Abstract
Aims
To explore the pharmacodynamics of mycophenolic acid (MPA) through inosine monophosphate dehydrogenase (IMPDH) capacity measurement and purine levels in peripheral blood mononuclear cells (PBMC) longitudinally during the first year after renal transplantation (TX).
Methods
PBMC were isolated from renal recipients 0–4 days prior to and 6–9 days, 5–7 weeks and 1 year after TX (before and 1.5 hours after dose). IMPDH capacity and purine (guanine and adenine) levels were measured in stimulated and nonstimulated PBMC.
Results
Twenty‐nine patients completed the follow‐up period, of whom 24 received MPA. In stimulated PBMC, the IMPDH capacity (pmol 10−6 cells min−1) was median (interquartile range) 127 (95.8–147) before TX and thereafter 44.9 (19.2–93.2) predose and 12.1 (4.64–23.6) 1.5 hours postdose across study days after TX. The corresponding IMPDH capacity in nonstimulated PBMC was 5.71 (3.79–6.93), 3.35 (2.31–5.62) and 2.71 (1.38–4.08), respectively. Predose IMPDH capacity in nonstimulated PBMC increased with time, reaching pre‐TX values at 1 year. In stimulated PBMC, both purines were reduced before (median 39% reduction across days after TX) and after (69% reduction) dose compared to before TX. No alteration in the purine levels was observed in nonstimulated PBMC. Patients needing dose reductions during the first year had lower pre‐dose IMPDH capacity in nonstimulated PBMC (1.87 vs 3.00 pmol 10−6 cells min−1, P = .049) at 6–9 days.
Conclusion
The inhibitory effect of MPA was stronger in stimulated PBMC. Nonstimulated PBMC became less sensitive to MPA during the first year after TX. Early IMPDH capacity appeared to be predictive of dose reductions.
Keywords: biomarkers, immunosuppression, therapeutic drug monitoring, transplantation
1.
What is already known about the subject
The prodrug mycophenolate mofetil (MMF) and its metabolite mycophenolic acid (MPA) are used after solid organ transplantation, impeding the lymphocyte proliferation through inhibition of inosine monophosphate dehydrogenase (IMPDH) and the de novo purine synthesis.
IMPDH monitoring in renal transplant patients has shown potential utility, but longitudinal studies are lacking and there are few studies on the pharmacodynamic monitoring in activated lymphocytes.
Adverse effects are a common reason for MMF dose reduction and have been associated with impaired outcome.
What this study adds
MPA is a more potent inhibitor of IMPDH in activated peripheral blood mononuclear cells compared with nonactivated cells.
Over time, circulating lymphocytes become less sensitive to MPA.
The IMPDH capacity in peripheral blood mononuclear cells is low early after renal transplantation in patients needing later MMF dose reduction.
2. INTRODUCTION
Proliferation of activated B and T cells is a vital step in an adaptive immune response, and the purine nucleotides of guanine and adenine are in this respect essential for the (deoxy)ribonucleic acid synthesis. Mammalian cells with a low proliferation rate rely mainly on the salvage pathway for these purines,1 whilst rapidly dividing lymphocytes are more dependent on de novo purine synthesis.2 Mycophenolic acid (MPA) is a reversible inhibitor of inosine monophosphate dehydrogenase (IMPDH), a rate‐limiting enzyme in the de novo synthesis of guanine nucleotides (Figure 1). MPA therefore inhibits proliferating lymphocytes and is frequently used as an immunosuppressant to prevent rejection after solid organ transplantation3, 4 and to treat certain autoimmune disorders.5, 6, 7
Figure 1.
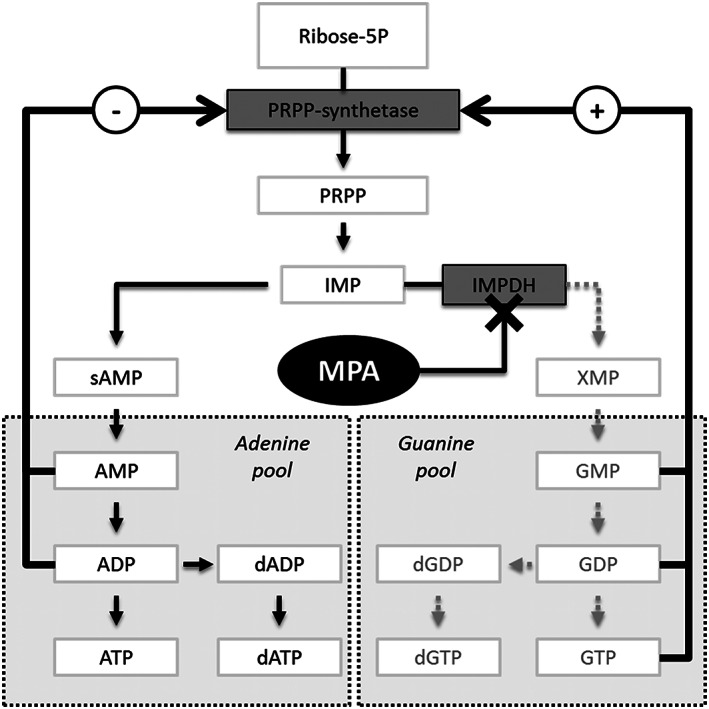
Ribose‐5P is synthesized to phosphoribosyl pyrophosphate (PRPP) by PRPP‐synthetase and further to inosine monophosphate (IMP). Mycophenolic acid (MPA) inhibits inosine monophosphate dehydrogenase (IMPDH) and thereby the conversion of IMP to xanthosine monophosphate (XMP), guanosine‐5′‐monophosphate (GMP), guanosine‐5′‐diphosphate (GDP), 2′‐deoxyguanosine‐5′‐diphosphate (dGDP), guanosine‐5′‐triphosphate (GTP) and 2′‐deoxyguanosine‐5′‐triphosphate (dGTP). AMP, (d)ADP, and (d)ATP are corresponding adenosine nucleotides
After renal transplantation, either the sodium salt of MPA or the prodrug mycophenolate mofetil (MFF) is used together with other immunosuppressants, commonly a calcineurin inhibitor and prednisolone. By including MMF in the immunosuppressive regimen after renal transplantation, the incidence of biopsy‐proven acute rejections (BPAR) within the first year was reduced from 56 to 30%.8 Reduction in dose, therapy interruption, or discontinuation of MMF or MPA treatment is associated with increased risk of BPAR.9 Nevertheless, it has been reported that up to half of patients starting on MMF need dose reductions within the first year after renal transplantation, most often due to haematological toxicities.10
An MPA area under the concentration vs time curve (AUC) below 30–40 mg h L−1,11, 12, 13, 14, 15 or an MPA trough concentration below 1.6 mg/L16, 17, 18 is associated with the risk of BPAR. Although some studies have shown a relationship between AUC19 or trough concentrations and MPA‐related adverse events,18 including leucopenia and anaemia,20 a clear relationship between MPA exposure and toxicity has not been determined.21, 22 As an alternative to MPA concentration‐based monitoring, assays for pharmacodynamic monitoring of MPA have been developed23, 24, 25, 26, 27 with the rationale being that there is an interindividual variation in the pharmacodynamic response to the same MPA exposure. While most assays quantify the effect of MPA on the target enzyme IMPDH, some assays monitor the levels of lymphocyte purine nucleotides.24, 28 Although the adenosine‐5′‐triphosphate (ATP) levels in ex‐vivo activated CD4+ cells have been suggested to reflect the immune status with the ability to predict BPAR,29 the application of purines as selective biomarkers for MPA has not been widely explored in a clinical setting.
In this study, we aimed to explore the potential applicability of molecular pharmacodynamic markers for monitoring MPA therapy. We performed a longitudinal study during the first year after renal transplantation. Samples were collected pre‐ and post‐transplant at the time of MPA trough and peak concentrations, and both IMPDH capacity and levels of adenine and guanine nucleotides were assessed in nonstimulated and ex vivo‐stimulated lymphocytes. In this explorative setting, associations between these biomarkers and 2 central clinical end points—BPAR and dose reduction—were examined.
3. METHODS
3.1. Nomenclature of targets and ligands
Key drugs and targets are named according to the Concise Guide to PHARMACOLOGY 2017/2018 source.30
3.2. Patients, treatment protocol and sampling
Patients admitted for renal transplantation, aged 18 years or older and receiving transplant from living donors, were included. The study was approved by the Regional Committee for Medical and Health Research Ethics (reference 2011/1282) and performed in accordance with the declaration of Helsinki. Written informed consents were provided from the participants. Data on tacrolimus (TAC) concentrations in peripheral blood mononuclear cells (PBMC)31 and the nuclear factor of activated T‐cell‐regulated gene expression32 are previously reported.
All patients received TAC and prednisolone. Patients with 1 or more human leucocyte antigen (HLA)‐mismatches were given 750 mg MMF twice daily. Standard risk patients (i.e patients without donor specific antibodies, panel reactive antibodies or ABO mismatch) were given TAC initially administered at 0.04 mg kg−1 twice daily and adjusted to obtain a predose concentration within 3–7 μg L−1 in whole blood during the first 180 days post‐transplant and thereafter 3–5 μg L−1. Standard risk patients received 250 mg methylprednisolone (350 mg if body weight was >90 kg) intravenously on the day of transplantation followed by peroral prednisolone once daily with a gradual reduction to 5 mg daily at 6 months. Basiliximab was given at the day of transplantation and at day 4 (20 mg each day). TAC, MMF and prednisolone were administered simultaneously.
One patient with pretransplant donor‐specific antibodies was classified as high risk with increased risk of graft rejection. This patient received enhanced immunosuppression according to protocol: TAC target level was initially 8–12 μg L−1 (day 0–30) and 6–10 μg L−1 thereafter. Methylprednisolone (350 mg) and prednisolone (80 mg) was given on the day of transplantation. Prednisolone was tapered to 10 mg by week 8. Rituximab (375 mg m−2) was given 30 days prior to transplantation and human normal immunoglobulin (400 mg kg−1 day−1) was given day 0–4 after transplantation. MMF treatment was the same for standard and high‐risk patients.
Blood samples were collected in the morning on 4 occasions: 0–4 days before transplantation, and 6–9 days, 5–7 weeks and 1 year after transplantation. On each post‐transplant occasion, blood samples were collected immediately prior to (t0) and 1.5 hours (t1.5) after the morning dose. At each sampling, blood was collected in 4‐mL EDTA tubes (Vacuette, Greiner Bio‐One, Monroe, NC, USA) for determination of MPA plasma concentration and in 4‐mL heparinized tubes (Vacuette) for isolation of PBMCs and subsequent measurement of the IMPDH capacity and the levels of guanine and adenine nucleotides.
As per hospital protocol, surveillance graft biopsies were collected and classified according to Banff criteria,33 at week 6, at 1 year or when a rejection was clinically suspected. The MMF dose was adjusted according to clinical judgment in standard practice.
3.3. Determination of MPA plasma concentration
Plasma was isolated from EDTA whole blood by centrifugation at 1500 g for 10 minutes at 4°C and stored at −80°C until analysis. Determination of plasma MPA has been described previously.34 Briefly, plasma samples were thawed and 200 μL of acidified methanol was added to 100 μL of sample for protein precipitation. After centrifugation at 4°C, the supernatant was diluted with equal volume of water. Separation and analysis were performed using reverse phase high performance liquid chromatography (Ultimate 3000, Thermo Fisher, Waltham, MA, USA). A 15‐cm phenyl column with 3‐μm particles (ACE Phenyl, Advanced Chromatography Technologies, Aberdeen, UK) was applied with mobile phase containing 53% methanol and phosphate buffer (pH 2.5). Detection was performed with UV absorbance at 215 nm.
3.4. PBMC isolation and stimulation
PBMCs were isolated from 4 mL heparinized whole blood with slight modifications to the protocol of the LeucoSep manufacturer (Greiner Bio‐One, Kremsmünster, Austria). The plasma was first separated by centrifugation (1000 g, 10 min, 4°C). Remaining blood cells were mixed with 6 mL cold phosphate‐buffered saline (PBS) without calcium or magnesium (BioWhittaker; Lonza, Basel, Switzerland) and thereafter transferred to prefilled LeucoSep‐tubes and centrifuged (1000 g, 10 min, 4°C). The resulting PBMC layer was transferred to a 14 mL round‐bottom polystyrene tube and washed by resuspension in 5 mL cold PBS and centrifuged (300 g, 10 min, 4°C). The supernatant was discarded and the remaining PBMC pellet was resuspended in 1 mL Roswell Park Memorial Institute medium (RPMI; Sigma Aldrich, St. Louis, MO, USA). An aliquot of 10 μL was used for cell counting on a Coulter counter (Counter Z‐series, Beckman Coulter, Brea, CA, USA) with particle size range 5–15 μm. The cell suspension was diluted with heparinized plasma from the original sample and RPMI, resulting in a final concentration of 20% (v/v) plasma and 1.6 × 106 cells mL−1. Diluted cell suspensions (200 μL aliquots) were transferred to 2 5 mL round‐bottom tubes. One aliquot was to added 200 μL of RPMI containing mitogens for activation of lymphocytes (100 ng mL−1 phorbol‐12‐myristate‐13‐acetate, 2.5 μg L−1 ionomycin, 0.030% v/v DMSO, 200 U L−1 penicillin–streptomycin), and the other aliquot was added to RPMI without mitogens (0.030% v/v DMSO, 200 U L−1 penicillin–streptomycin). The cell suspensions were thereafter incubated for 72 hours in a humid environment (37°C, 5% CO2) with cap allowing for gas exchange. After incubation, the cells were centrifuged (1000 g, 4°C, 5 min) and the supernatant was removed. The cells were resuspended in 500 μL cold PBS and transferred to 1.5 mL microcentrifuge tubes (LoBind; Eppendorf, Hamburg, Germany). The PBS supernatant was removed after centrifugation (2350 g, 2 min, 4°C) and the resulting cell pellet was lysed in 125 μL de‐ionized water (Milli‐Q; Merck, Darmstadt, Germany) during vortex. The lysates were stored at −80°C until assaying IMPDH capacity and purines.
3.5. Determination of IMPDH capacity and purines in PBMC
We used a previously reported assay for the simultaneous determination of guanine, adenine and IMPDH capacity.24 The samples were thawed at room temperature and homogenized by vortexing (30 s) and ultrasonication (120 s). Following centrifugation (1150 g, 2 min), 50 μL supernatant was added to each of 2 1.5‐mL polypropylene microcentrifuge tubes (aliquots A and B). To both A and B, 50 μL of aqueous buffer solution (250 μmol L−1 trishydrochloride, 7.5 mmol L−1 EDTA, 250 μmol L−1 KCl, 5.0 mmol L−1 DTT) was added. To A, 25 μL of an aqueous solution containing 9.0 mmol L−1 IMP and 2.0 mmol L−1 NAD+ was added, and 25 μL of deionized water was added to B. Both aliquots were briefly vortexed and aliquot A was incubated in a heated water bath (37°C) for 120 minutes to allow IMPDH‐mediated production of xanthosine monophosphate (XMP). Aliqout B was kept on the laboratory bench during incubation. The enzyme reaction was terminated by adding 20 μL of 4.0 mol L−1 perchloric acid and a brief vortex (added to aliquot A and B). A 20‐μL volume aqueous solution containing internal standards (25 μmol L−1 1,3‐15 N2‐xanthine, 25 μmol L−1 8‐13C‐7,9‐15 N2‐guanine, 25 μmol L−1 13C5‐adenine) was added to both aliquots. After vortex and centrifugation (9400 g, 4 min, 4°C) the supernatants were transferred to flat‐bottom glass inserts in liquid chromatography vials. The vials were placed on a heating block (60 minutes 100°C) to hydrolyse XMP, guanosine nucleotides (guanosine‐5′‐monophophate, guanosine‐5′‐diphosphate, guanosine‐5′‐triphosphate, 2′‐deoxy‐guanosine‐5′‐diphosphate and 2′‐deoxy‐guanosine‐5′‐triphosphate) and adenosine nucleotides (adenosine‐5′‐monophophate, adenosine‐5′‐diphosphate, adenosine‐5′‐triphosphate, 2′‐deoxy‐adenosine‐5′‐diphosphate and 2′‐deoxy‐adenosine‐5′‐triphosphate) to xanthine, guanine and adenine respectively. After cooling, 15 μL aqueous solution containing 4.0 mol L−1 potassium acetate was added to each vial. The vials were centrifuged (2000 g, 5 min, 4°C) and placed in the autosampler maintained at 4°C. Details regarding the liquid chromatography–tandem mass spectrometry conditions are described in the method publication.24 The IMPDH capacity was calculated using the following formula:
XANA and XANB was the amount of xanthine (pmol) in aliquot A and B, respectively, while N was the number of lysed cells (in millions) in the reaction and t was the duration of the enzyme reaction (min).
Since the assay measures the rate of XMP production in a setting where both substrate and co‐factor are added to saturate the enzyme reaction, the term IMPDH capacity was applied to underline that this was the maximum ex vivo production rate (not the in vivo IMPDH activity).
Guanine and adenine were measured in aliquot B and expressed as nmol 10−6 cells, reflecting the corresponding purine nucleotide pools.
3.6. IMPDH, purines and clinical outcome
Results from biopsy evaluations as well as any need for MMF dose reduction were collected from patient records. To assess the predictability of IMPDH‐capacity, guanine and adenine on the risk for BPAR these measurements were compared before transplantation between patients with and without BPAR within the first year after transplantation. To assess predictability whilst under MMF treatment we divided patients who had been BPAR free until 5–7 weeks into those who later did and did not have BPAR, and compared the biomarkers between these groups.
To explore the predictability of biomarkers measured early after transplantation on the need for later reduction of MMF dose, the biomarkers and MPA plasma concentrations 0–4 days before and 6–9 days after transplantation were compared between patients who later needed MMF dose reduction and patients who remained on the initial dose during the whole study.
3.7. Statistics and data sharing
The study was designed as an exploratory study to describe the molecular pharmacodynamics of MPA and to identify the potential associations between the investigated biomarkers and clinical outcome.
Continuous variables were compared using a t‐test when data was normally distributed or when ln‐transformation resulted in normal distribution. Continuous variables not being normally distributed were compared using Wilcoxon signed rank test for paired data or Mann–Whitney U test for unpaired data. Pearson correlation was used to correlate IMPDH capacities in stimulated and nonstimulated PBMC and to correlate adenine and guanine. Spearman correlation was used to correlate IMPDH capacity and MPA plasma concentrations.
Multigroup comparison between continuous variables collected at different times was performed using Skillings–Mack test to allow for non‐normal distribution and missing data.
Statistical significance was considered with 2 tails at P < 0.05.
All statistical tests were performed in R v.1.1.447.
Research data are not shared.
4. RESULTS
4.1. Patients and clinical outcome
Out of 33 patients initially included in the study, 4 were excluded before the first sampling (2 transplantations were postponed, 1 switched to cyclosporine, 1 was lost to study follow‐up). Among the remaining 29 patients, 5 patients received a zero HLA‐mismatched graft and, according to protocol, were not started on MMF at the time of transplantation. From patients receiving MMF at the time of sampling, IMPDH capacity and purine levels were measured in 94% (n = 158) of the maximum possible number (n = 168) of samples. In addition, a total of 29 samples from patients not receiving MMF (n = 5) were analysed for IMPDH and purines. Complete datasets from 13 patients using MMF throughout the study, and not receiving MMF before the pretransplant sample, were available. A total of 8 BPARs (2 with clinical manifestations) were recorded in 7 patients. One patient was diagnosed with BPAR at day 4 and 1 year, 1 patient at day 5, 1 patient at 60 days, 1 patient at day 34 and 3 patients at 1 year. One of the patients with BPAR at 5–7 weeks received a zero HLA‐mismatch graft and did not receive MMF, but was started on MMF after the BPAR episode.
Of the 24 patients given MMF at transplantation, 6 (25%) required a dose reduction during the first year after transplantation. Three patients required a dose reduction at 8, 80 and 111 days after transplantation, but received full dose at 1 year. The remaining 3 patients had reduced dose at 1 year.
4.2. MPA concentrations
Plasma concentrations of MPA are summarized in Table 1. Predose concentrations varied between study days (P < .001) and were lower at 6–9 days compared to the other study days (P < .010), whilst no difference between 5–7 weeks and 1 year could be shown (P = .948). No difference between MPA t1.5 concentrations was observed with respect to time after transplantation (P = .405).
Table 1.
MPA plasma concentrations and biomarkers measured in peripheral blood mononuclear cells from 24 patients before and after renal transplantation (median, quartiles)
| 0–4 days before treatment | 6–9 days t0 | 6–9 days t1.5 | 5–7 weeks t0 | 5–7 weeks t1.5 | 1 year t0 | 1 year t1.5 | P‐value across study days (Skillings–Mack) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Number of patients | 19 | 22 | 22 | 24 | 24 | 23 | 24 | t0 | t1.5 | |
| Plasma MPA (mg/L) | ‐ | 1.47 (1.14–2.39) | 5.27 (3.84–7.59) | 2.75 (1.75–3.26) | 7.02 (4.56–9.95) | 1.98 (1.77–4.09) | 6.47 (4.77–7.98) | <.001 | .405 | |
| IMPDH capacity (pmol 10−6 cells min−1) | Stimulated | 127 (95.8–147) | 33.1 (13.4–50.7) | 5.11 *** (2.30–25.1) | 35.3 (16.1–83.4) | 10.7 *** (5.72–19.5) | 84.8 (43.6–99.3) | 18.4 *** (7.34–31.2) | .041 | .526 |
| Nonstimulated | 5.71 (3.79–6.93) | 2.55 (1.78–4.83) | 1.98 * (1.27–3.66) | 3.17 (2.41–4.37) | 2.97 * (1.73–3.90) | 4.67 (3.06–8.32) | 2.77 *** (1.89–4.42) | .032 | .343 | |
| Guanine (nmol 10−6 cells) | Stimulated | 4.06 (2.68–5.69) | 1.14 (0.61–2.14) | 0.45 *** (0.33–0.88) | 1.06 (0.59–2.41) | 0.56 *** (0.34–1.25) | 1.83 (1.26–2.51) | 0.99 *** (0.47–1.33) | .198 | .171 |
| Nonstimulated | 0.89 (0.74–1.16) | 0.65 (0.49–0.88) | 0.58ns (0.48–0.96) | 0.91 (0.44–1.18) | 0.69ns (0.46–1.29) | 0.96 (0.55–1.10) | 0.61ns (0.52–0.99) | .49 | .57 | |
| Adenine (nmol 10−6 cells) | Stimulated | 6.41 (4.63–7.19) | 2.63 (1.47–3.63) | 1.44 *** (0.75–2.48) | 2.59 (1.67–4.44) | 1.37 *** (1.12–2.97) | 4.69 (3.71–5.53) | 2.41 *** (1.79–2.92) | .024 | .009 |
| Nonstimulated | 2.00 (1.67–2.51) | 1.33 (1.21–1.97) | 1.46ns (1.09–1.89) | 1.62 (1.33–1.97) | 1.90ns (1.50–2.70) | 1.89 (1.28–2.66) | 1.75ns (1.19–2.55) | .063 | .381 | |
IMPDH capacity and levels of purines measured in peripheral blood mononuclear cells from renal transplant recipients before and after transplantation. Stimulated; mitogens stimulated PBMC. IMPDH, inosine monophosphate dehydrogenase; MPA; mycophenolic acid; t0, immediately before next dose; t1.5, 1.5 hours after dose. Differences between t0 and t1.5 tested using t‐test on ln‐transformed values (IMPDH) or Wilcoxon signed rank test (guanine and adenine). Significantly lower than t0; *P < .05, ***P < .01, ns; not significant. Variations between days tested using Skillings–Mack test.
4.3. IMPDH capacity
The IMPDH capacity in ex vivo stimulated and nonstimulated PBMC is shown in Table 1 and Table 2. In pretransplant samples taken before commencement of any immunosuppressant therapies (n = 23), mitogen stimulation increased the IMPDH capacity on average 22‐fold (range 6–45‐fold). In samples collected from patients receiving MMF after transplantation, mitogen stimulation increased predose IMPDH capacity on average 15‐fold (range 1.1–60‐fold) and postdose IMPDH capacity increased on average 7.1‐fold (range 0.7–59‐fold), compared to no stimulation. Before transplantation, the interindividual variation (CV %) in IMPDH capacity was 40% in stimulated PBMC and 62% in nonstimulated PBMC. In the stimulated PBMC post‐transplantation, the interindividual variation was highest 6–9 days after transplantation being (before/after dose) 111/124%, and thereafter 82/112% at 5–7 weeks and 59% /72% at 1 year. The interindividual variations in IMPDH capacity in nonstimulated PBMC showed a similar pattern: 100/102% at 6–9 days, 71/68% at 5–7 weeks and 62% /74% at 1 year.
Table 2.
Inosine monophosphate dehydrogenase (IMPDH) capacity and purines in peripheral blood mononuclear cells (PBMC) from renal transplant patients with zero human leucocyte antigen‐mismatch graft and not receiving mycophenolate mofetil (median, range)
| 0–4 days before transplantation | 6–9 days t0 | 6–9 days t1.5 | 5–7 weeks t0 | 5–7 weeks t1.5 | 1 year t0 | 1 year t1.5 | ||
|---|---|---|---|---|---|---|---|---|
| (n = 5) | (n = 5) | (n = 5) | (n = 5*) | (n = 5) | (n = 4) | (n = 4) | ||
| IMPDH capacity (pmol 10−6 cells min−1) | Stimulated | 172 (41.0–208) | 134 (73.4–253) | 149 (116–180) | 186 (103–239) | 166 (93.5–216) | 194 (140–220) | 214 (118–269) |
| Nonstimulated | 8.36 (2.88–20.6) | 5.15 (3.45–16.5) | 5.57 (2.32–7.47) | 11.1 (4.32–15.4) | 7.11 (1.74–12.6) | 10.7 (3.58–11.8) | 10.5 (2.79–27.3) | |
| Guanine (nmol 10−6 cells) | Stimulated | 5.23 (4.06–6.84) | 5.27 (3.36–7.54) | 4.73 (3.87–5.74) | 5.27 (4.45–12.4) | 5.55 (3.95–11.1) | 6.65 (4.84–6.95) | 6.66 (2.75–8.65) |
| Nonstimulated | 1.69 (0.52–1.90) | 0.79 (0.38–2.91) | 0.77 (0.34–1.14) | 1.32 (0.39–2.02) | 1.25 (0.32–3.76) | 1.08 (0.48–2.20) | 1.35 (0.41–2.96) | |
| Adenine (nmol 10−6 cells) | Stimulated | 5.74 (4.14–7.89) | 7.7 (5.35–11.0) | 7.73 (4.73–9.41) | 8.13 (5.04–9.22) | 6.45 (4.61–9.77) | 6.45 (5.20–8.96) | 5.38 (5.12–9.06) |
| Nonstimulated | 2.62 (0.92–4.88) | 1.75 (1.33–6.09) | 2.11 (1.22–2.53) | 2.81 (1.16–3.76) | 2.54 (1.14–5.04) | 2.67 (1.54–3.39) | 2.67 (1.51–4.20) | |
IMPDH capacity and levels of purines measured in PBMC from renal transplant recipients not receiving mycophenolate mofetil. Stimulated; mitogens stimulated PBMC. t0; immediately before next dose, t1.5; 1.5 hours after dose. *n = 4 for nonstimulated IMPDH.
Predose IMPDH capacity varied between study days both in stimulated (p = 0.041) and nonstimulated PBMC (P = .032). At 1 year, the predose IMPDH capacity was higher compared to 6–9 days after transplantation, both in stimulated and nonstimulated PBMC (P < .05). Figure 2 shows the IMPDH capacity in stimulated and nonstimulated PBMC at the different sampling times after transplantation as a percentage of pretransplantation values. In patients receiving MMF after transplantation, the IMPDH capacity in nonstimulated PBMC measured before dose at 1 year after transplantation was comparable to pretransplant values measured in patient not receiving MMF at the pretransplant time point (Figure 2, Panel B).
Figure 2.
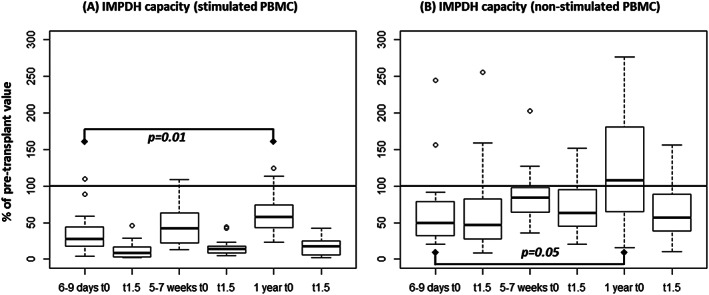
(A) Mitogen‐stimulated peripheral blood mononuclear cells (PBMC); (B) nonstimulated PBMC. t0; before morning dose of mycophenolate mofetil, t1.5; 1.5 hours after administration of mycophenolate mofetil (n = 13). IMPDH, inosine monophosphate dehydrogenase
After dose, the IMPDH capacity in nonstimulated PBMC was median 75, 73 and 57% of the predose levels at 6–9 days, 5–7 weeks and 1 year, respectively (P < .033). In the stimulated PBMC, the IMPDH capacity after dose was median 26, 34 and 21% of the predose levels at 6–9 days, 5–7 weeks and 1 year, respectively (P < .001).
Correlation between MPA plasma concentration and IMPDH capacity in either stimulated or nonstimulated PBMC was only observed at 1 year in stimulated cells. There was no correlation at any time point in nonstimulated cells (Figure S1).
At each sampling time point, there was a positive correlation between the IMPDH capacity in stimulated and nonstimulated PBMC (P < .036). The IMPDH capacity in nonstimulated PBMC explained between 22 and 51% of the capacity in stimulated PBMC.
The IMPDH capacity in patients with no HLA‐mismatch and not using MMF (n = 5) is shown in Table 2. After tacrolimus and prednisolone administration, the IMDPH capacity was on average (10–90 percentile) 102% (76–134%) of the predose capacity in stimulated PBMC (n = 14 comparisons) and 99% (50–141%) in nonstimulated PBMC (n = 13 comparisons).
4.4. Guanine and adenine
The results on guanine and adenine pools in PBMC are summarized in Table 1 and Table 2. Before transplantation and initiation of MMF, the mitogenic stimulation increased the guanine level mean 4.6‐fold (range 2.3–7.9, n = 19, P < .001) and the adenine level increased mean 3.3‐fold (range 1.4–6.6, n = 19, P < .001), compared to no stimulation. Following transplantation, the overall predose levels of guanine and adenine increased in a similar manner upon stimulation; mean 2.3‐fold (range 0.4–16‐fold) for guanine and 2.1‐fold (range 0.4–12 fold) for adenine (n = 69). After dose, the only significant increase after stimulation was seen in adenine at 1 year (mean 1.5‐fold increase, P= .008).
Compared to pretransplant levels, guanine and adenine was lower in stimulated PBMC at all sampling times after transplantation (P < .007). In nonstimulated PBMC, no reduction from baseline was observed following transplantation (guanine; P = .96, adenine; P = .36). In nonstimulated PBMC, no change in guanine or adenine was observed after dose (P > .055) relative to predose. In stimulated PBMC, the levels of both purines were reduced from predose to postdose at all sampling days (P < .001). Correlation between adenine and guanine is shown in Figure 3. In both stimulated and nonstimulated PBMC, guanine and adenine were positively correlated (R2 0.49–0.82, P < .001) at all time‐points.
Figure 3.
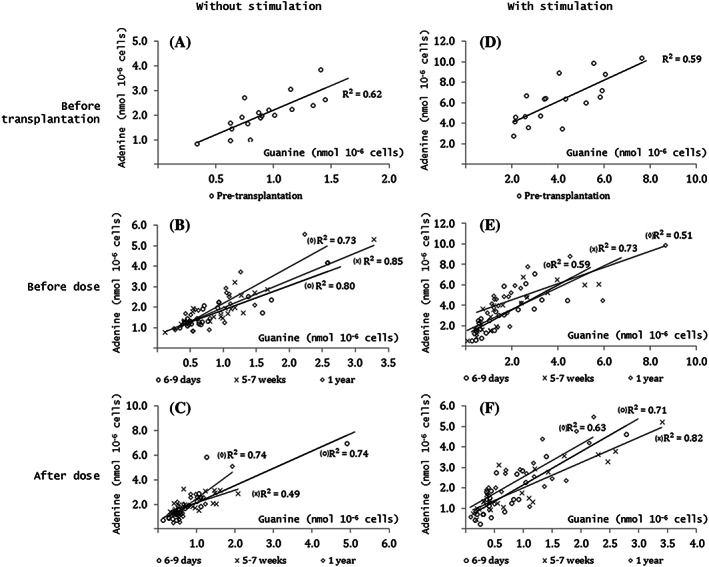
Guanine and adenine measured in peripheral blood mononuclear cells (PBMC) with mitogen‐stimulation (D, E, F) and without stimulation (A, B, C) from renal transplant patient measured 0–4 days before transplantation (A, D) and before (B, E) and after dose (C, F) at 6–9 days (o), 5–7 weeks (x) and at 1 year (◊) after transplantation. All correlation; P < .001 (Pearson)
4.5. Biomarkers and clinical outcome
IMPDH capacity measured prior to transplantation did not differ between patients with and without BPAR, either in stimulated (p = 0.71) or nonstimulated (P = .49) PBMC (Table S1). Different aspects of IMPDH‐capacity measured 5–7 weeks after transplantation (absolute capacity, capacity as a percentage of pretransplant and capacity postdose as a percentage of predose) was compared between patients who had been BPAR free until that time point, but who later did or did not have a BPAR episode. No differences were observed between the groups (Table S1). Similarly, neither adenine nor guanine was associated with BPAR (Table S2).
Pretransplantation measurements were available for 5 of the 6 patients who later needed reduced MFF dose. At 6–9 days, these were available for all 6. All dose reduction occurred after these sampling times. Although patients who later needed dose reduction trended towards lower IMPDH‐capacity in stimulated cells both 0–4 days before (median 69 vs 131 pmol 10−6 cells min−1, P = .13) and 6–9 days after transplantation (19.0 vs 38.1 pmol 10−6 cells min−1, P = .13) as well as higher MPA C0 levels 6–9 days after transplantation (2.46 vs 1.37 mg L−1, P = .224), these were not significantly different. In the nonstimulated PBMC, a similar difference in the IMPDH capacity was observed before transplantation (3.56 vs 5.88 pmol 10−6 cells min−1, P = .19), but reached statistical significance in predose samples 6–9 days after transplantation (1.87 vs 3.00 pmol 10−6 cells min−1, P = .049). Receiver operating characteristic curve analysis for these time points is shown in Figure 4. At these time points, plasma MPA concentrations, guanine or adenine levels did not have a predictive value for prospective dose reduction (Figure S1).
Figure 4.
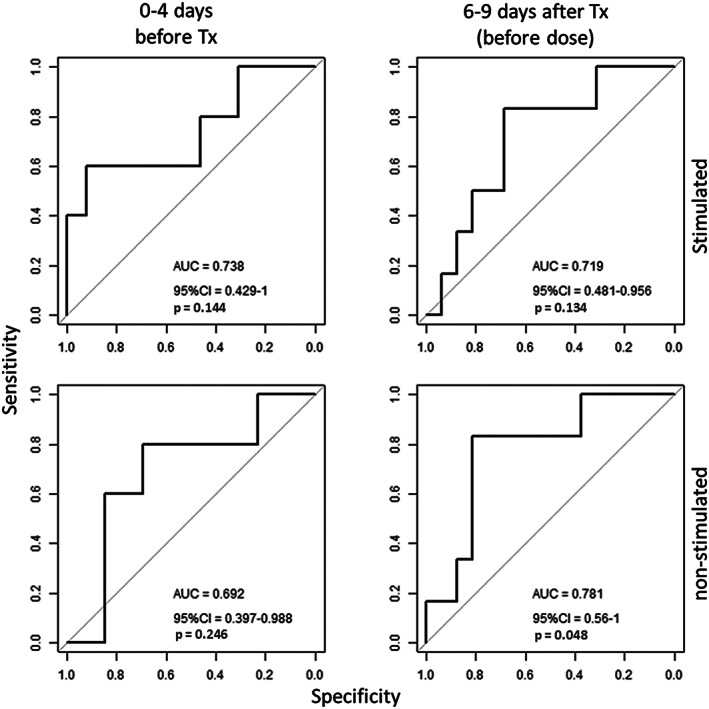
Receiver operating characteristic curve analysis of inosine monophosphate dehydrogenase capacity measured in mitogen‐stimulated and nonstimulated peripheral blood mononuclear cells (PBMC) collected 0–4 days prior to and 6–9 days (before dosage) after transplantation to predict the need for dose reduction within the first year after transplantation (Tx)
To explore if there were overall differences during the first year after transplantation in IMPDH‐capacity, guanine, adenine or MPA levels between patients with and without the need of MMF dose reduction a singular value was calculated for each variable. A predose and postdose average was calculated by averaging these values across 6–9 days, 5–7 weeks and 1 year for each patient. Patients needing dose reduction had a higher average MPA serum concentration and also lower average IMPDH‐capacity and adenine in both stimulated and nonstimulated PBMC, whilst guanine was lower in the stimulated PBMC only (Table 3).
Table 3.
Across 1 year, mycophenolic acid (MPA) plasma concentration and biomarkers in peripheral blood mononuclear cells (PBMC) in patients requiring reduction of the mycophenolate mofetil dose (median, quartiles)
| Patients with dose reduction (n = 6) | Patients without dose reduction (n = 18) | P* | ||
|---|---|---|---|---|
| Trough plasma MPA (mg L−1) | 3.22 (2.11–4.38) | 1.79 (1.50–2.79) | .036 | |
| Stimulated PBMC | IMPDH capacity (pmol 10−6 cells min−1) | 26.0 (11.2–43.5) | 65.3 (4.2–96.0) | .042 |
| Guanine (nmol 10−6 cells) | 0.91 (0.62–1.37) | 2.18 (1.12–2.65) | .042 | |
| Adenine (nmol 10−6 cells) | 2.27 (1.34–3.27) | 4.50 (2.40–5.15) | .042 | |
| Nonstimulated PBMC | IMPDH capacity (pmol 10−6 cells min−1) | 2.96 (2.34–3.12) | 4.50 (3.30–6.16) | .025 |
| Guanine (nmol 10−6 cells) | 0.78 (0.54–0.85) | 0.85 (0.70–1.20) | .48 | |
| Adenine (nmol 10−6 cells) | 1.45 (1.33–1.49) | 2.08 (1.52–2.34) | .042 | |
Median (quartiles) of average predose measurements at 6–9 days, 5–7 weeks and 1 year. Omitting samples taken at time points when the mycophenolate mofetil dose was reduced. *Differences in cross‐year averages between patients with and without dose reduction tested using Mann–Whitney U test
IMPDH, inosine monophosphate dehydrogenase
5. DISCUSSION
In this exploratory study, we have characterized potential biomarkers for MPA, the active metabolite of the immunosuppressive drug MMF. We have measured its direct pharmacodynamic effect on the target enzyme and the downstream alterations of purine levels. A novel aspect of our clinical study is that biomarkers were measured in both mitogen‐stimulated and nonstimulated PBMC. For all biomarkers, MPA showed a stronger inhibitory effect in stimulated PBMC compared to nonstimulated PBMC: The IMPDH capacity was only reduced 20–44% in nonstimulated PBMC when patients were given MMF, whilst the reduction in stimulated PBMC was 66–79%. An explanation for this difference in inhibition could be that MPA inhibits IMPDH2 more potently than IMPDH135 and the former is more abundantly expressed in activated PBMC.36, 37 Also, MPA may indirectly inhibit the expression of IMPDH during activation. One limitation of the study was the timing of cell counting. For both stimulated and nonstimulated PBMC this was done prior to incubation. Some cell proliferation may have occurred during the incubation period, with less proliferation taking place in the postdose sample where MPA is present at higher concentration. The IMPDH‐capacity and purines are normalized to the preincubation cell number, therefore the apparent stronger inhibitory effect MPA had on stimulated PBMC at 1.5 hours postdose could be related to subdued proliferation.
During PBMC isolation, resuspension and 72 hour incubation, MPA could redistribute to the extracellular space and underestimate the inhibitory effect of MPA. However, the reduction in IMPDH‐capacity from pre‐ to postdose of 25–43% in nonstimulated cells is comparable to other studies without prolonged incubation.38, 39, 40 This indicates that incubation of cells with 20% of a patient's own plasma may adequately counter the efflux of MPA, although it is important to note that measuring IMPDH‐capacity ex vivo will not represent the IMPDH‐activity in vivo directly, but has to be considered as a model.
Although MPA lowered IMPDH capacity in nonstimulated PBMC, no alteration of adenine or guanine in these cells could be shown, indicating that purine levels are maintained in the resting lymphocyte by the salvage pathway and less by the de novo synthesis.41 We have previously shown unaltered levels of guanine nucleotides in resting lymphocytes following a single MMF dose in healthy individuals42 and this has also been observed in heart transplant recipients.41
Although IMPDH is not directly involved in the synthesis of adenosine phosphates (see Figure 1), there was a markedly decrease in adenine levels in stimulated PBMC after initiation of MMF‐treatment and a further decrease from predose to postdose. Allison et al. described a feed‐back mechanism were GMP, GDP and GTP stimulated further synthesis of IMP from ribose‐5‐phosphate via 5‐phosphoribosyl pyrophosphate synthetase. When this induction of 5‐phosphoribosyl pyrophosphate synthetase is removed due to depletion of guanosine phosphates, the level of IMP may also be reduced causing depletion of adenosine phosphates.43 In addition, the conversion of IMP to AMP has previously been shown to be GMP dependent44 and depletion of GMP by IMDPH inhibition is therefore expected to result in simultaneous decrease in adenosine phosphate levels. Qui et al. observed a similar parallel decrease in a primary human T‐cell model in vitro 45 and we have previously shown this to be the case in MOLT‐4 leukaemia cells.46 The current study supports that this also occurs in PBMC in vivo (see Figure 3). However, inhibition of proliferation due to higher MPA content in the postdose sample could also explain the differences in purine levels in stimulated cells.
Our study indicated that patients needing reduction of the MMF dose within the first year have lower IMPDH capacity and levels of purines (Figure 4 and Table 3), in concordance with previous studies.47 These biomarkers appeared with potentially relevant predictive values, measured before and 1 week after transplantation. They should be further investigated as possible biomarkers to identify those patients who could benefit of less MMF to avoid adverse effects (e.g. diarrhoea or leucopenia). Although patients who needed a reduction of MMF dose had generally higher plasma MPA concentrations throughout the year (Table 3), plasma concentrations were not predictive of the need for dose reduction when measured early (6–9 days) after transplantation (Figure S2). However, IMPDH capacity measured at 6–9 days was related to dose reduction.
There was no clear association between MPA concentration in plasma and IMPDH‐capacity (Figure S1). A possible explanation could be that MPA concentrations in plasma poorly reflects the concentration inside PBMC, and that MPA concentration measured in PBMC might correlate with IMPDH‐capacity. Md Dom et al. measured IMPDH‐capacity, as well as MPA concentration in plasma and PBMC.48 There was no clear correlation between MPA concentrations in PBMC, MPA concentration in plasma, or IMPDH‐capacity, indicating that the varying IMPDH‐capacity during MMF/MPA therapy is due to varying enzyme capacity or sensitivity, rather than distribution kinetics.
Unfortunately, we could not demonstrate any association between BPAR and IMPDH or purine levels. MPA is given as part of a multiple drug regimen after renal transplantation and under‐immunosuppression with MPA could be compensated for with sufficient exposure to tacrolimus or prednisolone. Our study was designed as an exploratory study and was not powered to document differences in rejection rates. Given the limited number of patients and wide variation in IMPDH capacity and purines there is a risk for Type II statistical error. Other studies have shown an association between low IMPDH inhibition and increased risk of BPAR,47, 49 demonstrating the value of IMPDH measurements.
Currently, there is no consensus on how to measure the effect of MPA on IMPDH. Most assays measure the rate of XMP production where both substrate (IMP) and co‐substrate (NAD+) are in saturated concentrations, but there are several different ways of normalizing the results to amount of sample material. We have shown that adenine and guanine pools are highly regulated (Figure 3) indicating that adenine levels are also influenced by IMPDH activity. Several studies normalize the XMP production to AMP50, 51, 52, 53, 54, 55, 56 since this is technically attractive. However, careful interpretation is suggested in light of co‐regulation between the guanine and adenine pools in lymphocytes. Normalization to protein amount38, 39, 40, 47, 49, 57, 58, 59 or number of cells60 are other options.
In the present study, there was an increase in IMPDH capacity in both ex vivo‐stimulated and nonstimulated PBMC from 1 week to 1 year after transplantation (Figure 2 and Table 1). Chiarelli et al.39 reported a similar increase in nonstimulated PBMC when stable renal transplant recipients were monitored for 15 months. Tang et al. observed no increase in predose IMPDH‐capacity from day 6 to week 20 after transplantation,50 suggesting that this increase mainly occurs after week 20.
Since there are 2 IMPDH enzymes (IMPDH1 and 2), and MPA inhibits IMPDH2 more potently than IMPDH1, an explanation for decreased MPA sensitivity over time could be due to a larger proportion of total IMPDH being IMPDH1 than IMPDH2. However, we61 and others62 have shown that expression of IMPDH1 decreases in the post‐transplantation time frame, possibly related to decreasing doses of methylprednisolone or prednisolone. The predose IMPDH capacity in nonstimulated PBMC at 1 year was comparable to the pretransplant level (Figure 2 B), suggesting that patients could have insufficient immunosuppressive treatment. However, the IMPDH capacity in stimulated PBMC was still markedly decreased at 1 year. Since immunological rejection is mediated through the activated lymphocyte, this may explain why MMF still has a therapeutic value after prolonged treatment.
In conclusion, by measuring the molecular pharmacodynamic response to MPA in renal transplant patients over a prolonged period, we have shown that MPA inhibits activated lymphocytes to a larger degree than resting lymphocytes. In resting lymphocytes, the nucleotide pool appeared to be unaffected by MMF treatment, as it is probably being maintained through the salvage pathway. Low IMPDH capacity before and early after initiation of MMF treatment appeared to predict the need for dose reduction, suggesting that this biomarker should be further investigated in relation to patients at risk for MPA overexposure.
COMPETING INTERESTS
The authors have no conflicts of interest to declare.
CONTRIBUTORS
St.B., Sa.B. and N.T.V. participated in research design. R.A.K. performed data analysis and wrote the manuscript. N.T.V., K.H., A.M.A. and C.B.N. participated in performance of research. K.M. and M.H.S. recruited patients. All authors were involved in the discussion of results, critical revision of the manuscript and approval of the final version.
Supporting information
TABLE S1 Inosine monophosphate dehydrogenase capacity in peripheral blood mononuclear cells from patients with and without rejection occurring after sampling
TABLE S2 Guanine and adenine in peripheral blood mononuclear cells from patients with and without rejection occurring after sampling
FIGURE S1 Correlation between inosine monophosphate dehydrogenase and mycophenolic acid in renal transplant patients
FIGURE S2 Receiver operating characteristic curves for pre‐/peri‐guanine, adenine and plasma mycophenolic acid and the risk for mycophenolate mofetil dose reduction
ACKNOWLEDGEMENTS
The authors are grateful to Margrete Kasbo and Elisabet Dahl Johansson for substantial contribution in collecting and handling samples.
Klaasen RA, Bergan S, Bremer S, et al. Pharmacodynamic assessment of mycophenolic acid in resting and activated target cell population during the first year after renal transplantation. Br J Clin Pharmacol. 2020;86 1100–1112. 10.1111/bcp.14218
The authors confirm that the Principal Investigator for this paper is Karsten Midtvedt MD PhD and that he had direct clinical responsibility for patients.
REFERENCES
- 1. Murray AW. The biological significance of purine salvage. Annu Rev Biochem. 1971;40:811‐826. [DOI] [PubMed] [Google Scholar]
- 2. Allison AC, Hovi T, Watts RW, Webster AD. The role of de novo purine synthesis in lymphocyte transformation. Ciba Found Symp. 1977;48:207‐224. [DOI] [PubMed] [Google Scholar]
- 3. Silva HT Jr, Yang HC, Meier‐Kriesche HU, et al. Long‐term follow‐up of a phase III clinical trial comparing tacrolimus extended‐release/MMF, tacrolimus/MMF, and cyclosporine/MMF in de novo kidney transplant recipients. Transplantation. 2014;97(6):636‐641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vincenti F, Rostaing L, Grinyo J, et al. Belatacept and long‐term outcomes in kidney transplantation. N Engl J Med. 2016;374(4):333‐343. [DOI] [PubMed] [Google Scholar]
- 5. Appel GB, Contreras G, Dooley MA, et al. Mycophenolate mofetil versus cyclophosphamide for induction treatment of lupus nephritis. J am Soc Nephrol. 2009;20(5):1103‐1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Howard J, Hoffbrand AV, Prentice HG, Mehta A. Mycophenolate mofetil for the treatment of refractory auto‐immune haemolytic anaemia and auto‐immune thrombocytopenia purpura. Br J Haematol. 2002;117(3):712‐715. [DOI] [PubMed] [Google Scholar]
- 7. Kotb R, Pinganaud C, Trichet C, et al. Efficacy of mycophenolate mofetil in adult refractory auto‐immune cytopenias: a single center preliminary study. Eur J Haematol. 2005;75(1):60‐64. [DOI] [PubMed] [Google Scholar]
- 8. European Mycophenolate Mofetil Cooperative Study Group . Placebo‐controlled study of mycophenolate mofetil combined with cyclosporin and corticosteroids for prevention of acute rejection. Lancet. 1995;345:1321‐1325. [PubMed] [Google Scholar]
- 9. Langone A, Doria C, Greenstein S, et al. Does reduction in mycophenolic acid dose compromise efficacy regardless of tacrolimus exposure level? An analysis of prospective data from the mycophenolic renal transplant (MORE) registry. Clin Transplant. 2013;27(1):15‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vanhove T, Kuypers D, Claes KJ, et al. Reasons for dose reduction of mycophenolate mofetil during the first year after renal transplantation and its impact on graft outcome. Transpl Int. 2013;26(8):813‐821. [DOI] [PubMed] [Google Scholar]
- 11. Le Meur Y, Buchler M, Thierry A, et al. Individualized mycophenolate mofetil dosing based on drug exposure significantly improves patient outcomes after renal transplantation. Am J Transplant. 2007;7(11):2496‐2503. [DOI] [PubMed] [Google Scholar]
- 12. van Gelder T, Silva HT, de Fijter JW, et al. Comparing mycophenolate mofetil regimens for de novo renal transplant recipients: the fixed‐dose concentration‐controlled trial. Transplantation. 2008;86(8):1043‐1051. [DOI] [PubMed] [Google Scholar]
- 13. van Gelder T, Hilbrands LB, Vanrenterghem Y, et al. A randomized double‐blind, multicenter plasma concentration controlled study of the safety and efficacy of oral mycophenolate mofetil for the prevention of acute rejection after kidney transplantation. Transplantation. 1999;68(2):261‐266. [DOI] [PubMed] [Google Scholar]
- 14. Hale MD, Nicholls AJ, Bullingham RE, et al. The pharmacokinetic‐pharmacodynamic relationship for mycophenolate mofetil in renal transplantation. Clin Pharmacol Ther. 1998;64(6):672‐683. [DOI] [PubMed] [Google Scholar]
- 15. Kiberd BA, Lawen J, Fraser AD, Keough‐Ryan T, Belitsky P. Early adequate mycophenolic acid exposure is associated with less rejection in kidney transplantation. Am J Transplant. 2004;4(7):1079‐1083. [DOI] [PubMed] [Google Scholar]
- 16. Gaston RS, Kaplan B, Shah T, et al. Fixed‐ or controlled‐dose mycophenolate mofetil with standard‐ or reduced‐dose calcineurin inhibitors: the Opticept trial. Am J Transplant. 2009;9(7):1607‐1619. [DOI] [PubMed] [Google Scholar]
- 17. Rhu J, Lee KW, Park H, Park JB, Kim SJ, Choi GS. Clinical implication of mycophenolic acid trough concentration monitoring in kidney transplant patients on a tacrolimus triple maintenance regimen: a single‐center experience. Ann Transplant. 2017;22:707‐718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Borrows R, Chusney G, Loucaidou M, et al. Mycophenolic acid 12‐h trough level monitoring in renal transplantation: association with acute rejection and toxicity. Am J Transplant. 2006;6(1):121‐128. [DOI] [PubMed] [Google Scholar]
- 19. Mourad M, Malaise J, Chaib Eddour D, et al. Correlation of mycophenolic acid pharmacokinetic parameters with side effects in kidney transplant patients treated with mycophenolate mofetil. Clin Chem. 2001;47(1):88‐94. [PubMed] [Google Scholar]
- 20. Kuypers DR, de Jonge H, Naesens M, et al. Current target ranges of mycophenolic acid exposure and drug‐related adverse events: a 5‐year, open‐label, prospective, clinical follow‐up study in renal allograft recipients. Clin Ther. 2008;30(4):673‐683. [DOI] [PubMed] [Google Scholar]
- 21. Le Meur Y, Borrows R, Pescovitz MD, et al. Therapeutic drug monitoring of mycophenolates in kidney transplantation: report of the transplantation society consensus meeting. Transplant Rev (Orlando). 2011;25(2):58‐64. [DOI] [PubMed] [Google Scholar]
- 22. Jeong H, Kaplan B. Therapeutic monitoring of mycophenolate mofetil. Clin J am Soc Nephrol. 2007;2(1):184‐191. [DOI] [PubMed] [Google Scholar]
- 23. Storck M, Abendroth D, Albrecht W, Sollinger HW. IMPDH activity in whole blood and isolated blood cell fraction for monitoring of CellCept‐mediated immunosuppression. Transplant Proc. 1999;31(1‐2):1115‐1116. [DOI] [PubMed] [Google Scholar]
- 24. Vethe NT, Ali AM, Reine PA, et al. Simultaneous quantification of IMPDH activity and purine bases in lymphocytes using LC‐MS/MS: assessment of biomarker responses to mycophenolic acid. Ther Drug Monit. 2013;36:108‐118. [DOI] [PubMed] [Google Scholar]
- 25. Sombogaard F, Peeters AM, Baan CC, et al. Inosine monophosphate dehydrogenase messenger RNA expression is correlated to clinical outcomes in mycophenolate mofetil‐treated kidney transplant patients, whereas inosine monophosphate dehydrogenase activity is not. Ther Drug Monit. 2009;31(5):549‐556. [DOI] [PubMed] [Google Scholar]
- 26. Laverdiere I, Caron P, Couture F, Guillemette C, Levesque E. Liquid chromatography‐coupled tandem mass spectrometry based assay to evaluate inosine‐5′‐monophosphate dehydrogenase activity in peripheral blood mononuclear cells from stem cell transplant recipients. Anal Chem. 2012;84(1):216‐223. [DOI] [PubMed] [Google Scholar]
- 27. Glander P, Sombogaard F, Budde K, et al. Improved assay for the nonradioactive determination of inosine 5′‐monophosphate dehydrogenase activity in peripheral blood mononuclear cells. Ther Drug Monit. 2009;31(3):351‐359. [DOI] [PubMed] [Google Scholar]
- 28. Daxecker H, Raab M, Cichna M, Markl P, Muller MM. Determination of the effects of mycophenolic acid on the nucleotide pool of human peripheral blood mononuclear cells in vitro by high‐performance liquid chromatography. Clin Chim Acta. 2001;310:81‐87. [DOI] [PubMed] [Google Scholar]
- 29. Perez‐Flores I, Sanchez‐Fructuoso A, Santiago JL, et al. Intracellular ATP levels in CD4+ lymphocytes are a risk marker of rejection and infection in renal graft recipients. Transplant Proc. 2009;41(6):2106‐2108. [DOI] [PubMed] [Google Scholar]
- 30. Alexander SP, Kelly E, Marrion NV, et al. THE CONCISE GUIDE TO PHARMACOLOGY 2017/18: overview. Br J Pharmacol. 2017;174(Suppl 1):S1‐s16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Klaasen RA, Bergan S, Bremer S, et al. Longitudinal study of tacrolimus in lymphocytes during the first year after kidney transplantation. Ther Drug Monit. 2018;40(5):558‐566. [DOI] [PubMed] [Google Scholar]
- 32. Bremer S, Vethe NT, Skauby M, et al. NFAT‐regulated cytokine gene expression during tacrolimus therapy early after renal transplantation. Br J Clin Pharmacol. 2017;83(11):2494‐2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Solez K, Colvin RB, Racusen LC, et al. Banff 07 classification of renal allograft pathology: updates and future directions. Am J Transplant. 2008;8(4):753‐760. [DOI] [PubMed] [Google Scholar]
- 34. Reine PA, Vethe NT, Kongsgaard UE, et al. Mycophenolate pharmacokinetics and inosine monophosphate dehydrogenase activity in liver transplant recipients with an emphasis on therapeutic drug monitoring. Scand J Clin Lab Invest. 2013;73(2):117‐124. [DOI] [PubMed] [Google Scholar]
- 35. Carr SF, Papp E, Wu JC, Natsumeda Y. Characterization of human type I and type II IMP dehydrogenases. J Biol Chem. 1993;268(36):27286‐27290. [PubMed] [Google Scholar]
- 36. Jain J, Almquist SJ, Ford PJ, et al. Regulation of inosine monophosphate dehydrogenase type I and type II isoforms in human lymphocytes. Biochem Pharmacol. 2004;67(4):767‐776. [DOI] [PubMed] [Google Scholar]
- 37. Dayton JS, Lindsten T, Thompson CB, Mitchell BS. Effects of human T lymphocyte activation on inosine monophosphate dehydrogenase expression. J Immunol. 1994;152:984‐991. [PubMed] [Google Scholar]
- 38. Fukuda T, Goebel J, Thogersen H, et al. Inosine monophosphate dehydrogenase (IMPDH) activity as a pharmacodynamic biomarker of mycophenolic acid effects in pediatric kidney transplant recipients. J Clin Pharmacol. 2011;51(3):309‐320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chiarelli LR, Molinaro M, Libetta C, et al. Inosine monophosphate dehydrogenase variability in renal transplant patients on long‐term mycophenolate mofetil therapy. Br J Clin Pharmacol. 2010;69(1):38‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Molinaro M, Chiarelli LR, Biancone L, et al. Monitoring of inosine monophosphate dehydrogenase activity and expression during the early period of mycophenolate mofetil therapy in de novo renal transplant patients. Drug Metab Pharmacokinet. 2013;28(2):109‐117. [DOI] [PubMed] [Google Scholar]
- 41. Devyatko E, Zuckermann A, Bohdjalian A, et al. Activation of the purine salvage pathway in mononuclear cells of cardiac recipients treated with mycophenolate mofetil. Transplantation. 2006;82(1):113‐118. [DOI] [PubMed] [Google Scholar]
- 42. Vethe NT, Bremer S, Rootwelt H, Bergan S. Pharmacodynamics of mycophenolic acid in CD4+ cells: a single‐dose study of IMPDH and purine nucleotide responses in healthy individuals. Ther Drug Monit. 2008;30(6):647‐655. [DOI] [PubMed] [Google Scholar]
- 43. Allison AC, Kowalski WJ, Muller CD, Eugui EM. Mechanisms of action of mycophenolic acid. Ann N Y Acad Sci. 1993;696:63‐87. [DOI] [PubMed] [Google Scholar]
- 44. Lowe JK, Brox L, Henderson JF. Consequences of inhibition of guanine nucleotide synthesis by mycophenolic acid and virazole. Cancer Res. 1977;37(3):736‐743. [PubMed] [Google Scholar]
- 45. Qiu Y, Fairbanks LD, Ruckermann K, et al. Mycophenolic acid‐induced GTP depletion also affects ATP and pyrimidine synthesis in mitogen‐stimulated primary human T‐lymphocytes. Transplantation. 2000;69(5):890‐897. [DOI] [PubMed] [Google Scholar]
- 46. Vethe NT, Bremer S, Bergan S. IMP dehydrogenase basal activity in MOLT‐4 human leukaemia cells is altered by mycophenolic acid and 6‐thioguanosine. Scand J Clin Lab Invest. 2008;68(4):277‐285. [DOI] [PubMed] [Google Scholar]
- 47. Glander P, Hambach P, Braun KP, et al. Pre‐transplant inosine monophosphate dehydrogenase activity is associated with clinical outcome after renal transplantation. Am J Transplant. 2004;4(12):2045‐2051. [DOI] [PubMed] [Google Scholar]
- 48. Md Dom ZI, Coller JK, Carroll RP, et al. Mycophenolic acid concentrations in peripheral blood mononuclear cells are associated with the incidence of rejection in renal transplant recipients. Br J Clin Pharmacol. 2018;84(10):2433‐2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Raggi MC, Siebert SB, Steimer W, Schuster T, Stangl MJ, Abendroth DK. Customized mycophenolate dosing based on measuring inosine‐monophosphate dehydrogenase activity significantly improves patients' outcomes after renal transplantation. Transplantation. 2010;90(12):1536‐1541. [DOI] [PubMed] [Google Scholar]
- 50. Tang JT, de Winter BC, Hesselink DA, Sombogaard F, Wang LL, van Gelder T. The pharmacokinetics and pharmacodynamics of mycophenolate mofetil in younger and elderly renal transplant recipients. Br J Clin Pharmacol. 2017;83(4):812‐822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Liu F, Xu L, Sheng C, Qiu X, Zhang M, Jiao Z. Optimization and application of an HPLC method for quantification of inosine‐5′‐monophosphate dehydrogenase activity as a pharmacodynamic biomarker of mycophenolic acid in Chinese renal transplant patients. Clin Chim Acta. 2018;485:333‐339. [DOI] [PubMed] [Google Scholar]
- 52. Rath T, Kupper M. Comparison of inosine‐monophosphate‐dehydrogenase activity in patients with enteric‐coated mycophenolate sodium or mycophenolate mofetil after renal transplantation. Transplant Proc. 2009;41(6):2524‐2528. [DOI] [PubMed] [Google Scholar]
- 53. Sombogaard F, van Schaik RH, Mathot RA, et al. Interpatient variability in IMPDH activity in MMF‐treated renal transplant patients is correlated with IMPDH type II 3757T > C polymorphism. Pharmacogenet Genomics. 2009;19(8):626‐634. [DOI] [PubMed] [Google Scholar]
- 54. Dostalek M, Gohh RY, Akhlaghi F. Inosine monophosphate dehydrogenase expression and activity are significantly lower in kidney transplant recipients with diabetes mellitus. Ther Drug Monit. 2013;35(3):374‐383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rother A, Glander P, Vitt E, et al. Inosine monophosphate dehydrogenase activity in paediatrics: age‐related regulation and response to mycophenolic acid. Eur J Clin Pharmacol. 2012;68(6):913‐922. [DOI] [PubMed] [Google Scholar]
- 56. Yoshimura K, Yano I, Yamamoto T, et al. Population pharmacokinetics and pharmacodynamics of mycophenolic acid using the prospective data in patients undergoing hematopoietic stem cell transplantation. Bone Marrow Transplant. 2018;53(1):44‐51. [DOI] [PubMed] [Google Scholar]
- 57. Thi MT, Mourad M, Capron A, Tshinanu FM, Vincent MF, Wallemacq P. Plasma and intracellular pharmacokinetic‐pharmacodynamic analysis of mycophenolic acid in de novo kidney transplant patients. Clin Biochem. 2015;48(6):401‐405. [DOI] [PubMed] [Google Scholar]
- 58. Smits TA, Cox S, Fukuda T, et al. Effects of unbound mycophenolic acid on inosine monophosphate dehydrogenase inhibition in pediatric kidney transplant patients. Ther Drug Monit. 2014;36(6):716‐723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Dong M, Fukuda T, Cox S, et al. Population pharmacokinetic‐pharmacodynamic modelling of mycophenolic acid in paediatric renal transplant recipients in the early post‐transplant period. Br J Clin Pharmacol. 2014;78(5):1102‐1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Vethe NT, Mandla R, Line PD, Midtvedt K, Hartmann A, Bergan S. Inosine monophosphate dehydrogenase activity in renal allograft recipients during mycophenolate treatment. Scand J Clin Lab Invest. 2006;66(1):31‐44. [DOI] [PubMed] [Google Scholar]
- 61. Bremer S, Mandla R, Vethe NT, et al. Expression of IMPDH1 and IMPDH2 after transplantation and initiation of immunosuppression. Transplantation. 2008;85(1):55‐61. [DOI] [PubMed] [Google Scholar]
- 62. Sanquer S, Maison P, Tomkiewicz C, et al. Expression of inosine monophosphate dehydrogenase type I and type II after mycophenolate mofetil treatment: a 2‐year follow‐up in kidney transplantation. Clin Pharmacol Ther. 2008;83(2):328‐335. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TABLE S1 Inosine monophosphate dehydrogenase capacity in peripheral blood mononuclear cells from patients with and without rejection occurring after sampling
TABLE S2 Guanine and adenine in peripheral blood mononuclear cells from patients with and without rejection occurring after sampling
FIGURE S1 Correlation between inosine monophosphate dehydrogenase and mycophenolic acid in renal transplant patients
FIGURE S2 Receiver operating characteristic curves for pre‐/peri‐guanine, adenine and plasma mycophenolic acid and the risk for mycophenolate mofetil dose reduction


