Abstract
Aims
To determine the effects of statins on postprandial lipaemia (PPL) and to study if exercise could enhance statin actions.
Methods
Ten hypercholesteraemic (blood cholesterol 204 ± 36 mg dL−1; low‐density lipoprotein–cholesterol 129 ± 32 36 mg dL−1) overweight (body mass index 30 ± 4 kg m−2), metabolic syndrome individuals chronically medicated with statins (>6 months) underwent 5‐hour PPL tests in 4 occasions in a randomized order: (i) substituting their habitual statin medication by placebo for 96 hours (PLAC trial); (ii) taking their habitual statin medicine (STA trial); (iii) placebo combined with a bout of intense aerobic exercise (EXER+PLAC trial); and (iv) combining exercise and statin medicine (EXER+STA trial).
Results
Before the fat meal, statin withdrawal (i.e. PLAC and EXER+PLAC) increased blood triglycerides (TG; 24%), low‐density lipoprotein–cholesterol (31%) and total cholesterol (19%; all P < .05) evidencing treatment compliance. After the meal, statin withdrawal increased 5‐hour postprandial TG (PPTG) compared to its matched trials (94% higher PLAC vs STA and 45% higher EXER+PLAC vs EXER+STA; P < .05). EXER+PLAC trial did not lower PPTG below PLAC (i.e. incremental AUC of 609 ± 152 vs 826 ± 190 mg dL−1 5 h; P = .09). Adding exercise to statin did not result in larger reductions in PPTG (i.e. EXER+STA vs STA incremental area under the curve of 421 ± 87 vs 421 ± 84 mg dL−1 5 h; P = .99).
Conclusion
In hypercholesteraemic metabolic syndrome individuals, chronic statin therapy blunts the elevations in TG after a fat meal (i.e. incremental area under the curve of PPTG) reducing the cardiovascular risk associated to their atherogenic dyslipidaemia. However, a single bout of intense aerobic exercise before the high fat meal, does not reduce PPTG but also does not interfere with the effects of statin treatment.
Keywords: aerobic exercise, hydroxymethylglutaryl‐CoA reductase inhibitor, hypercholesterolaemia, metabolic syndrome X, postprandial lipaemia
What is known about this subject
Hypercholesteraemic individuals are treated with statins to lower their blood fasting cholesterol and triglycerides levels.
The cardiovascular risk associated with atherogenic dyslipidaemia increases after a fat meal due to the elevations in postprandial triglycerides (PPTG).
It is unclear if statins lower not only fasting TG but also PPTG in hypercholesteraemic individuals.
What this study adds
In hypercholesteraemic individuals, chronic statin therapy blunts the elevations in TG after a fat meal (i.e. incremental area under the curve of PPTG) reducing their atherogenic cardiovascular risk.
A single bout of exercise that elicited moderate energy expenditure (302 kcal) 20 minutes prior to a high fat meal ingestion does not reduces PPTG.
However, exercise prior to a high fat meal ingestion does not interfere with statin PPTG reduction.
1. INTRODUCTION
Triglyceride (TG)‐depleted remnants of postprandial chylomicrons are believed to be the blood particles with the largest atherogenic potential. Accordingly, the risk for atherogenic plaque formation is elevated after meal ingestion due to the elevations in plasma concentration of these TG‐rich lipoprotein remnants. Using a standard fat meal ingestion,1 the magnitude and exposure time to high levels of circulating TGs can be assessed (i.e. postprandial triglyceridaemia; PPTG). It has been reported that hypercholesteraemic individuals have larger and more prolonged PPTG evidencing a problem in the oxidation‐storage of the ingested fat.2 By contrast, aerobic exercise training of sufficient volume could improve blood atherogenic lipid profile lowering TGs3 and increasing high‐density lipoprotein cholesterol (HDL‐c) largely when exercise is combined with dietary omega‐3‐fatty acids at least in metabolic syndrome (MetS) individuals.4, 5 Nonetheless, exercise training affects energy balance favouring body weight and abdominal fat losses,6 which would influence blood lipids. The study of a single exercise session avoids the confounding effects of exercise training on body fat losses. Encouragingly, in hypercholesteraemic men, a single bout of prolonged aerobic exercise lowers blood TG from 24 to 48 hours after exercise.7 Furthermore, completion of a single bout of exercise prior to a high fat meal ingestion reduces postprandial lipaemia (PPL8), which may help to prevent the development cardiovascular diseases (CVD) in healthy populations. Further, a bout of exercise could lower PPL in individuals with increased risk for CVD such as those with obesity, the MetS and hypercholesterolaemia, which may confer exercise a clinically relevant role.9, 10, 11, 12
Individuals at risk of developing CVD are commonly treated with the statins. Statins (i.e. hydroxymethylglutaryl‐coenzyme A reductase inhibitors) competitively inhibit the rate‐limiting step in liver cholesterol synthesis. The reduced intracellular hepatic cholesterol production, induces low‐density lipoprotein (LDL)‐receptor expression in hepatocyte cell surface, increasing LDL–cholesterol (LDL‐c) extraction from the blood and so reducing its concentration.13 The overexpression of liver LDL receptors increases the catabolism of all Apo‐B containing lipoproteins including TG‐rich very low‐density lipoproteins (VLDL, chylomicrons) resulting in lower TG concentration.14 Therefore, the ability of statins to lower blood TG has been considered a secondary effect of statins. However, this effect is of great importance since there is enough evidence that hypertriglyceridaemia is an independent risk factor for coronary heart diseases.15 These findings are allowing to expand statin prescription to patients with lipid disorders other than hypercholesterolaemia. Statins not only decrease fasting cholesterol and TG in hypertriglyceridaemic patients but have the potential to lower PPTG16 which is the situation with the highest risk for atherogenic plaque formation.
Guidelines from the American Diabetes Association state that in diabetic individuals older than 40 years, statin prescription should be added to lifestyle counselling to lower LDL‐c below 100 mg dL−1. 17 Statins are prescribed in conjunction with medical advice to reduce body weight, smoking, saturated fat and alcohol intake, while increasing physical activity and exercise. Most of the literature studying interactions between statins therapy and exercise has focused on statins side‐effects such as myopathy and rabdomiolysis18 which are important because they could prevent individuals from engaging in an exercise programme.19 The effects of statins on the main metabolic adaptations to training (i.e. increased fat metabolism) has received much less attention. Apparently, statin use reduces skeletal muscle coenzyme Q10 content which could reduce mitochondrial oxidative capacity20 and potentially fat oxidation. The literature is unclear on the effects of prolonged statin use on fat oxidation during exercise with reports of depressed21 or unchanged fat oxidation.22, 23, 24
Although exercise affects different bodily organs, its main effects are on skeletal and cardiac muscle and its associated vasculature. In contrast, most of the ingested statin is biotransformed in the liver with less than 5% reaching the systemic circulation.25 Thus, it is possible that since exercise and statins have different organ targets, their actions on postprandial TG secretion and clearance could be additive. If both therapies have additive effects, exercise could lower the need for statin medication in mild hypercholesteraemic individuals. While some studies have addressed if exercise training potentiate statin reductions in blood lipids26 scarce information is available on the combined effects of 1 bout of exercise and statins treatment on PPL.
Hypercholesterolaemia is a common component of MetS and it is strongly associated with the risk of suffering CVD27 in this population. Pharmacological (statin) and lifestyle (exercise) therapies are used to reduce cardiovascular disease risk in these individuals. However, it is unclear what are the effects of these therapies during the blood lipid elevations after a meal (i.e. PPL), a situation that greatly increases CVD risks. The objective of this study was to determine if chronic statin therapy could blunt the elevations in lipids after a fat meal (PPL) and if exercise could enhance pharmacological hypercholesterolaemia treatment with statins. The hypotheses were that statins would reduce PPL and that exercise will act additively resulting in larger PPL lowering effects.
2. METHODS
2.1. Participants and preliminary testing
Ten subjects (1 woman and 9 men) with average age of 61 ± 7 years, body mass index of 30 ± 4 kg m−2 and hypercholesterolaemia treatment with statins were recruited. All subjects were diagnosed with MetS based on accumulation of 3 or more risk factors28 as displayed in Table 1. Subjects were physically active and medicated with statins (ator‐, pita‐, sim‐, rosuvastatin) for at least 6 months prior to the onset of the study. All participants were under the supervision of their primary care physicians. Doctors followed the Spanish National Institute of Health guidelines for management of lipids as cardiovascular risk factor. Those guidelines require lifestyle advice progressing to pharmacological prescription with statins in accordance with blood lipid levels (i.e. any combination of 2 of the following, total cholesterol > 200 mg dL−1; LDL‐c > 125 mg dL−1; HDL‐c < 40 mg dL−1 in men and < 50 mg dL−1 in women29). Participants' statin prescriptions differed in type and dosage (Table 2), but statins were the only lipid therapy medications used by participants. Six subjects were on treatment with metformin for diabetes or prediabetes and 3 were not medicated but had fasting blood glucose in the prediabetic range. Individuals signed a witnessed, informed consent of the protocol approved by the local Hospital's Ethics Committee in accordance with the declaration of Helsinki. Subjects underwent medical physical examination and completed a maximal cardiopulmonary graded exercise test on an electronically braked cycle ergometer (Ergoselect 200, Ergoline, Germany) with electrocardiography monitoring (Quark T12, Cosmed, Italy) to screen for myocardial diseases and determine their maximal oxygen consumption (VO2MAX). The graded exercise test started with a 3‐minute warm‐up at 15 W for women and 20 W for men, followed by stages of 15–20 W min−1 for women and men, respectively, until exhaustion. After a cool down and 15 minutes of passive rest including rehydration, a short (2–3 min) confirmatory test was performed at 110% of the maximum load reached during the previous ramp test.30 The VO2MAX obtained was used to set exercise intensity. This is a substudy, part of a larger clinical trial evaluating the effects of 4‐month exercise training and habitual medication in 160 individuals with MetS (http://ClinicalTrials.gov Identifier: NCT03019796). The data presented correspond to the 10 individuals in which we tested the effects of a high fat meal. Thus, there has been no selection bias with regard to the overall study population since we are presenting all the data collected on PPL.
Table 1.
Anthropometric and metabolic syndrome factors of participants
| Variable | |
|---|---|
| Age (y) | 61 ± 7 |
| Body mass index (kg m−2) | 30 ± 4 |
| Body weight (kg) | 86 ± 4 |
| Body fat (%) | 32 ± 6 |
| Waist circumference (cm) | 105 ± 6 |
| Fasting glucose (mg dL–1) | 120 ± 27 |
| Triglycerides (mg dL–1) | 107 ± 54 |
| HDL‐c (mg dL–1) | 49 ± 14 |
| Resting SBP (mmHg) | 128 ± 10 |
| Resting DBP (mmHg) | 79 ± 7 |
| VO2 max (mL kg−1 min−1) | 31 ± 6 |
| Heart rate max (beats min−1) | 156 ± 13 |
| Years under statin treatment | 5 ± 2 |
Data are mean ± standard deviation for 10 metabolic syndrome patients with hypercholesterolaemia
HDL‐c, high‐density lipoprotein–cholesterol; SBP, systolic blood pressure; DBP, diastoli blood pressure
Table 2.
Statin use and pharmacokinetics data
| Subjects | Dose (mg d–1) | Bioavailability | Half‐life (h) | Renal excretion (%) | Solubility | |
|---|---|---|---|---|---|---|
| Atorvastatina | 4 | 20–80 | 12% | 14 | 5 | Lipophilic |
| Pitavastatina | 2 | 2–2 | 80% | 11 | NA | Lipophilic |
| Simvastatina | 2 | 10–20 | 5% | 2 | 13 | Lipophilic |
| Rosuvastatina | 2 | 10–20 | 20% | 19 | 10 | Hydrophilic |
Schachter64
2.2. Experimental design
Using repeated‐measures crossover, randomized control trial design, subjects completed 4 trials. Random order sequence was generated using the macro feature of Excel (Microsoft Office) without repetition. The team physician performed the randomization and concealed it to the rest of the team until data analysis completion. Upon study enrolment, participants turned in their statin prescription drugs to the team physician for masking. Masking consisted on embedding participants' prescription drug into larger capsules. Identical capsules were used for placebo but filled with dextrose. Prescription and placebo capsules were placed into plastic pill bottles wearing an alphanumeric code only known to the physician. On the morning of every fifth day, for the duration of the experiment (4 weeks), participants turned in their empty research pill bottle to receive a new prescription bottle. In that way, we altered participants' drug intake between placebo and statins in a double blinded fashion. Placebo was taken for 4 days (i.e. 96 h) prior to the trials, because this time exceeds by 5‐fold the longer lasting statin half‐life of subjects (i.e. rosuvastatin; Table 2). During the first trial, subjects filled out an activity/diet diary and were instructed to replicate those for the 48 hours prior to every trial. Habitual physical activity but not training was allowed during those for 48 hours preceding the trials. Subjects underwent 4 trials to measure PPL after a high‐fat meal under the following conditions: (i) substituting their habitual statin medication by placebo medicine (PLAC trial); (ii) taking their habitual statin medicine (STA trial); (iii) placebo medicine combined with a bout of intense aerobic exercise (EXER+PLAC trial); and (iv) combining exercise and statin medicine (EXER+STA trial). Trials were separated by at least a week among them and commenced between 7 and 8 AM in the same day of the week for each subject.
2.3. Experimental trials
Subjects arrived at the laboratory between 7 and 8 AM after an 10–12‐hour overnight fast preceded by a standardized 322‐kcal dinner (325 g of precooked pork tenderloin in mushroom sauce with 4 g of fat, 9.5 g of carbohydrate, 6 g of protein per 100 g, 500 mL of water and a medium size apple). Upon arrival, subjects' body weight (Hawk, Mettler Toledo, USA), composition and total body water (BIA using Tanita BC‐418‐MA, Japan) were assessed. Subjects lay on a gurney while a catheter (20‐gauge, BD Insyte, Becton and Dickinson, Spain) was inserted in an antecubital vein and a 3‐way stopcock attached (Luer‐lock, CPK IV, Farmaban, Spain). After 20 minutes of lying in a quiet laboratory (22 ± 1°C and 25 ± 6% humidity) resting metabolic rate was assessed for 20 minutes using indirect calorimetry (Quark b2, Cosmed, Italy). A blood sample was then withdrawn (i.e. −60 min blood sample) and blood pressures (electrocardiography‐gated electro‐sphygmomanometer; Tango, Suntec Medical; NC; USA) measured in triplicate. In trials with exercise (EXER+PLAC and EXER+STA trial), subjects pedalled continuously for 41 minutes at alternating intensities. After a 5‐minute warm up, the subject completed 3 bouts of 7 minutes of increasing intensity (i.e. 40–70–85% VO2MAX) interspersed with 5 minutes of low‐intensity pedalling, to end with a 5‐minute warm‐down (40% VO2MAX). We chose this interval aerobic exercise scheme because a recent meta‐analysis review deems that high‐intensity interval training is more effective for reducing PPTG.31 The exercise bout entailed 41 minutes of moderately high average intensity cycling (i.e. 72% of HRMAX). This bout was taxing enough for our subjects and increases either in duration or intensity would have resulted in undue fatigue. Thus, we think that our exercise bout represented a tolerable exercise bout that could be implemented on these individual's health promotion exercise training routines.
During exercise, VO2, VCO2 and respiratory exchange ratio (RER) of the last minute of each stage was averaged to calculate, energy expenditure.32 Carbohydrate and fat oxidation were calculated according to Frayn's equations33 with protein oxidation considered negligible:
Carbohydrate oxidation rate (μmol kg−1 min−1) = ([(4.585 × VCO2) − (3.226 × VO2)]/180) × 1000/body weight.
Fat oxidation rate (μmol kg−1 min−1) = ([(1.695 × VO2) – (1.701 × VCO2)]/860) × 1000/body weight, where, VO2 and VCO2 are in mL min−1.
After each workload a blood sample was drawn (5 mL). In the nonexercise trials (STA and PLAC trials), subjects remained supine during those 41 minutes. After exercise subjects returned to the gurney and after 20 minutes of supine rest a blood sample was drawn (i.e. 0‐min blood sample). This blood sample was used as baseline for the calculations of incremental area under the curve for blood TGs (iAUC of TG; see description below).
2.4. High‐fat meal and resting metabolic rates
Subjects sat and ingested within 10 minutes a high‐fat liquid meal consisting on 244 g of Oreo frozen cheesecake (Granderroble Deserts, Spain) containing, per 100 g, 24 g of dairy‐derived fat (0.71 ± 0.03 g kg−1 body weight), 34 g of simple sugar carbohydrate (1.06 ± 0.04 g kg−1) and 5 g of milk‐derived protein (0.20 ± 0.01 g kg−1). The cheesecake was blended with 150 mL of skimmed milk (Asturiana, Spain) for a total volume of 394 ± 21 mL. The meal amounted to 11.6 kcal kg−1 body weight for a group average of 995 ± 82 kcal. We chose this meal because it was commercially available, the source of fat (i.e. mostly dairy) is what is commonly used in fat meal experiments and we could deliver in a sizable volume (394 mL) >0.7 g of fat kg−1, which is the threshold for high‐fat meal consideration.8 During the 5 hours that followed the high fat test meal ingestion, subjects remained seated in the laboratory and blood samples were collected hourly (i.e. samples 60, 120, 180, 240 and 300 min) while the catheter was maintained patent by flushing with 5 mL of 0.9% saline (Grifols, Spain) after each blood collection. After 60, 180 and 300 minutes of the high‐fat meal ingestion, resting metabolic rate32 and fat oxidation33 were assessed using indirect calorimetry (Quark b2, Cosmed, Italy) for 20 minutes while subjects rested supine on the gurney.
Data from blood TG concentration was modelled by the trapezoidal rule to calculate the incremental area under the curve (iAUC) as follows34:
2.5. Blood analysis
The 5‐mL blood samples were collected in tubes with a clot activator (Vacutainer; Becton‐Dickinson, USA) to upon centrifugation, obtain serum. Serum was analysed for TG, total cholesterol and HDL‐c. LDL‐c was calculated using Friedewald formula.35 Serum glucose and insulin concentrations were analysed in all samples. Glucose was analysed using glucose oxidase peroxidase method with intra–interassay coefficient of variation (iCV) of 0.9–1.2%, blood TGs with the glycerol‐3‐phosphate oxidize method (iCV; 0.8–1.7%), total serum cholesterol by an enzymatic method with a single aqueous reagent (iCV; 1.1–1.4%) and HDL‐c using the accelerator selective detergent method (iCV; 1.7–2.9%). These analyses were run in an automated analyser (Mindray BS 400 Medical Instrumentation, Shenzhen, China). Insulin concentration was analysed by chemiluminescence (Architect System Insulin, Abbott Diagnostics Division, Germany).
2.6. Statistical analysis
Shapiro–Wilk test confirmed that all dependent variables of interest were normally distributed. Power analysis suggested that 10 participants would be required as determined by using the variance in postprandial TG iAUC at an effect size of β = 0.80 and an α = 0.05. Data collected on arrival at the laboratory (anthropometrics, blood lipid, heart rate, blood pressure and resting energy expenditure) were analysed using 1‐way (treatment) repeated‐measures ANOVA. Data collected overtime was analysed using 2‐factor (exercise × drug) repeated measures ANOVA. After a significant F test, pairwise differences were identified using posthoc Tukey's HSD. Data are presented as means ± standard deviation unless otherwise noted. Associations between variables were identified with the Pearson's product moment correlation coefficient. All analyses were performed with SPSS version 21 (Chicago, IL, USA). Statistical significance level was set at P ≤ 0.05.
3. RESULTS
3.1. Baseline conditions
Subjects' baseline body weight (range 84.2 ± 5.9 to 85.4 ± 5.6 kg; P = .181), body fat, total body water (i.e. surrogate of hydration status) and blood pressure (systolic between 126 ± 11 and 129 ± 13 mmHg; P = .323) were not different among trials. Further, resting heart rate (range 56 ± 6 to 57 ± 7 beats min−1; P = .573), resting VO2 (range 239 ± 30 to 255 ± 33 mL O2 min−1; P = .187) and fat oxidation (range 1.13 ± 0.11 to 1.22 ± 0.28 μmol kg min−1; P = .474) were at baseline similar among trials with no effect of the 4 days of statin withdrawal (i.e. PLAC and EXER+PLAC; Figure 3B).
Figure 3.
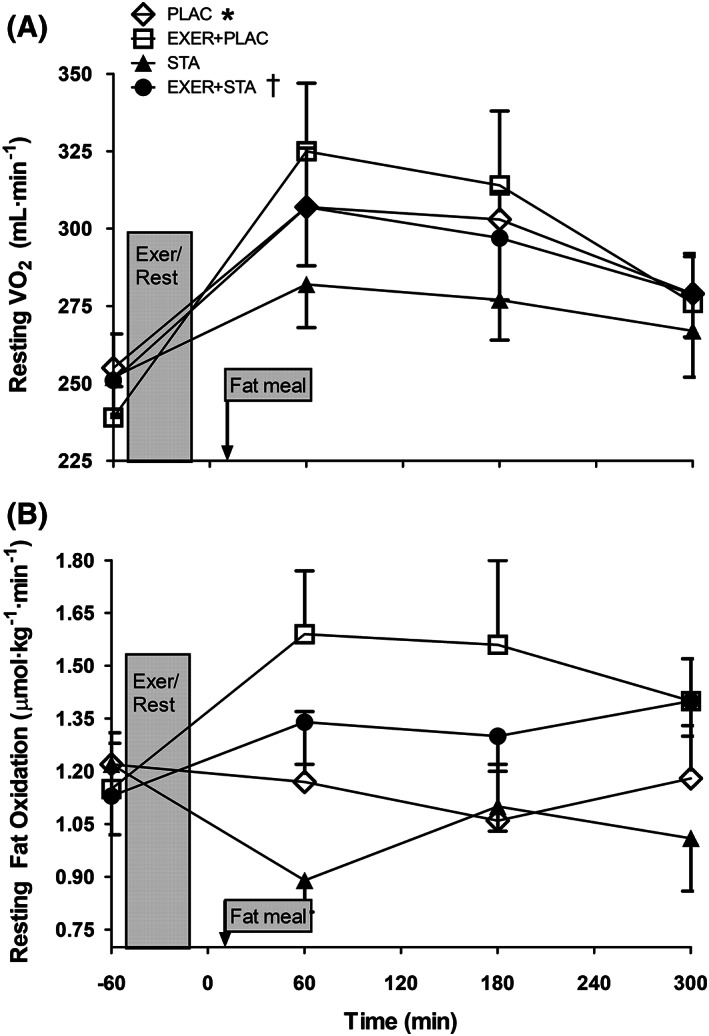
(A) Resting energy expenditure and (B) fat oxidation prior to and 1, 3 and 5 hours after treatments for the 4 experimental trials. Data are presented as mean ± standard error of the mean for 10 hypercholesteraemic metabolic syndrome individuals. * Area under the curve values were significantly higher when statin was withdrawn in comparison to its matched trial with statins (P < .05). † Area under the curve values were significantly higher in the trial with exercise in comparison to its matched trial without exercise (P < .05)
3.2. Basal blood lipid concentration
While statin withdrawal in the PLAC and EXER+PLAC trials did not affect resting body weight, metabolic rate (Figure 3A), blood pressure, heart rate or these responses during exercise, it did raise blood lipid concentration. At the onset of the experiment (i.e. −60 min), blood TGs were higher in EXER+PLAC compared to EXER+STA (i.e. 124 ± 59 vs 95 ± 42 mg dL−1; P = .003) but were similar in PLAC when compared to STA (i.e. 127 ± 56 vs 113 ± 57 mg dL−1; P = .249; Figure 1A). Likewise, total blood cholesterol and LDL‐c were higher when statins were withdrawn (PLAC and EXER+PLAC; Figure 2) evidencing subjects' compliance with the experimental treatments.
Figure 1.
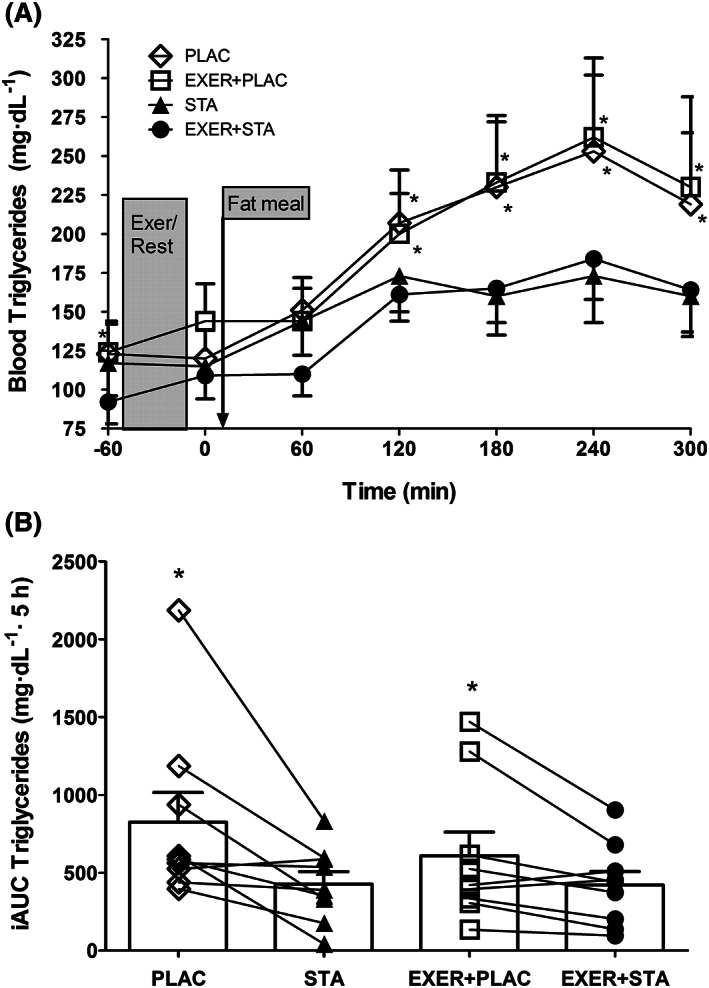
Postprandial blood triglyceride response to a high fat meal: (A) hourly concentrations and (B) incremental area under the curve (iAUC) for the 4 experimental trials (i.e. with and without exercise and statin). Data are presented as mean ± standard error of the mean for 10 hypercholesteraemic metabolic syndrome individuals. * significantly higher when statin medicine was withdrawn compared to its matched trial with statins (i.e. PLAC vs EXER+PLAC and STA vs EXER+STA; P < .05)
Figure 2.
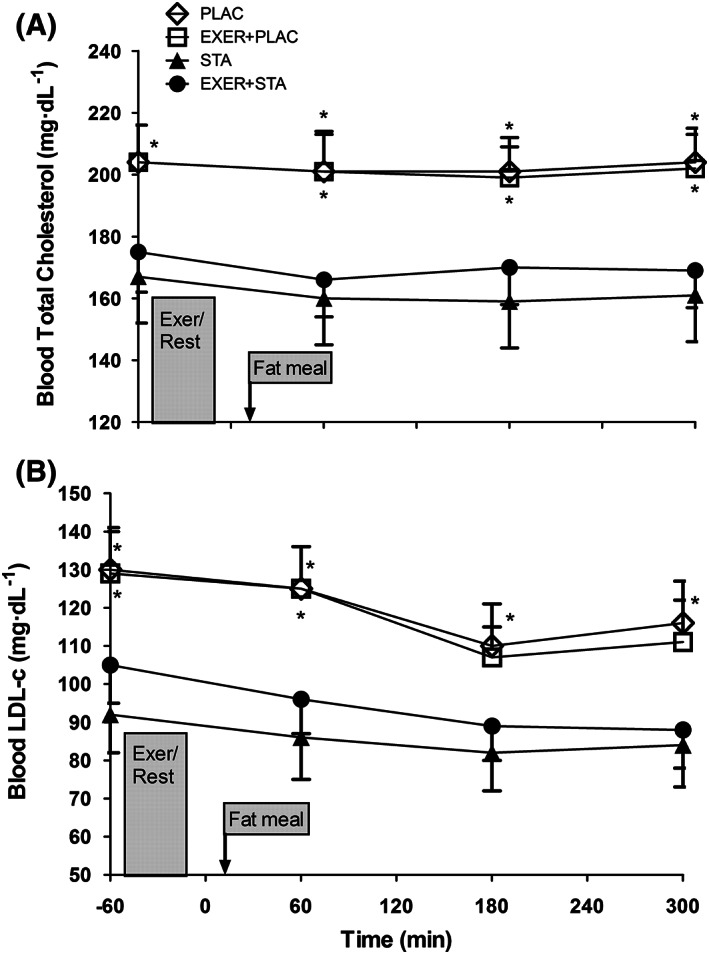
Blood serum concentration of (A) total cholesterol and (B) low‐density lipoprotein–cholesterol (LDL‐c) response to the 4 experimental trials (i.e. with and without exercise and statin). Data are presented as mean ± standard error of the mean for 10 hypercholesteraemic metabolic syndrome individuals. * significantly higher when statin medicine was withdrawn compared to its matched trial with statins (i.e. PLAC vs EXER+PLAC and STA vs EXER+STA; P < .05)
3.3. Postprandial lipid responses
In Figure 1A, we do not show the TG response during exercise because free glycerol from lipolysis would artificially inflate TG concentrations since the assay is based on glycerol concentration. In all trials the fat meal elevated blood TGs although the effects were significantly blunted by statin medication (Figure 1A). When the incremental area under the curve (iAUC) for TG was calculated, the trials with statin displayed reduced TG elevations during the 5 hours postprandial (i.e. iAUC for PPTG). Conversely, statin withdrawal increased iAUC for PPTG by 94% in the trials without exercise (i.e. STA vs PLAC) and by 44% in the trials involving exercise (i.e. EXER+STA vs EXER+PLAC; Figure 1B). Blood total cholesterol and LDL‐c were significantly increased when statins were withdrawn (Figure 2A and B at −60 min). However, neither exercise nor the fat meal affected blood total cholesterol or LDL‐c. Blood HDL‐c concentration was not altered by 4 days of statin withdrawal when comparing STA to PLAC trials (48 ± 14 vs 47 ± 16 mg dL−1; P = .898) or EXER+STA to EXER+PLAC (51 ± 15 vs 47 ± 14 mg dL−1; P = .401). The fat meal or the 41 minutes of aerobic exercise did not affect the levels of HDL‐c that remained at basal levels in all trials.
3.4. Exercise responses
The average workload in the 2 trials involving exercise (i.e. EXER+PLAC and EXER+STA) was similar (83 ± 4 W), eliciting similar exercise heart rates when exercising at 40% VO2MAX (90 ± 9 vs 89 ± 9 beats min−1; P = .390), 70% VO2MAX (126 ± 17 vs 124 ± 17 beats min−1; P = .183) and 85% VO2MAX (148 ± 15 vs 145 ± 14 beats min−1; P = .256). The average intensity for the whole bout of exercise in both trials was 72% of HR max. Oxygen consumption rates were also similar among the exercising trials (EXER+PLAC vs EXER+STA) resulting in similar total energy expenditure (i.e. 299 ± 43 vs 304 ± 40 kcal; P = .564). Total fat (i.e. 5.8 ± 4.5 vs 6.3 ± 4.7 g; P = .504) and carbohydrate (i.e. 63.2 ± 18.6 vs 63.0 ± 18.3 g; P = .947) oxidation were also similar in both exercise trials.
3.5. Postprandial carbohydrate responses
The fat meal also contained carbohydrates (i.e. 59 ± 5 g) and thus raised blood glucose and insulin concentrations (i.e. average peak glucose 180 ± 53 mg dL−1 and insulin 45 ± 19 μIU mL−1). In the 2 trials involving exercise (EXER+PLAC and EXER+STA), blood glucose and insulin were elevated 20 minutes after exercise (i.e. glucose 150 ± 48 mg dL−1) in comparison to the trials without exercise (i.e. glucose 120 ± 22 mg dL−1; P < .05). Thus, exercise caused transient hyperinsulinaemia/hyperglycaemia.
3.6. Resting metabolic rates and fat oxidation
Before exercise and fat meal ingestion resting energy expenditure (REE, 1.2 ± 0.2 kcal min−1) and fat oxidation (FOx, 1.18 ± 0.11 μmol kg−1 min−1) were similar among trials with no effect of 4 days statin withdrawal. In the trials without exercise, the thermic effect of the food increased REE but the effect was blunted by statin (i.e. 506 ± 57 vs 464 ± 66 kcal in 5 h for PLAC vs STA; P = .048; Figure 3A). Exercise also increased REE by the effect known as excess postexercise oxygen consumption (EPOC) but in the statin trials the increase started from a lower level and thus reached lower values (i.e. 464 ± 66 vs 498 ± 80 kcal in 5 h for STA vs EXER+STA; P = .045; Figure 3A). FOx was elevated in the exercise trials compared to their nonexercise matched trials. FOx for the 5 hours postexercise follow‐up was 25 ± 7 vs 31 ± 9 g for PLAC vs EXER+PLAC and 24 ± 3 vs 28 ± 5 g for STA vs EXER+STA (all P < .05; Figure 3B).
4. DISCUSSION
Epidemiological studies suggest an association between elevated PPTG blood concentrations and the development of atherosclerotic cardiovascular diseases.36 Humans spend a good portion of the day in a postprandial state and thus strategies to reduce PPTG could aid in prevention of cardiovascular disease development. Prescription of statin has the potential to reduce not only fasting cholesterol but also PPTG in hypertriglyceridaemic individuals.16 However, in the study by Parhofer and co‐workers, subjects were evaluated once prior to and once following 1 month of statin therapy. In contrast, our subjects were chronically medicated with statins for an average of 5 years before the experiment. It is not uncommon that continuous drug use lowers its biological response (drug desensitization). Thus, our design better addresses the question of if prolonged statin therapy consistently reduces PPTG. Furthermore, in our data, continued statin use normalized fasting TG below the hypertriglyceridaemia threshold for MetS (i.e. 107 ± 54 mg dL−1; Table 1; below 150 mg dL−1),28 while it persisted above that threshold in Parhofer et al.’s, data (i.e. >160 mg dL−1).16 It is more likely that statins could lower elevated rather than normalized TG concentration. Thus, our first finding is of clinical relevance since statins significantly lowered PPTG (iAUC of TG 31–49% lower; Figure 1B) even when fasting TG levels were below the MetS threshold for hypertriglyceridaemia (i.e. <150 mg dL−1).
We also tested the combined effects of pharmacological (i.e. statin) and nonpharmacological (i.e. exercise) therapies on PPL in our individuals chronically medicated with statins. We hypothesized that a bout of exercise would act additively with statins to reduce PPTG. Our data refuted our hypothesis. Several studies suggest that for exercise to reduce PPTG it must induce a large enough energy deficit31 requiring at least moderate intensity exercise.37 Using selective replenishing of substrates after exercise by altering diet composition, Harrison and coworkers found that carbohydrate deficit is specifically needed for exercise to lower PPTG.38 According to those findings, we prescribed a bout of exercise to our subjects that elicited moderate energy expenditure (302 kcal) mostly fuelled by carbohydrate oxidation (i.e. 63 ± 18 g deficit). Further, we provided the test meal 20 min after finishing exercise avoiding exogenous muscle and liver replenishment of carbohydrate before the test meal. Despite our experimental design being geared to potentiate and maintain carbohydrate deficit, PPTG was not lowered by exercise (i.e. EXER+PLAC vs PLAC, Figure 1). By contrast, there are studies finding that carbohydrate replacement does not significantly attenuate PPTG39, 40 and that energy deficit is the main determinant of PPTG reductions.41 Thus, it is also possible that the moderate level of energy expenditure we achieved with exercise (302 kcal) may have not been enough stimulus to lower PPTG.
TGs are transported in blood combined with phospholipids, proteins and cholesterol in complex macromolecules known as lipoproteins. Thus, PPTG reductions after exercise should involve changes in lipoprotein metabolism. Exercise may reduce chylomicron secretion into blood from fat digestion,42 it may reduce hepatic VLDL‐TG secretion43, 44 or it may increase skeletal muscle TG uptake45 by activating endothelial lipoprotein lipase. Regarding this last mechanism, some studies sustain that the effects of exercise on PPTG appear after a latency time needed for endothelial lipoprotein lipase (LPL) to reach its maximal activity. Although muscle LPL activity peaks 18 hours after exercise,46 increased LPL activity is observed after only 4 hours47 and even immediately after exercise.7, 46 Furthermore, a recent study suggests that LPL activity does not predict VLDL‐TG oxidation since muscle LPL activity is in excess of what is required for lipid uptake and oxidation already at rest.48
Conversely, a study using an in vitro assay found that prior exercise increased the affinity of postprandial VLDL for LPL‐mediated clearance (i.e. TG hydrolysis49) explaining how exercise could increase VLDL‐TG clearance without necessarily increasing LPL activity.38, 50 Thus, it is unclear if our lack of effect of exercise on PPTG was due to the temporal proximity of exercise to the fat test meal (i.e. 20 min) giving insufficient time for endothelial LPL activation. By contrast, PPTG reductions has been reported in studies where the high fat meal was ingested within 1 hour after exercise termination,12, 37, 51, 52 which is similar to our ingestion time. Lastly, subjects were required to avoid exercise in the 48 hours preceding each trial and only habitual (domestic) physical activity was allowed. It has been recently evidenced that prolonged inactivity (4 days of prolonged sitting) prior to an exercise bout also fails to improve PPL in healthy‐young individuals.53 It is possible that in our older MetS individuals 2 days of inactivity could lead to a similar situation. Therefore, there may be 2 possibilities as to why exercise failed to improve PPL; short time after exercise and/or being inactive (prolonged sitting) in 2 days prior testing.
Before the high fat meal challenge, statin withdrawal for 4 days elevated blood lipid levels (i.e. −60 min blood sample in Figure 1 and 2). The increase in blood lipids upon statin withdrawal gauges the pharmacological power of the current prescription of statin type and dose in our subjects. All 10 subjects responded with elevations in TG and total blood cholesterol when comparing the PLAC to the STA trial. Thus, statins were active in all our subjects, since 4 days withdrawal was enough to evidence their antiatherogenic actions. Before the fat meal, statin withdrawal (i.e. PLAC and EXER+PLAC) increased blood TGs (24%), LDL‐c (31%) and total cholesterol (19%; all P < .05) evidencing treatment compliance. Statins reduced LDL‐c almost 1 mmol L−1 (i.e. 30 mg dL−1) an amount that has been related in epidemiological studies with a 25% reduction in cardiovascular related morbidity and mortality.54
Our fat meal (0.71 g kg−1) produced the typical elevations and subsequent reductions in blood TG (Figure 1) suggesting increased delivery and then increased clearance of blood TG. However, postprandial total cholesterol and LDL‐c did not respond to the fat meal ingestion or to exercise (Figure 2). In contrast, Ghafouri et al., 49 observed in healthy overweight men, reductions in postprandial concentrations of small dense LDL‐c, induced by 90 minutes of treadmill walking the day before. Our unchanged total LDL‐c suggests that either we are not monitoring the correct fraction of LDL‐c or that, in our individuals, the liver is not very actively manufacturing cholesterol or assembling LDL‐c from the fat meal chylomicron remnants. It has been reported that chylomicron remnants from a fat meal contribute to only 10% of liver VLDL‐TG synthesis. 55 Thus, it seems that intestinally derived chylomicrons can be the main source of the postprandial elevations in blood TG. Conversely, LPL activity in adipose tissue and muscle could be responsible for increasing TG clearance and the subsequent reductions in blood TG. Of note, total cholesterol and LDL‐c remained reduced with statin during the postprandial 5‐hours, suggesting that fat ingestion does not alter statin inhibition of the rate‐limiting step in liver cholesterol synthesis.
Hypertriglyceridaemia is an abnormality that confers a high atherogenic potential, particularly in the context of diabetes.56 To counteract their increased atherogenic cardiovascular risk, many hypertriglyceridaemic diabetic patients are treated with statins. Six of our 10 subjects had insulin resistance and were medicated with metformin while 3 of the remaining 4 had impaired fasting glucose (i.e. fasting glucose >100 mg dL−1) surpassing as a group the prediabetic threshold (Table 1). It has been reported that exercise fails to significantly improve PPL in type II diabetes individuals.9, 10 Furthermore, the high intensity of the latter segment of our exercise bout (7 min at 85% VO2MAX) resulted in hyperglycaemia and increased insulin concentration upon exercise discontinuation a phenomenon previously described in healthy population.57 Insulin resistance involves skeletal muscle, liver and/or adipose tissue. Insulin resistant individuals have inability to correctly suppress adipose tissue lipolysis upon elevations in blood insulin.58 Thus, it is possible that our prediabetic subjects were unable to suppress adipose tissue lipolysis despite elevations in insulin, maintaining a high supply of fatty acids to the liver and thus VLDL‐TG production.58, 59 Thus, either the diabetic condition of our subjects or the lack of reduction of insulin with exercise may have prevented exercise from lowering PPTG.
The exercise preceding the fat test meal (EXER+PLAC and EXER+STA) resulted in 6 g of fat oxidation. Exercise increased fat oxidation in the postprandial period with an extra 5 g oxidized (Figure 3B). However, the increased fat oxidation in the exercise trials did not reflect on reduced blood PPTG. It has been reported that during postexercise recovery, intramuscular TGs are oxidized to supply energy for muscle glycogen storage.46 It is likely that a larger glycogen depletion is required to increase postexercise intramuscular TG oxidation and in turn increase blood TG clearance to replenish them. Chronic statin use in our subject did not reduce fat oxidation during exercise coinciding with previous reports in healthy normolipidaemic22, 23 and hypercholesteraemic men.24 However, after exercise, statins blunted the meal thermic effect of food (Figure 3A). Exercise produced similar magnitude of EPOC in the statin and nonstatin trials. However, since the statin trials started from a lower baseline level, 5‐hour energy expenditure (i.e. thermic effect of food and EPOC combined) was lower. Statin reduced resting energy expenditure by 42 kcal in 5‐hour, and thus small effects on 24‐hour energy balance are expected.
A large epidemiological study reveals that fitness levels and statin act independently to lower LDL‐c and mortality hazard ratios in military veterans.60 However, intervention studies combining aerobic training and statins are contradictory. In one study, statins reduced serum TGs by 14%, but exercise training did not add to that reduction.26 Statin use may restrain the improvements in mitochondrial oxidative capacity with exercise training.20, 61 Although this could potentially reduce the capacity to oxidize fat, that effect has not been reported.24, 62 To our knowledge, there are no studies addressing the acute effect of a bout of exercise on PPTG in individuals chronically treated with statins. We did not observe an additive effect of a bout of exercise on statin reductions of PPTG. However, our results do not discard that another exercise/statin combination may result in reductions in PPTG.
Our study is not free of limitations. To perform an ecological study, we did not prescribe the statin pharmacological treatment but recruited subjects chronically treated with statin (>6 months). Subjects underwent a medicine washout period of 96 hours, which exceeded the half‐life of all prescriptions (Table 2). Although the withdrawal increased blood TGs, cholesterol and LDL‐c in the placebo trials (Figures 1, 2), we did not investigate if some drug effect may have remained.63 An alternative study design is to initiate statin treatment in borderline hypercholesteraemic participants. The advantage of our design, using subjects own prescribed medications, is that we could gauge the real‐life substitutive potential of exercise. Another limitation is that our study does not assess the mechanism or locus of the statin induced lowering of blood lipids. Finally, we are unsure if the other medication that subjects were taking (i.e. metformin in 60% of the sample) interacted with statins and thus altered its actions. Conversely, our study is relevant because we sought to lower PPTG in a population with overweight, MetS, hypercholesterolaemia and prediabetes and thus increased risk for suffering CVD. The study is novel on investigating the combined effects of statins and exercise on PPTG. The main strength of this study is its cross‐over, double‐blind randomized order control design with a large enough sample size to assess the efficacy of exercise and/or statin on PPTG.
In summary, our data suggest that in hypercholesteraemic, overweight MetS individuals, their chronic habitual statin therapy dose that normalizes blood fasting TG (Table 1) is enough to also reduce their lipaemic response to a fat meal (PPTG) lessening the CVD risk associated with atherogenic dyslipidaemia. A bout of intense aerobic exercise (i.e. 41 min at an average of 72% HR max) 20 minutes before the fat meal ingestion, does not lower PPL and thus cannot be substitutive of statin treatment. Our data support the efficacy of prolonged statin therapy on lowering not only fasting by also PPTG, while a bout of exercise is not substitutive of statin effects in hypercholesteraemic MetS individuals.
COMPETING INTERESTS
The authors report no potential conflicts of interest. The team physician had direct clinical responsibility for patients.
CONTRIBUTORS
Mora‐Rodriguez R. 1,2,3,5, Ortega J.F. 1,2,3,6, Morales‐Palomo F. 1,2,3,4,6, Ramirez‐Jimenez M. 1,2,3,4,6, Moreno‐Cabañas, A. 1,2,3,6
1. Formulated the research question; 2. Designed the study; 3. Collected data; 4. Analysed the data; 5. Wrote the article; 6. Revised the article.
ACKNOWLEDGEMENTS
The authors recognize the invaluable contribution of the subjects of the study. This work was partially funded by a grant from the Spanish Ministry of Economy and Competitivity (DEP‐2017‐83244‐R).
Mora‐Rodriguez R, Ortega JF, Morales‐Palomo F, Ramirez‐Jimenez M, Moreno‐Cabañas A. Effects of statin therapy and exercise on postprandial triglycerides in overweight individuals with hypercholesterolaemia. Br J Clin Pharmacol. 2020;86 1089–1099. 10.1111/bcp.14217
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon request.
REFERENCES
- 1. Kolovou GD, Mikhailidis DP, Kovar J, et al. Assessment and clinical relevance of non‐fasting and postprandial triglycerides: an expert panel statement. Curr Vasc Pharmacol. 2011;9(3):258‐270. [DOI] [PubMed] [Google Scholar]
- 2. Wideman L, Kaminsky LA, Whaley MH. Postprandial lipemia in obese men with abdominal fat patterning. J Sports Med Phys Fitness. 1996;36(3):204‐210. [PubMed] [Google Scholar]
- 3. Halbert JA, Silagy CA, Finucane P, Withers RT, Hamdorf PA. Exercise training and blood lipids in hyperlipidemic and normolipidemic adults: a meta‐analysis of randomized, controlled trials. Eur J Clin Nutr. 1999;53(7):514‐522. [DOI] [PubMed] [Google Scholar]
- 4. Mora‐Rodriguez R, Ortega JF, Hamouti N, et al. Time‐course effects of aerobic interval training and detraining in patients with metabolic syndrome. Nutrition, metabolism, and cardiovascular diseases: NMCD. 2014;24(7):792‐798. [DOI] [PubMed] [Google Scholar]
- 5. Ortega JF, Morales‐Palomo F, Fernandez‐Elias V, et al. Dietary supplementation with omega‐3 fatty acids and oleate enhances exercise training effects in patients with metabolic syndrome. Obesity (Silver Spring). 2016;24(8):1704‐1711. [DOI] [PubMed] [Google Scholar]
- 6. Mora‐Rodriguez R, Ortega JF, Guio de Prada V, et al. Effects of simultaneous or sequential weight loss diet and aerobic interval training on metabolic syndrome. Int J Sports Med. 2016;37(4):274‐281. [DOI] [PubMed] [Google Scholar]
- 7. Grandjean PW, Crouse SF, Rohack JJ. Influence of cholesterol status on blood lipid and lipoprotein enzyme responses to aerobic exercise. J Appl Physiol. 1985) 2000;89:472‐480. [DOI] [PubMed] [Google Scholar]
- 8. Maraki MI, Sidossis LS. The latest on the effect of prior exercise on postprandial lipaemia. Sports Med. 2013;43:463‐481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dalgaard M, Thomsen C, Hermansen K. Effects of one single bout of low‐intensity exercise on postprandial lipaemia in type 2 diabetic men. Br J Nutr. 2004;92(3):469‐476. [DOI] [PubMed] [Google Scholar]
- 10. Gill JM, Al‐Mamari A, Ferrell WR, et al. Effect of prior moderate exercise on postprandial metabolism in men with type 2 diabetes: heterogeneity of responses. Atherosclerosis. 2007;194(1):134‐143. [DOI] [PubMed] [Google Scholar]
- 11. Mestek ML, Plaisance EP, Ratcliff LA, Taylor JK, Wee SO, Grandjean PW. Aerobic exercise and postprandial lipemia in men with the metabolic syndrome. Med Sci Sports Exerc. 2008;40(12):2105‐2111. [DOI] [PubMed] [Google Scholar]
- 12. Zhang JQ, Ji LL, Fretwell VS, Nunez G. Effect of exercise on postprandial lipemia in men with hypertriglyceridemia. Eur J Appl Physiol. 2006;98:575‐582. [DOI] [PubMed] [Google Scholar]
- 13. Endo A. The discovery and development of HMG‐CoA reductase inhibitors. J Lipid Res. 1992;33(11):1569‐1582. [PubMed] [Google Scholar]
- 14. Beisiegel U. Lipoprotein metabolism. Eur Heart J. 1998;19(Suppl A):A20‐A23. [PubMed] [Google Scholar]
- 15. Hokanson JE, Austin MA. Plasma triglyceride level is a risk factor for cardiovascular disease independent of high‐density lipoprotein cholesterol level: a meta‐analysis of population‐based prospective studies. J Cardiovasc Risk. 1996;3(2):213‐219. [PubMed] [Google Scholar]
- 16. Parhofer KG, Laubach E, Barrett PH. Effect of atorvastatin on postprandial lipoprotein metabolism in hypertriglyceridemic patients. J Lipid Res. 2003;44(6):1192‐1198. [DOI] [PubMed] [Google Scholar]
- 17. Eldor R, Raz I. American Diabetes Association indications for statins in diabetes: is there evidence? Diabetes Care. 2009;32(Suppl 2):S384‐S391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Phillips PS, Haas RH, Bannykh S, et al. Statin‐associated myopathy with normal creatine kinase levels. Ann Intern Med. 2002;137(7):581‐585. [DOI] [PubMed] [Google Scholar]
- 19. Christensen CL, Wulff Helge J, Krasnik A, et al. LIFESTAT ‐ living with statins: an interdisciplinary project on the use of statins as a cholesterol‐lowering treatment and for cardiovascular risk reduction. Scand J Public Health. 2016;44(5):534‐539. [DOI] [PubMed] [Google Scholar]
- 20. Larsen S, Stride N, Hey‐Mogensen M, et al. Simvastatin effects on skeletal muscle: relation to decreased mitochondrial function and glucose intolerance. J Am Coll Cardiol. 2013;61(1):44‐53. [DOI] [PubMed] [Google Scholar]
- 21. Limprasertkul A, Fisher NM, Awad AB, Pendergast DR. Statin therapy depresses fat metabolism in older individuals. J Am Coll Nutr. 2012;31(1):32‐38. [DOI] [PubMed] [Google Scholar]
- 22. Eagles CJ, Kendall MJ, Maxwell S. A comparison of the effects of fluvastatin and bezafibrate on exercise metabolism: a placebo‐controlled study in healthy normolipidaemic subjects. Br J Clin Pharmacol. 1996;41(5):381‐387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Head A, Jakeman PM, Kendall MJ, Cramb R, Maxwell S. The impact of a short course of three lipid lowering drugs on fat oxidation during exercise in healthy volunteers. Postgrad Med J. 1993;69(809):197‐203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Larsen S, Vigelso A, Dandanell S, Prats C, Dela F, Helge JW. Simvastatin‐induced insulin resistance may be linked to decreased lipid uptake and lipid synthesis in human skeletal muscle: the LIFESTAT study. J Diabetes Res. 2018;2018:9257874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Reinoso RF, Sanchez Navarro A, Garcia MJ, Prous JR. Preclinical pharmacokinetics of statins. Methods Find Exp Clin Pharmacol. 2002;24(9):593‐613. [PubMed] [Google Scholar]
- 26. Walsh JH, Yong G, Cheetham C, et al. Effects of exercise training on conduit and resistance vessel function in treated and untreated hypercholesterolaemic subjects. Eur Heart J. 2003;24(18):1681‐1689. [DOI] [PubMed] [Google Scholar]
- 27. Mottillo S, Filion KB, Genest J, et al. The metabolic syndrome and cardiovascular risk a systematic review and meta‐analysis. J Am Coll Cardiol. 2010;56(14):1113‐1132. [DOI] [PubMed] [Google Scholar]
- 28. Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640‐1645. [DOI] [PubMed] [Google Scholar]
- 29. Vilaseca Canals J, Espinas BJ. Guıa terapeutica en Atencion Primaria, basada en la evidencia. 6th Editon ed. Barcelona (Spain): semFYC ediciones; 2003. [Google Scholar]
- 30. Poole DC, Jones AM. Measurement of the maximum oxygen uptake VO2max: VO2peak is no longer acceptable. J Appl Physiol. 2017;122(4):997‐1002. [DOI] [PubMed] [Google Scholar]
- 31. Freese EC, Gist NH, Cureton KJ. Effect of prior exercise on postprandial lipemia: an updated quantitative review. J Appl Physiol. 1985) 2014;116:67‐75. [DOI] [PubMed] [Google Scholar]
- 32. Weir JB. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol 1949;109(1‐2):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Frayn KN. Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol Respir Environ Exerc Physiol. 1983;55(2):628‐634. [DOI] [PubMed] [Google Scholar]
- 34. Matthews JN, Altman DG, Campbell MJ, Royston P. Analysis of serial measurements in medical research. BMJ. 1990;300:230‐235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low‐density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499‐502. [PubMed] [Google Scholar]
- 36. Nordestgaard BG, Benn M, Schnohr P, Tybjaerg‐Hansen A. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA. 2007;298(3):299‐308. [DOI] [PubMed] [Google Scholar]
- 37. Katsanos CS, Grandjean PW, Moffatt RJ. Effects of low and moderate exercise intensity on postprandial lipemia and postheparin plasma lipoprotein lipase activity in physically active men. J Appl Physiol (1985). 2004;96(1):181‐188. [DOI] [PubMed] [Google Scholar]
- 38. Harrison M, O'Gorman DJ, McCaffrey N, et al. Influence of acute exercise with and without carbohydrate replacement on postprandial lipid metabolism. J Appl Physiol (1985). 2009;106(3):943‐949. [DOI] [PubMed] [Google Scholar]
- 39. Chiu CH, Burns SF, Yang TJ, et al. Energy replacement using glucose does not increase postprandial lipemia after moderate intensity exercise. Lipids Health Dis. 2014;13(1):177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Freese EC, Levine AS, Chapman DP, Hausman DB, Cureton KJ. Effects of acute sprint interval cycling and energy replacement on postprandial lipemia. J Appl Physiol (1985). 2011;111(6):1584‐1589. [DOI] [PubMed] [Google Scholar]
- 41. Burton FL, Malkova D, Caslake MJ, Gill JM. Energy replacement attenuates the effects of prior moderate exercise on postprandial metabolism in overweight/obese men. Int J Obes (Lond). 2008;32(3):481‐489. [DOI] [PubMed] [Google Scholar]
- 42. Kolifa M, Petridou A, Mougios V. Effect of prior exercise on lipemia after a meal of moderate fat content. Eur J Clin Nutr. 2004;58(10):1327‐1335. [DOI] [PubMed] [Google Scholar]
- 43. Gill JM, Mees GP, Frayn KN, Hardman AE. Moderate exercise, postprandial lipaemia and triacylglycerol clearance. Eur J Clin Invest. 2001;31(3):201‐207. [DOI] [PubMed] [Google Scholar]
- 44. Malkova D, Evans RD, Frayn KN, Humphreys SM, Jones PR, Hardman AE. Prior exercise and postprandial substrate extraction across the human leg. Am J Physiol Endocrinol Metab. 2000;279:E1020‐E1028. [DOI] [PubMed] [Google Scholar]
- 45. Annuzzi G, Jansson E, Kaijser L, Holmquist L, Carlson LA. Increased removal rate of exogenous triglycerides after prolonged exercise in man: time course and effect of exercise duration. Metabolism. 1987;36(5):438‐443. [DOI] [PubMed] [Google Scholar]
- 46. Kiens B, Richter EA. Utilization of skeletal muscle triacylglycerol during postexercise recovery in humans. Am J Physiol. 1998;275:E332‐E337. [DOI] [PubMed] [Google Scholar]
- 47. Kiens B, Lithell H, Mikines KJ, Richter EA. Effects of insulin and exercise on muscle lipoprotein lipase activity in man and its relation to insulin action. J Clin Invest. 1989;84:1124‐1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sondergaard E, Andersen IR, Sorensen LP, Gormsen LC, Nielsen S. Lipoprotein lipase activity does not predict very low‐density lipoprotein‐triglyceride fatty acid oxidation during exercise. Scand J Med Sci Sports. 2017;27(5):474‐481. [DOI] [PubMed] [Google Scholar]
- 49. Ghafouri K, Cooney J, Bedford DK, Wilson J, Caslake MJ, Gill JM. Moderate exercise increases affinity of large very low‐density lipoproteins for hydrolysis by lipoprotein lipase. J Clin Endocrinol Metab. 2015;100(6):2205‐2213. [DOI] [PubMed] [Google Scholar]
- 50. Malkova D, Gill JM. Effects of exercise on postprandial lipoprotein metabolism. Future Lipidol. 2006;1:743‐755. [Google Scholar]
- 51. Davitt PM, Arent SM, Tuazon MA, Golem DL, Henderson GC. Postprandial triglyceride and free fatty acid metabolism in obese women after either endurance or resistance exercise. J Appl Physiol (1985). 2013;114(12):1743‐1754. [DOI] [PubMed] [Google Scholar]
- 52. Plaisance EP, Mestek ML, Mahurin AJ, Taylor JK, Moncada‐Jimenez J, Grandjean PW. Postprandial triglyceride responses to aerobic exercise and extended‐release niacin. Am J Clin Nutr. 2008;88(1):30‐37. [DOI] [PubMed] [Google Scholar]
- 53. Akins JD, Crawford CK, Burton HM, Wolfe AS, Vardarli E, Coyle EF. Inactivity induces resistance to the metabolic benefits following acute exercise. J Appl Physiol (1985) 2019; 126: 1088–94, 4, 1094. [DOI] [PubMed] [Google Scholar]
- 54. Collins R, Reith C, Emberson J, et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet. 2016;388(10059):2532‐2561. [DOI] [PubMed] [Google Scholar]
- 55. Barrows BR, Timlin MT, Parks EJ. Spillover of dietary fatty acids and use of serum nonesterified fatty acids for the synthesis of VLDL‐triacylglycerol under two different feeding regimens. Diabetes. 2005;54(9):2668‐2673. [DOI] [PubMed] [Google Scholar]
- 56. Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. JAMA. 2002;287:2570‐2581. [DOI] [PubMed] [Google Scholar]
- 57. Marliss EB, Simantirakis E, Miles PD, et al. Glucoregulatory and hormonal responses to repeated bouts of intense exercise in normal male subjects. J Appl Physiol (1985). 1991;71(3):924‐933. [DOI] [PubMed] [Google Scholar]
- 58. Bush NC, Basu R, Rizza RA, Nair KS, Khosla S, Jensen MD. Insulin‐mediated FFA suppression is associated with triglyceridemia and insulin sensitivity independent of adiposity. J Clin Endocrinol Metab. 2012;97(11):4130‐4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. 2005;115(5):1343‐1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kokkinos PF, Faselis C, Myers J, Panagiotakos D, Doumas M. Interactive effects of fitness and statin treatment on mortality risk in veterans with dyslipidaemia: a cohort study. Lancet. 2013;381(9864):394‐399. [DOI] [PubMed] [Google Scholar]
- 61. Mikus CR, Boyle LJ, Borengasser SJ, et al. Simvastatin impairs exercise training adaptations. J Am Coll Cardiol. 2013;62(8):709‐714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Meex RC, Phielix E, Schrauwen‐Hinderling VB, et al. The use of statins potentiates the insulin‐sensitizing effect of exercise training in obese males with and without type 2 diabetes. Clin Sci (Lond). 2010;119(7):293‐301. [DOI] [PubMed] [Google Scholar]
- 63. Kim J, Ahn BJ, Chae HS, et al. A population pharmacokinetic‐pharmacodynamic model for simvastatin that predicts low‐density lipoprotein‐cholesterol reduction in patients with primary hyperlipidaemia. Basic Clin Pharmacol Toxicol. 2011;109(3):156‐163. [DOI] [PubMed] [Google Scholar]
- 64. Schachter M. Chemical, pharmacokinetic and pharmacodynamic properties of statins: an update. Fundam Clin Pharmacol. 2005;19: 117‐125. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon request.


