Abstract
PURPOSE
Platinum-based chemotherapy for first-line treatment of metastatic urothelial cancer is typically administered for a fixed duration followed by observation until progression. “Switch maintenance” therapy with PD-1 blockade at the time of chemotherapy cessation may be attractive for mechanistic and pragmatic reasons.
PATIENTS AND METHODS
Patients with metastatic urothelial cancer achieving at least stable disease on first-line platinum-based chemotherapy were enrolled. Patients were randomly assigned double-blind 1:1 to switch maintenance pembrolizumab 200 mg intravenously once every 3 weeks versus placebo for up to 24 months. Patients with disease progression on placebo could cross over to pembrolizumab. The primary objective was to determine the progression-free survival. Secondary objectives included determining overall survival as well as treatment outcomes according to PD-L1 combined positive score (CPS).
RESULTS
Between December 2015 and November 2018, 108 patients were randomly assigned to pembrolizumab (n = 55) or placebo (n = 53). The objective response rate was 23% with pembrolizumab and 10% with placebo. Treatment-emergent grade 3-4 adverse events occurred in 59% receiving pembrolizumab and 38% of patients receiving placebo. Progression-free survival was significantly longer with maintenance pembrolizumab versus placebo (5.4 months [95% CI, 3.1 to 7.3 months] v 3.0 months [95% CI; 2.7 to 5.5 months]; hazard ratio, 0.65; log-rank P = .04; maximum efficiency robust test P = .039). Median overall survival was 22 months (95% CI, 12.9 months to not reached) with pembrolizumab and 18.7 months (95% CI, 11.4 months to not reached) with placebo. There was no significant interaction between PD-L1 CPS ≥ 10 and treatment arm for progression-free survival or overall survival.
CONCLUSION
Switch maintenance pembrolizumab leads to additional objective responses in patients achieving at least stable disease with first-line platinum-based chemotherapy and prolongs progression-free survival in patients with metastatic urothelial cancer.
INTRODUCTION
Platinum-based combination chemotherapy has been standard first-line treatment of metastatic urothelial cancer for decades.1 Cisplatin-based regimens, or carboplatin-based regimens for patients deemed cisplatin ineligible,2 are typically administered for approximately 6 cycles and then discontinued, given concerns for cumulative toxicities in the setting of diminishing benefit.3 However, the vast majority of patients experience disease progression soon after completing first-line chemotherapy, with a median progression-free survival of approximately 3 months.4
CONTEXT
Key Objectives
To define the impact of switch maintenance pembrolizumab versus placebo chemotherapy in patients with metastatic urothelial cancer with at least stable disease after first-line chemotherapy.
Knowledge Generated
Switch maintenance pembrolizumab significantly improves progression-free survival in patients with metastatic urothelial cancer completing first-line chemotherapy.
Relevance
Sequential integration of chemotherapy and immune checkpoint blockade using a switch maintenance approach may improve outcomes in patients with metastatic urothelial cancer.
Immune checkpoint blockade with anti–PD-1 or PD-L1 antibodies has changed the treatment landscape for metastatic urothelial cancer. Five PD-1/PD-L1 inhibitors have received regulatory agency approval for the treatment of metastatic urothelial cancer on the basis of trials demonstrating durable responses achieved in a subset of patients in the context of a relatively favorable tolerability profile.5-9 A randomized phase III trial in patients with metastatic urothelial cancer progressing despite prior platinum-based chemotherapy reported a significant improvement in overall survival (OS) with the PD-1 inhibitor pembrolizumab versus second-line chemotherapy.5
The initiation of immune checkpoint blockade immediately after cessation of first-line platinum-based chemotherapy, as “switch maintenance” therapy, may be an attractive strategy for both scientific and pragmatic reasons.10 Initial chemotherapy could potentially induce immunogenic cell death or depletion of suppressive immune cell populations such as myeloid-derived suppressor cells, thereby enhancing the effects of subsequent immune checkpoint blockade.11 Alternatively, switch maintenance immune checkpoint blockade could potentially confer benefit largely for practical reasons. Chemotherapy and immune checkpoint blockade are non–cross resistant, and observational studies reveal that only approximately 30%-50% of patients with metastatic urothelial cancer initiating first-line chemotherapy are able to receive subsequent lines of systemic therapy.12,13 Therefore, earlier use of immune checkpoint blockade may simply increase the likelihood that individual patients are exposed to potentially active therapy.
PATIENTS AND METHODS
Study Design and Treatment
Hoosier Cancer Research Network GU14-182 is an investigator-initiated multicenter double-blind randomized phase II trial. Patients with metastatic urothelial cancer achieving at least stable disease on first-line cisplatin- or carboplatin-based combination chemotherapy regimens were eligible for enrollment. Patients were randomly assigned to receive pembrolizumab 200 mg intravenously every 3 weeks versus placebo, in the absence of prohibitive toxicities or disease progression, for up to 24 months. Random assignment was stratified based on the presence of visceral metastatic disease (lung, liver, or bone or other solid organs) at the time of initiation of first-line chemotherapy and response to first-line chemotherapy (complete and partial response v stable disease). At the time of disease progression, patients randomly assigned to placebo could cross over to receive open-label pembrolizumab.
The study was conducted in accordance with the Declaration of Helsinki. The protocol was approved by local ethics committees at each participating site, and informed consent was provided by all patients before enrollment. The trial was registered at ClinicalTrials.gov (ClinicalTrials.gov identifier: NCT02500121).
Patients
Eligible patients were ≥ 18 years of age, with metastatic urothelial cancer. Patients were required to have received up to 8 cycles of first-line platinum-based combination chemotherapy for metastatic urothelial cancer, to have achieved at least stable disease, and to commence study treatment within 2-6 weeks after receiving their last dose of first-line chemotherapy. Urothelial cancer with variant histology was permitted provided that the predominant component was urothelial cancer. Measurable disease was not required, because patients could have achieved a complete response with first-line chemotherapy. Patients were required to have adequate organ function and an Eastern Cooperative Oncology Group performance status of ≤ 1. Exclusion criteria included: active brain metastases, chronic use of immunosuppressive drugs, and prior treatment with immune checkpoint blockade.
Disease Assessments
Tumor assessments were conducted using cross-sectional imaging of the chest, abdomen, and pelvis after every 4 cycles until evidence of disease progression. Response and progression-free survival (PFS) were investigator assessed and were determined both by RECIST 1.1 and by immune-related RECIST (irRECIST).14 The RECIST 1.1 and irRECIST classification of response and progression differ only in that confirmation of progression is required per irRECIST. That is, for patients continuing in study treatment post progression according to RECIST 1.1, repeat imaging 4-6 weeks later was required to confirm progression. The initial date of progression, if confirmed on the subsequent imaging study, was considered the date of progression. Patients were eligible to continue study treatment post RECIST 1.1–defined progression provided the absence of (1) signs and symptoms of progression, (2) decline in performance status, (3) rapid disease progression on imaging, or (4) site of progression that might result in the near-term need for urgent intervention.
Adverse events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (v4.0). Adverse events suspected related to pembrolizumab were managed according to algorithms on the basis of the specific toxicity as defined in the protocol.
Unblinding of Study Treatment and Crossover
Patients were unblinded from study treatment if there was evidence of disease progression, treatment discontinuation due to unacceptable toxicity, or a medical event in which knowledge of the treatment was deemed critical to the subject’s clinical management. At the time of unblinding for disease progression, patients found to be on placebo could cross over to receive open-label pembrolizumab 200 mg intravenously every 3 weeks, in the absence of prohibitive toxicities or disease progression, for up to 24 months.
PD-L1 Testing
Immunohistochemistry for PD-L1 was performed in a central laboratory (QualTek Molecular Laboratories, Newtown, PA) using the antibody clone 22C3 as previously described.15 PD-L1 expression was quantified by a single genitourinary pathologist (G.K.H.) using the combined positive score (CPS), defined as the percentage of PD-L1–expressing tumor and infiltrating immune cells relative to the total number of tumor cells.16 A cut point of CPS ≥ 10 was used to define “high” PD-L1 expression as per prior studies in urothelial cancer.16
Statistical Analysis
The primary end point was PFS, defined as the time from random assignment to death or progression (whichever occurred first), according to irRECIST, with pembrolizumab versus placebo. When the study was initially designed in 2014, we sought to enroll 200 patients to detect an improvement in PFS with a hazard ratio (HR) of 0.6. However, the study was amended in 2017, after enrollment of 70 patients, on the basis of two considerations: (1) the study was accruing at slower than the projected rate, and (2) concerns regarding whether the HR is an optimal approach to measure the potential benefits conferred by immune checkpoint blockade. Specifically, the results of several randomized studies exploring immune checkpoint blockade in various solid tumors had emerged at the time, demonstrating survival curves that routinely violated the proportional hazards assumption and suggesting the benefits of immune checkpoint blockade were most apparent during later phases of the survival curve.5,17 Therefore, we amended the study on the basis of assumptions related to three theoretical phases of treatment effect. We assumed that during months 0-2 there would be no treatment effect, during months 2-3 there would be a minor treatment effect, and that after month 3 the full effect would be achieved (ie, HR of 1 before month 2 and a target HR value 𝜃 < 1 after month 3). We set the target value 𝜃 of the full treatment effect as HR = 0.462, extrapolating from Kaplan-Meier curves from emerging studies.5 The sample size was determined by inverting the test statistic proposed by Zucker and Lakatos.18 With a type I error as 0.05 and power as 80%, this amended analysis plan required a sample size of least 104 patients. No interim analyses had taken place when the study was amended, and the study remained blinded.
To compare the two treatment arms, we used both the standard log-rank test and the test proposed by Zucker and Lakatos,18 labeled as the maximum efficiency robust test (MERT). Hazard ratios for PFS and OS were estimated from the Cox model. However, because of the violation of the proportional hazards assumption, we also used the restricted mean survival time (RMST) as an alternative to the hazard ratio.19,20 Differences in RMST for both PFS and OS at 24 months were compared between the treatment arms.
The relationship between CPS for PD-L1 and treatment arm on PFS and OS was explored using interaction tests from the Cox model.
RESULTS
Patients
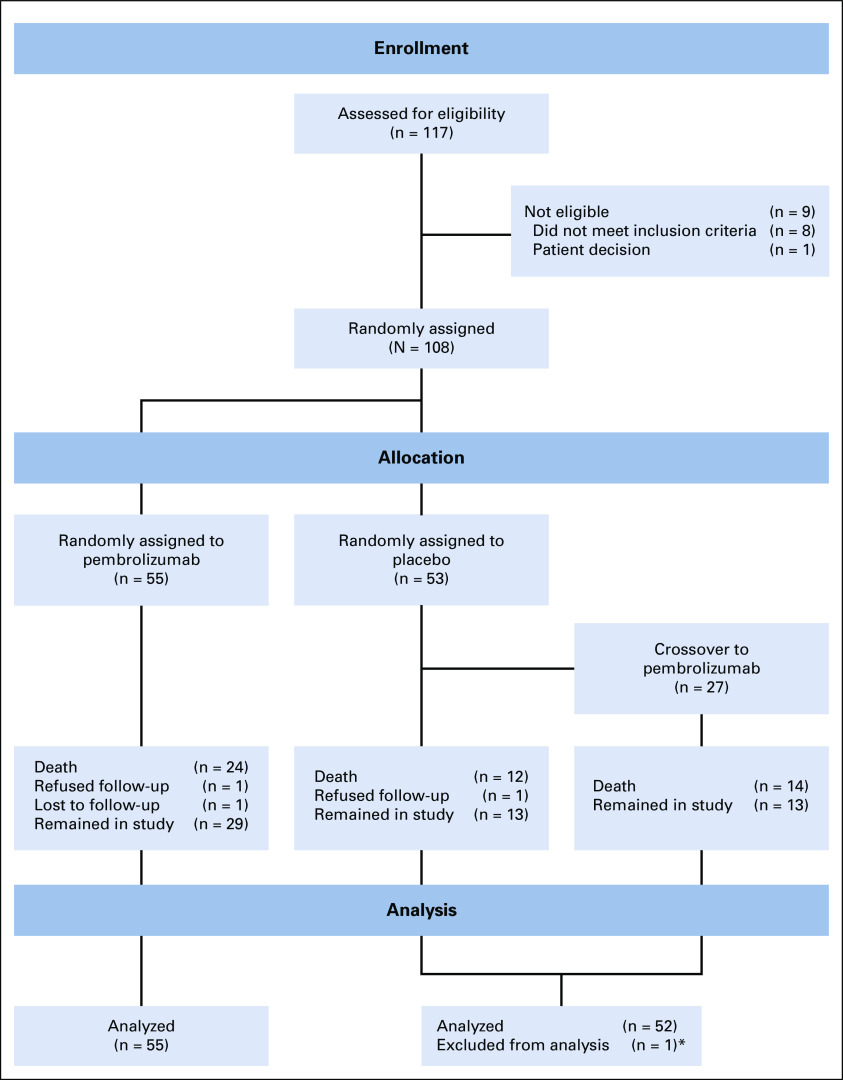
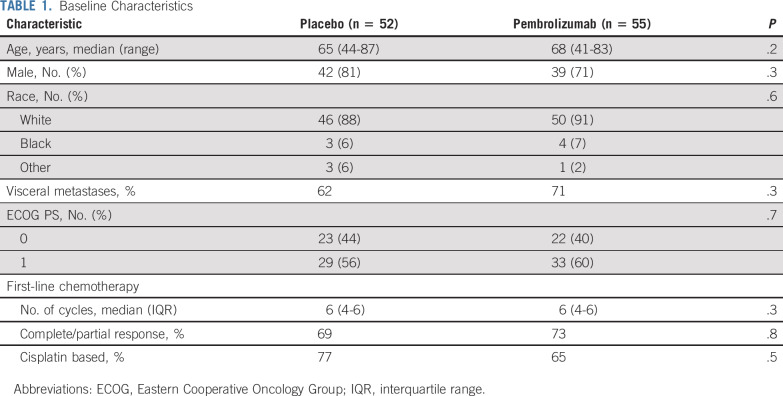
Between 2015 and 2018, 117 patients were screened and 108 patients were randomly assigned to placebo (n = 53) versus pembrolizumab (n = 55); one patient randomly assigned to placebo was excluded from the analysis because of inadvertent receipt of pembrolizumab rather than placebo intermittently during the initial several cycles of study treatment (Fig 1). The baseline characteristics are shown in Table 1. The distribution of baseline characteristics was similar in patients randomly assigned to pembrolizumab compared with placebo (Table 1).
FIG 1.
CONSORT diagram. (*) One patient randomly assigned to placebo was excluded from the analysis because of inconsistent receipt of pembrolizumab rather than placebo during the initial several cycles of study treatment.
TABLE 1.
Baseline Characteristics
Treatment
Patients assigned to pembrolizumab received a median of 8 cycles (interquartile range, 4-15 cycles), and patients assigned to placebo received a median of 6 cycles (interquartile range, 4-9 cycles). The most common reasons for treatment discontinuation included progression of disease (70%) and adverse events (17%).
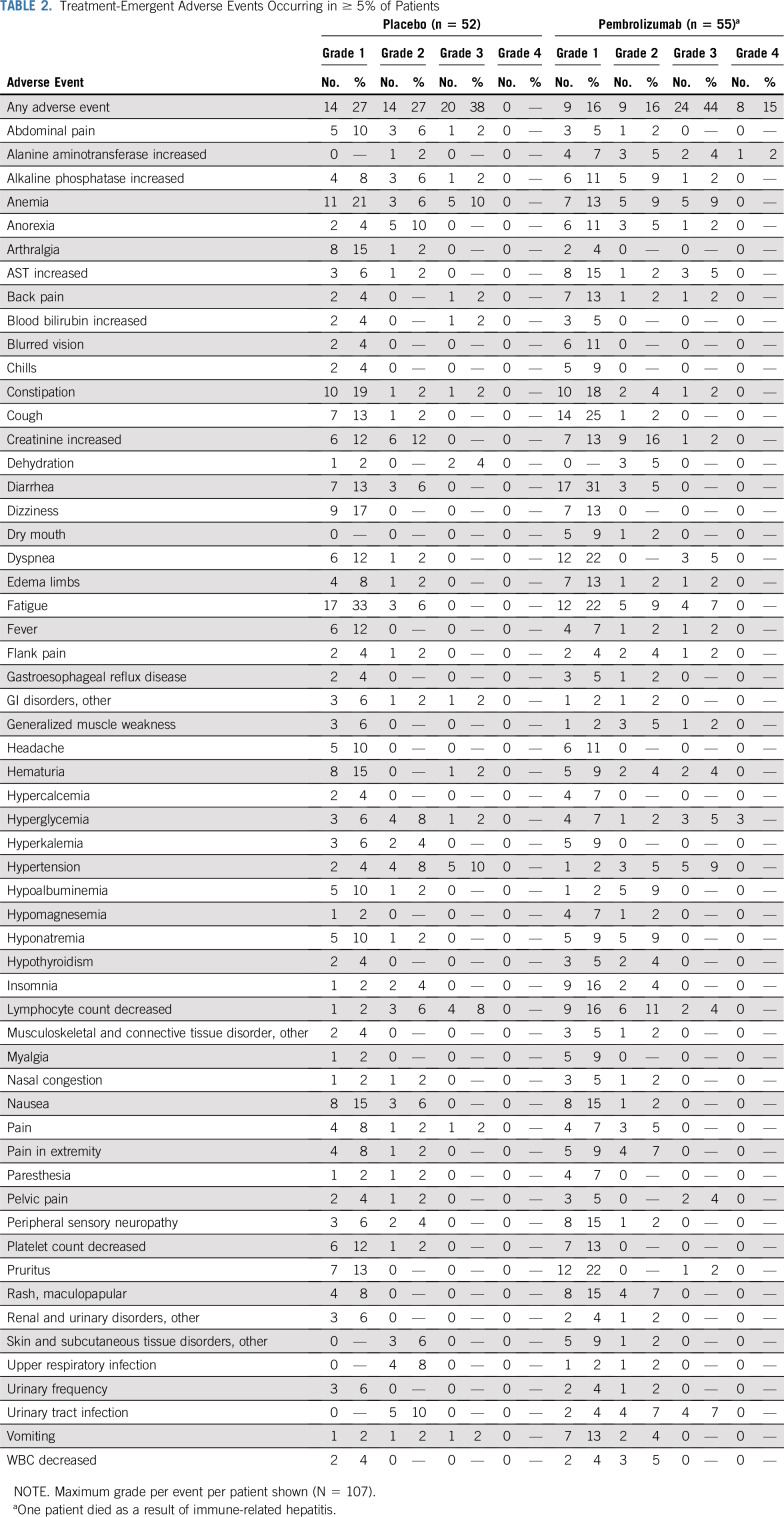
Safety
Treatment-emergent grade 3-4 adverse events occurred in 59% of patients receiving pembrolizumab and 38% of patients receiving placebo (Table 2). Immune-related adverse events requiring systemic steroid treatment occurred in 20% of patients randomly assigned to initial pembrolizumab. There was one fatal treatment-related adverse event (hepatitis) in the pembrolizumab arm.
TABLE 2.
Treatment-Emergent Adverse Events Occurring in ≥ 5% of Patients
Objective Response Rate
Patients with a complete radiographic response to first-line chemotherapy on study entry were not considered assessable for objective response with pembrolizumab or placebo (Table 3). After excluding such patients, the objective response rate in patients randomly assigned to placebo was 10%, and in patients randomly assigned to pembrolizumab it was 23%. There were no complete responses achieved in the placebo arm, whereas the complete response rate with pembrolizumab was 9%.
TABLE 3.
Objective Response Rate (RECIST 1.1)

PFS and OS
After a median follow-up of 12.9 months (range, 0.9-34.5 months), 50/107 patients have died. Given that no patients received treatment beyond initial RECIST 1.1–defined progression, the PFS on the basis of irRECIST and RECIST was identical. The PFS was significantly longer in patients randomly assigned to pembrolizumab versus placebo (MERT P = .039; Fig 2A). The difference in 24-month restricted mean progression-free survival time (RMPFST) with pembrolizumab versus placebo adjusted for the two stratification factors (response to first-line chemotherapy and presence of visceral metastases) was 3.4 months (95% CI, 0.7 to 6.2 months; P = .015). The PFS curves stratified by response to first-line chemotherapy, presence or absence of visceral metastases, and first-line cisplatin- versus carboplatin-based chemotherapy are shown in the Data Supplement. As a secondary end point, the HR was calculated given general familiarity with this measure. The median PFS was 5.4 months with pembrolizumab (95% CI, 3.1 to 7.3 months) and 3.0 months with placebo (95% CI, 2.7 to 5.5 months), with an HR of 0.65 (log-rank P value = .04).
FIG 2.
Kaplan-Meier curves for (A) progression-free survival (PFS), and (B) overall survival (OS) in patients treated with pembrolizumab versus placebo (N = 107). MERT, maximum efficiency robust test.
The median OS was 22 months (95% CI, 12.9 months to not reached) in patients randomly assigned to pembrolizumab and 18.7 months (95% CI, 11.4 months to not reached) in patients randomly assigned to placebo (HR, 0.91; 95% CI, 0.52 to 1.59; Fig 2B). The difference in 24-month RMST with pembrolizumab versus placebo adjusted for the two stratification factors (response to first-line chemotherapy and presence of visceral metastases) was 0.4 months (95% CI, −2.8 to 3.6 months; P = .8). The OS curves stratified by clinical characteristics are shown in the Data Supplement. The OS of patients randomly assigned to maintenance pembrolizumab versus placebo with patients on placebo censored at crossover is shown in the Data Supplement. To further explore the potential effect of pembrolizumab maintenance in the context of crossover, we included treatment as a time-dependent covariate in the Cox model for OS (HR, 1.2; 95% CI, 0.62 to 2.38; P = .6).
Outcomes on the Basis of PD-L1 Expression
Among the 107 evaluable patients, archival tumor tissue was available for PD-L1 testing from 94 patients. The anatomic sites from which the tumor tissue was derived are listed in the Data Supplement. The CPS was ≥ 10 in 14/47 (30%) of specimens from patients randomly assigned to pembrolizumab and 14/47 (30%) of specimens from patients randomly assigned to placebo. There was no significant interaction between PD-L1 CPS ≥ 10 and treatment arm on PFS or OS (P = .8 and .9, respectively); additional cut points of CPS ≥ 1, 5, and 15 were also explored (Data Supplement).
Crossover From Placebo to Pembrolizumab
Among 52 patients initially randomly assigned to placebo who experienced disease progression by the time of the data lock, 27 crossed over to receive pembrolizumab and 12 did not cross over (7 patients died before receiving any further systemic therapy, and 5 patients opted for further treatment off study). The objective response rate with pembrolizumab among these 27 patients was 22%. The median PFS from crossover was 2.7 months (95% CI, 2.5 to 9.3 months) and median OS from crossover was 15.8 months (95% CI, 8 months to not reached).
DISCUSSION
Metastatic urothelial cancer is a relatively chemotherapy-sensitive solid tumor. However, the vast majority of patients experience disease progression despite first-line platinum-based chemotherapy while on treatment, or within months of completing treatment, highlighting the need for better therapeutic strategies. PD-1/PD-L1 blockade has changed the landscape of treatment of patients experiencing progression despite platinum-based chemotherapy, with durable responses achieved in a subset of patients.5-9 Here, we show that earlier use of PD-1 blockade with pembrolizumab, at the time of cessation of first-line chemotherapy, leads to additional objective responses and significantly prolongs PFS compared with placebo. Importantly, in this placebo-controlled double-blind trial, the adverse event profile of pembrolizumab was similar to that reported in prior studies.21
For both pragmatic and scientific reasons, we modified our original sample size and analysis plan during enrollment, adapting to emerging data from clinical trials exploring immune checkpoint blockade in urothelial cancer and other solid tumors.5 Specifically, we were concerned that the HR would not represent a meaningful summary measure, given multiple prior studies with immune checkpoint blockade where the proportional hazards assumption has been invalid, with crossing of the survival curves.5,17 We assumed no difference in PFS during the early follow-up period, and more robust differences with later follow-up, and used a weighted log-rank test placing more emphasis on the later follow-up period. Nonetheless, we did observe a significant improvement in PFS with pembrolizumab versus placebo both using this weighted approach as specified in our primary analysis or by using a more traditional log-rank test. We also observed an improvement in outcomes with pembrolizumab using the restricted mean PFS time. This latter approach, which is a measure of the difference in the area under the PFS curve until a defined time-point with pembrolizumab versus placebo, has recently received increased attention as a potentially more intuitive and clinically relevant approach to survival analyses in immunotherapy trials.20,22,23
At the time that we initially conceived the current trial, there were no immune checkpoint inhibitors approved by regulatory authorities for the treatment of urothelial cancer. However, we anticipated this was highly likely to occur during the course of the trial, and given the primary end point of PFS, for practical reasons related to accrual, and to ensure detailed capture of post-progression therapies, crossover from placebo to pembrolizumab at the time of progression was integrated into the design. Remarkably, despite follow-up every 3 weeks of this clinical trial cohort, at the time of the data lock 13% of patients randomly assigned to placebo (including patients who had achieved stable disease and partial responses, but not complete responses, with first-line chemotherapy) had died before receiving any second-line systemic therapy, a potential concern that has been highlighted in prior observational studies.12,13 These findings underscore at least one of the potential rationales underlying a switch maintenance approach with an active non–cross-resistant therapeutic class.
Only three prospective randomized trials, to our knowledge, have previously explored a switch maintenance strategy in patients with metastatic urothelial cancer (Data Supplement). In a small randomized phase II trial, Grivas et al4 reported no difference in PFS in patients randomly assigned to sunitinib versus placebo. A randomized phase II trial of switch maintenance vinflunine did report a significant improvement in PFS.24 Notably, enrollment in that trial was limited to patients who had received 6 cycles of first-line gemcitabine plus cisplatin, and randomization was versus supportive care without placebo control. In the only phase III trial of switch maintenance therapy in patients with metastatic urothelial cancer reported to date, Powles et al25 compared lapatinib with placebo in a cohort of patients selected for enrollment on the basis of tumor overexpression of HER-1 or HER-2 by immunohistochemistry; there was no significant difference in PFS or OS between the treatment arms.
PD-L1 testing did not clearly enrich for patients for whom switch maintenance pembrolizumab was beneficial. Though limited by the small sample size of the subsets in the current study, the PD-L1 biomarker findings are consistent with other studies of PD-1/PD-L1 blockade in the post-platinum setting in urothelial cancer.21 Whether related to tumor evolution and/or chemotherapy-related tumor/host modulation, PD-L1 testing may play a more important role in the chemotherapy-naïve setting in metastatic urothelial cancer. Furthermore, the study was not designed or powered to determine whether patient subsets on the basis of clinical characteristics (eg, response to first-line chemotherapy) derive differential benefit from maintenance therapy.
The ultimate measures of benefit from a new treatment approach relate to improvements in how patients feel, function, or survive. Furthermore, the risk of financial toxicity must be carefully considered in the context of a disease state in which observation is the standard approach. OS in the current study was not significantly different in patients randomly assigned to maintenance pembrolizumab versus placebo. However, OS was a secondary end point, the study was not adequately powered to detect a survival improvement, and the survival outcomes of patients in both arms were favorable relative to data from the pre–PD-1/PD-L1 blockade era (Data Supplement). Importantly, switch maintenance PD-L1 blockade was recently reported in a press release to improve OS in a phase III study (ClinicalTrials.gov identifier: NCT02603432).26 Additional ongoing randomized phase III trials, including the recently reported IMvigor130 study (ClinicalTrials.gov identifier: NCT02807636),27 are exploring even earlier use of PD-1/PD-L1 blockade in metastatic urothelial cancer, administered concurrently with first-line chemotherapy and continuing as maintenance. Theoretical advantages to a sequential (ie, switch maintenance), compared with concurrent, combination approach include the lack of concomitant administration of immune suppressive chemotherapy and corticosteroid antiemetic prophylaxis, although a sustained negative impact of concurrent chemotherapy on lymphocyte subsets, at least as measured in peripheral blood, has not been well established.28 Ultimately, the outcomes of the several pending randomized trials will together shape the near-term landscape of first-line treatment of metastatic urothelial cancer, a disease state characterized by a paucity of advances in decades.
PRIOR PRESENTATION
Presented in part as an oral presentation at the American Society of Clinical Oncology Annual Meeting, Chicago, IL, May 31-June 4, 2019.
SUPPORT
Supported by Merck and by the Tisch Cancer Institute Cancer Center Grant No. P30 CA196521.
CLINICAL TRIAL INFORMATION
AUTHOR CONTRIBUTIONS
Conception and design: Matthew D. Galsky, Noah M. Hahn, Menggang Yu
Financial support: Matthew D. Galsky
Administrative support: Matthew D. Galsky, David I. Quinn, Noah M. Hahn, Sumanta K. Pal
Provision of study material or patients: Matthew D. Galsky, Amir Mortazavi, Saby George, Sumati Gupta, Mark T. Fleming, Robert S. Alter, Jue Wang, Shilpa Gupta, Nancy Davis, Joel Picus, George Philips, David I. Quinn, Noah M. Hahn, Sumanta K. Pal
Collection and assembly of data: Matthew D. Galsky, Amir Mortazavi, Matthew I. Milowsky, Saby George, Sumati Gupta, Long H. Dang, Radhika Walling, Robert S. Alter, Mohamad Kassar, Jue Wang, Shilpa Gupta, Joel Picus, George Philips, David I. Quinn, G. Kenneth Haines III, Noah M. Hahn, Sumanta K. Pal
Data analysis and interpretation: Matthew D. Galsky, Amir Mortazavi, Matthew I. Milowsky, Saby George, Sumati Gupta, Mark T. Fleming, Long H. Dang, Daniel M. Geynisman, Jue Wang, Shilpa Gupta, Nancy Davis, Joel Picus, George Philips, David I. Quinn, Noah M. Hahn, Qianqian Zhao, Menggang Yu, Sumanta K. Pal
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Randomized Double-Blind Phase II Study of Maintenance Pembrolizumab Versus Placebo After First-Line Chemotherapy in Patients With Metastatic Urothelial Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Matthew D. Galsky
Stock and Other Ownership Interests: Rappta Therapeutics
Consulting or Advisory Role: BioMotiv, Janssen, Dendreon, Merck, GlaxoSmithKline, Lilly, Astellas Pharma, Genentech, Bristol-Myers Squibb, Novartis, Pfizer, EMD Serono, AstraZeneca, Seattle Genetics, Incyte, Aileron Therapeutics, Dracen Pharmaceuticals, Inovio Pharmaceuticals, NuMab, Dragonfly Therapeutics
Research Funding: Janssen Oncology (Inst), Dendreon (Inst), Novartis (Inst), Bristol-Myers Squibb (Inst), Merck (Inst), AstraZeneca (Inst), Genentech/Roche (Inst)
Patents, Royalties, Other Intellectual Property: Methods and compositions for treating cancer and related methods. Mount Sinai School of Medicine, July 2012, Application number: 20120322792.
Amir Mortazavi
Honoraria: Motive Medical Intelligence
Consulting or Advisory Role: Seattle Genetics, Debiopharm Group
Research Funding: Acerta Pharma (Inst), Genentech/Roche (Inst), Merck (Inst), Novartis (Inst), Seattle Genetics (Inst), Mirati Therapeutics (Inst), Bristol-Myers Squibb (Inst), Roche (Inst), Astellas Pharma (Inst)
Matthew I. Milowsky
Consulting or Advisory Role: BioClin Therapeutics
Research Funding: Merck (Inst), Acerta Pharma (Inst), Roche/Genentech (Inst), Bristol-Myers Squibb (Inst), Seattle Genetics (Inst), Astellas Pharma (Inst), Clovis Oncology (Inst), Inovio Pharmaceuticals (Inst), AstraZeneca (Inst), X4 Pharmaceuticals (Inst), Mirati Therapeutics (Inst), Boehringer Ingelheim (Inst), Constellation Pharmaceuticals (Inst), Jounce Therapeutics (Inst), Syndax (Inst), Innocrin Pharma (Inst), MedImmune (Inst), Cerulean Pharma (Inst), Incyte (Inst)
Other Relationship: Asieris Pharmaceuticals
Saby George
Consulting or Advisory Role: Bristol-Myers Squibb, Bayer, Pfizer, Exelixis, Janssen Oncology, Corvus Pharmaceuticals, Genentech/Roche, Sanofi/Genzyme, EMD Serono
Research Funding: Pfizer (Inst), Merck (Inst), Agensys (Inst), Novartis (Inst), Bristol-Myers Squibb (Inst), Bayer (Inst), Eisai (Inst), Seattle Genetics/Astellas (Inst), Calithera Biosciences (Inst), Immunomedics (Inst), Corvus Pharmaceuticals (Inst)
Sumati Gupta
Stock and Other Ownership Interests: Salarius Pharmaceuticals (I)
Research Funding: Mirati Therapeutics (Inst), Novartis (Inst), Pfizer (Inst), Viralytics (Inst), Hoosier Cancer Research Network (Inst), Rexahn Pharmaceuticals (Inst), Five Prime Therapeutics (Inst), Incyte (Inst), MedImmune (Inst), Merck (Inst), Bristol-Myers Squibb (Inst), Clovis Oncology (Inst), LSK (Inst), QED (Inst)
Mark T. Fleming
Employment: Virginia Oncology Associates
Consulting or Advisory Role: Janssen Oncology
Speakers' Bureau: Genentech, Janssen Oncology
Travel, Accommodations, Expenses: Medivation/Astellas, Genentech
Long H. Dang
Patents, Royalties, Other Intellectual Property: We discovered Clostridium novyi NT for cancer treatment. It is patented at Johns Hopkins, licensed to BioMed Valley Discoveries, and is currently in clinical trials.
Daniel M. Geynisman
Employment: 2nd.MD
Consulting or Advisory Role: Pfizer, Exelixis, AstraZeneca, Seattle Genetics/Astellas, Eisai
Research Funding: Genentech (Inst), Merck (Inst), Calithera Biosciences (Inst), Astellas Pharma (Inst)
Robert S. Alter
Consulting or Advisory Role: Eisai, Bayer
Speakers' Bureau: Astellas Pharma, Janssen Oncology
Jue Wang
Employment: Genentech
Stock and Other Ownership Interests: Genentech
Shilpa Gupta
Stock and Other Ownership Interests: Nektar
Honoraria: Janssen Oncology, AstraZeneca, Exelixis, Bristol-Myers Squibb, Merck Sharp & Dohme, Seattle Genetics
Speakers' Bureau: Bristol-Myers Squibb, Janssen Oncology
Research Funding: Astellas Medivation (Inst), Pfizer (Inst), MedImmune (Inst), Merck (Inst), Moderna Therapeutics (Inst), Bristol-Myers Squibb (Inst), Incyte (Inst)
Nancy Davis
Research Funding: AstraZeneca (Inst), Hoffman-LaRoche (Inst), Pfizer (Inst), Merck (Inst), Incyte (Inst), Mirati Therapeutics (Inst), Seattle Genetics/Astellas (Inst), Calithera Biosciences (Inst), Taris BioMedical (Inst), Immunomedics (Inst), Bristol-Myers Squibb (Inst), Jounce Therapeutics (Inst), Exelixis (Inst)
Travel, Accommodations, Expenses: Taris BioMedical, Calithera Biosciences, Bristol-Myers Squibb, Jounce Therapeutics
Joel Picus
Consulting or Advisory Role: Novo Nordisk, Sanofi
Research Funding: BioClin Therapeutics (Inst), Agensys (Inst), Mirati Therapeutics (Inst), Innocrin Pharma (Inst), Rexahn Pharmaceuticals (Inst), Endocyte (Inst), Seattle Genetics (Inst), BioClin Therapeutics (Inst), TRACON Pharma (Inst), eFFECTOR Therapeutics (Inst)
George Philips
Consulting or Advisory Role: Pfizer/EMD Serono
David I. Quinn
Honoraria: Bayer, Astellas Pharma, Pfizer, Genentech/Roche, Merck Sharp & Dohme, Bristol-Myers Squibb, AstraZeneca, Exelixis, Janssen Oncology, Novartis, Mundipharma, Pharmacyclics, Clovis Oncology, Seattle Genetics
Consulting or Advisory Role: Astellas Pharma, Pfizer, Bristol-Myers Squibb, Genentech/Roche, Merck Sharp & Dohme, Bayer, Exelixis, AstraZeneca, Janssen Oncology, Eisai, Novartis, US Biotest, Clovis Oncology, Seattle Genetics, AstraZeneca
Research Funding: Millennium (Inst), Genentech/Roche (Inst), Sanofi (Inst), GlaxoSmithKline (Inst), Merck (Inst), Pfizer (Inst)
Travel, Accommodations, Expenses: Merck, Roche, Bayer, Bristol-Myers Squibb Japan, Exelixis, Astellas Pharma
Uncompensated Relationships: Eisai, US Biotest
Noah M. Hahn
Honoraria: Bladder Cancer Academy, PeerView, PlatformQ Health
Consulting or Advisory Role: Bristol-Myers Squibb, AstraZeneca/MedImmune, Pieris Pharmaceuticals, Inovio Pharmaceuticals, Genentech/Roche, Health Advances, Merck, Ferring, Principia, Champions Oncology, Taris BioMedical, Seattle Genetics/Astellas, Incyte, TransMed, Rexahn Pharmaceuticals, CicloMed, Janssen, Celgene, GlaxoSmithKline, Mirati Therapeutics
Research Funding: Genentech/Roche (Inst), Merck (Inst), Bristol-Myers Squibb (Inst), AstraZeneca/MedImmune (Inst), Principa Biopharma (Inst), Acerta Pharma (Inst), Incyte (Inst), Seattle Genetics/Astellas (Inst), Astex Pharmaceuticals (Inst), Pieris Pharmaceuticals (Inst)
Sumanta K. Pal
Consulting or Advisory Role: Pfizer, Novartis, Aveo, Myriad Pharmaceuticals, Genentech, Exelixis, Bristol-Myers Squibb, Astellas Pharma, Ipsen, Eisai
Research Funding: Medivation
No other potential conflicts of interest were reported.
REFERENCES
- 1. von der Maase H, Sengelov L, Roberts JT, et al: Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol 23:4602-4608, 2005. [DOI] [PubMed]
- 2. Galsky MD, Hahn NM, Rosenberg J, et al: Treatment of patients with metastatic urothelial cancer “unfit” for cisplatin-based chemotherapy. J Clin Oncol 29:2432-2438, 2011. [DOI] [PubMed]
- 3.von der Maase H, Hansen SW, Roberts JT, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: Results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol. 2000;18:3068–3077. doi: 10.1200/JCO.2000.18.17.3068. [DOI] [PubMed] [Google Scholar]
- 4.Grivas PD, Daignault S, Tagawa ST, et al. Double-blind, randomized, phase 2 trial of maintenance sunitinib versus placebo after response to chemotherapy in patients with advanced urothelial carcinoma. Cancer. 2014;120:692–701. doi: 10.1002/cncr.28477. [DOI] [PubMed] [Google Scholar]
- 5. Bellmunt J, de Wit R, Vaughn DJ, et al: Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med 376:1015-1026, 2017. [DOI] [PMC free article] [PubMed]
- 6.Rosenberg JE, Hoffman-Censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: A single-arm, multicentre, phase 2 trial. Lancet. 2016;387:1909–1920. doi: 10.1016/S0140-6736(16)00561-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharma P, Retz M, Siefker-Radtke A, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): A multicentre, single-arm, phase 2 trial. Lancet Oncol. 2017;18:312–322. doi: 10.1016/S1470-2045(17)30065-7. [DOI] [PubMed] [Google Scholar]
- 8.Powles T, O’Donnell PH, Massard C, et al. Efficacy and safety of durvalumab in locally advanced or metastatic urothelial carcinoma: Updated results from a phase 1/2 open-label study. JAMA Oncol. 2017;3:e172411. doi: 10.1001/jamaoncol.2017.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patel MR, Ellerton J, Infante JR, et al. Avelumab in metastatic urothelial carcinoma after platinum failure (JAVELIN Solid Tumor): Pooled results from two expansion cohorts of an open-label, phase 1 trial. Lancet Oncol. 2018;19:51–64. doi: 10.1016/S1470-2045(17)30900-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collazo-Lorduy A, Galsky MD. Combining chemotherapy and immune checkpoint blockade. Curr Opin Urol. 2016;26:508–513. doi: 10.1097/MOU.0000000000000333. [DOI] [PubMed] [Google Scholar]
- 11.Hato SV, Khong A, de Vries IJ, et al. Molecular pathways: The immunogenic effects of platinum-based chemotherapeutics. Clin Cancer Res. 2014;20:2831–2837. doi: 10.1158/1078-0432.CCR-13-3141. [DOI] [PubMed] [Google Scholar]
- 12. Galsky MD, Chowdhury S, Bellmunt J, et al: Treatment patterns and outcomes in “real world” patients (pts) with metastatic urothelial cancer (UC). J Clin Oncol 31, 2013 (15_suppl; abstr 4525) [Google Scholar]
- 13.Flannery K, Boyd M, Black-Shinn J, et al. Outcomes in patients with metastatic bladder cancer in the USA: A retrospective electronic medical record study. Future Oncol. 2019;15:1323–1334. doi: 10.2217/fon-2018-0654. [DOI] [PubMed] [Google Scholar]
- 14.Nishino M, Giobbie-Hurder A, Gargano M, et al. Developing a common language for tumor response to immunotherapy: Immune-related response criteria using unidimensional measurements. Clin Cancer Res. 2013;19:3936–3943. doi: 10.1158/1078-0432.CCR-13-0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dolled-Filhart M, Locke D, Murphy T, et al. Development of a prototype immunohistochemistry assay to measure programmed death ligand-1 expression in tumor tissue. Arch Pathol Lab Med. 2016;140:1259–1266. doi: 10.5858/arpa.2015-0544-OA. [DOI] [PubMed] [Google Scholar]
- 16.Balar AV, Castellano D, O’Donnell PH, et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): A multicentre, single-arm, phase 2 study. Lancet Oncol. 2017;18:1483–1492. doi: 10.1016/S1470-2045(17)30616-2. [DOI] [PubMed] [Google Scholar]
- 17.Powles T, Durán I, van der Heijden MS, et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): A multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2018;391:748–757. doi: 10.1016/S0140-6736(17)33297-X. [DOI] [PubMed] [Google Scholar]
- 18. Zucker DM, Lakatos E: Weighted log rank type statistics for comparing survival curves when there is a time lag in the effectiveness of treatment. Biometrika 77:853-864, 1990. [Google Scholar]
- 19.Uno H, Claggett B, Tian L, et al. Moving beyond the hazard ratio in quantifying the between-group difference in survival analysis. J Clin Oncol. 2014;32:2380–2385. doi: 10.1200/JCO.2014.55.2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pak K, Uno H, Kim DH, et al. Interpretability of cancer clinical trial results using restricted mean survival time as an alternative to the hazard ratio. JAMA Oncol. 2017;3:1692–1696. doi: 10.1001/jamaoncol.2017.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bellmunt J, de Wit R, Vaughn DJ, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. 2017;376:1015–1026. doi: 10.1056/NEJMoa1613683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Z-X, Wu H-X, Xie L, et al. Correlation of milestone restricted mean survival time ratio with overall survival hazard ratio in randomized clinical trials of immune checkpoint inhibitors: A systematic review and meta-analysis. JAMA Netw Open. 2019;2:e193433. doi: 10.1001/jamanetworkopen.2019.3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Royston P, Parmar MK. Restricted mean survival time: An alternative to the hazard ratio for the design and analysis of randomized trials with a time-to-event outcome. BMC Med Res Methodol. 2013;13:152. doi: 10.1186/1471-2288-13-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.García-Donas J, Font A, Pérez-Valderrama B, et al. Maintenance therapy with vinflunine plus best supportive care versus best supportive care alone in patients with advanced urothelial carcinoma with a response after first-line chemotherapy (MAJA; SOGUG 2011/02): A multicentre, randomised, controlled, open-label, phase 2 trial. Lancet Oncol. 2017;18:672–681a. doi: 10.1016/S1470-2045(17)30242-5. [DOI] [PubMed] [Google Scholar]
- 25.Powles T, Huddart RA, Elliott T, et al. Phase III, double-blind, randomized trial that compared maintenance lapatinib versus placebo after first-line chemotherapy in patients with human epidermal growth factor receptor 1/2-positive metastatic bladder cancer. J Clin Oncol. 2017;35:48–55. doi: 10.1200/JCO.2015.66.3468. [DOI] [PubMed] [Google Scholar]
- 26. ASCO Post: JAVELIN Bladder 100 study of avelumab for urothelial cancer meets primary endpoint. https://www.ascopost.com/news/january-2020/javelin-bladder-100-study-of-avelumab-for-urothelial-cancer-meets-primary-endpoint/
- 27. Grande E, Galsky MD, Arranz JÁ, et al: IMvigor130: Efficacy and safety from a Phase III study of atezolizumab as monotherapy or in combination with platinum-based chemotherapy (PBC) vs placebo + PBC in previously untreated locally advanced or metastatic urothelial carcinoma. Ann Oncol 30:v851-v934, 2019 (suppl 5) [Google Scholar]
- 28.Galsky MD, Wang H, Hahn NM, et al. Phase 2 trial of gemcitabine, cisplatin, plus ipilimumab in patients with metastatic urothelial cancer and impact of DNA damage response gene mutations on outcomes. Eur Urol. 2018;73:751–759. doi: 10.1016/j.eururo.2017.12.001. [DOI] [PubMed] [Google Scholar]