Abstract
Rice is the most tolerant staple crop to aluminum (Al) toxicity, which is a limiting stress for grain production worldwide. This Al tolerance is the result of combined mechanisms that are triggered in part by the transcription factor ASR5. ASRs are dual target proteins that participate as chaperones in the cytoplasm and as transcription factors in the nucleus. Moreover, these proteins respond to biotic and abiotic stresses, including salt, drought and Al. Rice plants with silenced ASR genes are highly sensitive to Al. ASR5, a well-characterized protein, binds to specific cis elements in Al responsive genes and regulates their expression. Because the Al sensitive phenotype found in silenced rice plants could be due to the mutual silencing of ASR1 and ASR5, we investigated the effect of the specific silencing of ASR5. Plants with artificial microRNA silencing of ASR5 present a non-transformed phenotype in response to Al due to the induction of ASR1. ASR1 has the same subcellular localization as ASR5, binds to ASR5 cis-regulatory elements, regulates ASR5 regulated genes in a non-preferential manner and might replace ASR5 under certain conditions. Our results indicate that ASR1 and ASR5 act in concert and complementarily to regulate gene expression in response to Al.
Keywords: aluminum, ASR, transcription factor, artificial microRNA
Introduction
Since their discovery in the 1990s (Iusem et al. 1993), ASR genes (ABA-stress and ripening) have been reported in several plant species but are absent in Arabidopsis thaliana (González and Iusem 2014). ASR expression is induced by abscisic acid (Joo et al. 2013; Vaidyanathan et al. 1999), fruit ripening (Maskin et al. 2001; Dóczi et al. 2005) and biotic and abiotic stresses (Wang et al., 2011; Hu et al., 2013; Liu et al., 2010). In the nucleus, ASR proteins bind to DNA in a Zn2+ dependent manner to regulate specific promoters (Rom et al. 2006; Goldgur et al. 2007; Çakir et al. 2003; Saumonneau et al. 2008; Frankel et al. 2007; Arenhart et al. 2014; Ricardi et al. 2014). In rice, six copies of ASR are found in different chromosomes. However, a consensual nomenclature is lacking (Frankel et al. 2006; Joo et al., 2013; Philippe et al., 2010). In rice, ASR5 and ASR1 are the two most abundant members at the transcript level in roots and leaves (Arenhart et al. 2013). Both are induced by aluminum (Al), an abiotic stress that severely impacts crop production. Under acidic conditions, the trivalent Al3+ ions are dissolved from clay minerals and incorporated into the plant, inhibiting root growth and function (Kochian et al. 2015).
In addition to ASR, at least one other transcription factor plays a role in Al tolerance in rice. ART1 (Aluminum Resistance Transcription Factor 1) is constitutively expressed in the roots and is not induced by Al. However, ART1 knockout mutants exhibit high Al sensitivity (Yamaji et al. 2009). Furthermore, both ART1 and ASR5 regulate important genes in the response to Al (Yamaji et al. 2009; Arenhart et al. 2014), including STAR1 (sensitive to aluminum rhizotoxicity 1), an ATP-binding protein that together with STAR2 transports UDP-glucose, masking Al binding sites in the cell wall (Huang et al., 2009).
Rice is the most Al-tolerant crop under both field and controlled conditions (Foy 1988; Famoso et al. 2010), employing several strategies to cope with Al toxicity. Recently, a conserved DNA binding motif for rice ASR5 and tomato ASR1 was identified (Arenhart et al. 2014; Ricardi et al. 2014). This consensus sequence (A/GGCCCAA/T) is present in the promoter of many Al responsive genes in rice (Arenhart et al. 2014), including the ASR members ASR1 and ASR5. However, it is not clear whether ASR genes are auto-regulated.
The clear role of ASR genes in Al tolerance in rice was demonstrated when ASR silenced plants (RNAi_ASR) became highly sensitive to Al stress (Arenhart et al. 2013). However, it was not possible to describe the specific role of ASR5, as the RNAi silenced both ASR1 and ASR5.
In this study, we show that the use of an artificial microRNA in plants to specifically silence ASR5 induces ASR1 to compensate and regulate ASR5-regulated genes, demonstrating a complementary function of ASR genes in rice. Furthermore, ASR1 and ASR5 both regulate each other and auto-regulate themselves, indicating the complex regulation of these genes by a positive feedback loop control.
Material and Methods
Real-time RT–qPCR
RNA from amiRNA plants was extracted with TRIzol (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. cDNA was synthesized using M-MLV RT reverse transcriptase (Promega, Madison, WI, USA). For the real-time RT–qPCR assays, the original cDNA reaction solution was diluted 100×. The protocol applied for real-time RT–qPCR can be summarized as follows: an initial step of 5 min at 94°C was followed by 40 cycles of 10 s at 94°C, 15 s at 60°C, and 15 s at 72°C. The melting curves of the RT–qPCR samples were analyzed to assure that a single and expected peak was obtained. Relative changes in gene expression levels were calculated using the 2–ΔΔCt method (Livak and Schmittgen 2001). All reactions were performed in four technical replicates. Quantitative PCR was conducted using the specific primer pairs listed in Table S1. Real-time RT–qPCR was performed in a StepOne Applied Biosystems real-time cycler™. FDH (LOC_Os02g57040) and Actin2 (LOC_Os08g29650) were used as reference genes. For ASR genes, we used the nomenclature according to Frankel et al (Frankel et al. 2006).
Construction of the amiRNA vector and plant transformation
A chimeric gene producing an artificial microRNA (Schwab et al. 2006) was designed using the Osa-MIR528 precursor as a stemloop backbone (Warthmann et al. 2008). The mature miRNA for the specific silencing of ASR5 (LOC_Os11g06710) was designed using the WMD2 web tool (http://wmd2.weigelworld.org). The 21mer (amiRNA) mimics the foldback structure of the endogenous osa-MIR528. The primary amiRNA was engineered from pNW55, replacing the 21 bases of the natural osa-MIR528 miRNA as well as the partially complementary region of the miRNA by modification PCRs. For the amiRNA construct, three fragments including the multiple cloning sites (MCS) were PCR amplified from the template clone pNW55 using a total of six primers (Table S1). Four of these primers were designed such that the miRNA and miRNA* sequences were replaced by the desired 21mer. The amiRNA construct was generated by three modification PCRs using primers G-4368+II, I+IV and III+G-4369 on pNW55 as template, yielding fragments of 256, 87 and 259 bp, respectively. The three resulting fragments were gel purified (Life Technologies®) and then fused by one PCR with the two flanking primers G-4368 and G-4369 on a mixture of 1 μl from each previous PCR as template. The primer G-4368 was modified to amplify the CACC sequence necessary for pENTR recombination. The 558 bp fusion product was gel purified (Life Technologies®), cloned into pENTR D-TOPO Vector (Invitrogen, Carlsbad, CA, USA), and recombined into the modified binary vector pH7WG2D (Karimi, Inzé, and Depicker 2002). Restriction enzymes were used to replace the 35S promoter with the maize ubiquitin promoter in this binary vector. Agrobacterium-mediated transformation of rice calli was performed as described previously (Upadhyaya et al. 2000) using the Nipponbare cultivar. Regenerated plants were selected using hygromicin, and T2 generation plants were used in the analyses.
Morin treatment and relative root elongation
For Morin treatment, rice seeds from Japonica Nipponbare (NT and RNAi_ASR), Indica Br-Irga and amiRNA backgrounds were germinated on filter paper for 4 days in the dark at 28°C. The seedlings were grown in a 0.5 mM CaCl2 solution for 2 days in a growth chamber at 28°C under 12 hours of light. The seedlings were then exposed to the same solution plus AlCl3 (50 μM) for 1 and 6 hours at pH 4.5. After the exposure time, the roots of the seedling (control and Al-treated) were incubated for 30 minutes with Morin (100 μM) and visualized using a fluorescence microscope. For relative root elongation, the seedlings were grown in a 0.5 mM CaCl2 solution for 2 days in a growth chamber at 28°C under 12 hours of light and then exposed to the same solution plus AlCl3 (150 μM) for 24 hours at pH 4.5. Relative root elongation was defined as the percentage of the root elongated by AlCl3 compared with the control (AlCl3-free).
Subcellular localization
For the transient expression of ASR1 and ASR5 in Arabidopsis protoplasts, the complete coding sequences of both were fused to the GFP coding sequence at the N-terminus and cloned into the Gateway vector pART7-YFP (Galvan-Ampudia and Offringa 2006). The ampliffed cDNA was introduced into the appropriate plasmids by Gateway technology. The resulting vector was used to perform protoplast transformation.
Transient gene expression assays
Protoplast isolation and PEG transformation were performed using the tape method (F.-H. Wu et al. 2009). Plasmid DNAs were extracted using the QIAGEN Plasmid Maxi Kit (Qiagen, Hilden, Germany). Approximately 1×104 isolated mesophyll protoplasts were transfected with 10 μg of the plasmids 35S::Renilla Luciferase, pGusXXproAsr1_500, pGusXXproAsr5_1000, pGus_STAR1, pART35S::ASR1 and pART35S::ASR5 according to each experiment and incubated for 48 h at 25 °C in the dark. The empty vector pGusXX and positive control pGusSH (35S::Gus) were used. Primers are listed in Table S1. Protoplasts were harvested via centrifugation and lysed in 100 μl of CCLR buffer (25 mM K-phosphate pH 7.5, 1 mM EDTA, 7 mM 2-mercaptoethanol, 1 % triton X-100, 1 % Glycerol). Renilla activity was measured using Coelenterazine (Sigma), while GUS activity was measured using MUG (4-methylumbelliferyl-β-D-glucuronide) and MU (4-methylumbelliferone). pGusXX and pGusSH plasmids are described in Pasquali et al. 1994 (Pasquali, Ouwerkerk, and Memelink 1994). Please see Table S1 for primers list.
Pull-down assay
Pull-down assays were performed as previously described (K. K. Wu 2006). Biotin-labeled forward and reverse primers for the STAR1 promoter (LOC_Os06g48060) were used to amplify fragments via PCR employing rice genomic DNA as a template. The amplified product was bound to streptavidin-agarose beads and used to precipitate the ASR1 (LOC_Os02g33820) and ASR5 proteins. A Western blot assay was subsequently performed. The primers used in these assays are described in Table S1.
Western blot
Aliquots of ASR proteins were loaded and separated by SDS-PAGE in a 15% gel. The ASR5 protein was detected with a rabbit polyclonal ASR5 antibody (1:500 dilution). The ASR1_GST protein was detected with a rabbit polyclonal GST antibody (1:500 dilution). Goat anti-rabbit IgG (1:1000) conjugated to alkaline phosphatase was used as the secondary antibody. The bands were detected with a premixed BCIP/NBT substrate solution (Sigma-Aldrich, St. Louis, MO, USA) and recorded on X-ray film.
Characterization of the rice ssp Indica response to Al
The Indica Br-Irga background strain was germinated on filter paper for four days in the dark at 28°C. The seedlings were grown in a CaCl2 0.5 mM solution for one week in a growth chamber at 28°C under 12 hours of light. The seedlings were then exposed to the same solution plus AlCl3 (150 μM) for three days at pH 4.5.
Yeast two-hybrid assay
To investigate the interaction between the ASR5 and ASR1 proteins, the coding sequences of both genes were amplified via PCR using specific primers (Table S1). The yeast strain AH109 was co-transformed with pXDGATcy86 (GAL-4-binding domain) and pGADT7 (GAL-4 activation domain) plasmids containing these genes. The lithium acetate yeast transformation method was applied with some modifications to introduce the constructs into the cells (Gietz and Woods 2002). Briefly, AH109 yeast cells were incubated overnight at room temperature in a solution containing 1 μg of each plasmid, single stranded carrier DNA and LiAc, 10X TE and PEG4000 50% (1:1:8). The next morning, the mixture was incubated for 15 min at 42°C and the yeast was plated on selective medium.
Results
ASR1 compensates for ASR5 silencing in rice plants
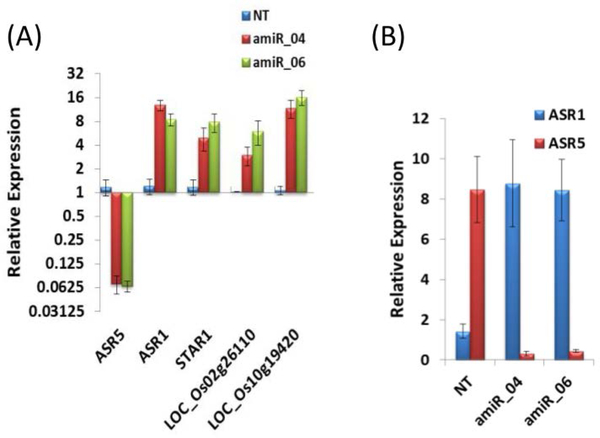
In a previous study, we showed that ASR-silenced plants present higher sensitivity to Al (Arenhart et al. 2013). Because these plants were silenced for both the ASR1 and ASR5 genes, we generated plants silenced specifically for ASR5 using an artificial microRNA (Schwab et al. 2006; Warthmann et al. 2008). Two transgenic lines (amiR_04 and amiR_06) were obtained that did not present any apparent contrasting phenotype to non-transformed (NT) plants. At the molecular level, amiRNA plants exhibited a strong decrease in ASR5 transcript in comparison to NT plants (Figure 1A). However, these plants exhibited higher levels of ASR1 transcript compared with NT plants. Moreover, the silencing of ASR5 and the consequent induction of ASR1 led to the induction of ASR5-regulated genes (Figure 1A). Furthermore, ASR1 transcripts increased to the same levels as ASR5 in NT plants (Figure 1B).
Figure 1. Quantitative Real Time RT-PCR of artificial microRNA plants.
Total RNA was extracted from the roots and used to synthesize cDNA. The relative expression was plotted using the FDH and Actin 2 gene expression levels as references. The roots of the Non-transformed (NT-Nipponbare cultivar), and amiRNA plants amiR_04 and amiR_06 were collected after 8 days. (A) Expression levels of ASR5, ASR1, STAR1, LOC_Os02g26110 and LOC_Os10g19420 in amiRNA plants compared to NT plants. (B) Expression levels of ASR1 and ASR5 in amiRNA plants compared to NT plants. Values represent the means +/− SD (N=4).
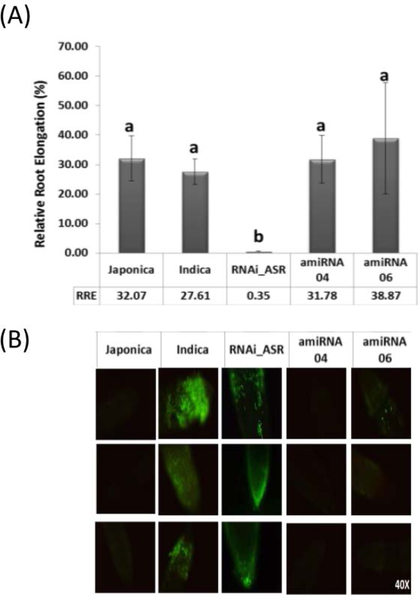
The plants harboring artificial microRNAs targeting ASR5 also presented a similar phenotype to NT plants (ssp Japonica background Nipponbare and ssp Indica background Br-Irga) in response to Al, showing a higher tolerance to Al compared with ASR silenced plants (RNAi_ASR) (Figure 2A). The use of Morin staining confirmed that amiRNA plants accumulated less Al than RNAi_ASR and Indica plants (a rice subspecies sensitive to Al – Supplementary Figure 1), being comparable to the Japonica phenotype (Figure 2B – Supplementary Figure 2). These results suggest that ASR1 can compensate for ASR5 silencing and indicates that ASR1 has a complementary role with ASR5.
Figure 2. amiRNAs plants have wild type phenotype.
(A) Relative Root Elongation for Non transformed rice ssp Japonica cv Nipponbare, ssp Indica cv BrIrga, RNAi_ASR and amiRNA plants after 24 hours of aluminum exposure (150μM). (B) Morin staining showing Al accumulation in the roots from the above plants treated with AlCl3 (50 μM) for 6 hours at pH 4.5. n =10. Bars with different letters are significantly different (ANOVA, P < 0.05).
ASR1 and ASR5 are localized in the same cellular compartments
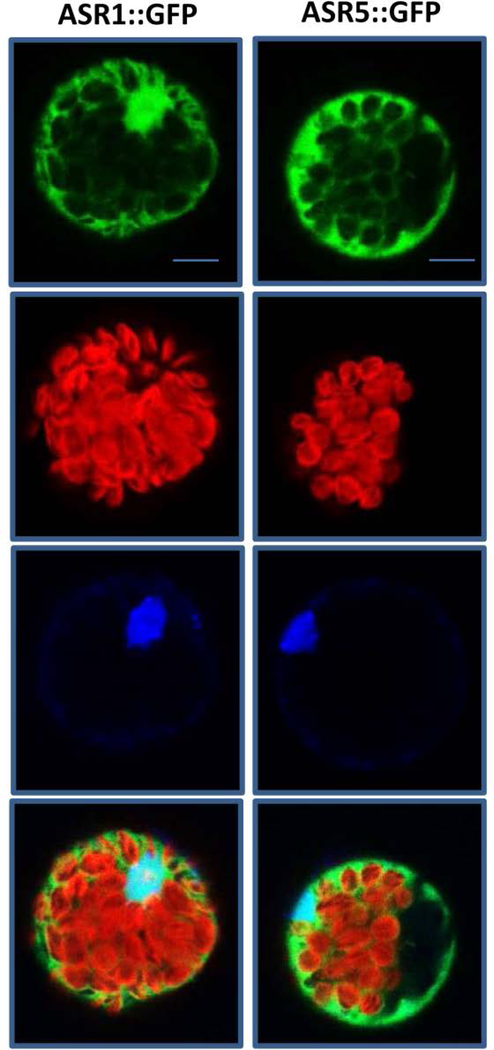
To investigate the subcellular localization of both proteins, the full-length cDNA of ASR1 and ASR5 were fused at their N-termini to the coding sequence of GFP. Transient expression using Arabidopsis protoplasts showed nuclear and cytoplasmic localization for both proteins (Figure 3). Alignment of the amino acid sequences also shows a close identity (74%) between ASR1 and ASR5 (data not show).
Figure 3. ASR1 and ASR5 are localized in nuclei and cytoplasm.
Localization of 35S-ASR1::GFP and 35S-ASR5::GFP in Arabidopsis protoplasts. From upper to lower – green fluorescent signal from GFP constructs, fluorescence of chlorophyll, DAPI staining fluorescence and merged images using a confocal laser scanning microscope. Bar = 10 μM.
Promoter analyses of ASR1 and ASR5 genes
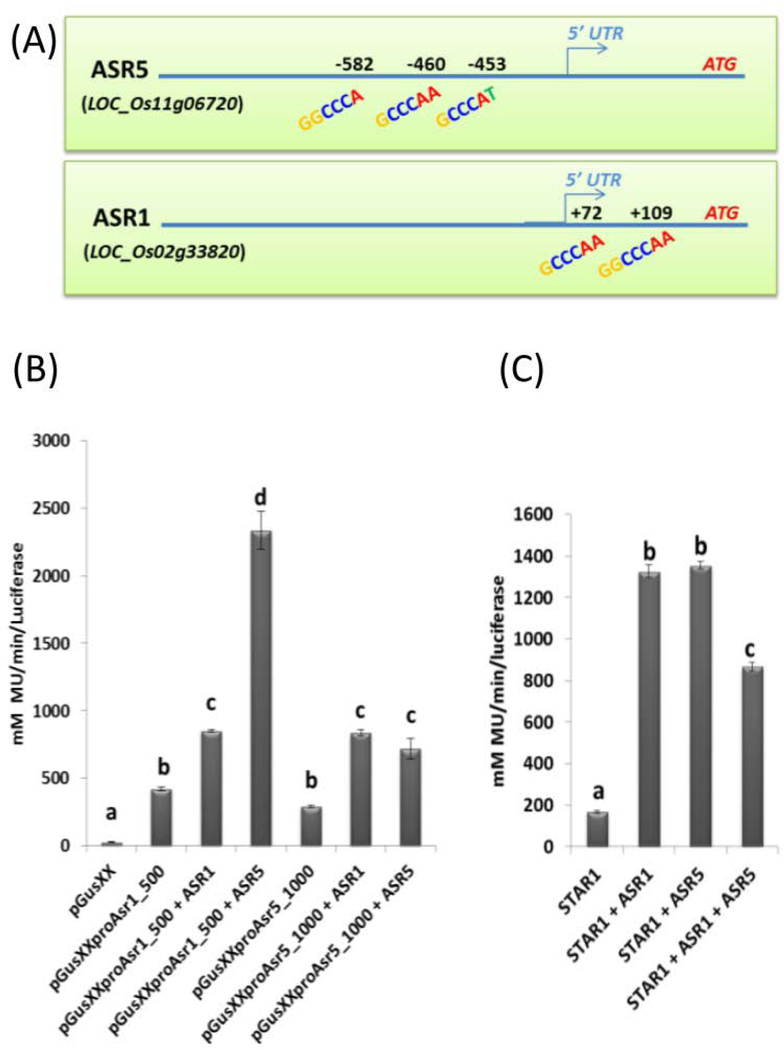
We further analyzed the promoter regions of ASR1 and ASR5. Interestingly, the ASR1 promoter region contains two ASR binding motifs located at −149 and −187 bp. ASR5 contains three ASR binding motifs located at −550, −557 and −679 bp before the start codon (Figure 4A). Transactivation assays using the ASR1 and ASR5 regions covering these motifs were used to drive GUS expression. ASR1 can auto-regulate itself, but ASR5 seemed to play a major role in ASR1 regulation (Figure 4B). When the ASR5 promoter region was used, both ASR1 and ASR5 were able to regulate GUS expression, suggesting that ASR1 and ASR5 regulate each other and auto-regulate themselves. To test if ASR1 and ASR5 can act synergistically to promote gene expression, both proteins were co-transformed with the STAR1 promoter region containing the previously described ASR5 binding motif (Arenhart et al. 2014). However, the addition of both proteins did not increase GUS expression driven by STAR1 promoter (Figure 4C), which suggests that they do not act synergistically.
Figure 4. A redundant function for ASR1 and ASR5.
(A) ASR5 binding motifs found in ASR1 and ASR5 promoter regions. (B) Transient gene expression assays showing regulation of ASR1 and ASR5 by ASR1 and ASR5 using GUS/Luciferase assay. (C) Transient gene expression assays showing regulation of STAR1 by ASR1 and ASR5 using GUS/Luciferase assay. pGusXX = empty vector; pGusXXproAsr1_500 = 500 base pairs from ASR1 promoter fused to GUS; pGusXXproAsr5_1000 = 1000 base pairs from ASR5 promoter fused to GUS. The bars containing different letters are significantly different (ANOVA, P < 0.05). Bars with different letters are significantly different (ANOVA, P < 0.05).
ASR1 binds to the ASR5 binding motif and regulates gene expression
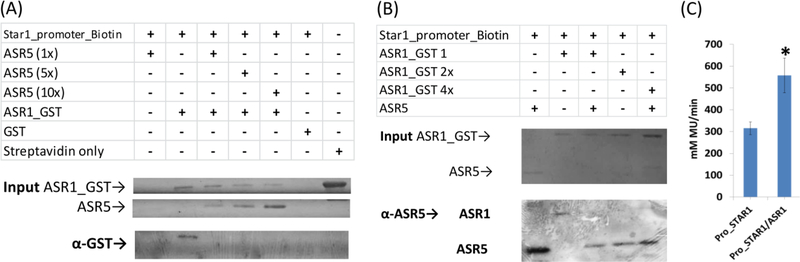
To confirm our hypothesis that ASR1 acts complementarily to ASR5, we analyzed the binding of ASR1 to the STAR1 promoter through an in vitro DNA pull down assay (K. K. Wu 2006). ASR1 bound to the STAR1 DNA, but ASR5 strongly competed with ASR1 (Figure 5). ASR5 protein in the same concentration as ASR1 was sufficient to inhibit ASR1 binding (Figure 5A). A four-fold increase in the ASR1 protein was not sufficient to compete with and displace ASR5 from its binding at the STAR1 promoter (Figure 5B). Moreover, transient gene expression assays demonstrated the regulation of STAR1 by ASR1 (Figure 5C). To verify a possible physical interaction between ASR1 and ASR5, a two-hybrid system assay was performed, but no interaction was found between the proteins (Supplementary Figure 3).
Figure 5. ASR1 binds to the STAR1 promoter in vitro in a non-preferential manner.
(A) SAPA pull-down system showing that ASR1-GST binds to the STAR1 DNA biotinylated fragment. ASR5 was used as competitor. (B) SAPA pull-down system showing that ASR5 binds preferentially to the STAR1 DNA biotinylated fragment. ASR1-GST was used as competitor. GST alone was used as a negative control. (C) Transient gene expression assays showing regulation of STAR1 by ASR1 using GUS/Luciferase assay. Values represent the means +/− SD (N=4). Asterisks indicate significantly different means: (*) P<0.05.
These results indicated that rice ASR1 acts in a complementary way to ASR5 and can also activate STAR1 expression through a direct interaction with the cis-elements in the STAR1 promoter in response to Al (Figure 6).
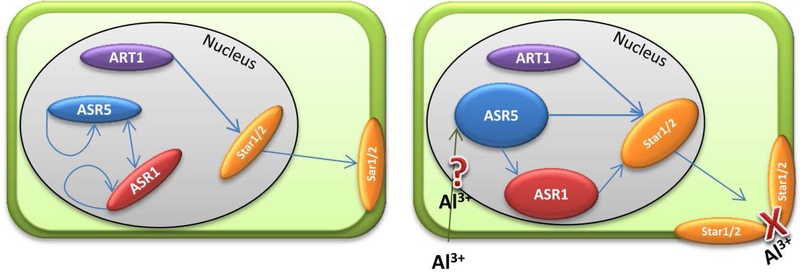
Figure 6. A proposed model for the ASR1/ASR5-STAR1 promoter interaction.
ASR1 and ASR5 are auto-regulated. In response to Al, ASR5 increase their transcript levels by an unknown pathway, regulates positively ASR1 and together with ART1, increase STAR1/STAR2 complex to mask Al binding sites in the cell wall.
Discussion
Aluminum is a highly abundant metal in the earth’s crust, mostly in nontoxic forms. Its solubilization to toxic forms occurs in acid soils, a major worldwide problem. As one of the most Al tolerant crops, rice employs different strategies (internal and external mechanisms), and key genes such as ASR5 are indispensable. ASR5 regulates at least 36 genes in response to Al, including STAR1 (Arenhart et al. 2014), which complexes with STAR2 to transport UDP-glucose, which is implicated in cell wall modifications to mask Al binding sites in the cell wall (Huang et al. 2009).
In rice, plants silenced for ASR5 present a sensitive phenotype in response to Al. However, not only ASR5 but also ASR1 was silenced in these plants (Arenhart et al. 2013). To more fully understand the role of ASR5 in the Al response, we generated amiRNA plants silenced specifically for ASR5. Two transgenic lines with no apparent phenotype compared to NT plants exhibit a remarkable silencing of this gene (Figure 1A). However, to compensate for ASR5 silencing, ASR1 transcript levels were increased in amiRNA plants to the same level that ASR5 was expressed in NT plants (Figure 1B). Moreover, other ASR5 regulated genes were induced in these plants (Figure 1A). These results led us to propose that ASR1 might act as a transcription factor and compensate for ASR5 silencing in amiRNA plants. To corroborate this finding, amiRNA plants exhibited a NT phenotype in response to Al compared with RNAi_ASR plants (Figure 2). Moreover, ASR1 exhibited the same subcellular localization as ASR5 (Figure 3).
We also tested whether ASR1 and ASR5 could regulate each other and auto-regulate themselves, as ASR5 binding motifs were found in both promoter regions (Figure 4A). Arabidopsis protoplasts were used in this assay because this species lacks ASR genes, decreasing unspecific transactivation. Addition of ASR1 and ASR5 in the promoter assays (ASR1 promoter and ASR5 promoter) induced Gus expression and indicated that these proteins regulated each other and auto-regulated themselves (Figure 4B). In fact, ASR1 was a target gene found in an ASR5 ChIP-Seq experiment in response to Al (Arenhart et al. 2014). Auto-regulation, a mechanism allowing the maintenance of the transcription factor levels and refining their action, has been used from bacteriophages to humans (Crews and Pearson 2009). A positive auto-regulation has been shown in rice for the OSH1 gene, which binds to the promoter of five other family members (Tsuda et al. 2011).
To test whether ASR1 regulates the expression of ASR5 regulated genes, ASR1 was co-transformed with the STAR1 promoter. This STAR1 promoter region was previously used to demonstrate specific ASR5 binding in response to Al (Arenhart et al. 2014). These analyses showed that ASR1 bound to the ASR5 motif and regulated gene expression (Figure 4C). The possibility of ASR1 and ASR5 acting synergistically was tested, but no increase in expression or physical interaction occurred (Figure 4C and Supplementary Figure 3). ASR proteins form homodimers in tomato (Goldgur et al., 2007) but not in rice ASR5 (Arenhart et al. 2014). Moreover, the grape ASR VvASR interacts with a DREB transcription factor in the nucleus (Saumonneau et al. 2008). Even as a monomer, we cannot discard that ASR5 can interact with another partner for gene regulation. Until now, we are the only group who tested possible interactors of rice ASR proteins. Because ASR1 and ASR5 do not act synergistically, a pull down approach was used to test for binding site competition between these proteins. ASR1 was able to bind and to regulate gene expression in vitro and in vivo at the ASR5 cis-element contained in the STAR1 promoter (Figure 5A, B and C). However, in our approach ASR5 bound preferentially to the STAR1 promoter.
Taken together, our results show that ASR1 and ASR5 auto-regulate themselves. In response to Al, the ASR5 transcript levels increased by an unknown mechanism, positively regulating ASR1 and together with ART1 increasing the STAR1/STAR2 complex to mask Al binding sites in the cell wall. This hypothetical model does not exclude other potential roles for ASR1 and ASR5, as it is well known that ASR participates in processes from plant development to environmental stresses. These results indicated that rice ASR1 complements ASR5 and can also activate STAR1 expression through a direct interaction with cis-elements in the STAR1 promoter in response to Al.
We conclude that ASR1 and ASR5 have complementary and compensatory functions regarding the Al response. ASR1 has the same subcellular localization as ASR5, binds to ASR5 DNA cis-regulatory elements and consequently also regulates ASR5 regulated genes in a non-preferential manner in response to Al.
Supplementary Material
Acknowledgments
This work was supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES: www.capes.gov.br), Fundação de apoio a Pesquisa do Rio Grande do Sul (FAPERGS), and the Brazilian National Council of Technological and Scientific Development (CNPq). This research was partially supported by a grant from the NIH (R01GM066258) to Z-Y.Wang.
References
- Arenhart Rafael Augusto, Bai Yang, Oliveira Luiz Felipe, Neto Lauro Bucker, Schunemann M, Maraschin FS, Mariath J, et al. 2014. “New Insights into Aluminum Tolerance in Rice: The ASR5 Protein Binds the STAR1 Promoter and Other Aluminum-Responsive Genes.” Molecular Plant 7 (4): 709–21. doi:doi: 10.1093/mp/sst160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arenhart Rafael Augusto, De Lima Julio César, Pedron Marcelo, Carvalho Fabricio E L, Da Silveira Joaquim Albenisio Gomes, Rosa Silvia Barcelos, Caverzan Andreia, et al. 2013. “Involvement of ASR Genes in Aluminium Tolerance Mechanisms in Rice.” Plant, Cell & Environment 36 (1) (January): 52–67. doi: 10.1111/j.1365-3040.2012.02553.x. http://www.ncbi.nlm.nih.gov/pubmed/22676236. [DOI] [PubMed] [Google Scholar]
- Crews Stephen T, and Joseph C Pearson 2009. “Transcriptional Autoregulation in Development.” Current Biology 19 (6): 1–10. doi: 10.1016/j.cub.2009.01.015.Transcriptional. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dóczi Róbert, Kondrák Mihály, Kovács Gabriella, Beczner Farkas, and Zsófia Bánfalvi. 2005. “Conservation of the Drought-inducible DS2 Genes and Divergences from Their ASR Paralogues in Solanaceous Species.” Plant Physiology and Biochemistry : PPB / Société Française De Physiologie Végétale 43 (3) (March): 269–76. doi: 10.1016/j.plaphy.2005.02.002. http://www.ncbi.nlm.nih.gov/pubmed/15854835. [DOI] [PubMed] [Google Scholar]
- Famoso Adam N, Clark Randy T, Shaff Jon E, Craft Eric, McCouch Susan R, and Kochian Leon V. 2010. “Development of a Novel Aluminum Tolerance Phenotyping Platform Used for Comparisons of Cereal Aluminum Tolerance and Investigations into Rice Aluminum Tolerance Mechanisms.” Plant Physiology 153 (4) (August): 1678–91. doi: 10.1104/pp.110.156794. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2923895&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foy CD. 1988. “Plant Adaptation to Acid, Aluminum-toxic Soils.” Soil Sci Plant Anal 19: 959–987. [Google Scholar]
- Frankel Nicolás, Carrari Fernando, Hasson Esteban, and Iusem Norberto D. 2006. “Evolutionary History of the Asr Gene Family.” Gene 378: 74–83. doi: 10.1016/j.gene.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Frankel Nicolás, Adriano Nunes-Nesi Ilse Balbo, Mazuch Jeannine, Centeno Danilo, Iusem Norberto D, Fernie Alisdair R, and Fernando Carrari 2007. “ci21A/Asr1 Expression Influences Glucose Accumulation in Potato Tubers.” Plant Molecular Biology 63 (5) (March): 719–30. doi:10.1007/s11103-006-9120-0 . 10.1007/s11103-006-9120-0http://www.ncbi.nlm.nih.gov/pubmed/17211513. http://www.ncbi.nlm.nih.gov/pubmed/17211513 . [DOI] [PubMed] [Google Scholar]
- Galvan-Ampudia CS, and Offringa R. 2006. “Plant Evolution: AGC Kinases Tell the Auxin Tale.” Trends in Plant Science 12: 541–547. [DOI] [PubMed] [Google Scholar]
- Gietz RD, and Woods RA. 2002. “Transformation of Yeast by the Liac/SS Carrier DNA/PEG Method.” Methods in Enzymology 350: 87–96. [DOI] [PubMed] [Google Scholar]
- Goldgur Yehuda, Rom Slava, Ghirlando Rodolfo, Shkolnik Doron, Shadrin Natalia, Konrad Zvia, and Bar-Zvi. Dudy 2007. “Desiccation and Zinc Binding Induce Transition of Tomato Abscisic Acid Stress Ripening 1, a Water Stress-and Salt Stress-regulated Plant-specific Protein, from Unfolded to Folded State.” Plant Physiology 143 (2) (February): 617–28. doi: 10.1104/pp.106.092965. http://www.ncbi.nlm.nih.gov/pubmed/17189335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González Rodrigo M, and Iusem Norberto D. 2014. “Twenty Years of Research on Asr (ABA-stress-ripening) Genes and Proteins.” Planta (February 15). doi: 10.1007/s00425-014-2039-9. http://www.ncbi.nlm.nih.gov/pubmed/24531839. [DOI] [PubMed] [Google Scholar]
- Hsu Yi-Feng, Yu Shu-Chuan, Yang Chin-Ying, and Wang Co-Shine. 2011. “Lily ASR Protein-conferred Cold and Freezing Resistance in Arabidopsis.” Plant Physiology and Biochemistry 49 (9) (September): 937–45. doi: 10.1016/j.plaphy.2011.07.002. http://www.ncbi.nlm.nih.gov/pubmed/21803593. [DOI] [PubMed] [Google Scholar]
- Hu Wei, Huang Chao, Deng Xiaomin, Zhou Shiyi, Chen Lihong, Li Yin, Wang Cheng, et al. 2013. “TaASR1, a Transcription Factor Gene in Wheat, Confers Drought Stress Tolerance in Transgenic Tobacco.” Plant, Cell & Environment 36 (8) (August): 1449–64. doi: 10.1111/pce.12074. http://www.ncbi.nlm.nih.gov/pubmed/23356734. [DOI] [PubMed] [Google Scholar]
- Huang CF, Yamaji Naoki, Mitani Namiki, Yano Masahiro, Nagamura Yoshiaki, and Ma Jian Feng. 2009. “A Bacterial-type ABC Transporter Is Involved in Aluminum Tolerance in Rice.” The Plant Cell 21 (2) (February): 655–67. doi: 10.1105/tpc.108.064543. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2660611&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iusem ND, Bartholomew DM, Hitz WD, and Scolnik PA. 1993. “Tomato (Lycopersicon Esculentum) Transcrip Induced by Water Deficit and Ripening.” Plant Physiology 102: 1353–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo Joungsu, Youn Hab Lee Yeon-ki Kim, Nahm Baek Hie, and Song Sang Ik. 2013. “Abiotic Stress Responsive Rice ASR1 and ASR3 Exhibit Different Tissue-Dependent Sugar And.” Molecules and Cells: 1–10. doi: 10.1007/s10059-013-0036-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi Mansour, Inzé Dirk, and Depicker Ann. 2002. “Gateway Vectors for Agrobacterium-mediated Plant Transformation.” Trends in Plant Science 7 (5): 193–195. [DOI] [PubMed] [Google Scholar]
- Kochian Leon V, Piñeros Miguel a, Liu Jiping, and Magalhaes Jurandir V. 2015. “Plant Adaptation to Acid Soils: The Molecular Basis for Crop Aluminum Resistance.” Annual Review of Plant Biology 11:1 (January) (January 22): 1–28. doi: 10.1146/annurev-arplant-043014-114822. http://www.ncbi.nlm.nih.gov/pubmed/25621514. [DOI] [PubMed] [Google Scholar]
- Konrad Zvia, and Dudy Bar-Zvi. 2008. “Synergism Between the Chaperone-like Activity of the Stress Regulated ASR1 Protein and the Osmolyte Glycine-betaine.” Planta 227 (6) (May): 1213–9. doi: 10.1007/s00425-008-0693-5. http://www.ncbi.nlm.nih.gov/pubmed/18270732. [DOI] [PubMed] [Google Scholar]
- Liu Hai-Yan, Dai Jin-Ran, Feng Dong-Ru, Liu Bing, Wang Hong-Bin, and Wang Jin-Fa. 2010. “Characterization of a Novel Plantain Asr Gene, MpAsr, That Is Regulated in Response to Infection of Fusarium Oxysporum F. Sp. Cubense and Abiotic Stresses.” Journal of Integrative Plant Biology 52 (3) (March): 315–23. doi: 10.1111/j.1744-7909.2010.00912.x. http://www.ncbi.nlm.nih.gov/pubmed/20377692. [DOI] [PubMed] [Google Scholar]
- Livak Kenneth J, and Schmittgen Thomas D 2001. “Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2 Ϫ ⌬⌬ C T Method.” Methods 25: 402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Maskin Laura, Frankel Nicolás, Gudesblat Gustavo, Demergasso María J, Pietrasanta Lía I, and Iusem Norberto D. 2007. “Dimerization and DNA-binding of ASR1, a Small Hydrophilic Protein Abundant in Plant Tissues Suffering from Water Loss.” Biochemical and Biophysical Research Communications 352 (4) (January): 831–5. doi: 10.1016/j.bbrc.2006.11.115. http://www.ncbi.nlm.nih.gov/pubmed/17157822. [DOI] [PubMed] [Google Scholar]
- Maskin Laura, Gudesblat Gustavo E., Moreno Javier E., Carrari Fernando O., Frankel Nicolás, Sambade Adrián, Rossi Magdalena, and Iusem Norberto D.. 2001. “Differential Expression of the Members of the Asr Gene Family in Tomato (Lycopersicon Esculentum).” Plant Science 161 (4) (September): 739–746. doi: 10.1016/S0168-9452(01)00464-2. http://linkinghub.elsevier.com/retrieve/pii/S0168945201004642. [DOI] [Google Scholar]
- Pasquali Giancarlo, Ouwerkerk Pieter B. F., and Memelink Johan. 1994. “Versatile Transformation Vectors to Assay the Promoter Activity of DNA Element in Plants.” Gene 149: 373–374. [DOI] [PubMed] [Google Scholar]
- Philippe Romain, Courtois Brigitte, Kenneth L Mcnally Pierre Mournet, Redouane El-malki Marie Christine Le, Denis Paslier, Claire Fabre, Brunel Dominique, and Dominique Jean-christophe Glaszmann. 2010. “Structure, Allelic Diversity and Selection of Asr Genes, Candidate for Drought Tolerance, in Oryza Sativa L. and Wild Relatives.” Theoretical and Applied Genetics 121: 769–787. doi: 10.1007/s00122-010-1348-z. [DOI] [PubMed] [Google Scholar]
- Ricardi MM, González RM, Zhong S, Domínguez PG, Duffy T, Turjanski PG, Salter JDS, et al. 2014. “Genome-wide Data ( ChIP-seq ) Enabled Identification of Cell Wall-related and Aquaporin Genes as Targets of Tomato ASR1, a Drought Stress-responsive Transcription Factor.” BMC Plant Biology 14 (29). doi: 10.1186/1471-2229-14-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rom Slava, Gilad Ayelet, Kalifa Yossi, Konrad Zvia, Mark M Karpasas Yehuda Goldgur, and Dudy Bar-Zvi. 2006. “Mapping the DNA-and Zinc-binding Domains of ASR1 (abscisic Acid Stress Ripening), an Abiotic-stress Regulated Plant Specific Protein.” Biochimie 88 (6) (June): 621–8. doi: 10.1016/j.biochi.2005.11.008. http://www.ncbi.nlm.nih.gov/pubmed/16387406. [DOI] [PubMed] [Google Scholar]
- Saumonneau Amélie, Agasse Alice, Bidoyen Marie-Thérèse, Lallemand Magali, Cantereau Anne, Medici Anna, Laloi Maryse, and Atanassova Rossitza. 2008. “Interaction of Grape ASR Proteins with a DREB Transcription Factor in the Nucleus.” FEBS Letters 582 (23–24) (October): 3281–7. doi: 10.1016/j.febslet.2008.09.015. http://www.ncbi.nlm.nih.gov/pubmed/18804467. [DOI] [PubMed] [Google Scholar]
- Schwab Rebecca, Ossowski Stephan, Riester Markus, Warthmann Norman, and Weigel Detlef. 2006. “Highly Specific Gene Silencing by Artificial MicroRNAs in Arabidopsis” 18 (May): 1121–1133. doi: 10.1105/tpc.105.039834.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda Katsutoshi, Ito Yukihiro, Sato Yutaka, and Kurata Nori. 2011. “Positive Autoregulation of a KNOX Gene Is Essential for Shoot Apical Meristem Maintenance in Rice.” The Plant Cell 23 (12) (December): 4368–81. doi: 10.1105/tpc.111.090050. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3269871&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyaya NM, Surin B Ramm, Gaudron J, Schunmann PHD, Taylor W, Waterhouse PM, and Wang MB. 2000. “Agrobacterium-mediated Transformation of Australian Rice Cultivars Jarrah and Amaroo Using Modiffed Promoters and Selectable Markers.” Aust J Plant Physiol 27: 201–210. [Google Scholar]
- Vaidyanathan R, Kuruvilla S, and Thomas G. 1999. “Characterization and Expression Pattern of an Abscisic Acid and Osmotic Stress Responsive Gene from Rice.” Plant Science 140 (1) (January): 21–30. doi: 10.1016/S0168-9452(98)00194-0. http://linkinghub.elsevier.com/retrieve/pii/S0168945298001940. [DOI] [Google Scholar]
- Warthmann Norman, Chen Hao, Ossowski Stephan, Weigel Detlef, and Philippe Hervé. 2008. “Highly Specific Gene Silencing by Artificial miRNAs in Rice.” PloS One 3 (3) (January): e1829. doi: 10.1371/journal.pone.0001829. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2262943&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Fu-Hui, Shen Shu-Chen, Lee Lan-Ying, Lee Shu-Hong, Chan Ming-Tsar, and Lin Choun-Sea. 2009. “Tape-Arabidopsis Sandwich -a Simpler Arabidopsis Protoplast Isolation Method.” Plant Methods 5 (January): 16. doi: 10.1186/1746-4811-5-16. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2794253&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Kenneth K. 2006. “Analysis of protein-DNA Binding by Streptavidin – Agarose Pulldown.” Methods in Molecular Biology 338 (1): 281–290. [DOI] [PubMed] [Google Scholar]
- Yamaji Naoki, Chao Feng Huang Sakiko Nagao, Yano Masahiro, Sato Yutaka, Nagamura Yoshiaki, and Ma Jian Feng. 2009. “A Zinc Finger Transcription Factor ART1 Regulates Multiple Genes Implicated in Aluminum Tolerance in Rice.” The Plant Cell 21 (10) (October): 3339–49. doi: 10.1105/tpc.109.070771. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2782276&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Çakir Birsen, Agasse Alice, Gaillard Cécile, Saumonneau Amélie, Delrot Serge, and Atanassova Rossitza. 2003. “A Grape ASR Protein Involved in Sugar and Abscisic Acid Signaling.” The Plant Cell 15 (September): 2165–2180. doi: 10.1105/tpc.013854.contain. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.