class switched to IgG2a and chimerized with human IgG1-Fc (ch14.18, dinutuximab) and manufactured in SP2/0mouse myeloma cells. Ch14.18 was later produced in Chinese hamster ovary (CHO) cells and renamed ch14.18/CHO (dinutuximab-β).61 Although dinutuximab families have efficient ADCC activity, mouse 3F8 has strong CMC activity as a result of the difference between human IgG1 and mouse IgG3.59,62 Regarding toxicities, both antibodies induce neuropathic pain in nearly all patients; fever and allergic reactions are also common. Motor neuropathy, ophthalmoplegia, and transverse myelitis seemed to be more prevalent with dinutuximab,3,63 whereas hypertension and posterior reversible encephalopathy syndrome were more noticeable for 3F8.64 The difference in toxicity profile is partly explained by the difference in plasma half-life of 3F8 versus dinutuximab (2 v 8-10 days, respectively). Despite these differences, the clinical impact on survival appeared similar.2,3,65 Postconsolidation treatment with 3F8 plus granulocyte-macrophage colony-stimulating factor (GM-CSF) improved overall survival to > 65% among patients with high-risk metastatic NB.2 Dinutuximab (Unituxin; United Therapeutics, Silver Spring, MD) plus interleukin (IL)-2, GM-CSF, and 13-cis-retinoic acids also significantly improved survival when compared with standard of care.3 A subsequent randomized study using dinutuximab-β showed no benefit of IL-2 over mAb alone,61 suggesting that NK-ADCC may not be the dominant contributor to clinical benefit of anti-GD2 mAbs. The unexpected impact on survival after mouse 3F8, which has stronger CMC but substantially inferior ADCC compared with dinutuximab and naxitamab (humanized 3F8 [hu3F8]), suggests that complement activation pathways could be important in the immunotherapy of NB. This high sensitivity of NB to CMC is partly attributed to low expression of complement decay-accelerating factor (DAF or CD55) on NB cells.59,60
Although active against minimal residual disease (MRD), anti-GD2 mAbs have been less successful against bulky soft tissue tumors, and neuropathic pain and on-target off-tumor adverse effects (because of the presence of GD2 on peripheral pain fibers) have been major management challenges. Furthermore, antidrug antibodies (ADAs), including human antimouse antibodies or human antichimeric antibodies, are causing treatment delays or even terminations and, most importantly, abrogating the antitumor effect. Naxitamab was created to reduce these ADAs while enhancing ADCC through the human IgG1-Fc, as well as retaining CMC potency through its high affinity for GD2.66 Phase I and II trials of hu3F8 (ClinicalTrials.gov identifiers: NCT01419834, NCT01757626, and NCT03033303) have confirmed its low immunogenicity, favorable pharmacokinetics (4 days instead of 8-10 days), and improved toxicity profile.66-68 Another humanized anti-GD2 mAb with K322A point mutation, hu14.18K322A, was developed to increase ADCC by lowering fucosylation and to remove CMC to reduce the adverse effect of pain. Reduced fucosylation of the carbohydrate attached to the Asn297 glycosylation site of the Fc region can greatly enhance ADCC by increasing FcγRIIIA/B binding,69 while alanine substitution at K322 significantly decreases complement activation.70
Arming IgG Antibodies With Conjugates
Another strategy to enhance IgG functions is to arm them with therapeutic agents such as drugs,71 radionuclides,72 or cytokines.73 Inactive prodrugs selectively delivered by antibodies can be activated in the tumor stroma or after internalization. The most common conjugates are microtubule inhibitors and DNA-damaging agents. Microtubule inhibitors, including auristatins and maytansines, bind tubulin, destabilize microtubules, and cause G2/M phase cell cycle arrest. DNA-damaging agents such as anthracyclines, calicheamicin, duocarmycin, and pyrrolobenzodiazepines (PBDs) function by binding the minor groove of DNA and cause DNA strand scission, alkylation, or cross-linking. Antibody-drug conjugates targeting neural cell adhesion molecule (NCAM; CD56), HuN901-DM1, maytansinoid (DM1)-conjugated anti-NCAM mAb (lorvotuzumab, hN901), showed antitumor activity against NB,74 and lorvotuzumab mertansine (IMGN901) is in a phase II clinical trial for relapsed or refractory solid tumors including NB (ClinicalTrials.gov identifier: NCT02452554). In addition, m906, another human anti-NCAM mAb, was conjugated to the cytotoxic drug PBD and showed antitumor effect against CD56+ NB in vitro.75 For anti-GD2 antibodies, pegylated anti-GD2 immunoliposomes for targeted delivery of the survivin inhibitor sepantronium bromide (YM155) were successfully formulated to improve serum half-life and tumor accumulation of YM155.76 Other pegylated anti-GD2 etoposide-loaded immunoliposomes have also shown antitumor potential in preclinical studies.77
Built on centuries of knowledge in radiation biology, radionuclides are powerful payloads with major therapeutic and diagnostic potential. Using antibodies as delivery vehicles, RIT exploits radionuclides that emit α- or β-particles or Auger electrons, with the potential to rival the precision and intensity of external-beam radiation.72,78 Early studies showing clinical benefit in non-Hodgkin lymphoma have resulted in FDA approval of both 131I-tositumomab (Bexxar; GlaxoSmithKline, London, United Kingdom) and 90Y-ibritumomab tiuxetan (Zevalin; Acrotech Biopharma, East Windsor, NJ). However, clinical development in solid tumors has lagged behind, mostly because of the unfavorable pharmacokinetics of large molecules, such as IgG, with slow clearance or of small molecules, such as single-chain Fv, with rapid renal clearance leading to insufficient tumor uptake.72 131I-labeled GD2 or B7-H3 mAbs have been tested for NB, but systemic administration has encountered 2 major drawbacks, namely myelotoxicity and insufficient tumor dose, which is a limitation of IgG pharmacokinetics where the TI (payload area under curve for tumor v that for blood or normal tissues) is at best 5:1.72 To increase the TI and to avoid liver uptake of intravenous 8H9, compartmental RIT was adopted among patients with CNS metastasis.4,79,80 131I-3F8 and 131I-omburtamab have been administered intrathecally to overcome the blood-brain barrier and to achieve a high TI for the treatment of recurrent leptomeningeal disease. In a phase I trial, intra-Ommaya 131I-3F8 for GD2-positive CNS disease achieved high TI with major antitumor responses.79 Intra-Ommaya 131I-omburtamab administered as part of a salvage regimen produced long-term survival after CNS relapse.4 In addition, convection-enhanced delivery of 124I-omburtamab directly into diffuse intrinsic pontine glioma showed favorable dosimetry with a potential for escalation to curative doses.80 α-Particle–emitting actinium-225 [225Ac] has also been conjugated to 3F8 (225Ac-1,4,7,10-tetra-azacyclododecane [DOTA]-3F8; 225Ac-3F8) and administered intrathecally without toxicities, which improved survival in a xenograft model of meningeal carcinomatosis.81
Another class of ligands targetable by mAbs are cytokines that can enhance both the afferent and the efferent arms of the immune response. The expectation is to deliver cytokines into the tumor, avoiding systemic toxicities.73 Different cytokines have been tested, including IL-2, IL-12, IL-13, IL-15, and GM-CSF, each fused to the amino and/or carboxy terminus of the IgG, and each showing antitumor benefits in preclinical studies.82 Hu14.18-IL2 (EMD273063) immunocytokine is a genetic fusion protein where IL-2 is attached to the carboxy terminus of each of the IgG heavy chain on hu14.18. A phase II study of hu14.18-IL2 in relapsed or refractory NB has shown antitumor effect in patients with MRD in the bone marrow, but the response was difficult to separate from hu14.18 alone.83 Intratumoral injection of hu14.18-IL2 in preclinical models achieved better immunocytokine retention and induced more potent antitumor responses than systemic injection by activating intratumoral NK cells and T cells.84,85 Moreover, IL-15/IL-15Rα fusion protein (RLI) linked to the carboxy terminus of the heavy chain of anti-GD2 IgG showed superior antitumor effect compared with RLI or antibody alone.86
BsAbs
Unlike classic mAbs, BsAbs possess 2 binding specificities, built chemically or genetically based on a wide selection of structural platforms.1,87 NK cell–engaging BsAbs have 2 specificities, one toward a tumor target and the other toward an NK-activating receptor such as CD16. T-cell BsAbs have the second specificity at the activating receptor CD3 and recruit polyclonal T cells without the restriction of HLA to overcome the low clonal frequency of classic cytotoxic T cells in tumor. BsAbs can be structurally grouped into the following 2 general classes: those built on the IgG framework (IgG-like BsAbs) and those built using antibody fragments such as a single-chain fragment (non–IgG-like BsAbs).1 The most common non–IgG-like format is the tandem single-chain variable fragment (scFv; bispecific T-cell engager [BiTE; Amgen, Thousand Oaks, CA]) used in blinatumomab, the first BsAb to receive FDA approval.88 Non–IgG-like BsAbs usually have short serum half-lives as a result of their small size (< 65 kDa) and absent interaction with neonatal Fc receptor (FcRn). Although their small size facilitates fast tissue penetration, their fast clearance requires repeated daily injections. Besides BiTE, various formats such as diabody, tandem diabody, DART, tandem triple scFv, and, dock-and-rock, Fab3 have been developed; however, most have encountered short half-lives as potential limitations.89 IgG-like BsAbs are larger molecules (> 150 kDa) with longer serum half-lives because of their size above the renal clearance threshold and recycling through the FcRn-IgG complex.90 The presence of Fc in IgG-like BsAb has other advantages over non-IgG BsAbs, such as structural symmetry, ease of manufacturing, drug stability during formulation, and distribution in vivo.1,87 Yet, because the Fc domain is associated with undesirable cytokine release syndrome and interferes with T-cell infiltration into tumor,91 silencing the Fc function is now routinely adopted in building IgG-like BsAbs. Other IgG-like BsAb formats include additional single-chain or disulfide stabilized Fvs or Fabs fused to the N or C termini of IgGs, resulting in tetravalent molecules with bivalent binding specificities.87,89
A number of BsAbs targeting GD2 have been built. At first, a bispecific Fab × Fab anti-GD2/anti-FcγRI (CD64) antibody was developed to engage antigen-presenting cells, monocytes, and macrophages against NB.92 BsAbs containing anti-GD2 murine 5F11-scFv and anti-CD3 huOKT3-scFv (BiTE) recruited T cells and demonstrated antitumor effect against NB.93 Substituting 5F11-scFv with the higher affinity hu3F8-scFv significantly improved T-cell activation and tumor cell killing in vitro.94 Exploiting the IgG-like platform, a chemically conjugated anti-GD2 BsAb was developed,95 and a phase I/II clinical trial using BsAb-armed T cells is ongoing (ClinicalTrials.gov identifier: NCT02173093). Using genetic engineering, a more recent IgG-like anti-GD2 BsAb, hu3F8-BsAb, where the anti-CD3 huOKT3-scFv is linked to the carboxyl end of the light chain of hu3F8 IgG1 [IgG(L)-scFv], has been developed. Hu3F8-BsAb had N297A aglycosylation and K322A mutation of the Fc region to prevent FcγRs binding to reduce complement activation and cytokine storm.34,91 Its high tumor killing potency (femtomolar half-maximal effective concentration [EC50]), wide margin of safety (105-fold EC50 selectivity of tumor v normal tissue), ability to drive circulating T cells into solid tumors, and absence of neurotoxicity in preclinical models warranted the initiation of its clinical trial (ClinicalTrials.gov identifier: NCT03860207).34 In parallel, pretargeted RIT (PRIT) using radiolabeled hu3F8-C825 BsAb, where anti-CD3 scFv is replaced by an anti-DOTA(metal) scFv (C825), achieved high TI (> 100:1) and cured NB tumors without toxicities in preclinical models.96,97 This PRIT can adapt therapeutic β-emitters (177Lu and 90Y), α-emitters (225Ac, 212Pb), or diagnostic emitters (66Ga, 89Zr) and expand its clinical application.
ANTIBODY-BASED THERAPY OF NB AT THE CROSSROADS: A NEW PERSPECTIVE
Limitations of GD2 Immunotherapy
Two anti-GD2 mAb families, 3F8/hu3F8 (naxitamab) and ch14.18 (dinutuximab)/dinutuximab-β/hu14.18-K322A, have produced long-term cures among patients with high-risk metastatic NB. Antibody engineering through humanization and Fc modification to optimize their structure and function can reduce immunogenicity, improve effectiveness, and decrease on-target off-tumor adverse effects.67,98,99 Engaging T cells using T-BsAbs also improved the potency of GD2 immunotherapy, and furthermore, the combination of BiTE-expressing oncolytic virus with CAR T-cell therapy has demonstrated successful outcomes for patients with advanced solid tumors.100 Attaching payloads to IgGs enabled the delivery of therapeutic agents to the tumor even more efficiently. Of note, PRIT based on BsAb structure has produced cures in preclinical models without physical, chemical, or histologic toxicities and may provide an alternative to dose-intensive chemotherapy, which is deemed necessary for rapidly progressing metastatic NB.
Damaged Immune System
Partly because of intensive chemotherapy, immune effector cells in patients with NB are insufficient or incapacitated. Supplemented cytokines such as GM-CSF and IL-2 have been instrumental in enhancing myeloid cell–associated ADCC in NB.3,101,102 Although IL-2 seemed to have failed in augmenting NK cell function,61 IL-15 is a viable alternative given its pleiotropic effects on NK cells and T cells.103 Immunocytokines have shown early promise, but competing affinities for cytokine receptor versus tumor target can derail the intended driver function of IgGs, such that cytokines fail to accumulate in the tumor.104 Intratumoral injection of immunocytokine may be an alternative with the potential for inducing adaptive immunity.105
Suppressive TME
Among the key elements of the TME, TAMs, MDSCs, and immune checkpoints provide viable options to counter immune evasion.106,107 Anti-CD105 antibody to deplete tumor-infiltrating myeloid cells has shown synergy with dinutuximab to overcome immunosuppressive TME.108 The histone deacetylase inhibitor vorinostat decreases MDSCs and increases macrophage effector cells, which express high levels of FcγRs, thereby enhancing anti-GD2 mAb potency.109 NK cell or myeloid cell inhibitory receptors, as members of immune checkpoints, provide biologic reasons for treatment failures as well as predictive biomarkers for clinical response. The sensitivity of NB to NK-ADCC and myeloid-ADCC derives partly from the downregulation or absence of HLA, hence missing-self recognition by inhibitory killer cell immunoglobulin-like receptors (KIRs) or inhibitory leukocyte immunoglobulin-like receptor subfamily B receptors (LILRBs).110,111 For NK cells, checkpoint receptors and molecules include KIRs, CD94/NKG2A,TIGIT, CD96, TIM-3, CTLA-4, LAG-3, and PD-1; for macrophages, CD47 is the most studied.112 Inhibition of NK checkpoints has the potential to reverse NK cell dysfunction and to boost antitumor activity, both in preclinical (anti-TIGIT and anti-CD96) and clinical studies (anti-NKG2A and anti-KIR).113-115 The PD-1/PD-L1 axis also acts as a checkpoint in regulating NK-ADCC in NB,26,116 and its modulation by nivolumab is being tested in combination with dinutuximab-β both in preclinical and clinical studies (ClinicalTrials.gov identifier: NCT02914405).116 More recently, the gut microbiome might offer another tool to reboot or recruit antitumor responses through direct or indirect effects on antigen presentation, effector cell function, and vaccine efficacy.117-119 In the phase I GD2 vaccine study, the effect of microbiome on anti-GD2 antibody titer is actively being investigated (ClinicalTrials.gov identifier: NCT00911560).
Biomarkers to Guide Treatment
The missing KIR ligand for NK-ADCC is associated with improved survival in patients treated with anti-GD2 IgGs, and KIR polymorphism KIR3LD1 and HLA-B allele combinations have been implicated as strong prognostic factors.120,121 Moreover, FcγR2A polymorphisms,122 the proportion of GD2-positive tumor cells in tumor,123 and quantitation of bone marrow MRD by quantitative reverse transcription polymerase chain reaction124,125 can be highly prognostic for survival after anti-GD2 immunotherapy. The utility of MRD measured early on after 2 cycles of immunotherapy was particularly relevant to provide rationale for stopping futile toxic therapies.124 MRD panels including patient-specific DNA markers using whole-genome sequencing126 and circulating microRNA127 may provide additional insights into prognosis and treatment responses. With the clinical introduction of BsAbs with or without checkpoint inhibitors, other biomarkers for both response and toxicities could be highly relevant.128
Chemoimmunotherapy
Induction and stem-cell transplantation followed by anti-GD2 antibody therapy has produced long-term cures.3 Under the hypothesis that chemotherapy-induced microvascular or TME modification could enhance IgG-mediated antitumor response, moving anti-GD2 antibody hu14.18K322A or 3F8 up front to be administered concurrently with induction chemotherapy is feasible.129,130 Hu14.18K322A incorporated into induction chemotherapy significantly improved early responses, reduced tumor volumes, and improved 2-year event-free survival (ClinicalTrials.gov identifier: NCT01857934).131 For relapsed or refractory diseases, dinutuximab plus GM-CSF, when combined with irinotecan and temozolomide, and hu14.18K322A plus GM-CSF combined with chemotherapy and haploidentical NK cells have produced favorable response rates and survival.129,130
Alternative Targets
GD2 has provided a proof of principle for antibody-based targeting of NB. If it represents the tip of the iceberg, uncovering novel high-payoff targets should continue. So far NB antigens targeted by antibodies have included surface receptors or ligands shared with the neural crest (eg, GD2, CD56, L1CAM, ALK, and polysialic acid), immune checkpoint (eg, B7-H3), and signaling receptors (eg, glypicans).132-135 Internal antigens, classically recognized only by T cells when presented as peptides buried in the HLA pocket, have just recently become druggable with T-cell receptor mimic antibodies.136,137 These antigens include oncoproteins unique to NB (eg, MYCN),138 cancer testis antigens (eg, PRAME),139-142 transcription factors (eg, WT1),143 or telomerase.144 Multiomics approaches continue to uncover both cell surface and internal proteins as potential therapeutic targets.132,145,146 However, the low density of these peptide-MHC complexes, their HLA allele restriction, potential tissue cross-reactivity, and tumor downregulation of HLA class I could limit their utility in clinical applications that rely on CMC and ADCC. Because normal tissue expression of antibody targets can influence the pharmacokinetics of mAbs, monitoring of their biodistribution in preclinical models and in patients should help prioritize their clinical development. Unexpected liver or lung uptakes have blunted enthusiasm for some antibodies in pediatrics; for example, a phase I trial of anti-CD99 MAB-O13 for Ewing sarcoma was terminated because of liver and lung uptake associated with hypotension and chills (Memorial Sloan Kettering Institutional Review Board No. 90140), whereas liver uptake after intravenous anti–B7-H3 antibody forced its clinical development toward compartmental approaches (ClinicalTrials.gov identifier: NCT00582608). In vitro cytotoxicity directed at GD2, whether through CMC, ADCC, or antibody-dependent T-cell–mediated cytotoxicity, tends to be substantially stronger than that observed against other surface antigens, most likely attributable to its unique properties for immunotherapy. Despite the cross-reactivity to neural tissues, irreversible or chronic neurologic damage has rarely been reported through decades of clinical development, allowing GD2 to stand out among NCI priority antigens for immunotherapy.28
Integration of immunotherapy Into the Standard of Care
Finally, integrating antibody-based immunotherapy into the overall standard of care is still challenging. Many variables can affect the clinical outcome, such as passive versus active immunotherapy, up front versus sequential combinations, the type of chemotherapy, and the timing and the dose of radiation. These combinations are best optimized in appropriate animal models.101 Yet, because most biologics are designed for human use, they are highly immunogenic in immunocompetent animals, hence the limitation of transgenic mouse or dog models. Immune-deficient mice engrafted with human cells can be constrained by graft-versus-host reactions that can confound both efficacy and toxicity measurements. In addition, NB xenografts and PDXs typically become admixed with substantial murine stroma content, thereby confounding conclusions on the TME. Despite these limitations, for diseases as rare as NB, skipping animal models and adopting a trial-and-error clinical approach is highly inefficient and should be discouraged. Here, a scientific consensus is sorely needed.
CONCLUSION
Cancer immunotherapy will improve long-term patient survival while reducing acute or chronic toxicities from genotoxic therapies. High-risk NB is one of the few cancers transformed by immunotherapy, changing its natural history from a uniformly lethal disease to a potentially curable one in more than half of patients. Yet, our understanding of immunobiology of NB and anti-GD2 therapy needs to be improved, with implications for future antibody-based therapies in NB and cancer immunotherapy in general. With the advances in protein engineering, novel antibody formats have the potential to deliver high-dose radiation to achieve responses without long-term toxicities, offering powerful alternatives to dose-intensive chemotherapy deemed necessary to treat rapidly growing NB. The combination of Fc-dependent and T-cell–mediated antibody approaches plus high-TI antibody-targeting strategies should change the outlook for children devastated by metastatic NB.
Appendix
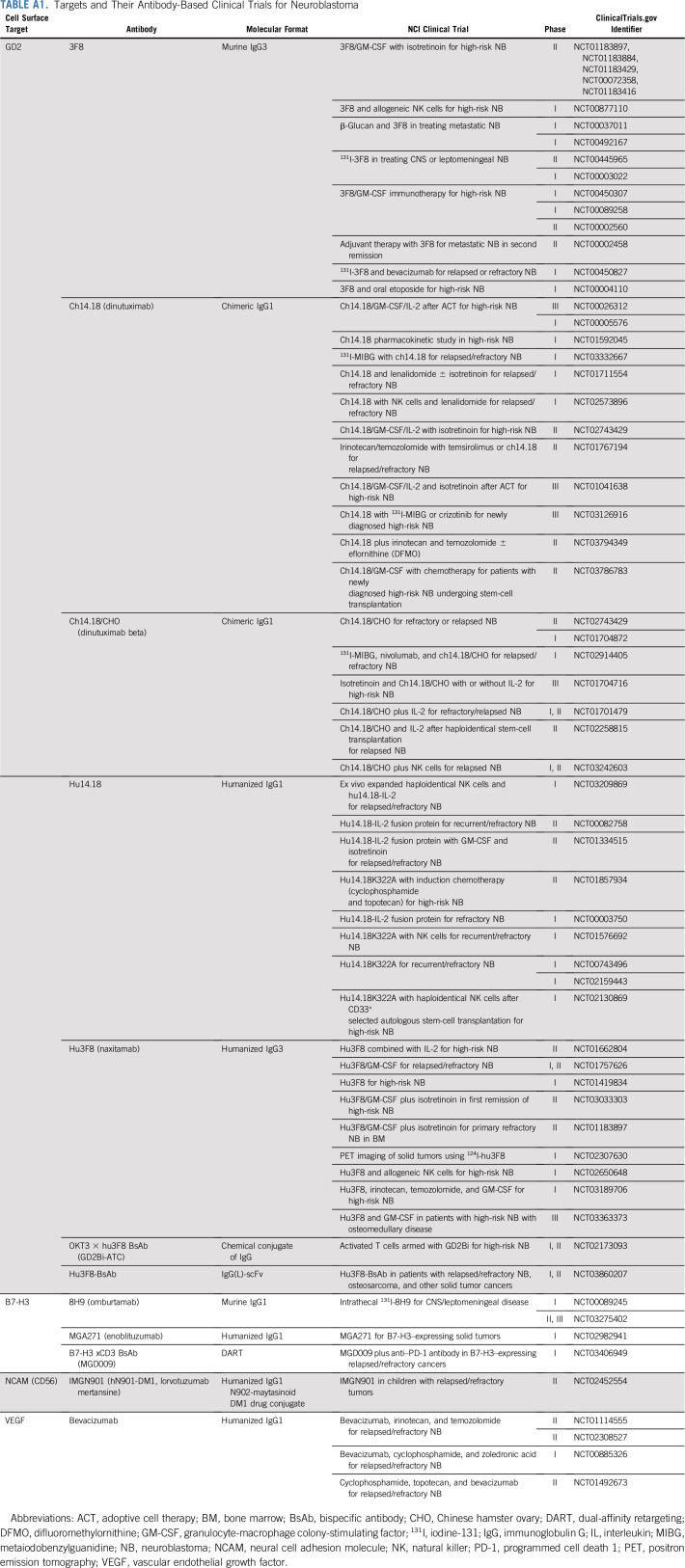
TABLE A1.
Targets and Their Antibody-Based Clinical Trials for Neuroblastoma
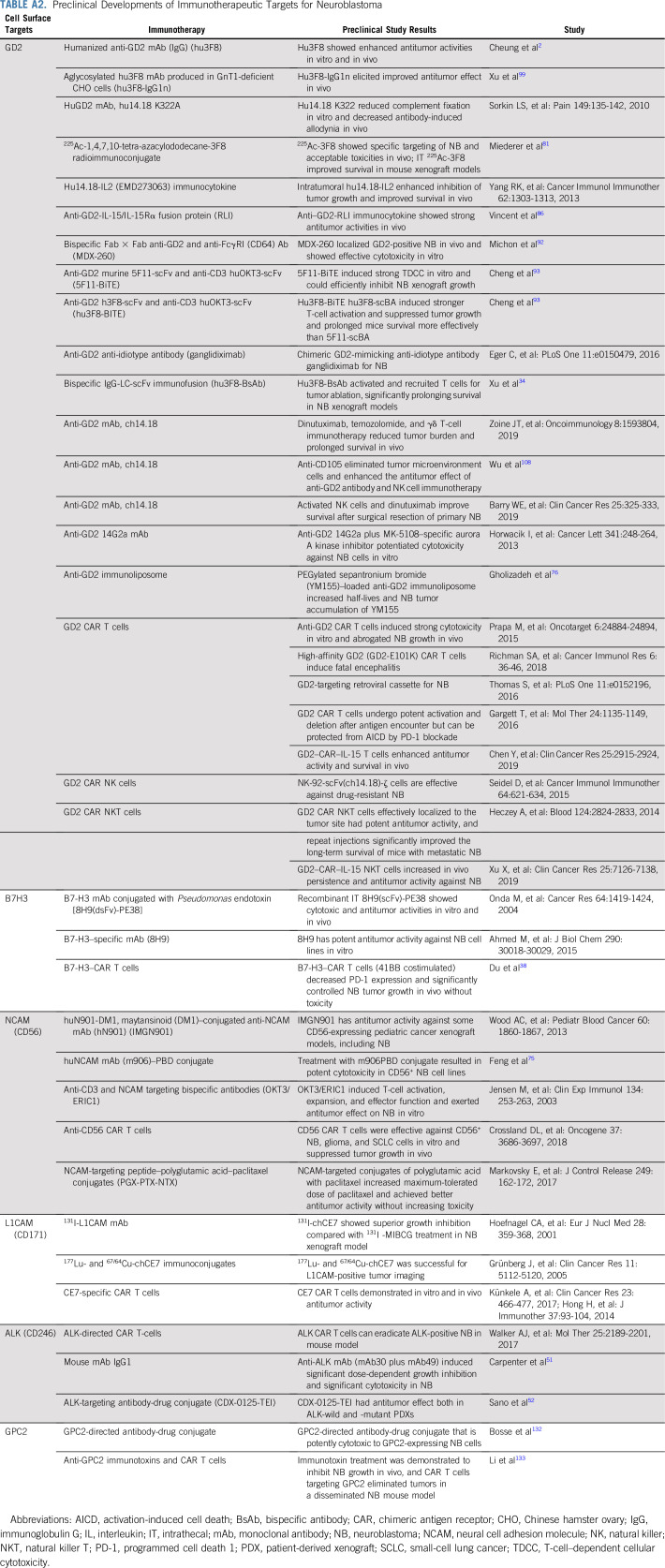
TABLE A2.
Preclinical Developments of Immunotherapeutic Targets for Neuroblastoma
SUPPORT
Supported in part by funds from Enid A. Haupt Endowed Chair, the Robert Steel Foundation, and Kids Walk for Kids With Cancer.
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Financial support: Nai-Kong V. Cheung
Administrative support: Nai-Kong V. Cheung
Collection and assembly of data: All authors
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Targets and Antibody Formats for Immunotherapy of Neuroblastoma
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Nai-Kong V. Cheung
Stock and Other Ownership Interests: Ymabs Therapeutics
Consulting or Advisory Role: AstraZeneca/MedImmune, Abpro, Eureka Therapeutics
Research Funding: Ymabs Therapeutics (Inst), Abpro (Inst)
Patents, Royalties, Other Intellectual Property: scFv constructs of anti-GD2 antibodies (Inst), therapy-enhancing glucan (Inst), use of monoclonal antibody (mAb) 8H9 (Inst), methods for preparing and using scFv (Inst), GD2 peptide mimics (Inst), methods for detecting minimal residual disease (Inst), anti-GD2 antibodies (Inst), generation and use of HLA-A2–restricted peptide-specific mAbs and chimeric antibody receptors (Inst), high-affinity anti-GD2 antibodies (Inst), multimerization technologies (Inst), bispecific HER2 and CD3 binding molecules (Inst), affinity matured hu8H9 (Inst), anti-chondroitin sulfate proteoglycan 4 antibodies and uses thereof (Inst), ROR2 antibodies (Inst), T-cell receptor–like antibody agents specific for Epstein-Barr virus latent membrane protein 2A peptide presented by human HLA (Inst), anti-CD33 antibody agents (Inst), anti-KIR3DL1 antibodies (Inst), modular self-assembly disassembly (SADA) technologies (Inst), A33-C825 conjugate for pretargeted radioimmunotherapy and application as a theranostic product (Inst), anti-L1-CAM antibodies and uses thereof (Inst), Anti-A33 antibodies and uses thereof (Inst), DOTA BsAb for new humanized next-generation anti-GPA33 antibodies with Fc-enhanced function or bispecific properties (Inst), Herceptin-C825 conjugate for pretargeted radioimmunotherapy and application as a theranostic product (Inst), anti-polysialic acid antibodies and uses thereof (Inst), methods of enhancing immunogenicity of poorly immunogenic antispecific vaccines using oral yeast β-glucans (Inst), Small-molecule hapten chelates for pretargeted radioimmunotherapy with anti-DOTA (lanthanide) bispecific antibodies (Proteus) (Inst), a N-acetylgalactosamino dendron-clearing agent for DOTA-pretargeted radioimmunotherapy (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Wu Z, Cheung NV. T cell engaging bispecific antibody (T-BsAb): From technology to therapeutics. Pharmacol Ther. 2018;182:161–175. doi: 10.1016/j.pharmthera.2017.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheung NK, Cheung IY, Kushner BH, et al. Murine anti-GD2 monoclonal antibody 3F8 combined with granulocyte-macrophage colony-stimulating factor and 13-cis-retinoic acid in high-risk patients with stage 4 neuroblastoma in first remission. J Clin Oncol. 2012;30:3264–3270. doi: 10.1200/JCO.2011.41.3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu AL, Gilman AL, Ozkaynak MF, et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med. 2010;363:1324–1334. doi: 10.1056/NEJMoa0911123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kramer K, Kushner BH, Modak S, et al. Compartmental intrathecal radioimmunotherapy: Results for treatment for metastatic CNS neuroblastoma. J Neurooncol. 2010;97:409–418. doi: 10.1007/s11060-009-0038-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kushner BH, Cheung IY, Modak S, et al. Phase I trial of a bivalent gangliosides vaccine in combination with β-glucan for high-risk neuroblastoma in second or later remission. Clin Cancer Res. 2014;20:1375–1382. doi: 10.1158/1078-0432.CCR-13-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leung W, Heslop HE. Adoptive immunotherapy with antigen-specific T cells expressing a native TCR. Cancer Immunol Res. 2019;7:528–533. doi: 10.1158/2326-6066.CIR-18-0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martinez-Quintanilla J, Seah I, Chua M, et al. Oncolytic viruses: Overcoming translational challenges. J Clin Invest. 2019;130:1407–1418. doi: 10.1172/JCI122287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elster JD, Krishnadas DK, Lucas KG. Dendritic cell vaccines: A review of recent developments and their potential pediatric application. Hum Vaccin Immunother. 2016;12:2232–2239. doi: 10.1080/21645515.2016.1179844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Richards RM, Sotillo E, Majzner RG. CAR T cell therapy for neuroblastoma. Front Immunol. 2018;9:2380. doi: 10.3389/fimmu.2018.02380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ambros PF, Ambros IM, Brodeur GM, et al. International consensus for neuroblastoma molecular diagnostics: Report from the International Neuroblastoma Risk Group (INRG) Biology Committee. Br J Cancer. 2009;100:1471–1482. doi: 10.1038/sj.bjc.6605014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Squire R, Fowler CL, Brooks SP, et al. The relationship of class I MHC antigen expression to stage IV-S disease and survival in neuroblastoma. J Pediatr Surg. 1990;25:381–386. doi: 10.1016/0022-3468(90)90375-j. [DOI] [PubMed] [Google Scholar]
- 12.Brodeur GM, Bagatell R. Mechanisms of neuroblastoma regression. Nat Rev Clin Oncol. 2014;11:704–713. doi: 10.1038/nrclinonc.2014.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mina M, Boldrini R, Citti A, et al. Tumor-infiltrating T lymphocytes improve clinical outcome of therapy-resistant neuroblastoma. Oncoimmunology. 2015;4:e1019981. doi: 10.1080/2162402X.2015.1019981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anand G, Bridge H, Rackstraw P, et al. Cerebellar and cortical abnormalities in paediatric opsoclonus-myoclonus syndrome. Dev Med Child Neurol. 2015;57:265–272. doi: 10.1111/dmcn.12594. [DOI] [PubMed] [Google Scholar]
- 15.Pranzatelli MR, Tate ED, McGee NR, et al. CSF neurofilament light chain is elevated in OMS (decreasing with immunotherapy) and other pediatric neuroinflammatory disorders. J Neuroimmunol. 2014;266:75–81. doi: 10.1016/j.jneuroim.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 16.Stefanowicz J, Izycka-Swieszewska E, Drozyńska E, et al. Neuroblastoma and opsoclonus-myoclonus-ataxia syndrome: Clinical and pathological characteristics. Folia Neuropathol. 2008;46:176–185. [PubMed] [Google Scholar]
- 17.Asgharzadeh S, Salo JA, Ji L, et al. Clinical significance of tumor-associated inflammatory cells in metastatic neuroblastoma. J Clin Oncol. 2012;30:3525–3532. doi: 10.1200/JCO.2011.40.9169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pelizzo G, Veschi V, Mantelli M, et al. Microenvironment in neuroblastoma: Isolation and characterization of tumor-derived mesenchymal stromal cells. BMC Cancer. 2018;18:1176. doi: 10.1186/s12885-018-5082-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jales A, Falahati R, Mari E, et al. Ganglioside-exposed dendritic cells inhibit T-cell effector function by promoting regulatory cell activity. Immunology. 2011;132:134–143. doi: 10.1111/j.1365-2567.2010.03348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wondimu A, Liu Y, Su Y, et al. Gangliosides drive the tumor infiltration and function of myeloid-derived suppressor cells. Cancer Res. 2014;74:5449–5457. doi: 10.1158/0008-5472.CAN-14-0927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shurin GV, Gerein V, Lotze MT, et al. Apoptosis induced in T cells by human neuroblastoma cells: Role of Fas ligand. Nat Immun. 1998;16:263–274. doi: 10.1159/000069452. [DOI] [PubMed] [Google Scholar]
- 22.Raffaghello L, Prigione I, Airoldi I, et al. Mechanisms of immune evasion of human neuroblastoma. Cancer Lett. 2005;228:155–161. doi: 10.1016/j.canlet.2004.11.064. [DOI] [PubMed] [Google Scholar]
- 23.Grayson G, Ladisch S. Immunosuppression by human gangliosides: II. Carbohydrate structure and inhibition of human NK activity. Cell Immunol. 1992;139:18–29. doi: 10.1016/0008-8749(92)90096-8. [DOI] [PubMed] [Google Scholar]
- 24.Perdicchio M, Ilarregui JM, Verstege MI, et al. Sialic acid-modified antigens impose tolerance via inhibition of T-cell proliferation and de novo induction of regulatory T cells. Proc Natl Acad Sci USA. 2016;113:3329–3334. doi: 10.1073/pnas.1507706113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nallasamy P, Chava S, Verma SS, et al. PD-L1, inflammation, non-coding RNAs, and neuroblastoma: Immuno-oncology perspective. Semin Cancer Biol. 2018;52:53–65. doi: 10.1016/j.semcancer.2017.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dondero A, Pastorino F, Della Chiesa M, et al. PD-L1 expression in metastatic neuroblastoma as an additional mechanism for limiting immune surveillance. Oncoimmunology. 2015;5:e1064578. doi: 10.1080/2162402X.2015.1064578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dobrenkov K, Cheung NK. GD2-targeted immunotherapy and radioimmunotherapy. Semin Oncol. 2014;41:589–612. doi: 10.1053/j.seminoncol.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheever MA, Allison JP, Ferris AS, et al. The prioritization of cancer antigens: A national cancer institute pilot project for the acceleration of translational research. Clin Cancer Res. 2009;15:5323–5337. doi: 10.1158/1078-0432.CCR-09-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lammie G, Cheung N, Gerald W, et al. Ganglioside gd(2) expression in the human nervous-system and in neuroblastomas: An immunohistochemical study. Int J Oncol. 1993;3:909–915. doi: 10.3892/ijo.3.5.909. [DOI] [PubMed] [Google Scholar]
- 30.Liu Y, Wondimu A, Yan S, et al. Tumor gangliosides accelerate murine tumor angiogenesis. Angiogenesis. 2014;17:563–571. doi: 10.1007/s10456-013-9403-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suzuki M, Cheung NK. Disialoganglioside GD2 as a therapeutic target for human diseases. Expert Opin Ther Targets. 2015;19:349–362. doi: 10.1517/14728222.2014.986459. [DOI] [PubMed] [Google Scholar]
- 32.Julien S, Bobowski M, Steenackers A, et al. How do gangliosides regulate RTKs signaling? Cells. 2013;2:751–767. doi: 10.3390/cells2040751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ahmed M, Cheung NK. Engineering anti-GD2 monoclonal antibodies for cancer immunotherapy. FEBS Lett. 2014;588:288–297. doi: 10.1016/j.febslet.2013.11.030. [DOI] [PubMed] [Google Scholar]
- 34.Xu H, Cheng M, Guo H, et al. Retargeting T cells to GD2 pentasaccharide on human tumors using bispecific humanized antibody. Cancer Immunol Res. 2015;3:266–277. doi: 10.1158/2326-6066.CIR-14-0230-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Louis CU, Savoldo B, Dotti G, et al. Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood. 2011;118:6050–6056. doi: 10.1182/blood-2011-05-354449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun TW, Gao Q, Qiu SJ, et al. B7-H3 is expressed in human hepatocellular carcinoma and is associated with tumor aggressiveness and postoperative recurrence. Cancer Immunol Immunother. 2012;61:2171–2182. doi: 10.1007/s00262-012-1278-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang P, Yu S, Li H, et al. ILT4 drives B7-H3 expression via PI3K/AKT/mTOR signalling and ILT4/B7-H3 co-expression correlates with poor prognosis in non-small cell lung cancer. FEBS Lett. 2015;589:2248–2256. doi: 10.1016/j.febslet.2015.06.037. [DOI] [PubMed] [Google Scholar]
- 38. doi: 10.1016/j.ccell.2019.01.002. Du H, Hirabayashi K, Ahn S, et al: Antitumor responses in the absence of toxicity in solid tumors by targeting B7-H3 via chimeric antigen receptor T cells. Cancer Cell 35:221-237.e8, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu H, Cheung IY, Guo HF, et al. MicroRNA miR-29 modulates expression of immunoinhibitory molecule B7-H3: Potential implications for immune based therapy of human solid tumors. Cancer Res. 2009;69:6275–6281. doi: 10.1158/0008-5472.CAN-08-4517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Castriconi R, Dondero A, Augugliaro R, et al. Identification of 4Ig-B7-H3 as a neuroblastoma-associated molecule that exerts a protective role from an NK cell-mediated lysis. Proc Natl Acad Sci USA. 2004;101:12640–12645. doi: 10.1073/pnas.0405025101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Modak S, Carrasquillo J, LaQuaglia M, et al. Intraperitoneal radioimmunotherapy for desmoplastic small round cell tumor: Results of a phase I study ( NCT01099644) Cancer Res. 2018 ;78 (abstr CT006) [Google Scholar]
- 42.Loo D, Alderson RF, Chen FZ, et al. Development of an Fc-enhanced anti-B7-H3 monoclonal antibody with potent antitumor activity. Clin Cancer Res. 2012;18:3834–3845. doi: 10.1158/1078-0432.CCR-12-0715. [DOI] [PubMed] [Google Scholar]
- 43.Wang L, Kang FB, Shan BE. B7-H3-mediated tumor immunology: Friend or foe? Int J Cancer. 2014;134:2764–2771. doi: 10.1002/ijc.28474. [DOI] [PubMed] [Google Scholar]
- 44.van Gaal JC, Flucke UE, Roeffen MH, et al. Anaplastic lymphoma kinase aberrations in rhabdomyosarcoma: Clinical and prognostic implications. J Clin Oncol. 2012;30:308–315. doi: 10.1200/JCO.2011.37.8588. [DOI] [PubMed] [Google Scholar]
- 45.Lamant L, Pulford K, Bischof D, et al. Expression of the ALK tyrosine kinase gene in neuroblastoma. Am J Pathol. 2000;156:1711–1721. doi: 10.1016/S0002-9440(10)65042-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bresler SC, Weiser DA, Huwe PJ, et al. ALK mutations confer differential oncogenic activation and sensitivity to ALK inhibition therapy in neuroblastoma. Cancer Cell. 2014;26:682–694. doi: 10.1016/j.ccell.2014.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schulte JH, Schulte S, Heukamp LC, et al. Targeted therapy for neuroblastoma: ALK inhibitors. Klin Padiatr. 2013;225:303–308. doi: 10.1055/s-0033-1357132. [DOI] [PubMed] [Google Scholar]
- 48.Chiarle R, Martinengo C, Mastini C, et al. The anaplastic lymphoma kinase is an effective oncoantigen for lymphoma vaccination. Nat Med. 2008;14:676–680. doi: 10.1038/nm1769. [DOI] [PubMed] [Google Scholar]
- 49.Ait-Tahar K, Damm-Welk C, Burkhardt B, et al. Correlation of the autoantibody response to the ALK oncoantigen in pediatric anaplastic lymphoma kinase-positive anaplastic large cell lymphoma with tumor dissemination and relapse risk. Blood. 2010;115:3314–3319. doi: 10.1182/blood-2009-11-251892. [DOI] [PubMed] [Google Scholar]
- 50.Toor JS, Rao AA, McShan AC, et al. A recurrent mutation in anaplastic lymphoma kinase with distinct neoepitope conformations. Front Immunol. 2018;9:99. doi: 10.3389/fimmu.2018.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carpenter EL, Haglund EA, Mace EM, et al. Antibody targeting of anaplastic lymphoma kinase induces cytotoxicity of human neuroblastoma. Oncogene. 2012;31:4859–4867. doi: 10.1038/onc.2011.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sano R, Krytska K, Larmour CE, et al. An antibody-drug conjugate directed to the ALK receptor demonstrates efficacy in preclinical models of neuroblastoma. Sci Transl Med. 2019;11:eaau9732. doi: 10.1126/scitranslmed.aau9732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Köhler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- 54.Mujoo K, Kipps TJ, Yang HM, et al. Functional properties and effect on growth suppression of human neuroblastoma tumors by isotype switch variants of monoclonal antiganglioside GD2 antibody 14.18. Cancer Res. 1989;49:2857–2861. [PubMed] [Google Scholar]
- 55.Perez Horta Z, Goldberg JL, Sondel PM. Anti-GD2 mAbs and next-generation mAb-based agents for cancer therapy. Immunotherapy. 2016;8:1097–1117. doi: 10.2217/imt-2016-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Munn DH, Cheung NK. Interleukin-2 enhancement of monoclonal antibody-mediated cellular cytotoxicity against human melanoma. Cancer Res. 1987;47:6600–6605. [PubMed] [Google Scholar]
- 57.Kushner BH, Cheung NK. GM-CSF enhances 3F8 monoclonal antibody-dependent cellular cytotoxicity against human melanoma and neuroblastoma. Blood. 1989;73:1936–1941. [PubMed] [Google Scholar]
- 58.Munn DH, Cheung NK. Antibody-dependent antitumor cytotoxicity by human monocytes cultured with recombinant macrophage colony-stimulating factor: Induction of efficient antibody-mediated antitumor cytotoxicity not detected by isotope release assays. J Exp Med. 1989;170:511–526. doi: 10.1084/jem.170.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cheung NK, Walter EI, Smith-Mensah WH, et al. Decay-accelerating factor protects human tumor cells from complement-mediated cytotoxicity in vitro. J Clin Invest. 1988;81:1122–1128. doi: 10.1172/JCI113426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen S, Caragine T, Cheung NK, et al. CD59 expressed on a tumor cell surface modulates decay-accelerating factor expression and enhances tumor growth in a rat model of human neuroblastoma. Cancer Res. 2000;60:3013–3018. [PubMed] [Google Scholar]
- 61.Ladenstein R, Pötschger U, Valteau-Couanet D, et al. Interleukin 2 with anti-GD2 antibody ch14.18/CHO (dinutuximab beta) in patients with high-risk neuroblastoma (HR-NBL1/SIOPEN): A multicentre, randomised, phase 3 trial. Lancet Oncol. 2018;19:1617–1629. doi: 10.1016/S1470-2045(18)30578-3. [DOI] [PubMed] [Google Scholar]
- 62.Saarinen UM, Coccia PF, Gerson SL, et al. Eradication of neuroblastoma cells in vitro by monoclonal antibody and human complement: Method for purging autologous bone marrow. Cancer Res. 1985;45:5969–5975. [PubMed] [Google Scholar]
- 63.Ding YY, Panzer J, Maris JM, et al. Transverse myelitis as an unexpected complication following treatment with dinutuximab in pediatric patients with high-risk neuroblastoma: A case series. Pediatr Blood Cancer. doi: 10.1002/pbc.26732. [epub ahead of print on July 27, 2017] [DOI] [PubMed] [Google Scholar]
- 64.Kushner BH, Modak S, Basu EM, et al. Posterior reversible encephalopathy syndrome in neuroblastoma patients receiving anti-G 3F8 monoclonal antibody. Cancer. 2013;119:2789–2795. doi: 10.1002/cncr.28137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ladenstein R, Weixler S, Baykan B, et al. Ch14.18 antibody produced in CHO cells in relapsed or refractory stage 4 neuroblastoma patients: A SIOPEN phase 1 study. MAbs. 2013;5:801–809. doi: 10.4161/mabs.25215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cheung NK, Guo H, Hu J, et al. Humanizing murine IgG3 anti-GD2 antibody m3F8 substantially improves antibody-dependent cell-mediated cytotoxicity while retaining targeting in vivo. Oncoimmunology. 2012;1:477–486. doi: 10.4161/onci.19864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cheung IY, Kushner BH, Modak S, et al. Phase I trial of anti-GD2 monoclonal antibody hu3F8 plus GM-CSF: Impact of body weight, immunogenicity and anti-GD2 response on pharmacokinetics and survival. Oncoimmunology. 2017;6:e1358331. doi: 10.1080/2162402X.2017.1358331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. doi: 10.1001/jamaoncol.2018.4005. Kushner BH, Cheung IY, Modak S, et al: Humanized 3F8 anti-GD2 monoclonal antibody dosing with granulocyte-macrophage colony-stimulating factor in patients with resistant neuroblastoma: A phase 1 clinical trial. JAMA Oncol 4:1729-1735, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sopp J, Cragg MS. Deleting malignant B cells with second-generation anti-CD20 antibodies. J Clin Oncol. 2018;36:2323–2325. doi: 10.1200/JCO.2018.78.7390. [DOI] [PubMed] [Google Scholar]
- 70.Idusogie EE, Presta LG, Gazzano-Santoro H, et al. Mapping of the C1q binding site on Rituxan, a chimeric antibody with a human IgG1 Fc. J Immunol. 2000;164:4178–4184. doi: 10.4049/jimmunol.164.8.4178. [DOI] [PubMed] [Google Scholar]
- 71.Beck A, Goetsch L, Dumontet C, et al. Strategies and challenges for the next generation of antibody-drug conjugates. Nat Rev Drug Discov. 2017;16:315–337. doi: 10.1038/nrd.2016.268. [DOI] [PubMed] [Google Scholar]
- 72.Larson SM, Carrasquillo JA, Cheung NK, et al. Radioimmunotherapy of human tumours. Nat Rev Cancer. 2015;15:347–360. doi: 10.1038/nrc3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Neri D. Antibody-cytokine fusions: Versatile products for the modulation of anticancer immunity. Cancer Immunol Res. 2019;7:348–354. doi: 10.1158/2326-6066.CIR-18-0622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Smith SV. Technology evaluation: huN901-DM1, ImmunoGen. Curr Opin Mol Ther. 2005;7:394–401. [PubMed] [Google Scholar]
- 75.Feng Y, Wang Y, Zhu Z, et al. Differential killing of CD56-expressing cells by drug-conjugated human antibodies targeting membrane-distal and membrane-proximal non-overlapping epitopes. MAbs. 2016;8:799–810. doi: 10.1080/19420862.2016.1155014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gholizadeh S, Dolman EM, Wieriks R, et al. Anti-GD2 immunoliposomes for targeted delivery of the survivin inhibitor sepantronium bromide (YM155) to neuroblastoma tumor cells. Pharm Res. 2018;35:85. doi: 10.1007/s11095-018-2373-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Brown BS, Patanam T, Mobli K, et al. Etoposide-loaded immunoliposomes as active targeting agents for GD2-positive malignancies. Cancer Biol Ther. 2014;15:851–861. doi: 10.4161/cbt.28875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Martins CD, Kramer-Marek G, Oyen WJG. Radioimmunotherapy for delivery of cytotoxic radioisotopes: Current status and challenges. Expert Opin Drug Deliv. 2018;15:185–196. doi: 10.1080/17425247.2018.1378180. [DOI] [PubMed] [Google Scholar]
- 79.Kramer K, Humm JL, Souweidane MM, et al. Phase I study of targeted radioimmunotherapy for leptomeningeal cancers using intra-Ommaya 131-I-3F8. J Clin Oncol. 2007;25:5465–5470. doi: 10.1200/JCO.2007.11.1807. [DOI] [PubMed] [Google Scholar]
- 80.Souweidane MM, Kramer K, Pandit-Taskar N, et al. Convection-enhanced delivery for diffuse intrinsic pontine glioma: A single-centre, dose-escalation, phase 1 trial. Lancet Oncol. 2018;19:1040–1050. doi: 10.1016/S1470-2045(18)30322-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Miederer M, McDevitt MR, Borchardt P, et al. Treatment of neuroblastoma meningeal carcinomatosis with intrathecal application of alpha-emitting atomic nanogenerators targeting disialo-ganglioside GD2. Clin Cancer Res. 2004;10:6985–6992. doi: 10.1158/1078-0432.CCR-04-0859. [DOI] [PubMed] [Google Scholar]
- 82.Lode HN, Reisfeld RA. Targeted cytokines for cancer immunotherapy. Immunol Res. 2000;21:279–288. doi: 10.1385/IR:21:2-3:279. [DOI] [PubMed] [Google Scholar]
- 83.Shusterman S, London WB, Gillies SD, et al. Antitumor activity of hu14.18-IL2 in patients with relapsed/refractory neuroblastoma: A Children’s Oncology Group (COG) phase II study. J Clin Oncol. 2010;28:4969–4975. doi: 10.1200/JCO.2009.27.8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yang RK, Kalogriopoulos NA, Rakhmilevich AL, et al. Intratumoral hu14.18-IL-2 (IC) induces local and systemic antitumor effects that involve both activated T and NK cells as well as enhanced IC retention. J Immunol. 2012;189:2656–2664. doi: 10.4049/jimmunol.1200934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mortara L, Balza E, Bruno A, et al. Anti-cancer therapies employing IL-2 cytokine tumor targeting: Contribution of innate, adaptive and immunosuppressive cells in the anti-tumor efficacy. Front Immunol. 2018;9:2905. doi: 10.3389/fimmu.2018.02905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vincent M, Bessard A, Cochonneau D, et al. Tumor targeting of the IL-15 superagonist RLI by an anti-GD2 antibody strongly enhances its antitumor potency. Int J Cancer. 2013;133:757–765. doi: 10.1002/ijc.28059. [DOI] [PubMed] [Google Scholar]
- 87.Brinkmann U, Kontermann RE. The making of bispecific antibodies. MAbs. 2017;9:182–212. doi: 10.1080/19420862.2016.1268307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kantarjian H, Stein A, Gökbuget N, et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med. 2017;376:836–847. doi: 10.1056/NEJMoa1609783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kontermann RE, Brinkmann U. Bispecific antibodies. Drug Discov Today. 2015;20:838–847. doi: 10.1016/j.drudis.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 90.Ward ES, Ober RJ. Targeting FcRn to generate antibody-based therapeutics. Trends Pharmacol Sci. 2018;39:892–904. doi: 10.1016/j.tips.2018.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang LL, Hoseini SS, Xu H, et al. Silencing Fc in T cell engaging bispecific antibodies is critical for T cell trafficking and anti-tumor potency. Cancer Immunol Res. 2019;7:2013–2024. doi: 10.1158/2326-6066.CIR-19-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Michon J, Perdereau B, Brixy F, et al. In vivo targeting of human neuroblastoma xenograft by anti-GD2/anti-Fc gamma RI (CD64) bispecific antibody. Eur J Cancer. 1995;31A:631–636. doi: 10.1016/0959-8049(95)00013-9. [DOI] [PubMed] [Google Scholar]
- 93.Cheng M, Ahmed M, Xu H, et al. Structural design of disialoganglioside GD2 and CD3-bispecific antibodies to redirect T cells for tumor therapy. Int J Cancer. 2015;136:476–486. doi: 10.1002/ijc.29007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cheng M, Santich BH, Xu H, et al. Successful engineering of a highly potent single-chain variable-fragment (scFv) bispecific antibody to target disialoganglioside (GD2) positive tumors. Oncoimmunology. 2016;5:e1168557. doi: 10.1080/2162402X.2016.1168557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yankelevich M, Kondadasula SV, Thakur A, et al. Anti-CD3 × anti-GD2 bispecific antibody redirects T-cell cytolytic activity to neuroblastoma targets. Pediatr Blood Cancer. 2012;59:1198–1205. doi: 10.1002/pbc.24237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cheal SM, Xu H, Guo HF, et al. Preclinical evaluation of multistep targeting of diasialoganglioside GD2 using an IgG-scFv bispecific antibody with high affinity for GD2 and DOTA metal complex. Mol Cancer Ther. 2014;13:1803–1812. doi: 10.1158/1535-7163.MCT-13-0933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Klein C, Cragg MS, Fuh G: Self-assembling and disassembling (SADA) bispecific antibodies (BsAb) for 2-step pretargeted radioimmunotherapy (PRIT). Presented at the Keystone Symposia on Molecular and Cellular Biology, Breckenridge, CO, April 7-11, 2019. [Google Scholar]
- 98.Navid F, Sondel PM, Barfield R, et al. Phase I trial of a novel anti-GD2 monoclonal antibody, Hu14.18K322A, designed to decrease toxicity in children with refractory or recurrent neuroblastoma. J Clin Oncol. 2014;32:1445–1452. doi: 10.1200/JCO.2013.50.4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Xu H, Guo H, Cheung IY, et al. Antitumor efficacy of anti-GD2 IgG1 is enhanced by Fc glyco-engineering. Cancer Immunol Res. 2016;4:631–638. doi: 10.1158/2326-6066.CIR-15-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wing A, Fajardo CA, Posey AD, Jr, et al. Improving CART-cell therapy of solid tumors with oncolytic virus-driven production of a bispecific T-cell engager. Cancer Immunol Res. 2018;6:605–616. doi: 10.1158/2326-6066.CIR-17-0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cheung NK, Dyer MA. Neuroblastoma: Developmental biology, cancer genomics and immunotherapy. Nat Rev Cancer. 2013;13:397–411. doi: 10.1038/nrc3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cheung NK, Cheung IY, Kramer K, et al. Key role for myeloid cells: Phase II results of anti-G(D2) antibody 3F8 plus granulocyte-macrophage colony-stimulating factor for chemoresistant osteomedullary neuroblastoma. Int J Cancer. 2014;135:2199–2205. doi: 10.1002/ijc.28851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Steel JC, Waldmann TA, Morris JC. Interleukin-15 biology and its therapeutic implications in cancer. Trends Pharmacol Sci. 2012;33:35–41. doi: 10.1016/j.tips.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tzeng A, Kwan BH, Opel CF, et al. Antigen specificity can be irrelevant to immunocytokine efficacy and biodistribution. Proc Natl Acad Sci USA. 2015;112:3320–3325. doi: 10.1073/pnas.1416159112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Morris ZS, Guy EI, Francis DM, et al. In situ tumor vaccination by combining local radiation and tumor-specific antibody or immunocytokine treatments. Cancer Res. 2016;76:3929–3941. doi: 10.1158/0008-5472.CAN-15-2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gabrilovich DI. Myeloid-derived suppressor cells. Cancer Immunol Res. 2017;5:3–8. doi: 10.1158/2326-6066.CIR-16-0297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Binnewies M, Roberts EW, Kersten K, et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med. 2018;24:541–550. doi: 10.1038/s41591-018-0014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wu HW, Sheard MA, Malvar J, et al. Anti-CD105 antibody eliminates tumor microenvironment cells and enhances anti-GD2 antibody immunotherapy of neuroblastoma with activated natural killer cells. Clin Cancer Res. 2019;25:4761–4774. doi: 10.1158/1078-0432.CCR-18-3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kroesen M, Büll C, Gielen PR, et al. Anti-GD2 mAb and vorinostat synergize in the treatment of neuroblastoma. Oncoimmunology. 2016;5:e1164919. doi: 10.1080/2162402X.2016.1164919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Boudreau JE, Giglio F, Gooley TA, et al. KIR3DL1/HLA-B subtypes govern acute myelogenous leukemia relapse after hematopoietic cell transplantation. J Clin Oncol. 2017;35:2268–2278. doi: 10.1200/JCO.2016.70.7059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.van der Touw W, Chen HM, Pan PY, et al. LILRB receptor-mediated regulation of myeloid cell maturation and function. Cancer Immunol Immunother. 2017;66:1079–1087. doi: 10.1007/s00262-017-2023-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Feng M, Jiang W, Kim BYS, et al. Phagocytosis checkpoints as new targets for cancer immunotherapy. Nat Rev Cancer. 2019;19:568–586. doi: 10.1038/s41568-019-0183-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Blake SJ, Dougall WC, Miles JJ, et al. Molecular pathways: Targeting CD96 and TIGIT for cancer immunotherapy. Clin Cancer Res. 2016;22:5183–5188. doi: 10.1158/1078-0432.CCR-16-0933. [DOI] [PubMed] [Google Scholar]
- 114. doi: 10.1016/j.cell.2018.10.014. Andre P, Denis C, Soulas C, et al: Anti-NKG2A mAb is a checkpoint inhibitor that promotes anti-tumor immunity by unleashing both T and NK cells. Cell 175:1731-1743.e13, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kohrt HE, Thielens A, Marabelle A, et al. Anti-KIR antibody enhancement of anti-lymphoma activity of natural killer cells as monotherapy and in combination with anti-CD20 antibodies. Blood. 2014;123:678–686. doi: 10.1182/blood-2013-08-519199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Siebert N, Zumpe M, Jüttner M, et al. PD-1 blockade augments anti-neuroblastoma immune response induced by anti-GD2 antibody ch14.18/CHO. Oncoimmunology. 2017;6:e1343775. doi: 10.1080/2162402X.2017.1343775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Iida N, Dzutsev A, Stewart CA, et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science. 2013;342:967–970. doi: 10.1126/science.1240527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gopalakrishnan V, Spencer CN, Nezi L, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 2018;359:97–103. doi: 10.1126/science.aan4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. doi: 10.1016/j.cell.2019.08.010. Hagan T, Cortese M, Rouphael N, et al: Antibiotics-driven gut microbiome perturbation alters immunity to vaccines in humans. Cell 178:1313-1328.e13, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Forlenza CJ, Boudreau JE, Zheng J, et al. KIR3DL1 allelic polymorphism and HLA-B epitopes modulate response to anti-GD2 monoclonal antibody in patients with neuroblastoma. J Clin Oncol. 2016;34:2443–2451. doi: 10.1200/JCO.2015.64.9558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Delgado DC, Hank JA, Kolesar J, et al. Genotypes of NK cell KIR receptors, their ligands, and Fcγ receptors in the response of neuroblastoma patients to Hu14.18-IL2 immunotherapy. Cancer Res. 2010;70:9554–9561. doi: 10.1158/0008-5472.CAN-10-2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cheung NK, Sowers R, Vickers AJ, et al. FCGR2A polymorphism is correlated with clinical outcome after immunotherapy of neuroblastoma with anti-GD2 antibody and granulocyte macrophage colony-stimulating factor. J Clin Oncol. 2006;24:2885–2890. doi: 10.1200/JCO.2005.04.6011. [DOI] [PubMed] [Google Scholar]
- 123.Terzic T, Cordeau M, Herblot S, et al. Expression of disialoganglioside (GD2) in neuroblastic tumors: A prognostic value for patients treated with anti-GD2 immunotherapy. Pediatr Dev Pathol. 2018;21:355–362. doi: 10.1177/1093526617723972. [DOI] [PubMed] [Google Scholar]
- 124.Cheung NK, Ostrovnaya I, Kuk D, et al. Bone marrow minimal residual disease was an early response marker and a consistent independent predictor of survival after anti-GD2 immunotherapy. J Clin Oncol. 2015;33:755–763. doi: 10.1200/JCO.2014.57.6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Beiske K, Burchill SA, Cheung IY, et al. Consensus criteria for sensitive detection of minimal neuroblastoma cells in bone marrow, blood and stem cell preparations by immunocytology and QRT-PCR: Recommendations by the International Neuroblastoma Risk Group Task Force. Br J Cancer. 2009;100:1627–1637. doi: 10.1038/sj.bjc.6605029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.van Wezel EM, Zwijnenburg D, Zappeij-Kannegieter L, et al. Whole-genome sequencing identifies patient-specific DNA minimal residual disease markers in neuroblastoma. J Mol Diagn. 2015;17:43–52. doi: 10.1016/j.jmoldx.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 127.Gholamin S, Mirzaei H, Razavi SM, et al. GD2-targeted immunotherapy and potential value of circulating microRNAs in neuroblastoma. J Cell Physiol. 2018;233:866–879. doi: 10.1002/jcp.25793. [DOI] [PubMed] [Google Scholar]
- 128.Havel JJ, Chowell D, Chan TA. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat Rev Cancer. 2019;19:133–150. doi: 10.1038/s41568-019-0116-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mody R, Naranjo A, Van Ryn C, et al. Irinotecan-temozolomide with temsirolimus or dinutuximab in children with refractory or relapsed neuroblastoma (COG ANBL1221): An open-label, randomised, phase 2 trial. Lancet Oncol. 2017;18:946–957. doi: 10.1016/S1470-2045(17)30355-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Federico SM, McCarville MB, Shulkin BL, et al. A pilot trial of humanized anti-GD2 monoclonal antibody (hu14.18K322A) with chemotherapy and natural killer cells in children with recurrent/refractory neuroblastoma. Clin Cancer Res. 2017;23:6441–6449. doi: 10.1158/1078-0432.CCR-17-0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Furman WL, Federico SM, McCarville MB, et al. A phase II trial of Hu14.18K322A in combination with induction chemotherapy in children with newly diagnosed high-risk neuroblastoma. Clin Cancer Res. 2019;25:6320–6328. doi: 10.1158/1078-0432.CCR-19-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. doi: 10.1016/j.ccell.2017.08.003. Bosse KR, Raman P, Zhu Z, et al: Identification of GPC2 as an oncoprotein and candidate immunotherapeutic target in high-risk neuroblastoma. Cancer Cell 32:295-309.e12, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Li N, Fu H, Hewitt SM, et al. Therapeutically targeting glypican-2 via single-domain antibody-based chimeric antigen receptors and immunotoxins in neuroblastoma. Proc Natl Acad Sci USA. 2017;114:E6623–E6631. doi: 10.1073/pnas.1706055114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ishiguro T, Sano Y, Komatsu SI, et al. An anti-glypican 3/CD3 bispecific T cell-redirecting antibody for treatment of solid tumors. Sci Transl Med. 2017;9:eaal4291. doi: 10.1126/scitranslmed.aal4291. [DOI] [PubMed] [Google Scholar]
- 135.Li W, Guo L, Rathi P, et al. Redirecting T cells to glypican-3 with 4-1BB.zeta CAR results in Th-1 polarization and potent anti-tumor activity. Hum Gene Ther. doi: 10.1089/hum.2016.025. 28:437-448, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Dubrovsky L, Dao T, Gejman RS, et al. T cell receptor mimic antibodies for cancer therapy. Oncoimmunology. 2015;5:e1049803. doi: 10.1080/2162402X.2015.1049803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ahmed M, Lopez-Albaitero A, Pankov D, et al. TCR-mimic bispecific antibodies targeting LMP2A show potent activity against EBV malignancies. JCI Insight. 2018;3:97805. doi: 10.1172/jci.insight.97805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sarkar AK, Nuchtern JG. Lysis of MYCN-amplified neuroblastoma cells by MYCN peptide-specific cytotoxic T lymphocytes. Cancer Res. 2000;60:1908–1913. [PubMed] [Google Scholar]
- 139.Wölfl M, Jungbluth AA, Garrido F, et al. Expression of MHC class I, MHC class II, and cancer germline antigens in neuroblastoma. Cancer Immunol Immunother. 2005;54:400–406. doi: 10.1007/s00262-004-0603-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Spel L, Boelens JJ, van der Steen DM, et al. Natural killer cells facilitate PRAME-specific T-cell reactivity against neuroblastoma. Oncotarget. 2015;6:35770–35781. doi: 10.18632/oncotarget.5657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chang AY, Dao T, Gejman RS, et al. A therapeutic T cell receptor mimic antibody targets tumor-associated PRAME peptide/HLA-I antigens. J Clin Invest. 2017;127:3557. doi: 10.1172/JCI96860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Pankov D, Sjöström L, Kalidindi T, et al. In vivo immuno-targeting of an extracellular epitope of membrane bound preferentially expressed antigen in melanoma (PRAME) Oncotarget. 2017;8:65917–65931. doi: 10.18632/oncotarget.19579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Dao T, Pankov D, Scott A, et al. Therapeutic bispecific T-cell engager antibody targeting the intracellular oncoprotein WT1. Nat Biotechnol. 2015;33:1079–1086. doi: 10.1038/nbt.3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lev A, Denkberg G, Cohen CJ, et al. Isolation and characterization of human recombinant antibodies endowed with the antigen-specific, major histocompatibility complex-restricted specificity of T cells directed toward the widely expressed tumor T-cell epitopes of the telomerase catalytic subunit. Cancer Res. 2002;62:3184–3194. [PubMed] [Google Scholar]
- 145. Yarmarkovich M, Marco MD, Nguyen S, et al: Novel MHC antigens in neuroblastoma and development of tumor-specific T-cells. Presented at the Advances in Neuroblastoma Research Association Conference, San Francisco, CA, May 9-12, 2018. [Google Scholar]
- 146.Machutta CA, Kollmann CS, Lind KE, et al. Prioritizing multiple therapeutic targets in parallel using automated DNA-encoded library screening. Nat Commun. 2017;8:16081. doi: 10.1038/ncomms16081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Pistoia V, Morandi F, Bianchi G, et al. Immunosuppressive microenvironment in neuroblastoma. Front Oncol. 2013;3:167. doi: 10.3389/fonc.2013.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]