Abstract
Purpose
This study aimed to evaluate the cost-effectiveness of treatment with retrograde intrarenal surgery (RIRS) versus repeated shock wave lithotripsy (SWL) in patients with renal calculi.
Materials and Methods
The non-retreatment rates (NRRs) and their respective real-world costs for RIRS and SWL were derived through retrospective analysis of health insurance claims data from 2015 to 2017. Decision tree modeling was performed to demonstrate the cost-effectiveness of RIRS. Furthermore, sensitivity analysis was performed to examine the robustness of the results.
Results
Analysis of the obtained data showed that NRRs of single SWL ranged from 46% to 56%, whereas NRRs of single RIRS ranged from 75% to 93%. Introducing RIRS early in the treatment sequence was observed to be favorable for the reduction of overall failure (overall NRR, 0.997) compared to the results of repeated SWL (overall NRR, 0.928). The implementation of decision tree modeling revealed that the cost per retreatment-avoided increased with the introduction of RIRS at an earlier time (first line, second line, third line, fourth line: 18640 USD, 10376 USD, 4294 USD, 3377 USD, respectively). Probabilistic modeling also indicated that the introduction of RIRS as the first line of treatment was least likely to be cost-effective, when compared to other options of introducing RIRS as the second, third, or fourth line of treatment.
Conclusion
Performing RIRS as early as possible can be recommended for eligible patients to reduce the overall failure, even if it is not as cost-effective as performing RIRS later.
Keywords: Lithotripsy, urology, cost-benefit analysis, kidney calculi
INTRODUCTION
Nephrolithiasis is one of the most common urinary diseases, and its prevalence has been increasing worldwide.1,2,3,4 In general, there is a consensus that the first line of treatment for patients with renal calculi of less than 20 mm is either shock wave lithotripsy (SWL) or retrograde intrarenal surgery (RIRS).5,6,7 According to a previous meta-analysis, the stone-free rate (SFR) of RIRS is higher than that of SWL; moreover, RIRS is generally followed by fewer retreatments than SWL.8,9,10,11,12
Generally, RIRS is performed at general hospitals as it requires hospitalization; at the hospital, general anesthesia is administered to the patient, and flexible ureteroscopy and endovision system facilities are available.13 In contrast, SWL, routinely performed at private clinics or low-volume hospitals, is preferred among patients due to its high accessibility, short procedural time, relatively inexpensive cost, and local anesthesia requirement.5,8,10,11,14,15,16,17 By examining extensive Korean claims data, Ko, et al.18 reported that SWL was frequently used as a method of treatment in South Korea but, due to relatively low SFRs, it resulted in hospital revisits, reoperations, patient inconvenience, and additional economic burden.
Considering that repeated SWL in private clinics and low-volume hospitals might incur unnecessary societal burden, SWL should be substituted with another procedure, the SFR of which is higher than that of SWL. A previous study reported that SWL was more cost-effective than RIRS.19 However, the study had a considerable limitation in that it compared the effectiveness of SWL with that of RIRS in the same line of treatment. In real-world clinical settings, however, diverse treatment sequences and intricate treatment plans may exist. Therefore, we compared the clinical and economic outcomes between RIRS and SWL by analyzing population-based data and evaluated the most cost-effective line of treatment for RIRS in the treatment sequence.
MATERIALS AND METHODS
Real clinical evidence generation
We analyzed the medical claims data for insurance reimbursement provided by the Health Insurance Review and Assessment Service (HIRA) to observe the outcomes of RIRS and SWL for renal stones. We then analyzed these two treatment modalities for their cost-effectiveness. As a public agency, HIRA conducts healthcare performance evaluation and determines whether or not medical claims need to be reimbursed. This repository of claims data covers approximately 98% of the Korean population and contains records of inpatient and outpatient utilization, medication use, and other healthcare resource use.20 We used the claims data from July 1, 2015 to June 30, 2017 (Fig. 1) in this study.
Fig. 1. Study design for real clinical evidence generation.
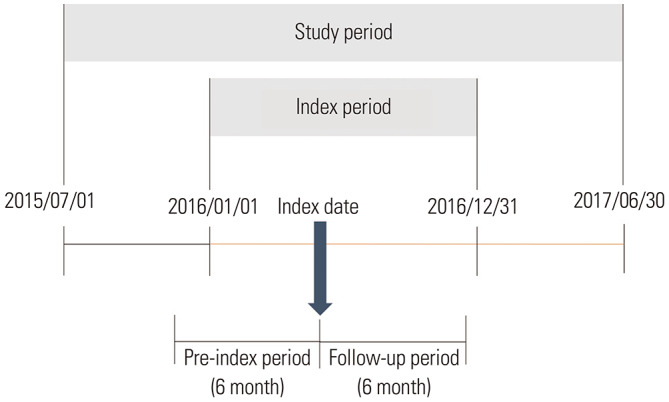
Since the aim of this study was to examine the clinical outcomes of SWL and RIRS, we only included patients who initiated treatment with either SWL or RIRS. Patients who had any record of SWL or RIRS from January 1, 2016 to December 31, 2016 were identified. Among the noted records for each patient, the first obtained record was selected and the date of record was defined as the index date. After defining the index date for each patient, we reviewed the claims data 6 months prior to the index date to include only incident patients. Those who had any record of treatment with modalities, including SWL, RIRS, and percutaneous nephrolithotomy (PCNL), within 6 months before the index date were excluded from the analysis.
The length of an episode to treat renal stones was defined as 6 months, as per expert opinion. If a record of SWL, RIRS, or PCNL existed after the index date within an episode, the record was classified as retreatment for the same renal stone. To include records related to only renal stones, claims data regarding reimbursement for exclusively renal stones were included [using the International Classification of Diseases, Tenth Revision (ICD-10) codes N-200]. The study protocol was reviewed by the Institutional Review Board of Gachon University (Approval No. 1044396-201708-HR-139-01).
Model overview
We used a decision tree model to investigate the cost-effectiveness of introducing RIRS in the treatment sequence from the payers' perspective in South Korea. The model was constructed based on a previous study,21 on the assumption that a maximum of four successive treatments can be performed in each patient.
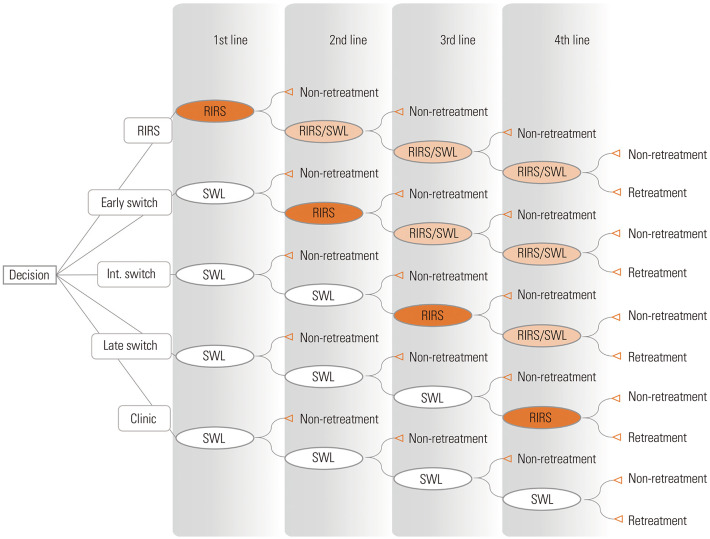
The treatment modalities, including SWL, RIRS, and PCNL, resulted in two possible outcomes: 1) “non-retreatment,” which probably meant a treatment success; and 2) “retreatment,” which indicated a treatment failure. After the failure of a previous treatment, the patient was expected to advance to the next line of treatment. As the data obtained from insurance claims do not provide information regarding the clinical setting, the current study used non-retreatment rate (NRR) rather than SFR, for the evaluation of treatment outcomes. Pilot analysis showed that the percentages of patients who underwent PCNL after first line SWL failure and after first line RIRS failure were 0.31% and 0.36%, respectively. As a cost-effectiveness model should be as simple as possible, PCNL was excluded from the model.22 The model is presented in Fig. 2. Oval shapes represent chance nodes. A chance node is a point where one or two alternative treatments (SWL or RIRS) are possible. In this study, we assumed five possible scenarios as follows. First, “clinic scenario” meant performing only SWL (maximum four times). Second, “RIRS scenario” meant performing RIRS as a first line treatment and, then performing RIRS or SWL as subsequent treatments. Third, “early switch scenario” meant performing RIRS as a second line of treatment after first SWL failure and, then performing RIRS or SWL as a subsequent treatment. Fourth, “intermediate switch scenario” meant performing RIRS as the third line treatment after two successive SWL failures and, then performing RIRS or SWL as a subsequent treatment. Fifth, “late switch scenario” meant performing RIRS as the fourth line of treatment after three successive SWL failures.
Fig. 2. Decision tree model. Each oval shape represents a chance node. Int. switch, intermediate switch; SWL, shock wave lithotripsy; RIRS, retrograde intrarenal surgery.
The authors assumed that the patients involved in the current study followed any of the aforementioned scenarios. The study was based on the assumption that each patient belonged to one of the following five mutually exclusive subgroups: 1) patients who did not undergo retreatment after the first line of treatment; 2) patients who did not undergo retreatment after the second line of treatment; 3) patients who did not undergo retreatment after the third line of treatment; 4) patients who did not undergo retreatment after the fourth line of treatment; and 5) patients who were not cured, even after four successive rounds of treatments. The expected NRR in each scenario was calculated by totaling the probabilities of belonging to any of the first four subgroups, which indicated the overall success rate of each scenario. The expected cost in each scenario was the weighted average of economic outcomes of the five mutually exclusive subgroups, which was calculated by multiplying the probability of being in a subgroup by the economic outcome of being that subgroup.
Model input
1) NRR: We defined SWL group as the group of patients who underwent SWL as the first line of treatment. Likewise, RIRS group was defined as the group of patients who underwent RIRS as the first line of treatment. NRRs were calculated for each group and each sequence. As the model assumed a maximum of fourth line of treatment, we estimated NRRs up to the fourth line of treatment. However, the claims data lacked information about renal stone size and location; therefore, we had to operationally define “non-retreatment” for the data. If a patient had no successive record of treatment within 6 months after the index date (i.e., the duration of an episode), as defined earlier, the patient was assumed to have undergone no further treatment. For example, if a patient had no further record of treatment within an episode after the index date of performing RIRS, the patient was considered to be cured by first line treatment with RIRS. We assumed that the NRRs of the fourth round of treatment were the same as those of the third, since the number of patients who underwent a fourth line of treatment was too small to allow NRR calculation.
2) Selection probability: Selection probability was applied on the chance node of the decision tree model. A patient was assumed to move to SWL or RIRS after failure according to the selection probability. As the sum of selection probability was 1 for each chance node, the selection probability of RIRS of each sequence was calculated by subtracting the value of SWL from 1. Similar to NRR, selection probabilities were also calculated separately for SWL and RIRS groups. The probability to select SWL as the second line of treatment, for example, was calculated by dividing “the number of patients who received SWL during the second line of treatment” by “the number of patients who underwent the second line of treatment.” Similar to the calculation of NRRs, we assumed that the selection probability for the fourth line of treatment was the same as that of the third line of treatment due to the limited sample size.
3) Cost: Costs were estimated separately according to the consequences and the sequence of each treatment. For example, in case of a patient who underwent SWL as the first line of treatment without success and subsequently underwent the second line of treatment of RIRS with success, the “cost of the first SWL that was termed a failure” and the “cost of the RIRS that was a success” were calculated separately. The former was the sum of the cost spent from the index date to the day before the date of second line of RIRS, and the latter was the cost incurred from the date of second line of RIRS to the end of the episode. After estimating the cost for each patient for each sequence, we derived the average cost by dividing the total cost by the number of patients for each sequence. Similar to NRR, the cost incurred from the fourth line of treatment was considered the same as that for the third line due to the limited sample size. All of the costs estimated in Korean won were converted to US dollars using a conversion rate of 1100 won/US dollar.
Cost-effectiveness analysis
The expected NRRs and the expected cost of each scenario was calculated by running the model with inputs, as mentioned above. Subsequently, the outcomes of RIRS including scenarios were compared with the outcomes of the clinical scenario. We also presented an incremental cost-effectiveness ratio (ICER), which is a summary measure to describe the economic value of an intervention compared to a comparator that is normally a standard of care.22 Generally, ICER is calculated by dividing the incremental cost by the incremental effectiveness in a cost-effectiveness analysis. Incremental cost refers to the cost incurred by substituting a comparator to a new intervention. Incremental effectiveness means the benefit derived by the substitution. In the current study, ICER was calculated by dividing the incremental cost by the incremental NRRs; that is, ICER indicated the monetary value per retreatment avoided. The clinic scenario was the comparator in all cost-effectiveness analysis. Therefore, the clinical and economic outcomes of the RIRS scenario, early switch scenario, intermediate switch scenario, and late switch scenario were compared to those of the clinic scenario.
Since the cost of RIRS and SWL are constantly changing in Korea, deterministic sensitivity analysis was performed to demonstrate the impact of the aforementioned changes in costs. Every year, the reimbursement plan for each treatment, including surgeries, is published by HIRA. Considering the increasing trend in the charges from 2014 to 2020, the cost of RIRS was assumed to increase by 70 USD annually, and the cost of the first session of SWL treatment was assumed to increase by 50 USD annually. The costs of the second, third, and fourth SWL treatments were assumed to rise by 30 USD annually. Probabilistic sensitivity analysis was performed to assess the acceptance probabilities of scenarios involving RIRS in the sequence compared to the clinic scenario under diverse willingness to pays (WTPs). In a cost-effectiveness analysis, an intervention was considered cost-effective if ICER was lower than WTP, indicating that the intervention could be accepted by insurers.22 Input parameters for probabilistic modeling were randomly selected from the means, standard errors, and distributions. Applying these random parameters, we ran the model 1000 times, which is termed as the Monte Carlo simulation.22 Through probabilistic modeling, we obtained 1000 ICERs for each comparison (e.g., RIRS scenario versus clinic scenario). If the 700 ICERs were under a certain WTP, the acceptance probability was considered as 70% under that WTP. As the standard errors for clinical outcomes could not be calculated, we assumed the standard errors to be 10% of the means. Acceptability curves were drawn with the obtained acceptance probabilities. Microsoft Excel 2016 (Microsoft Corporation, Redmond, WA, USA) was used for all analyses.
RESULTS
Among the 11700 patients who underwent treatment for renal stones in South Korea from 2015 to 2017, 10590 (90.5%) patients belonged to SWL group and only 1110 (9.5%) patients belonged to RIRS group. Most of the patients who failed at the first line of SWL treatment were subsequently treated by SWL again (98.2%). All of the patients in RIRS group were treated in a hospital, whereas only 31.7% in SWL group were treated in a hospital (Table 1).
Table 1. Demographics of the Study Population.
| SWL (n=10590) | RIRS (n=1110) | |
|---|---|---|
| Sex, n (%) | ||
| Male | 7086 (66.9) | 659 (59.4) |
| Female | 3504 (33.1) | 451 (40.6) |
| Age, n (%) | ||
| <45 yr | 3124 (29.5) | 248 (22.3) |
| 45–64 yr | 5537 (52.3) | 584 (52.6) |
| ≥65 yr | 1929 (18.2) | 278 (25.1) |
| Health insurance type, n (%) | ||
| Health insurance | 10015 (94.6) | 1073 (96.7) |
| Other | 575 (5.4) | 37 (3.3) |
| Charlson comorbidity score, n (%) | ||
| 0 | 8249 (77.9) | 788 (71.0) |
| 1 | 1969 (18.6) | 237 (21.4) |
| 2 | 320 (3.0) | 65 (5.9) |
| ≥3 | 52 (0.5) | 20 (1.8) |
| Type of medical institution, n (%) | ||
| Clinic | 7231 (68.3) | 0 (0.0) |
| Hospital | 176 (1.7) | 2 (0.2) |
| General hospital | 2044 (19.3) | 278 (25.0) |
| Tertiary general hospital | 1139 (10.8) | 830 (74.8) |
| Outcome of the first treatment, n (%) | ||
| Non-retreatment | 5493 (51.9) | 1031 (92.9) |
| Failure | 5097 (48.1) | 79 (7.1) |
| Type of treatment after failure | ||
| SWL | 5004 (98.2) | 37 (46.8) |
| RIRS | 62 (1.2) | 38 (48.1) |
| PCNL | 31 (0.6) | 4 (5.1) |
SWL, shock wave lithotripsy; RIRS, retrograde intrarenal surgery; PCNL, percutaneous nephrolithotomy.
As shown in Table 2, the NRRs of SWL were 56% or lower, whereas those of RIRS ranged from 75% to 93%. The average cost of SWL was lower than that of RIRS in every sequence (Table 3). Among all sequences, the cost of RIRS as the third line of treatment was the highest ($3832).
Table 2. Base Case Model Inputs.
| Parameters (n=11650) | Base value |
|---|---|
| Non-retreatment rate* | |
| SWL | |
| First line | 0.52 |
| Second line after SWL failure | 0.50 |
| Second line after RIRS failure | 0.56 |
| Third line after first SWL failure | 0.46 |
| Third line after first RIRS failure | 0.53 |
| Fourth line after first SWL failure | 0.46 |
| Fourth line after first RIRS failure | 0.53 |
| RIRS | |
| First line | 0.93 |
| Second line after SWL failure | 0.89 |
| Second line after RIRS failure | 0.92 |
| Third line after first SWL failure | 0.88 |
| Third line after first RIRS failure | 0.75 |
| Fourth line after first SWL failure | 0.88 |
| Fourth line after first RIRS failure | 0.75 |
| Selection probability† | |
| Second line SWL after RIRS failure | 0.49 |
| Third line SWL after two SWL failure | 0.99 |
| Third line SWL after first line SWL, second line RIRS failure | 0.71 |
| Third line SWL after first line RIRS, second line SWL failure | 0.88 |
| Third line SWL after two RIRS failure | 0.33 |
| Costs (USD)‡ | |
| Cost of first SWL (non-retreatment) | 840 |
| Cost of first SWL (failure) | 748 |
| Cost of first RIRS (non-retreatment) | 2555 |
| Cost of first RIRS (failure) | 2534 |
| Cost of second SWL (non-retreatment) | 540 |
| Cost of second SWL (failure) | 431 |
| Cost of second RIRS (non-retreatment) | 2400 |
| Cost of second RIRS (failure) | 2315 |
| Cost of third SWL (non-retreatment) | 492 |
| Cost of third SWL (failure) | 1508 |
| Cost of third RIRS (non-retreatment) | 2313 |
| Cost of third RIRS (failure) | 3832 |
SWL, shock wave lithotripsy; RIRS, retrograde intrarenal surgery.
*The non-retreatment rate or cost of fourth line of treatment was the same as the non-retreatment rate or cost of third line of treatment, †The selection probability of RIRS in each treatment sequence was calculated by subtracting the value of SWL from 1, ‡The cost in Korean won were converted to US dollars using the conversion rate of 1100 won/US dollar.
Table 3. Input Parameters for Deterministic Sensitivity Analyses.
| Input parameters | Cost (USD) | ||
|---|---|---|---|
| Base value | 1 year later | 2 years later | |
| Cost of first SWL (non-retreatment) | 840 | 890 | 940 |
| Cost of first SWL (failure) | 748 | 798 | 848 |
| Cost of first RIRS (non-retreatment) | 2555 | 2625 | 2695 |
| Cost of first RIRS (failure) | 2534 | 2604 | 2674 |
| Cost of second SWL (non-retreatment) | 540 | 570 | 600 |
| Cost of second SWL (failure) | 431 | 461 | 491 |
| Cost of second RIRS (non-retreatment) | 2400 | 2470 | 2540 |
| Cost of second RIRS (failure) | 2315 | 2385 | 2455 |
| Cost of third SWL (non-retreatment) | 492 | 522 | 552 |
| Cost of third SWL (failure) | 1508 | 1538 | 1568 |
| Cost of third RIRS (non-retreatment) | 2313 | 2383 | 2453 |
| Cost of third RIRS (failure) | 3832 | 3902 | 3972 |
SWL, shock wave lithotripsy; RIRS, retrograde intrarenal surgery.
On running the decision tree model with inputs derived from real-world data, the overall NRR in the clinic scenario was observed to be 0.928, whereas the overall NRRs of the other scenarios, including RIRS in their treatment sequences, were higher than that observed in the clinic scenario. The overall NRR was observed to increase up to 0.997, when RIRS was performed earlier (Table 4). The expected cost was also the highest in the RIRS scenario ($2692). On the contrary, the expected cost for each scenario decreased when RIRS was performed later. As a result, ICER was the lowest in the late switch scenario ($3377).
Table 4. Results of Decision Analyses.
| Expected cost (USD) | Expected NRR | Incremental value | |||
|---|---|---|---|---|---|
| Incremental cost (USD) | Incremental NRRs | ICER | |||
| Base case | |||||
| Clinic scenario (reference) | 1420 | 0.928 | - | - | - |
| RIRS scenario | 2692 | 0.997 | 1272 | 0.068 | 18640 |
| Early switch scenario | 2060 | 0.990 | 640 | 0.062 | 10376 |
| Intermediate switch scenario | 1662 | 0.985 | 242 | 0.056 | 4294 |
| Late switch scenario | 1610 | 0.985 | 190 | 0.056 | 3377 |
| 1 year later | |||||
| Clinic scenario (reference) | 1496 | 0.928 | - | - | - |
| RIRS scenario | 2766 | 0.997 | 1271 | 0.068 | 18624 |
| Early switch scenario | 2147 | 0.990 | 652 | 0.062 | 10559 |
| Intermediate switch scenario | 1745 | 0.985 | 249 | 0.056 | 4411 |
| Late switch scenario | 1691 | 0.985 | 195 | 0.056 | 3470 |
| 2 years later | |||||
| Clinic scenario (reference) | 1571 | 0.928 | - | - | - |
| RIRS scenario | 2841 | 0.997 | 1270 | 0.068 | 18609 |
| Early switch scenario | 2234 | 0.990 | 663 | 0.062 | 10742 |
| Intermediate switch scenario | 1827 | 0.985 | 255 | 0.056 | 4528 |
| Late switch scenario | 1772 | 0.985 | 201 | 0.056 | 3564 |
RIRS, retrograde intrarenal surgery; NRR, non-retreatment rate; ICER, incremental cost-effectiveness ratio.
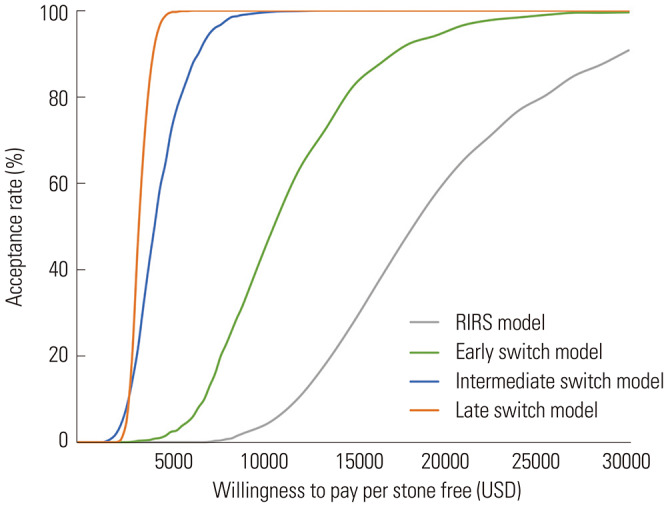
Deterministic sensitivity analysis showed that the order of ICERs of the scenarios did not change with the change in the costs of RIRS and SWL (Table 4). For sensitivity analysis, the acceptance curve was drawn using the values detailed in Table 5. Under varying WTPs, the late switch scenario was the most likely to be cost-effective, whereas the other scenarios, which involved RIRS in the earlier sequence, were less likely to be cost-effective (Fig. 3).
Table 5. Input Parameters for Probabilistic Sensitivity Analyses.
| Parameters | Base value | Standard error of the mean | Distribution |
|---|---|---|---|
| Non-retreatment rate | |||
| SWL | |||
| First line | 0.52 | 0.05* | Beta |
| Second line after SWL failure | 0.50 | 0.05* | Beta |
| Third line after initial SWL failure | 0.46 | 0.05* | Beta |
| RIRS | |||
| First line | 0.93 | 0.09* | Beta |
| Costs (USD) | |||
| Cost of first SWL (non-retreatment) | 840 | 7 | Gamma |
| Cost of first RIRS (non-retreatment) | 2555 | 32 | Gamma |
| Cost of second SWL (non-retreatment) | 540 | 13 | Gamma |
| Cost of second RIRS (non-retreatment) | 2400 | 204 | Gamma |
SWL, shock wave lithotripsy; RIRS, retrograde intrarenal surgery.
*Assumed to be 10% of the mean.
Fig. 3. Acceptability curve under diverse willingness-to-pay. RIRS, retrograde intrarenal surgery.
DISCUSSION
Compared to the non-conclusive pattern observed in SWL, the NRRs of RIRS were observed to increase when RIRS was performed earlier. Decision analysis demonstrated that the expected NRR was the highest in the RIRS scenario. However, the expected cost in the RIRS scenario was the highest, compared to those in the other scenarios. Consequently, ICER was the highest when the RIRS scenario was compared to the clinic scenario, indicating that performing RIRS earlier was not as cost-effective as performing it later. As shown by the acceptability curve, the late switch scenario was most likely to be cost-effective compared with the other scenarios.
Although the SFRs of RIRS were similar to the previously reported values, the SFRs of SWL derived from this study were lower than those obtained in previous clinical trials.11,12,23 The low SFRs may be due to the differences between clinical trials and real-world clinical settings. In clinical trials, clinicians sort out participants according to the predefined inclusion criteria to show the precise efficacy and effectiveness of a treatment.5 However, aspects other than the inclusion criteria, such as favorable accessibility of a clinic, short operative time, patients' experience, patients' preference on non-invasive treatments, decision of a physician, and a quick return to everyday life, may affect the NRRs of SWL.15,16,17,24,25 Also, in a real-world clinical setting, SWL can be performed in patients with renal stones that are larger than 20 mm, which deviates significantly from the guidelines.5 However, in the current study, both treatments of SWL and RIRS were analyzed with the same assumption; therefore, the NRRs of SWL and RIRS derived from this study can be considered as a reliable basis for the evaluation of cost-effectiveness of each treatment sequence at the population level. Moreover, the objective of this study was to analyze and validate the cost-effectiveness of performing RIRS in a real-world clinical setting. Therefore, determining ICER using real-world values was a meaningful strategy.
Based on the results of the current study, RIRS was the best treatment option to reduce treatment failure. Additionally, we observed that the expected NRR in the intermediate switch scenario was not significantly different from that of the late switch scenario, suggesting that the benefits of performing RIRS gets maximized when performed earlier in the sequence. Therefore, RIRS should be performed as early as possible to reduce retreatment at the population level. Despite the benefits of performing RIRS as described above, insurers cannot force physicians to perform RIRS for many reasons. First, RIRS cannot be performed at the level of private clinics due to the lack of infrastructure, which was demonstrated in the results of our study that RIRS was generally performed at the hospital level.14,26 Second, before performing RIRS in real clinical settings, the size, location, and density of the stone should be evaluated and documented, as these characteristics may impact the treatment outcomes.5,9,10,12 Early RIRS selection can be justified only if the related complications and the fitness for anesthesia and hospitalization are favorable for performing RIRS.15,16,17 Thus, even if the guidelines indicate that RIRS can be performed on a patient, in case of patients who are at high risk of anesthesia or are afraid of ureteral stent and urethral catheter, SWL can be performed as an alternative. Therefore, insurers should detail their reimbursement plan to encourage treatment with RIRS at an early stage for only eligible patients.
According to the results of our decision analysis, performing RIRS earlier on in the sequence led to higher expected costs. The negative effect of RIRS on the economic outcome was also shown by the acceptability curve, such that the RIRS scenario was less likely to be accepted than the late switch scenario under the same WTP. However, the expected cost was the cheapest in the clinic scenario. The cost of repeated SWL was lower due to the reimbursement policy in South Korea, which allows up to 50% discounted cost for subsequent SWL treatment. Our results from deterministic sensitivity analysis showed that even when the costs of treatments change, the order of ICER does not change. In real-world clinical settings, the entire population consists of the five scenarios that we assumed in this study. That is, some follow the RIRS scenario, whereas some follow the late switch scenario. Therefore, performing RIRS as the first line of treatment would be affordable at the population level, only if the patients who are eligible for RIRS follow the RIRS scenario. As the likelihood of performing RIRS at an earlier stage increases, the overall NRR and the total cost at the population level will also increase. Insurers should comprehensively consider the treatment accessibility, patient preference, and budget constraints to establish a new healthcare policy that would improve the health and economic outcomes at the population level. In addition, they should limit reimbursements in cases of repeated SWL that are not clinically indicated, and include a mandatory clause for the documentation of size, density, and location of the renal stone, before conducting any procedure.
This study had several limitations, as the data obtained from the insurance claims did not include the patients' social and physical conditions or details about the renal stones. As a result, it was not possible to reach conclusions regarding which patients should undergo RIRS at an early stage, based on the results of the present study. We believe that currently, treatment in accordance with the established guidelines is the most evidence-based. This study assumed a treatment to be successful when there were no records of further treatment. The aforementioned assumption and definition of a successful treatment may lead to bias, considering the actual clinical settings; in fact, even if some patients have remnant stones, follow-up observations may often be conducted for more than 6 months. However, the length of each episode (i.e., 6 months) was sufficient to detect stone remission. According to the European Association of Urology (EAU) guidelines, patients with kidney stones usually need repetitive follow-ups to deal with the infection and associated pain until the problematic stones are resolved.5 Also, if the situation does not warrant any treatment, the physician should follow up with the patient after 6 months, and then annually. Furthermore, the current study found that 90.8% of the patients who received SWL as the first line treatment presented with successful outcomes within 6 months after the index date. The mean time interval between the first SWL session and the first session of retreatment was 21.41 days, and the mean time interval between the first and second sessions of retreatment was 22 days, which indicates that the majority of treatments were done in the first 2 months after the index date. Meanwhile, among the 1110 patients who received RIRS as the first line of treatment, 99.0% presented with successful outcomes within 6 months. The mean of time interval between RIRS and first retreatment was 49.84 days, and the mean of time interval between the first and second retreatments was 56.47 days, which indicates that the majority of treatments, subsequent to the first RIRS, were done in the first 3 months after the index date. Therefore, we believed that the time interval was sufficient to detect the majority of cases of recurrent stones or retreatments that occur in the real world. Lastly, renal stone patients may also have ureteral stones or renal stones that exist in the other side of the kidneys. To overcome this limitation, we excluded the patients with a diagnostic code of ureteral stones, and only included the ones with renal stone disease (ICD-10 code, N200).
Our study is the first to incorporate real clinical values into decision modeling to examine the cost-effectiveness of introducing RIRS into the treatment sequence of renal stone disease. We traced all possible treatment sequences to estimate the values of parameters, such as NRRs, selection probability, and cost spent on each sequence, based on large claims data. We also conducted a sensitivity analysis to show the acceptability curve of each scenario involving RIRS on its sequence, compared to the clinic scenario under diverse WTPs. Although the reimbursement policy may differ by country, we believe that our findings would be useful for insurers around the world. Insurers should consider the clinical and financial impact of RIRS in advance, so that they can use our model to calculate ICER, which would be helpful in planning healthcare budget and revising patient reimbursement policy.
ACKNOWLEDGEMENTS
This study was supported by a grant from the Korea Health Technology R&D Project of the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HC17C0005).
Footnotes
The authors have no potential conflicts of interest to disclose.
- Conceptualization: Joo Yong Lee and Seon-Heui Lee.
- Data curation: So-Young Yang, Sun-Hong Kwon, and Hae Do Jung.
- Formal analysis: So-Young Yang and Sun-Hong Kwon.
- Funding acquisition: Seon-Heui Lee.
- Investigation: So-Young Yang and Hae Do Jung.
- Methodology: Eui-Kyung Lee and Seon-Heui Lee.
- Project administration: Seon-Heui Lee.
- Resources: Joo Yong Lee and Seon-Heui Lee.
- Software: So-Young Yang and Sun-Hong Kwon.
- Supervision: Seon-Heui Lee.
- Validation: Joo Yong Lee and Seon-Heui Lee.
- Visualization: So-Young Yang and Eui-Kyung Lee.
- Writing—original draft: So-Young Yang, Joo Yong Lee, and Seon-Heui Lee.
- Writing—review & editing: Hae Do Jung, Sun-Hong Kwon, Eui-Kyung Lee, Joo Yong Lee, and Seon-Heui Lee.
- Approval of final manuscript: all authors.
References
- 1.Stamatelou KK, Francis ME, Jones CA, Nyberg LM, Curhan GC. Time trends in reported prevalence of kidney stones in the United States: 1976-1994. Kidney Int. 2003;63:1817–1823. doi: 10.1046/j.1523-1755.2003.00917.x. [DOI] [PubMed] [Google Scholar]
- 2.Scales CD, Jr, Smith AC, Hanley JM, Saigal CS Urologic Diseases in America Project. Prevalence of kidney stones in the United States. Eur Urol. 2012;62:160–165. doi: 10.1016/j.eururo.2012.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raheem OA, Khandwala YS, Sur RL, Ghani KR, Denstedt JD. Burden of urolithiasis: trends in prevalence, treatments, and costs. Eur Urol Focus. 2017;3:18–26. doi: 10.1016/j.euf.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 4.Geraghty RM, Jones P, Somani BK. Worldwide trends of urinary stone disease treatment over the last two decades: a systematic review. J Endourol. 2017;31:547–556. doi: 10.1089/end.2016.0895. [DOI] [PubMed] [Google Scholar]
- 5.Türk C, Petřík A, Sarica K, Seitz C, Skolarikos A, Straub M, et al. EAU guidelines on interventional treatment for urolithiasis. Eur Urol. 2016;69:475–482. doi: 10.1016/j.eururo.2015.07.041. [DOI] [PubMed] [Google Scholar]
- 6.Donaldson JF, Lardas M, Scrimgeour D, Stewart F, MacLennan S, Lam TB, et al. Systematic review and meta-analysis of the clinical effectiveness of shock wave lithotripsy, retrograde intrarenal surgery, and percutaneous nephrolithotomy for lower-pole renal stones. Eur Urol. 2015;67:612–616. doi: 10.1016/j.eururo.2014.09.054. [DOI] [PubMed] [Google Scholar]
- 7.Chang KD, Lee JY, Park SY, Kang DH, Lee HH, Cho KS. Impact of pretreatment hydronephrosis on the success rate of shock wave lithotripsy in patients with ureteral stone. Yonsei Med J. 2017;58:1000–1005. doi: 10.3349/ymj.2017.58.5.1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manzoor S, Hashmi AH, Sohail MA, Mahar F, Bhatti S, Khuhro AQ. Extracorporeal shock wave lithotripsy (ESWL) vs. ureterorenoscopic (URS) manipulation in proximal ureteric stone. J Coll Physicians Surg Pak. 2013;23:726–730. doi: 10.2013/JCPSP.726730. [DOI] [PubMed] [Google Scholar]
- 9.Lawler AC, Ghiraldi EM, Tong C, Friedlander JI. Extracorporeal shock wave therapy: current perspectives and future directions. Curr Urol Rep. 2017;18:25. doi: 10.1007/s11934-017-0672-0. [DOI] [PubMed] [Google Scholar]
- 10.Perez Castro E, Osther PJ, Jinga V, Razvi H, Stravodimos KG, Parikh K, et al. Differences in ureteroscopic stone treatment and outcomes for distal, mid-, proximal, or multiple ureteral locations: the Clinical Research Office of the Endourological Society ureteroscopy global study. Eur Urol. 2014;66:102–109. doi: 10.1016/j.eururo.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 11.Zhang W, Zhou T, Wu T, Gao X, Peng Y, Xu C, et al. Retrograde intrarenal surgery versus percutaneous nephrolithotomy versus extracorporeal shockwave lithotripsy for treatment of lower pole renal stones: a meta-analysis and systematic review. J Endourol. 2015;29:745–759. doi: 10.1089/end.2014.0799. [DOI] [PubMed] [Google Scholar]
- 12.Zheng C, Yang H, Luo J, Xiong B, Wang H, Jiang Q. Extracorporeal shock wave lithotripsy versus retrograde intrarenal surgery for treatment for renal stones 1–2 cm: a meta-analysis. Urolithiasis. 2015;43:549–556. doi: 10.1007/s00240-015-0799-8. [DOI] [PubMed] [Google Scholar]
- 13.Jeong JY, Kim JC, Kang DH, Lee JY. Digital videoscopic retrograde intrarenal surgeries for renal stones: time-to-maximal stone length ratio analysis. Yonsei Med J. 2018;59:303–309. doi: 10.3349/ymj.2018.59.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ergin G, Kirac M, Kopru B, Ebiloglu T, Kibar Y, Biri H. Shock wave lithotripsy or retrograde intrarenal surgery: which one is more effective for 10-20-mm renal stones in children. Ir J Med Sci. 2018;187:1121–1126. doi: 10.1007/s11845-018-1776-3. [DOI] [PubMed] [Google Scholar]
- 15.Rassweiler J, Rassweiler MC, Klein J. New technology in ureteroscopy and percutaneous nephrolithotomy. Curr Opin Urol. 2016;26:95–106. doi: 10.1097/MOU.0000000000000240. [DOI] [PubMed] [Google Scholar]
- 16.Sarkissian C, Noble M, Li J, Monga M. Patient decision making for asymptomatic renal calculi: balancing benefit and risk. Urology. 2013;81:236–240. doi: 10.1016/j.urology.2012.10.032. [DOI] [PubMed] [Google Scholar]
- 17.Fann CY, Huang PC, Yen AM, Chen HH. Patient utility measurement for managing ureteral stones: a modified standard gamble approach. Value Health Reg Issues. 2012;1:87–92. doi: 10.1016/j.vhri.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 18.Ko WJ, Jung JH, Han HH, Hong JH. Analysis of the status and treatment pattern of patients with urinary tract stone. [accessed on 2018 January 3]. Available at: http://www.alio.go.kr/informationResearchView.do?seq=2285039.
- 19.Tarazona VC, Alba AB, de Pablos JAD, Mateu PB, Vivas-Consuelo D. Cost-effectiveness analysis of extracorporeal shock wave lithotripsy versus retrograde intrarenal surgery in the management of small moderated-sized renal stones. Value Health. 2017;20:A490 [Google Scholar]
- 20.Kim JA, Yoon S, Kim LY, Kim DS. Towards actualizing the value potential of Korea Health Insurance Review and Assessment (HIRA) data as a resource for health research: strengths, limitations, applications, and strategies for optimal use of HIRA data. J Korean Med Sci. 2017;32:718–728. doi: 10.3346/jkms.2017.32.5.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cone EB, Pareek G, Ursiny M, Eisner B. Cost-effectiveness comparison of ureteral calculi treated with ureteroscopic laser lithotripsy versus shockwave lithotripsy. World J Urol. 2017;35:161–166. doi: 10.1007/s00345-016-1842-2. [DOI] [PubMed] [Google Scholar]
- 22.Briggs A, Sculpher M, Claxton K. Decision modelling for health economic evaluation. New York: Oxford University Press; 2006. [Google Scholar]
- 23.Wu CF, Shee JJ, Lin WY, Lin CL, Chen CS. Comparison between extracorporeal shock wave lithotripsy and semirigid ureterorenoscope with holmium: YAG laser lithotripsy for treating large proximal ureteral stones. J Urol. 2004;172:1899–1902. doi: 10.1097/01.ju.0000142848.43880.b3. [DOI] [PubMed] [Google Scholar]
- 24.Childs MA, Rangel LJ, Lingeman JE, Krambeck AE. Factors influencing urologist treatment preference in surgical management of stone disease. Urology. 2012;79:996–1003. doi: 10.1016/j.urology.2011.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuo RL, Aslan P, Abrahamse PH, Matchar DB, Preminger GM. Incorporation of patient preferences in the treatment of upper urinary tract calculi: a decision analytical view. J Urol. 1999;162:1913–1918. doi: 10.1016/S0022-5347(05)68067-6. [DOI] [PubMed] [Google Scholar]
- 26.Bozzini G, Verze P, Arcaniolo D, Dal Piaz O, Buffi NM, Guazzoni G, et al. A prospective randomized comparison among SWL, PCNL and RIRS for lower calyceal stones less than 2 cm: a multicenter experience. A better understanding on the treatment options for lower pole stones. World J Urol. 2017;35:1967–1975. doi: 10.1007/s00345-017-2084-7. [DOI] [PubMed] [Google Scholar]