Abstract
Aims
L‐asparaginase is an essential medicine in the treatment of pediatric acute lymphoblastic leukemia (ALL) and the quality of generic formulations is an area of concern. We compared nine generic formulations of L‐asparaginase available in India with the innovator.
Methods
The quality of formulations was assessed by measuring 72‐hour trough asparaginase activity in children with ALL during induction following administration of 10,000 IU/m2 of L‐asparaginase. In‐vitro analysis of the label claim was assessed by measuring activity of three generic formulations. Liquid chromatography‐mass spectrometry (LC/MS) was used to determine the amount of host contaminant proteins (HCPs) in the formulations.
Results
Between March 2015 to June 2018, 240 samples from 195 patients were analyzed. The number of samples analyzed ranged from 7–66 per generic brand (median: 18) and seven of the innovator. The proportion of generic formulations that failed to achieve a predefined clinical threshold activity of 50 IU/L ranged from 16.7% (2/12) to 84.9% (28/33) in the highest activity to lowest activity generic respectively. On other hand, all innovator samples had activity greater than 50 IU/L. In‐vitro asparaginase activity in the three generic formulations tested ranged from 71.4–74.6% of the label claim (10,000 IU) compared to 93.5% for the innovator. LC/MS analysis of generic 5 identified 25 HCPs with a relative peptide count of 27.1% of the total peptides.
Conclusions
Generic formulations had lower asparaginase activity which raises serious clinical concerns regarding their quality. Until stringent regulatory enforcement improves the quality of these generics, dose adaptive strategies coupled with therapeutic drug monitoring need to be considered.
Keywords: Generics, Innovator, L‐asparaginase, Quality
What is already known about this subject?
The quality of generic L‐asparaginase is a matter of concern globally based on a study of one generic formulation.
Since a plethora of generic brands of L‐asparaginase are marketed in India, and because no clinical study has been conducted demonstrating their efficacy, there is lingering concern about their quality.
What this study adds
Compared to the innovator, the generic formulations had markedly inferior activity in vitro and in vivo.
Mass‐spectrometry analysis of one of the generic formulations showed high levels of host contaminating proteins.
Our study underscores the need for heightened regulatory oversight and enforcement to ensure patients' access to good quality products.
1. INTRODUCTION
L‐asparaginase is an essential drug in the treatment of paediatric acute lymphoblastic leukemia (ALL) and its addition to chemotherapy protocols has led to improved overall survival and decreased relapse in paediatric ALL.1 It is an enzyme isolated from the periplasmic region of Escherichia coli or Erwinia chrysanthemi, which degrades L‐asparagine to L‐aspartic acid and ammonia. As leukemic blasts have a reduced capacity to synthesize L‐asparagine, deprivation of this amino acid leads to cell cycle arrest and apoptosis of the leukemic cells.2
In India, at least ten generic formulations of L‐asparaginase have been approved by State Licensing Agencies (SLA) or by the Central Drugs Standard Control Organisation (CDSCO). The approval process of these biosimilars have been briefly detailed by the CDSCO in “Guidelines on Similar Biologic: Regulatory Requirements for Marketing Authorization in India, 2016.” However, a waiver of safety and efficacy studies can be applied for if the generic formulation can demonstrate comparability to the reference biologic using physicochemical and in vitro techniques, if it is comparable to the reference biologic preclinically, if pharmacokinetic/pharmacodynamics studies have preferentially been done in‐patient, and if a comprehensive post marketing surveillance plan is in place.3 As these guidelines were mainly drafted with a primary endpoint of safety and secondary endpoints of efficacy and immunogenicity, the post marketing surveillance of generic formulations is limited to 4 years after approval, after which there are no recommendations. There is a lingering concern, however, whether these regulations are sufficient to ensure that only good quality generic L‐asparaginase receive marketing approval, and more importantly, about the ability of current regulations to ensure that quality is maintained beyond the surveillance period.
As L‐asparaginase was approved by the CDSCO in 1973, and due to concern of possible differences in manufacturing practices of companies producing L‐asparaginase, we conducted a retrospective review of L‐asparaginase activity levels reported in pediatric ALL patients at our hospital during the induction phase of chemotherapy. We also compared in‐vitro label claims of several different off‐the‐shelf generic Indian formulations to assess L‐asparaginase activity levels and the presence of host contaminating proteins (HCP).
2. METHODS
2.1. Study population
Newly diagnosed paediatric acute lymphoblastic leukemia (ALL) cases treated at Tata Memorial Hospital, Mumbai during the period March 2015 to June 2018 were included in this retrospective analysis. Any paediatric ALL case who had a trough L‐asparaginase activity reported after the 1st and subsequent doses of L‐asparaginase were included and demographic variables were collected. Briefly, paediatric B cell ALL patients were all treated on our institutional protocol (ICICLE) which consisted of 10,000 units/m2 of L‐asparaginase for 4–8 doses along with vincristine, steroids for standard risk with the addition of daunorubicin for intermediate and high‐risk patients.4 Early T precursor, T cell ALL, T cell lymphoblastic lymphoma, and mixed phenotypic leukemia were treated with MCP841, an institutional protocol with a similar four drug induction including eight doses of 10,000 units/m2 of L‐asparaginase.5 Nine generic formulations of L‐asparaginase were available in our hospital during this period. The name of the generic formulation which was used in each case was captured from the patients' records. Ethics approval was obtained to retrospectively collect demographic and laboratory values of interest.
2.2. Drugs and reagents
The nine different generic formulations of L‐asparaginase (10,000 IU/vial) were procured by the hospital pharmacy. Reagents for asparaginase activity including L‐aspartic acid β‐hydroxamate (AHA), trichloroacetic acid (TCA), 8‐hydroxyquinoline, and reference standard L‐asparaginase were procured from Sigma Aldrich (Bangalore, India). Generic formulations 1 through 8 were obtained from pharmacies of Indian origin, and generic formulation 9 from an Asian based pharmaceutical company. Innovator L‐asparaginase was obtained directly from Medac.
2.3. In‐vitro analysis of L‐asparaginase activity
Three generic formulations (Generic 1, 3 and 5) and the innovator were obtained for in‐vitro analysis. Each formulation was individually dissolved in sterile water for injection according to instructions in the product insert. Reference standards for L‐asparaginase were prepared using water for injection in a range of 0.4–40 IU/ml. The diluted formulations were analysed in triplicate using a sensitive microplate reader based method as described below.6 Briefly, 200 μl of the synthetic substrate 1mM AHA was incubated with 10 μl of diluted formulations at 37°C for 30 minutes. The reaction was stopped by adding 24.5% TCA followed by addition of a solution of 8‐hydroxyquinoline, sodium bicarbonate and hydrogen peroxide. The intensity of the green colour of the product indooxine was measured at 710 nm in a 96‐well plate using a sensitive microplate reader. The activity of samples was determined using a 4‐parametric non‐linear regression equation.
2.4. In‐vivo analysis of L‐asparaginase activity
Dose and administration: L‐asparaginase was administered at a dose of 10,000 IU/m2 intramuscular every 3 days for 4–8 doses depending on risk as per the ICICLE or MCP841 protocol.4 Patients were administered different L‐asparaginase formulations, based on the treating oncologist's preference and availability in the hospital pharmacy at that time. Due to sporadic availability during certain times, at one point only three generic formulations were available in the hospital pharmacy. Since these generic formulations were used interchangeably, 34 patients had cross‐over from one generic formulation to another.
Pharmacokinetic Sampling: 3 ml of blood from each patient were collected in EDTA collection tubes 72 hours after the preceding dose of L‐asparaginase. Samples were placed on ice and centrifuged at 4°C/1008 rcf for 10 minutes. The plasma samples were stored at −80°C pending analysis. The actual time of L‐asparaginase administration and blood sampling were recorded.
Plasma L‐asparaginase activity: To determine L‐asparaginase activity, the method described by Lanvers et. al was used with modifications.6 Briefly, 20 μL of plasma standard having known asparaginase activity or patient plasma was taken in micro‐centrifuge tubesto which 180 μL of 10mM AHA solution was added, vortexed for 3 minutes and kept for incubation at 37°C for 30 minutes. The reaction was stopped by adding 100 μl of 24.5% TCA. The samples were centrifuged at 700 rcf for 5 minutes at room temperature. 10 μL of supernatant was added to 40 μL of milliQ water in pre‐labelled micro‐centrifuge tubes followed by 200 μL of freshly prepared 8‐ hydroxyquinoline solutions. After heating at 95°C for 1 minute, 100 μL from each micro‐centrifuge tube was transferred to unique wells in 96‐well plate and the absorbance of the product, indooxine, was recorded at 710 nm. Unlike Lanvers et al, who used two linear curves in the activity range of 2.5–75 and 75–1250 IU/L, a single 4‐parametric non‐linear regression equation, generally employed for immunoassays,7 was used to validate this method in the activity range of 2–2000 IU/L. The validation parameters are shown in supplementary Table S1. Inter‐day and intra‐day accuracy and precision was calculated using four quality control (QC) samplesi.e., 2 IU/L, 60 IU/L, 300 IU/L and 2000 IU/L corresponding to the lower limit of quantitation (LLOQ), low QC (MQC), medium QC (MQC) and high QC (HQC) respectively.
2.5. Thermal stability analysis of L‐asparaginase activity at room temperature
Two generic formulations (Generic 1, and 5) and the innovator formulation were reconstituted with sterile water for injection as recommended in the information insert available with the vial. Further, the formulations were diluted to a final concentration of 12.5 IU/ml. Equal amount of formulation was aliquoted in independent micro‐centrifuge tubes and was placed at room temperature for 2, 4, 8, and 24 hrs. In‐vitro asparaginase activity, as described above was performed at each above mentioned time points to determine the asparaginase activity of the formulations. The activity was also evaluated at 0 time point. Three replicates of each formulation were used to perform the assay.
2.6. Impurity profiling by liquid chromatography–tandem mass spectrometry (LC–MS/MS)
Generic 5 was analysed by LC–MS/MS using Qtrap machine. It was the only generic available in the hospital pharmacy at the time of this analysis. The sample was prepared using a method previously described by Zenatti et al.8 Briefly, the sample was reconstituted in normal saline from which 30 μg was aliquoted to which 10 μL urea 8 M and 0.4 μL 250mM Dithiothreitol (DTT) was added. For reduction, the sample was incubated at 56°C for 25 min, following which 0.57 μL 500mM iodoacetamide was added and the sample was incubated for 30 min at room temperature in the dark for alkylation. This was followed by the addition of 0.4 μL 250mM DTT and further incubated for 15 min. The sample was digested by the addition of 53.3 μL 50mM ammonium bicarbonate, 0.74 μL 100mM CaCl2 and 1 μg of trypsin and incubated at 37°C for 13 hours. The reaction was stopped by the addition of trifluoroacetic acid to a final concentration of 1%.
Samples were then dried in a speed vacuum concentrator for 2 hours and then stored in a ‐20°C refrigerator pending analysis. Reconstitution of the dried samples was done using 60 μL of 80% acetonitrile (ACN) and de‐salted using a Zip‐Tip. The sample was again dried in a speed vacuum concentrator for 30 minutes to remove the ACN and reconstituted with 0.1% formic acid.
The sample was analysed on a Triple ToF 5600 plus (SCIEX) connected to LC–MS/MS (Exigent). Through an autosampler, 6 μl (2 μg) of protein sample was injected into a NANOLC trap column (350 μm*0.5 mm; C18; particle size 3 μm, 120 Ao) to trap the protein and a 45 minute wash (0.1% formic acid) at a flow rate of 2 μl/min was given to remove the unwanted residues. The peptides were separated by a 0–95% gradient of acetonitrile in 0.1% formic acid using C18 NANOLC Column (75 μm*15 cm; 3C‐18; particle size‐ 3 μm, 120 Ao) for 146 minutes at a flow rate of 250 nl/min. The nanoelectrospray voltage was set to 2.2 kV and the scan MS spectra was set at a range of 350 to 1250 Daltons. The raw files were processed using Peak View 1.2, and the MS/MS spectra were searched using the Protein pilot software against the Uniprot SwissProt E. coli database.
2.7. Statistical analysis
Descriptive statistics were used to characterize the demographic features. Frequency distributions were tabulated for categorical variables, and means and standard deviations were calculated for continuous variables.
3. RESULTS
During the study period, 240 samples from 195 patients were analyzed. Selected demographic factors of the patient population in relation to each generic formulation are shown in Table 1. Overall, the age, gender distribution, BSA, and L‐asparaginase dose were grossly similar between all 9 generics. The majority of the samples were B cell ALL (n = 222 which were further stratified into standard (n = 67, 30.1%), intermediate (n = 87, 39.1%), and high (n = 68, 30.6%) risk based on the ICICLE risk stratification at diagnosis. The number of patients who had one, two, or three sequential samples were 161 (82.6%), 23 (11.8%), and 11 (5.6%) respectively.
Table 1.
Patient demographics
| Gen 1 | Gen 2 | Gen 3 | Gen 4 | Gen 5 | Gen 6 | Gen 7 | Gen 8 | Gen 9 | Innovator | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | 66 | 41 | 35 | 12 | 33 | 18 | 7 | 10 | 11 | 7 | |
| Malea (n,%) | 41 (62.1%) | 24 (58.5%) | 23 (65.7%) | 9 (75%) | 17 (51.5%) | 13 (72.2%) | 4 (57.1%) | 7 (70%) | 8 (72.7%) | 6 (85.7%) | |
| Femalea (n,%) | 25 (37.9%) | 17 (41.5%) | 12 (34.3%) | 3 (25%) | 16 (48.5%) | 5 (27.8%) | 3 (42.9%) | 3 (30%) | 3 (27.3%) | 1 (14.2%) | |
| Ageb (years) (range) | 4.9 (1–15) | 4.8 (1–15) | 5.5 (1–14) | 4.5 (2.1–14.5) | 4.0 (0.8–13) | 4.8 (1.1–14.8) | 4.6 (0.9–13) | 3.1 (1–6) | 3.0 (1–15) | 3.0 (3–5) | |
| BSA (m2) (range) | 0.77 (0.45–1.54) | 0.80 (0.48–1.45) | 0.75 (0.45–1.25) | 0.79 (0.44–1.55) | 0.71 (0.4–1.7) | 0.76 (0.44–1.28) | 0.70 (0.4–1.4) | 0.56 (0.35–0.75) | 0.74 (0.46–1.25) | 0.63 (0.6–0.73) | |
| Dose (IU) (range) | 7623.1 (3000–15000) | 7987.9 (5000–13400) | 7305.7 (4500–12500) | 7845.5 (4400–15500) | 7138.4 (4000–17000) | 7577.8 (4400–12800) | 7066.7 (4000–14000) | 5600 (3500–7500) | 7318.2 (4600–13000) | 6271 (5600–7300) | |
| B‐ALLc | HR (n,%) | 15 (25.4%) | 10 (27.8%) | 12 (36.4%) | 3 (27.3%) | 14 (45.2%) | 7 (38.9%) | 2 (33.3%) | 2 (20%) | 2 (18.2%) | 0 (0.0%) |
| IR (n,%) | 23 (39.0%) | 14 (38.9%) | 14 (42.4%) | 5 (45.5%) | 10 (32.3%) | 06 (33.3%) | 1 (16.7%) | 3 (30%) | 4 (36.4%) | 7 (100.0%) | |
| SR (n,%) | 21 (35.6%) | 12 (33.3%) | 7 (21.2%) | 3 (27.3%) | 7 (22.6%) | 5 (27.8%) | 3 (50%) | 5 (50%) | 5 (45.5%) | 0 (0.0%) | |
| T‐ALL | 5 | 4 | 2 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | |
| Othersd | 2 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | |
Based on the total number of samples drawn for each generic formulation and not the number of patients as some patients had multiple samples drawn.
median.
B‐ALL stratified per ICICLE risk stratification.
Includes Early T‐cell Precursor ALL and Mixed Phenotype Acute Leukemia.
3.1. In‐vitro asparaginase activity
In‐vitro asparaginase activity in the innovator was 93.5 ± 4.4% of the label claim (10,000 IU), whereas generics 1, 3, and 5 showed lower asparaginase activity levels of 74.6 ± 9.1%, 71.4 ± 1.6%, and 74.0 ± 2.0% respectively when compared to the label claim (Supplementary Figure S2).
3.2. Plasma asparaginase activity
3.2.1. Method validation parameters
The method had acceptable accuracy and precision in the calibration range of range of 2–2000 IU/L. A representative calibration curve of standard asparaginase activity versus absorbance is shown in supplementary Figure S1. The median coefficient of determination (R2) of three successive calibration runs was found to be 0.999 (0.998–0.999). The back calculated activity along with the accuracy and precision for each standard in three successive runs is shown in supplementary Table S1. The relative standard deviation (RSD) varied from 4.29 to 14.4% whereas the accuracy ranged from 94.16% to 109.61%. Inter‐day and intra‐day accuracy and precision for LLOQ, LQC, MQC and HQC samples is shown in Supplementary Table S2. The RSD varied from 3.35–11.03% for inter‐day and 2.19–10.33 for intra‐day runs. The accuracy was found in the range of 101.55 to 114.01% for inter‐day and 98.91 to 114.93% for intra‐day runs.
3.2.2. Asparaginase activity in patient samples
The plasma asparaginase activity for each generic formulation is displayed in Figure 1. The proportion of patients not achieving threshold values of 50 IU/L and 100 IU/L for each of the nine generic formulations is shown in Table 2. A higher proportion of samples drawn after administration of Indian generic formulations (Generics 1–8) were unable to achieve a clinically defined activity level of 100 IU/L or above (n = 171, 77.0%) when compared with Generic 9 (n = 3, 27.2%). A lower clinical cut‐off of 50 IU/L, required to achieve asparagine depletion9, was taken which again demonstrated an inability of Indian generic formulations to achieve adequate levels (n = 127, 57.2%) when compared to Generic 9 (n = 2, 16.7%).
Figure 1.
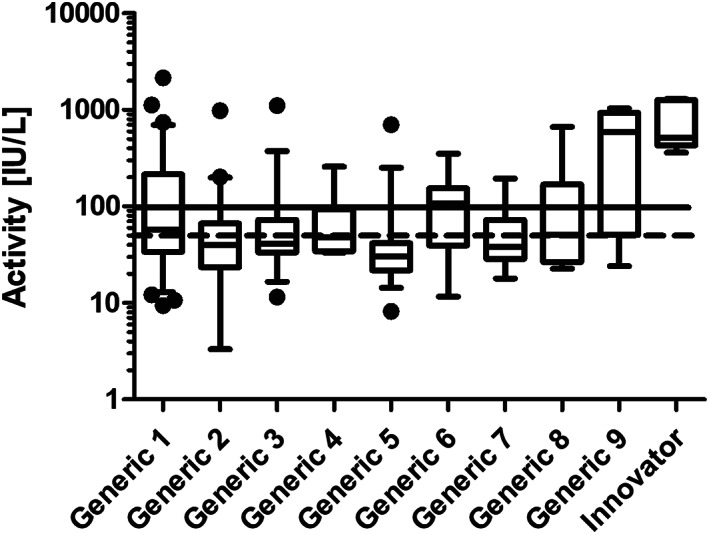
Box and whisker plots showing trough asparaginase activity in the clinical samples for each brand. The dotted line and solid line correspond to an activity of 50 IU/L and 100 IU/L respectively
Table 2.
Comparative in‐vivo asparaginase activity between generics
| Formulations | Total number of samples | Number of samples below 50 IU/L | Number of samples below 100 IU/L | Activity (IU/L) a |
|---|---|---|---|---|
| Innovator | 7 | 0 (0.0%) | 0 (0.0%) | 704.9 ± 400.3 |
| Generic 1 | 66 | 31 (46.97%) | 44 (66.67%) | 175.2 ± 317.7 |
| Generic 2 | 41 | 27 (65.85%) | 35 (85.37%) | 77.3 ± 152.7 |
| Generic 3 | 35 | 21 (60.00%) | 29 (82.86%) | 85.5 ± 181.5 |
| Generic 4 | 12 | 6 (50.00%) | 10 (83.33%) | 78.9 ± 71.0 |
| Generic 5 | 33 | 28 (84.85%) | 32 (96.97%) | 52.7 ± 117.0 |
| Generic 6 | 18 | 5 (27.78%) | 8 (44.44%) | 120.4 ± 99.9 |
| Generic 7 | 7 | 4 (57.00%) | 6 (86.0%) | 62.18 ± 61.0 |
| Generic 8 | 10 | 5 (50.00%) | 7 (70.00%) | 138.1 ± 202.6 |
| Generic 9b | 11 | 2 (16.67%) | 3 (27.27%) | 538.8 ± 404.3 |
| TOTAL of generics | 233 | 129 (55.13%) | 174 (74.36%) | 147.7 ± 151.8 |
Mean ± SD.
Generic 9 is a foreign based generic.
3.3. Thermal stability
Generics 1, 5 and the innovator had comparable thermal stability at room temperature and followed first order decline in asparaginase activity at the rate of 18.5%, 5.0% and 14.3% per hour respectively.
3.4. Impurity profiling by LC–MS/MS
Generic 5 was tested for host‐contaminating proteins (HCP) using LC–MS/MS and the results are displayed in Table 3. Twenty‐five spurious proteins were identified and the relative number of HCPs totaled 27.1% of the total peptide count. HCPs were found in several subcellular locations, of which 55% were periplasmic, 28.2% were cytoplasmic, 10.7% were cytoplasmic extracellular, and 6.1% were from the inner membrane.
Table 3.
LC–MS/MS of generic formulation 5
| N | % Cov | Accession # | Name | Location | Peptides(95%) |
|---|---|---|---|---|---|
| 1 | 99.7 | P00805 | L‐asparaginase 2 | Periplasmic | 352 |
| 2 | 43.1 | P23843 | Periplasmic oligopeptide‐binding protein | Periplasmic | 21 |
| 3 | 67 | P61889 | Malate dehydrogenase |
Cytoplasmic Extracellular |
14 |
| 4 | 53.2 | P0A867 | Transaldolase A | Cytoplasmic | 15 |
| 5 | 36.3 | P45523 | FKBP‐type peptidyl‐prolyl cis‐trans isomerase FkpA | Periplasmic | 8 |
| 6 | 28.6 | P33363 | Periplasmic beta‐glucosidase | Periplasmic | 11 |
| 7 | 17 | P33790 | Protein TraG | Inner membrane | 7 |
| 8 | 43.8 | P0AFH8 | Osmotically‐inducible protein Y | Periplasmic | 7 |
| 9 | 32.9 | P0A9B2 | Glyceraldehyde‐3‐phosphate dehydrogenase A | Cytoplasmic | 5 |
| 10 | 17.2 | P23847 | Periplasmic dipeptide transport protein | Periplasmic | 6 |
| 11 | 44.5 | P0AF93 | 2‐iminobutanoate/2‐iminopropanoate deaminase | Cytoplasmic | 5 |
| 12 | 22.9 | P0AEG6 | Thiol:Disulfide interchange protein DsbC | Periplasmic | 4 |
| 13 | 36.2 | P68066 | Autonomous glycyl radical cofactor | Cytoplasmic | 3 |
| 14 | 25.9 | P23827 | Ecotin | Periplasmic | 2 |
| 15 | 11.2 | P69441 | Adenylate kinase | Cytoplasmic | 2 |
| 16 | 14.5 | P0ACI6 | Regulatory protein AsnC | Cytoplasmic | 2 |
| 17 | 16.5 | P00448 | Superoxide dismutase [Mn] | Cytoplasmic | 2 |
| 18 | 13.9 | P0AEM9 | L‐cystine‐binding protein FliY | Periplasmic | 2 |
| 19 | 18.3 | P0A955 | KHG/KDPG aldolase | Cytoplasmic | 2 |
| 20 | 6.5 | P05458 | Protease 3 | Periplasmic | 2 |
| 21 | 17.5 | P0ABZ6 | Chaperone SurA | Periplasmic | 3 |
| 22 | 27.9 | P0AE22 | Class B acid phosphatase | Periplasmic | 4 |
| 23 | 5.1 | P40120 | Glucans biosynthesis protein D | Periplasmic | 1 |
| 24 | 0.6 | P46889 | DNA translocase FtsK | Inner membrane | 1 |
| 25 | 5.5 | P0AFM2 | Glycine betaine/proline betaine‐binding periplasmic protein | Periplasmic | 1 |
| 26 | 6.5 | P67662 | HTH‐type transcriptional activator AaeR | Cytoplasmic | 1 |
4. DISCUSSION
Our retrospective analysis found that of the 3 approved generic formulations we tested in‐vitro, none were within 90–110% of the label claim whereas the innovator was. The in‐vivo analysis also demonstrated that none of the 8 Indian generic formulations tested were able to consistently achieve a clinically defined threshold of 100 IU/L. Moreover, the generic formulation that achieved the highest activity irrespective of order of administration was one of the foreign generics, further highlighting the suboptimal quality in local L‐asparaginase formulations.
As lack of purification of L‐asparaginase has been cited as a reason for the inferior quality of generic formulations,10 we analysed a generic formulation for impurities using LC–MS/MS. The results clearly demonstrate a higher amount of host contaminating proteins (HCP) in the generic formulation when compared to published data of the innovator.11 The presence of cytoplasmic and membrane bound proteins possibly indicates a deficiency in the extraction process of the crude periplasmic protein. Additionally the high amount of HCPs could be due to insufficient purification of the final product.12 Though not examined in our study, high levels of HCPs can lead to drug antibody reactions and cause inactivation of L‐asparaginase along with a higher chance of hypersensitivity reactions.10 Our in‐vivo and LC–MS/MS findings similarly mirror published results from Brazil with minor differences in HCPs (i.e. beta lactamase) demonstrating the mounting concern worldwide regarding the efficacy of generic formulations.8, 13
Due to concerns raised over lack of cold chain between dispensations from the pharmacy to administration, we further investigated the thermal stability of generic formulations at room temperature and found the rate of decline in asparaginase activity was similar to the innovator. This suggests that the suboptimal in‐vitro and in‐vivo asparaginase activity is unlikely to be due to improper storage, as medications are dispensed to patient families in ice boxes, and administration practices are uniform in our hospital.
The Central Drugs Standard Control Organization (CDSCO) in its “Guidelines on Similar Biologic: Regulatory Requirements for Marketing Authorization in India, 2016” has listed criteria for which a waiver of safety and efficacy can be applied with the promise of periodic safety update reports (PSURs).3 In our study, we have demonstrated in‐vitro activity consistently below the label claim, which clearly doesn't meet minimal regulatory guidelines, and in‐vivo activity that is markedly different from the innovator and well below defined clinical thresholds in a majority of cases. This can happen as after approval, generic formulations do not have to submit efficacy reports, and PSURs must be submitted only up till 4 years after approval. Additionally, there is no strict definition of comparability as there is for oral formulations. It is left up to the SLA in many cases to determine the quality control measures necessary to approve a drug in their state which has led to heterogeneous practices throughout India and possibly a lax regulatory environment.14
Asparaginase activity in local generic versus foreign generic formulations has been raised as another area of concern in the quality of drugs. Out of the 9 generic formulations of L‐asparaginase tested, we were able to verify import licenses for the companies that distributed 3 of the formulations (generic 1, generic 4 and generic 9) and were able to demonstrate a difference between these 3 generics as well, with generic 9 performing better overall.15 As it is often the responsibility of SLAs to monitor and enforce regulatory guidelines (e.g. blacklisting companies), robust information networks besides online notices should be developed to disseminate critical information.16 Recent efforts by the CDSCO and independent SLAs such as independent state run drug testing facilities and more strict regulatory guidelines is a step in the right direction though it will likely take time to enforce these policies. Until that time, it may be of benefit to individually test batches of L‐asparaginase for activity before administering the drug to patients, since if in‐vitro levels are below 90% of the label claim, the in‐vivo activity will be less. Alternatively, individualized L‐asparaginase dose adjustment strategies to achieve asparaginase levels of 100–140 IU/L have demonstrated improved outcomes in pediatric ALL.17 The transition of most developed countries to pegylated L‐asparaginase is also a putative strategy that has been proven to be cost‐effective when compared to native L‐asparaginase.18, 19
The findings of this study also have global implications, as we were able to verify that 4 companies that produced Indian generic formulations 1,3,4,6 exported their product to 9 countries during 2016.20 Recent legislation has now made it easier to obtain no objection certificates from SLAs in an effort to increase exports of Indian generic formulations and help support local manufacturers. There is no question that the cost of innovator drugs are prohibitive to many developing countries and the need for biosimilars is paramount, but regulatory waivers need to be balanced by pharmacovigilance. This study had several limitations due to its retrospective design including the inability to uniformly evaluate all generic formulations, to test all generic formulations available in the market at that time, and to associate sub‐therapeutic levels with clinical outcomes. However, a large sample size and minimal variability in asparaginase activity amongst generic formulations along with consistency with in‐vitro and in‐vivo data add validity to our findings. Though we did not collect clinical outcomes in these patients, previous data has suggested that inability to achieve a clinical threshold of 100 IU/L can be linked to inferior outcomes.21 As B cell ALL patients made a large subset of our study group, we looked into the activity of L‐asparaginase based on different risk types due to concerns of asparaginase resistance (Supplementary Figure S3).22 Contrary to a recently published finding from Krishnan et al,23 we did not find a major difference in the asparaginase activity levels between different risk subtypes though these findings must be considered in the background of suboptimal activity of generics. To conclude, our findings of lower activity in all Indian generic formulations clearly demonstrates a serious clinical concern in the treatment of pediatric leukemia. Regulatory enforcement and periodic monitoring of generic formulations are necessary to ensure quality care of all patients and alternative strategies may need to be considered to ensure optimal dosing. Prospective studies aimed at determining acceptable in‐vitro levels and the effect of these generic formulations on clinical outcomes needs to be conducted.
COMPETING INTERESTS
The authors declare no conflict of interest.
CONTRIBUTORS
VG and SB conceived and supervised the study. SB was the Principal Investigator and handled all correspondence with the ethics committee.
SS analyzed in vitro asparaginase activity, thermal stability and impurity profiling of the formulations.
VP developed and validated the analytical method and analyzed asparaginase activity in patient samples.
AK, NRM, MP, CD and GN were responsible for data collection from medical records.
HS, SS, VP and VG analyzed the data and wrote the manuscript. All authors approved the manuscript.
Supporting information
Figure S1: Calibration curve of asparaginase activity is shown. The asparaginase activity of the patient samples was calculated using a four parametric non‐linear regression possibly because the substrate concentration was not close to Vmax of this Michalis‐Menten enzyme kinetics. Thus, when there was an abundance of asparaginase present (at the top end of the calibration curve), substrate was well depleted leading to less conversion to the measured analyte.
Figure S2: in vitro non clinical assessment of formulations of L‐asparaginase. Asparaginase activity was measured in triplicate from one vial each of innovator and generic brands respectively. Data is shown an Mean ± SD. The solid horizontal line represents the 90% activity of label claim.
Figure S3: Plot showing the asparaginase activity in patient samples of standard, intermediate and high risk groups. The difference in activity between the groups was not statistically significant.
Table S1: Back calculated activity, accuracy and precision of calibration standards
Table S2: Inter‐day and intra‐day accuracy and precision
ACKNOWLEDGEMENT
We would like to acknowledge the paediatric oncology department at Tata Memorial Hospital, especially Dr. Brijesh Arora, who started monitoring asparaginase levels in an effort to identify good quality medications.
Sankaran H, Sengupta S, Purohit V, et al. A comparison of asparaginase activity in generic formulations of E.coli derived L‐ asparaginase: In‐vitro study and retrospective analysis of asparaginase monitoring in pediatric patients with leukemia. Br J Clin Pharmacol. 2020;86 1081–1088. 10.1111/bcp.14216
Hari Sankaran, Soumika Sengupta and Vaitashi Purohit are joint first authors, Shripad Banavali and Vikram Gota should be considered as joint senior author
Principal Investigator statement: The authors confirm that the Principal Investigator for this paper is Dr. Shripad Banavali and that he had direct clinical responsibility for patients
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy and ethical restrictions.
REFERENCES
- 1. Pizzo PA, Poplack DG. In: Rabin K, Gramatges M, Margolin J, Poplack D, eds. Principles and Practice of Pediatric Oncology. 7th ed. Philadelphia, USA: Wolters Kluwer; 2016. [Google Scholar]
- 2. Pieters R, Hunger SP, Boos J, et al. L‐asparaginase treatment in acute lymphoblastic leukemia: a focus on Erwinia asparaginase. Cancer. 2011;117(2):238‐249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. GUIDELINES ON SIMILAR BIOLOGICS: Regulatory Requirements for Marketing Authorization in India, 2016, (2016). [DOI] [PubMed]
- 4. Narula G, Arora B, Subramanian PG, et al. Outcome of B‐ALL Treated with A Risk‐Stratified and Response‐Adapted Protocol: Interim Analysis of A Single‐Institution Pilot Study for A Multicenter Collaborative Protocol from India. 49th Congress of the International Society of Paediatric Oncology (SIOP) 10/12/17; Washington, DC: Pediatric Blood and Cancer; 2017.
- 5. Advani S, Pai S, Venzon D, et al. Acute lymphoblastic leukemia in India: an analysis of prognostic factors using a single treatment regimen. Ann Oncol. 1999;10(2):167‐176. [DOI] [PubMed] [Google Scholar]
- 6. Lanvers C, Pinheiro JPV, Hempel G, Wuerthwein G, Boos J. Analytical validation of a microplate reader‐based method for the therapeutic drug monitoring of L‐asparaginase in human serum. Anal Biochem. 2002;309(1):117‐126. [DOI] [PubMed] [Google Scholar]
- 7. Baud M. Data analysis, mathematical modeling Methods of Immunological Analysis. 1. New York, NY: VCH Publishers, Inc.; 1993:656‐671. [Google Scholar]
- 8. Zenatti PP, Migita NA, Cury NM, et al. Low bioavailability and high immunogenicity of a new brand of E. coli L‐Asparaginase with active host contaminating proteins. EBioMedicine. 2018;30:158‐166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rizzari C, Zucchetti M, Conter V, et al. L‐asparagine depletion and l‐asparaginase activity in children with acute lymphoblastic leukemia receiving i.m. or i.v. Erwinia C. or E. colil‐asparaginase as first exposure. Ann Oncol. 2000;11(2):189‐193. [DOI] [PubMed] [Google Scholar]
- 10. Bracewell DG, Francis R, Smales CM. The future of host cell protein (HCP) identification during process development and manufacturing linked to a risk‐based management for their control. Biotechnol Bioeng. 2015;112(9):1727‐1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pieters R, Appel I, Kuehnel HJ, et al. Pharmacokinetics, pharmacodynamics, efficacy, and safety of a new recombinant asparaginase preparation in children with previously untreated acute lymphoblastic leukemia: a randomized phase 2 clinical trial. Blood. 2008;112(13):4832‐4838. [DOI] [PubMed] [Google Scholar]
- 12. Tundisi LL, Coêlho DF, Zanchetta B, et al. L‐Asparaginase purification. Sep Purif Rev. 2016;46(1):35‐43. [Google Scholar]
- 13. Cecconello DK, Werlang ICR, Alegretti AP, et al. Monitoring asparaginase activity in middle‐income countries. Lancet Oncol. 2018;19(9):1149‐1150. [DOI] [PubMed] [Google Scholar]
- 14. Gota VS, Patial P. Toward better quality of anticancer generics in India. Indian J Cancer. 2014;51(3):366‐368. [DOI] [PubMed] [Google Scholar]
- 15. Organization CDSC . Import license issued 2015. 2015 [Available from: http://www.cdsco.nic.in/writereaddata/Import-license-issued-in-2015.xlsx.
- 16. E‐tender for the Procurement of Anti Cancer Drugs for RCC for the year 2019–20 – Technical Bid Evaluation – defects noticed/status– Published – reg., KMSCL/DRG/ED/833/2018 (5) (2019).
- 17. Vrooman LM, Stevenson KE, Supko JG, et al. Postinduction dexamethasone and individualized dosing of Escherichia Coli L‐asparaginase each improve outcome of children and adolescents with newly diagnosed acute lymphoblastic leukemia: results from a randomized study‐‐Dana‐Farber Cancer Institute ALL consortium protocol 00‐01. J Clin Oncol. 2013;31(9):1202‐1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kurre HA, Ettinger AG, Veenstra DL, et al. A Pharmacoeconomic analysis of Pegaspargase versus native Escherichia Coli L‐Asparaginase for the treatment of children with standard‐risk, acute lymphoblastic leukemia: the Children's cancer group study (CCG‐1962). J Pediatr Hematol Oncol. 2002;24(3):176‐181. [DOI] [PubMed] [Google Scholar]
- 19. Bauters T. V. Mondelaers, Moerloose BD, Robays H, Y. Benoit M. cost‐minimisation analysis of PEG‐L‐Asparaginase versus native L‐Asparaginase for the treatment of children with acute lymphoblastic leukaemia in Belgium. Pharmacotherapy. 2013;4(4):144‐147. [Google Scholar]
- 20. Solutions SE. L‐asparaginase Export Data http://www.seair.co.in2016 [Available from: https://www.seair.co.in/l-asparaginase-export-data.aspx.
- 21. van der Sluis IM, Vrooman LM, Pieters R, et al. Consensus expert recommendations for identification and management of asparaginase hypersensitivity and silent inactivation. Haematologica. 2016;101(3):279‐285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Offman MN, Krol M, Patel N, et al. Rational engineering of L‐asparaginase reveals importance of dual activity for cancer cell toxicity. Blood. 2011;117(5):1614‐1621. [DOI] [PubMed] [Google Scholar]
- 23. Sidhu J, Saha D, Roy P, et al. Therapeutic drug monitoring informs asparaginase dose scheduling in the InPOG‐ALL‐15‐01‐ICiCLe‐ALL‐14 trial. Pediatr Hemato Oncol J. 2017;2(2):S2‐S3. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Calibration curve of asparaginase activity is shown. The asparaginase activity of the patient samples was calculated using a four parametric non‐linear regression possibly because the substrate concentration was not close to Vmax of this Michalis‐Menten enzyme kinetics. Thus, when there was an abundance of asparaginase present (at the top end of the calibration curve), substrate was well depleted leading to less conversion to the measured analyte.
Figure S2: in vitro non clinical assessment of formulations of L‐asparaginase. Asparaginase activity was measured in triplicate from one vial each of innovator and generic brands respectively. Data is shown an Mean ± SD. The solid horizontal line represents the 90% activity of label claim.
Figure S3: Plot showing the asparaginase activity in patient samples of standard, intermediate and high risk groups. The difference in activity between the groups was not statistically significant.
Table S1: Back calculated activity, accuracy and precision of calibration standards
Table S2: Inter‐day and intra‐day accuracy and precision
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy and ethical restrictions.


