Abstract
Aims
Therapeutic drug monitoring (TDM) of trough serum infliximab concentrations has been mainly used in case of loss of response in patients with inflammatory bowel disease (IBD). The aim of this study was to evaluate the effectiveness and safety of a multidisciplinary early proactive TDM (mep‐TDM) programme for dose adjustment.
Methods
A 3‐year prospective study was conducted based on a sample of 81 patients who started treatment and were subsequently subjected to mep‐TDM with the first control at week 14. Data of a historical control group of 72 patients treated with infliximab and managed with empirical dosing were included. Effectiveness variables were treatment failure, IBD‐related surgery and IBD‐related hospitalization. Safety variables were serious infusion reactions (SIRs) and adverse reactions. Cox regression was used for survival analysis.
Results
In the mep‐TDM study group, compared to the control group, there was a significant reduction in the risk of treatment failure (hazard ratio [HR]: 0.51; 95% confidence interval [CI]: 0.27–0.92; P = .037), IBD‐related surgery (HR: 0.14; 95% CI: 0.03–0.65; P = .012) and hospitalization (HR: 0.38; 95% CI: 0.17–0.87; P = .022). SIRs were lower in the mep‐TDM group (2.5% vs 10.4%; P < .050); the incidence of adverse reactions was similar (3.7% vs 3.9%; p > .999).
Conclusion
This study found that compared to empirical dosing, mep‐TDM is associated with improved efficacy and safety of infliximab therapy, reduced IBD‐related hospitalization and surgery and incidence of SIRs, and increasing long‐term durability of treatment effects.
Keywords: inflammatory bowel diseases, infliximab, personalized medicine, therapeutic drug monitoring
What is already known about this subject
Therapeutic drug monitoring (TDM) of infliximab could be a useful tool for dose adjustment in the management of loss of response in inflammatory bowel disease.
There are limited data on the usefulness of proactive TDM in patients from the beginning of treatment.
What this study adds
Early proactive TDM improves the efficacy, safety and durability of treatments.
Patients with inflammatory bowel disease on an empirical dosage of infliximab have a high probability of presenting subtherapeutic concentrations, associated with loss of response.
Multidisciplinary teams, including experts with clinical pharmacokinetic experience to adequately interpret and present TDM results, contribute to achieve better outcomes.
1. INTRODUCTION
Inflammatory bowel disease (IBD) is a chronic process characterized by a disproportionate immune response that damages the tissues of the digestive tract and leads to injuries of different severity. Ulcerative colitis (UC) and Crohn's disease (CD) are the most common forms of IBD.1, 2 These pathologies share many similarities in terms of symptoms, risk factors and treatment, the main difference being the area of the digestive system where inflammation occurs: in UC only the mucosa of the large intestine or the colonic mucosa is affected, whereas in CD transmural lesions can occur in any part of the digestive tract.
The therapeutic use of anti‐tumour necrosis factor (anti‐TNF) monoclonal antibodies, such as infliximab, adalimumab, golimumab and certolizumab pegol, has dramatically changed the management of IBDs.3, 4 However, although a high percentage of patients (70–90%) initially respond to treatment, loss of response (LOR) rates after induction are high (20–50%).5, 6 There are several reasons for this lack of response, 1 being the formation of anti‐drug antibodies that bind to the epitope of the drug and form immune complexes that increase its clearance, leading to inferior clinical outcomes.5, 7, 8 Additionally, lack of response can be associated with non‐immune‐related factors such as high body mass index, hypoalbuminaemia, high disease burden, and the location and/or size of the affected surface.9, 10 Pharmacodynamic factors such as alternative pathway (re)activation of inflammation may also lead to nonresponse.4
Currently, in clinical practice, pharmacological options after failure of these drugs are limited.11 Once the lines of treatment have been exhausted, the only available alternative is surgery. Therefore, optimization of response in order to maintain treatment using standard anti‐TNFs in the early stages for as long as possible has gained relevance in recent years, becoming the subject of numerous studies.12, 13, 14
Therapeutic drug monitoring (TDM) of anti‐TNF has been traditionally used in patients with active inflammatory symptoms, this strategy being known as reactive TDM. Recent studies have assessed the usefulness of early proactive TDM15, 16, 17 following an approach that involves determination of trough serum infliximab concentrations (TSIC) in all patients from the beginning of the treatment to optimize the dose by reaching target TSIC. Since low TSIC have been associated with an increased risk of anti‐infliximab antibody (ATI) development, the potential clinical benefits of this type of monitoring include the prevention of immunogenicity and an increased probability of remaining longer on anti‐TNF therapy.10, 14
The objective of this study was to prospectively evaluate the long‐term effectiveness and safety of a multidisciplinary early proactive TDM programme (mep‐TDM) as a tool for infliximab dose adjustment in patients with IBD.
2. MATERIALS AND METHODS
2.1. Study design and population
In 2015, a mep‐TDM of infliximab programme aimed at treatment optimization in IBD patients was started at our Centre by the Gastroenterology Service in collaboration with the Pharmacokinetics Laboratory of the Pharmacy Service. Over the previous 3 years, 76 patients had been subjected to infliximab therapy with dose adjustments based only on clinical response as the standard of care. This group was used as control, and TSICs and ATIs were measured for all the patients under treatment with infliximab at the time of the beginning of the prospective study.
After the implementation of the mep‐TDM program, a 3‐year prospective, longitudinal, cohort study was conducted from September 2015 to September 2018 using data from patients aged >18 years who started infliximab treatment and had been diagnosed with moderate or severe CD and UC according to the following criteria: Partial Mayo Clinic Score > 4 for UC18 and Simplified Endoscopic Activity Score for CD >4 and/or Harvey–Bradshaw Index Score > 7 for CD.19, 20 The study was approved by the Local Ethics Committee and signed consent was obtained from all the patients who agreed to participate.
Patients with the following characteristics were excluded from the study: <14 weeks of treatment; isolated administration due to transfers to other centres; and isolated outbreaks. In all cases, the initial dose of infliximab was administered according to the recommendations provided in the drug data sheet: 5 mg kg−1 by intravenous infusion at weeks 0, 2, 6 and 14 (induction phase) and, subsequently, proactive TDM was used for dose adjustments. The use of concomitant immunosuppressants was allowed.
The following baseline variables were collected: age, sex, type of disease, extent of the disease, CD behaviour, age at diagnosis, age at start of infliximab, perianal fistulizing disease, concomitant use of immunomodulators (i.e. thiopurines or methotrexate), extraintestinal manifestations (musculoskeletal, dermatological, hepatopancreatobiliary or ocular), faecal calprotectin (FCP) and C‐reactive protein (CRP) serum levels. Disease extent and behaviour were defined according to the Montreal Classification.2
2.2. TDM strategy
During the study period, all patients who had started treatment with infliximab were proactively monitored, and the first TSIC was determined at week 14. TDM was repeated after 2 infliximab administrations in those patients who had required dose adjustment, to verify that target TSICs had been reached. Once TSICs were within therapeutic range, TDM was routinely performed every 6 months. Figure 1 shows the algorithm used for making therapeutic decisions based on TSICs and clinical response. This was adapted from the algorithm initially designed at our centre for TDM of anti‐TNF agents.12
Figure 1.
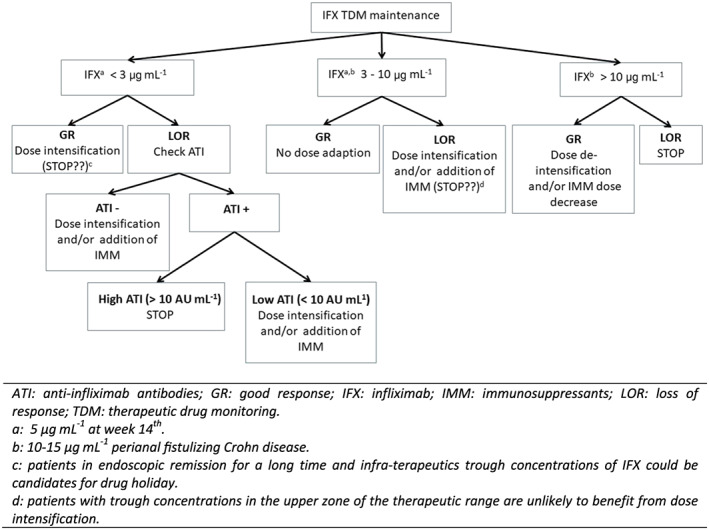
Algorithm designed to make therapeutic decisions based on trough serum concentrations of infliximab, presence of anti‐infliximab antibodies and treatment response. Response is based on clinical, endoscopic and biochemical outcomes. Adapted from reference 12
TSICs and ATIs were determined by ELISA (Promonitor) in the Pharmacokinetics Laboratory of our centre. In this technique, serum infliximab binds to TNF and is detected by an anti F (ab′)2‐infliximab, horseradish peroxidase‐labelled antibody. The disadvantages of this assay are that it is a drug‐sensitivity test that cannot detect drug‐bound ATI21 and that it lacks specificity to distinguish between biologically functional and nonfunctional ATIs.
The TSIC therapeutic range was established according to the available literature: 5–10 μg mL−1 at week 14 of treatment12 and 3–10 μg mL−1 during the maintenance phase.13, 14, 15, 22 Concentrations above 10 μg mL−1 were used in patients with CD and perianal fistulizing disease.23 ATIs were measured in patients with TSIC <1 μg mL−1.12, 24
TSICs were obtained in the hours prior to the administration of infliximab. However, in patients with inflammatory symptoms, mostly when low TSICs were suspected, additional samples were taken during the administration interval in order to make the optimal therapeutic decision.
Prior to the beginning of the study, a preliminary population pharmacokinetic model of infliximab in adult patients with IBD had been developed. For this purpose, a nonlinear mixed effects modelling using the first‐order conditional estimation method with interaction was used to develop the population pharmacokinetic model using NONMEM version 7.3.0 (Icon Development Solutions, Ellicott City, MD, USA). Patient characteristics and bi‐compartmental model specifications can be found in Appendix 1. In our preliminary pharmacokinetic model, body weight, FCP and ATIs proved to be the variables with the most significant impact on infliximab pharmacokinetics.
Optimal individualized dosage estimation was addressed using a Bayesian approach based on TSICs, demographical information and other patient characteristics to predict TSIC evolution over time. A priori information (IFX population pharmacokinetic parameters) was combined with a posteriori information (individual TSICs) to predict future concentrations.25 Individual pharmacokinetic parameters were estimated and subsequently used to predict the optimal individualized dosing (dose and interval) required to reach therapeutic TSICs. For this purpose, infliximab concentrations were simulated based on nonlinear mixed effects modelling (NONMEM version 7.3.0). Then, this information, together with the clinical and endoscopic outcomes, was used to draw up a pharmacokinetic report with the patient‐adapted dosage recommendation.
2.3. Therapeutic outcomes
Loss of response was defined as worsening or relapsing of symptoms, that is, Harvey–Bradshaw Index Score >4 in CD, partial Mayo clinic score > 2 in UC, biological activity (FCP >100 mg Kg−1 or CRP level > 0.5 mg dL−1), together with endoscopic or radiological findings of active disease. Endoscopies were performed according to clinical criteria.
The effectiveness variables were treatment failure (TF), IBD‐related surgery and IBD‐related hospitalization during the first 3 years from the beginning of treatment. The follow‐up period for IBD‐related surgery and IBD‐related hospitalization was extended 6 months beyond infliximab discontinuation provided that no other biological drug was administered in the meantime.
Reasons for TF were LOR despite therapeutic TSICs, severe infusion‐related reactions (SIRs), adverse reactions (ARs) or nonreversible ATIs.
IBD‐related surgery included total or partial bowel resection, ostomy, ileal pouch‐anal anastomosis. Fistula seton placement and abscess drainage were excluded from IBD‐related surgery. By contrast, IBD‐related hospitalization was defined as any hospitalization due to relapse, intestinal obstruction, fissure, symptomatic fistula, abscess or gastrointestinal symptoms secondary to IBD, such as abdominal pain, diarrhoea, constipation or gastrointestinal bleeding.
To evaluate the effect of mep‐TDM on drug safety, the number of patients with SIRs and ARs during the first 3 years after starting the treatment with infliximab was compared in both groups.
2.4. Statistical analysis
Descriptive statistics were provided using median or mean for the continuous variables, and frequency and percentage for the categorical variables. The continuous variables were compared using t‐test or Wilcoxon test, and the categorical variables using the χ2 test or Fisher's exact test, as appropriate.
Kaplan–Meier estimates were used to draw the cumulative and incidence curves of probability of TF, IBD‐related surgery and IBD‐related hospitalization. Curves were compared using the log‐rank test. Additionality, a subgroup analysis was made in order to evaluate the effectiveness variables on CD and UC patients.
Univariate and multivariate survival analysis using Cox proportional‐hazards regression was performed to determine the independent effects of different variables that could be associated with the therapeutic outcomes. The examined variables were sex, age at diagnosis, age at start of infliximab treatment, IBD subtype, UC extension, CD location and behaviour, perianal fistulizing disease, concomitant use of immunomodulators, and extraintestinal manifestations. Variables were eliminated from the multivariate model if Wald test results rendered them nonsignificant (P < .1). All P‐values were based on a 2‐sided hypothesis, and those <.05 were considered statistically significant.
2.5. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY,26 and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20.27
3. RESULTS
3.1. Therapeutic drug monitoring in empirically dosed patients
Prior to the beginning of the study, 52 patients were already under chronic infliximab therapy. The results obtained from these patients are shown in Figure 2. Dose adjustment according to standard care revealed that 48.1% of the patients had subtherapeutic TSICs and 13.5% had ATIs. The average TSIC (standard deviation) measured was 4.35 (4.40) μg mL−1.
Figure 2.
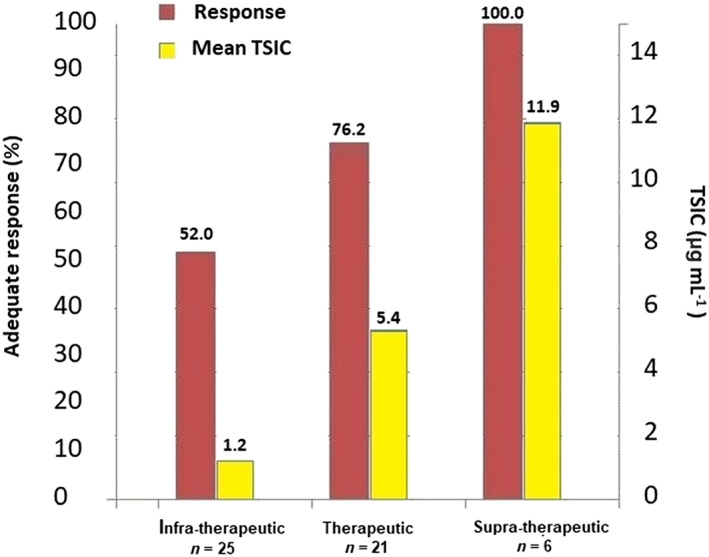
Serum infliximab concentrations and corresponding clinical response obtained in 52 patients of the control group managed with standard of care. TSIC: trough serum infliximab concentrations; supratherapeutic: TSIC <3 μg mL−1; therapeutic: TSIC 3–10 μg mL−1; supratherapeutic: TSIC >10 μg mL−1
These results were taken into account to manage the dosage regimens. Dose escalation (interval decrease and/or dose increase) was performed in 18 patients with subtherapeutic TSICs. Infliximab was discontinued in 7 patients with subtherapeutic TSICs and high levels of ATIs. Additionally, infliximab treatment was switched to adalimumab in 3 other patients with low TSICs who were already receiving exceptionally high intensified empirical doses. By contrast, a treatment de‐escalation was carried out in 6 patients with supratherapeutic TSICs. For this purpose, an extended interval was selected.
It must be pointed out that treatment was escalated in 4 patients with therapeutic levels of TSICs due to inadequate response, whereas infliximab treatment was switched to vedolizumab in 1 patient due to TSIC being in the upper limit of the therapeutic range and a lack of clinical control.
3.2. Early proactive therapeutic drug monitoring
Mep‐TDM was performed on 81 patients who started infliximab, and a total of 201 TSICs were determined over a 3‐year period. The median (interquartile range) follow‐up of patients was 82 (34–118) weeks. The clinical and demographic characteristics of the study and control group patients are presented in Table 1. As shown, patient characteristics at the time of inclusion in the study were similar in both groups and, therefore, comparable.
Table 1.
Baseline characteristics of patients included in the study
| Control group | Early proactive TDM group | P value | ||
|---|---|---|---|---|
| n | 76 | 81 | ||
| Sex, male (%) | 38 (50.0) | 48 (59.3) | .32 | |
| Age at diagnosis (y), median (IQR) | 29 (20–38) | 32 (20–42) | .22 | |
| Age at start of infliximab (y), median (IQR) | 38 (24–49) | 41 (29–50) | .17 | |
| Weight (kg), median (IQR) | 67.8 (57–80) | 69.4 (56–82) | .63 | |
| Body mass index (kg m −2 ), median (IQR) | 24.2 (21.1–27.5) | 24.6 (21.4–28.0) | .58 | |
| IBD type | CD, n (%) | 61 (80.3) | 56 (69.1) | .16 |
| UC, n (%) | 15 (19.7) | 25 (30.9) | ||
| CD Location a | L1 (ileal), n (%) | 30 (49.2) | 33 (58.9) | .71 |
| L2 (colonic), n (%) | 8 (13.1) | 4 (7.2) | ||
| L3 (ileocolonic), n (%) | 23 (37.7) | 19 (33.9) | ||
| L4 (upper GI disease), n (%) | 2 (3.3) | 1 (1.8) | ||
| CD behaviour | B1 (nonstricturing, nonpenetrating), n (%) | 23 (37.7) | 26 (46.4) | .62 |
| B2 (stricturing), n (%) | 8 (13.1) | 7 (12.5) | ||
| B3 (penetrating), n (%) | 30 (49.2) | 23 (41.1) | ||
| Perianal fistulizing disease, n (%) | 19 (25.0) | 14 (17.3) | .32 | |
| UC extent | E1 (proctitis), n (%) | 3 (20.0) | 9 (36.0) | .56 |
| E2 (left‐side colitis), n (%) | 3 (20.0) | 4 (16.0) | ||
| E3 (pancolitis), n (%) | 9 (60.0) | 12 (48.0) | ||
| Extraintestinal manifestations, n (%) | 28 (36.8) | 24 (29.6) | .72 | |
| Musculoskeletal, n (%) | 22 (28.9) | 19 (23.5) | ||
| Dermatologic, n (%) | 4 (5.3) | 1 (1.2) | ||
| Other, n (%) | 2 (2.6) | 4 (4.9) | ||
| Concomitant IMM at start of infliximab, n (%) | 59 (77.6) | 60 (74.1) | .73 | |
| Thiopurines (azathioprine, 6‐MP), n (%) | 53 (69.7) | 57 (7.4) | ||
| Methotrexate, n (%) | 6 (7.9) | 3 (3.7) | ||
| CRP at diagnosis (mg dL −1 ), median (IQR) | 1.1 (0.1–3.1) | 1.3 (0.1–3.7) | .72 | |
| FCP at diagnosis (mg kg −1 ), median (IQR) | NA | 222.0 (15–3590) | ‐ | |
ATI: antidrug antibody; CD: Crohn's disease; CRP: C‐reactive protein; FCP: faecal calprotectin; GI: gastrointestinal; IBD: inflammatory bowel disease; IMM: immunomodulators; NA: not available; TDM: therapeutic drug monitoring; UC: ulcerative colitis.
a Patients could present several locations (L) of the CD lesion.
Patients who started treatment with infliximab during the study were proactively monitored, with initial TDM at week 14. Table 2 shows the main data related to the mep‐TDM programme. Among the monitored patients, 33 (40.7%) and 6 (7.4%) required early dose adjustment because of low and high TSICs, respectively. Furthermore, 2 patients developed reversible ATIs, and 1 with therapeutic levels of TSIC presented primary TF and had to be treated with vedolizumab. Over the 3‐year follow‐up period, out of the patients subjected to mep‐TDM, 51 (63%) underwent dose escalation and 28 (35%) received de‐escalation in order to reach the therapeutic range. By contrast, 26 patients (32%) received no dose modification during the study.
Table 2.
Early proactive therapeutic drug monitoring outcomes
| n | 81 | |
|---|---|---|
| Duration of infliximab treatment (wk), median (IQR) | 82 (34–118) | |
| TSICs analysed during the study, n | 201 | |
| TSIC at week 14 (μg mL −1 ), median (IQR) | 5.8 (2.2–7.8) | |
| Therapeutic, n (%) | 42 (51.9) | |
| Supratherapeutic, n (%) | 6 (7.4) | |
| Subtherapeutic, n (%) | 33 (40.7) | |
| Immunogenic TSIC (< 1 μg mL −1 ), n (%) | 6 (7.4) | |
| ATI at week 14 , n (%) | 2 (2.5) | |
| Optimized treatments per patient (n y −1 ), median (IQR) | 1 (0–2) | |
| Optimized dose during the study (mg kg −1 ), median (IQR) | 5.1 (5.0–5.9) | |
| Optimized interval during the study (wk), median (IQR) | 7 (5–8) | |
| Non reversible ATI during the study, n (%) | 4 (4.9) | |
| Severe infusion‐related reactions, n (%) | 2 (2.5) | |
| Non infusion‐related adverse reactions, n (%) | 3 (3.7) | |
ATI: antidrug antibody; IQR: interquartile range; TSIC: trough serum infliximab concentrations.
Figure 3 shows the Kaplan–Meier cumulative probability curves for TF, IBD‐related surgery and IBD‐related hospitalization. Patients who underwent mep‐TDM had a statically higher probability of maintaining treatment with infliximab and a significantly lower cumulative probability of IBD‐related surgery and IBD‐related hospitalization than those who were not subjected to TDM. In the subgroup analysis, the survival curves of patients with CD and UC showed a similar behaviour and no statistically significant differences were found.
Figure 3.
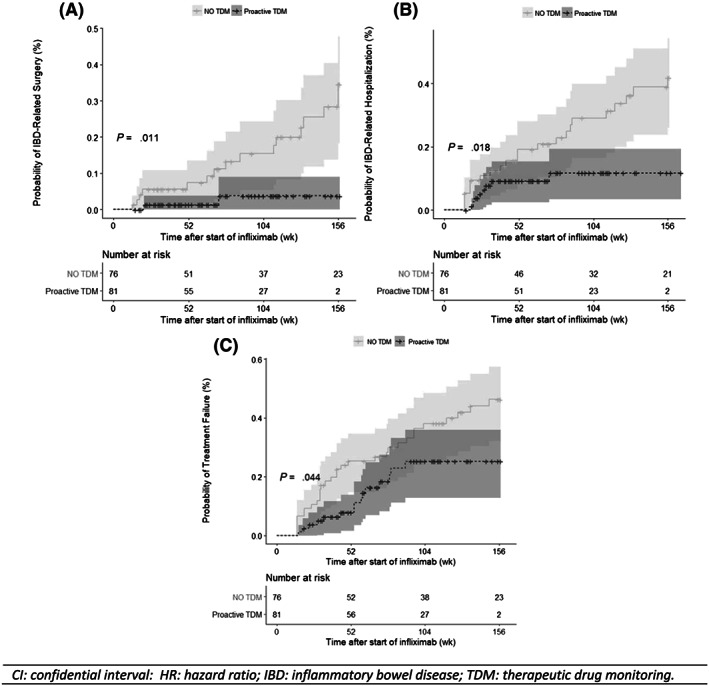
Kaplan–Meier cumulative probability curves for inflammatory bowel disease (IBD)‐related surgery (A) IBD‐related hospitalization (B) and probability of treatment failure with infliximab (C) in patients with early proactive therapeutic drug monitoring (TDM) and control group, respectively
A lower number of TFs was observed among the patients subjected to mep‐TDM: 15 (18.5%) of the patients in the study group vs 30 (39.5%) in the control group. Reasons for treatment discontinuation in the mep‐TDM group were: 8 inadequate clinical responses (9.9%), 4 ATIs (4.9%), 2 ARs (2.5%) and 1 SIR (1.2%). In the control group, 18 (23.7%), 7 (9.0%), 3 (3.9%) and 2 (2.6%) treatments were discontinued due to inadequate clinical response, SIRs, ARs and other, respectively. The median time for the appearance of ATIs in the study group was 27 weeks in a range between 15 and 42 weeks.
Table 3 shows the results from univariate and multivariate Cox analysis for TF. Univariate Cox analysis showed a lower probability of TF compared with the control group (hazard ratio [HR] = 0.53; 95% confidence interval [CI]: 0.28–0.97; P < .05) with 3‐year absolute risk reduction (ARR) of 23%. Mep‐TDM (HR = 0.51; 95% CI: 0.27–0.92; P < .05) and extraintestinal manifestations (HR = 1.72; 95% CI: 1.02–3.16; P < .05) were the only variables independently associated with TF.
Table 3.
Univariate and multivariate analysis of infliximab treatment failure
|
Univariate Cox analysis HR (95% CI) |
P |
Multivariate Cox analysis HR (95% CI) |
P | |
|---|---|---|---|---|
| Proactive TDM | 0.53 (0.28–0.97) | .042 | 0.51 (0.27–0.92) | .037 |
| Sex (ref male) | 1.02 (0.57–1.84) | .924 | ‐ | |
| Diagnosis (ref UC) | 0.79 (0.42–1.50) | .477 | ‐ | |
| Perianal fistulizing disease | 0.88 (0.43–1.83) | .734 | ‐ | |
| Age at diagnosis | 1.02 (0.68–1.52) | .916 | ‐ | |
| Age at start of infliximab | 1.33 (0.84–2.12) | .224 | ‐ | |
| CD localization (ref L1) | 0.99 (0.69–1.44) | .978 | ‐ | |
| CD behaviour (ref B1) | 0.91 (0.56–1.48) | .713 | ‐ | |
| UC extension (ref E1) | 1.64 (0.60–0.79) | .182 | ‐ | |
| Concomitant use of immunomodulators | 1.09 (0.54–2.20) | .806 | ‐ | |
| Extraintestinal manifestations | 1.75 (0.98–3.13) | .057 | 1.72 (1.02–3.16) | .044 |
CD: Crohn's disease; CI: confidence interval; HR: hazard ratio; TDM: therapeutic drug monitoring; UC: ulcerative colitis.
Patients who underwent mep‐TDM were less liable to require IBD‐related surgery (2 in the study group vs 16 in the control group) and 3‐year ARR was 25%. Multivariate analysis (Table 4) yielded significantly lower values for IBD‐related surgery with the use of mep‐TDM (HR = 0.14; 95% CI: 0.03–0.65; P < .05), whereas perianal fistulizing disease increased the risk (HR = 3.13; 95% CI: 1.17–8.42; P < .05).
Table 4.
Univariate and multivariate analysis of surgery related with inflammatory bowel disease
|
Univariate Cox analysis HR (95% CI) |
P |
Multivariate Cox analysis HR (95% CI) |
P | |
|---|---|---|---|---|
| Proactive TDM | 0.18 (0.04–0.79) | .023 | 0.14 (0.03–0.65) | .012 |
| Sex (ref male) | 2.28 (0.79–1.10) | .275 | ‐ | |
| Diagnosis (ref UC) | 1.62 (0.60–4.37) | .335 | ‐ | |
| Perianal fistulizing disease | 2.73 (1.08–6.95) | .024 | 3.13 (1.17–8.42) | .015 |
| Age at diagnosis | 1.25 (0.63–2.49) | .515 | ‐ | |
| Age at start of infliximab | 1.88 (0.80–4.39) | .146 | ‐ | |
| CD localization (ref L1) | 0.15 (0.03–0.69) | .015 | ‐ | |
| CD behaviour (ref B1) | 1.40 (0.23–8.30) | .708 | ‐ | |
| UC extension (ref E1) | 1.34 (0.66–2.72) | .411 | ‐ | |
| Concomitant use of immunomodulators | 2.18 (0.49–9.63) | .303 | ‐ | |
| Extraintestinal manifestations | 1.79 (0.67–4.78) | .244 | ‐ |
CD: Crohn's disease; CI: confidence interval; HR: hazard ratio; TDM: therapeutic drug monitoring; UC: ulcerative colitis.
Eight patients under mep‐TDM had to be admitted for IBD‐related hospitalization against 23 from the control group. By the end of the follow‐up period, the 3‐year ARR of IBD‐related hospitalization was 23% in the study group. Mep‐TDM was the only variable associated with IBD‐related hospitalizations (HR = 0.38; 95% CI: 0.17–0.87; P < .05). All univariate and multivariate analysis results are shown in Table 5.
Table 5.
Univariate and multivariate analysis of hospitalization related with inflammatory bowel disease
|
Univariate Cox analysis HR (95% CI) |
P |
Multivariate Cox analysis HR (95% CI) |
P | |
|---|---|---|---|---|
| Proactive TDM | 0.38 (0.17–0.87) | .022 | 0.38 (0.17–0.87) | .022 |
| Sex (ref male) | 0.88 (0.37–2.10) | .779 | ‐ | |
| Diagnosis (ref UC) | 1.24 (0.57–2.67) | .591 | ‐ | |
| Perianal fistulizing disease | 1.93 (0.91–4.10) | .088 | ‐ | |
| Age at diagnosis | 1.01 (0.59–1.73) | .962 | ‐ | |
| Age at start of infliximab | 1.02 (0.57–1.84) | .931 | ‐ | |
| CD localization (ref L1) | 0.81 (0.49–1.35) | .423 | ‐ | |
| CD behaviour (ref B1) | 2.86 (0.80–10.20) | .105 | ‐ | |
| UC extension (ref E1) | 1.10 (0.59–2.05) | .763 | ‐ | |
| Concomitant use of immunomodulators | 1.29 (0.49–3.42) | .611 | ‐ | |
| Extraintestinal manifestations | 1.11 (0.51–2.46) | .785 | ‐ |
CD: Crohn's disease; CI: confidence interval; HR: hazard ratio; TDM: therapeutic drug monitoring; UC: ulcerative colitis.
The apparently high percentage of patients receiving concomitant immunosuppressant therapy at the start of infliximab treatment led to the decision to analyse the effect of these drugs on the effectiveness variables. As shown in Tables 3, 4, 5, concomitant immunosuppressant therapy was not associated with TPF, IBD‐related hospitalization or surgery. In addition, a supplementary analysis comparing all the patients with concomitant immunosuppressant vs monotherapy infliximab yielded no statistically significant differences: HR: 1.09, 95% CI: 0.54–2.20, P = .81 for TF; HR: 2.58, 95% CI 0.59–11.25, P = .21 for IBD‐related surgery; and HR = 1.55, 95% CI: 0.59–4.04, P = .37 for IBD‐related hospitalization.
In relation to the effect of mep‐TDM on treatment safety, SIRs were significantly lower in patients under mep‐TDM (2.5 vs 10.4%; P = .048), but the onset of ARs was similar in both groups (3.7% and 3.9%, respectively; P > .999).
4. DISCUSSION
Pharmacokinetic variability is a well‐known key source of variability in anti‐TNF response.28 In addition, there is increasing evidence that higher infliximab levels are associated with sustained response and, likewise, low or undetectable TSICs increase the likelihood of LOR.29 Therefore, TDM is emerging as a useful therapeutic tool to facilitate personalization of these treatments.29, 30, 31
In our study, patients subjected to empirical therapy had a low probability of reaching therapeutic TSICs (40.4%). Furthermore, a positive correlation between TSICs and favourable therapeutic outcomes was observed (Figure 2). ATIs were also detected in a high number of patients (13.5%), which is probably connected to the high percentage (48.1%) of patients with subtherapeutic TSICs. These results are consistent with previous studies.29, 32, 33, 34 Our findings underscore the convenience of guiding therapeutic decisions through personalized dosing in order to reach the desired TSICs and maximize positive therapeutic outcomes.
From the start of the study, proactive TDM was considered as the dosing guide strategy for all the patients in our centre. We found that patients with mep‐TDM had 23% cumulative probability of TF 3 years from the beginning of the treatment. This probability was significantly lower (P < .05) than that observed in the control group (46%). In addition, cumulative risks of IBD‐related surgery and IBD‐related hospitalization became significantly reduced after implementing the mep‐TDM program, as presented in Figure 3: surgery from 28 to 3% (P < .05) and hospitalization from 32 to 9% (P < .05). The low percentage of patients with circulating ATIs (4.9%) observed after the implementation of TDM probably contributed towards achieving these better results.35
Papamichael et al.16 based on a sample of 130 patients with IBD under proactive TDM, estimated an accumulative probability of 85% of them remaining on infliximab 3 years after starting the treatment and a cumulative risk of IBD‐related surgery and IBD‐related hospitalization of 5 and 9%, respectively. In this study, the first TDM session was performed after a median time of >1 year of infliximab therapy. Therefore, the sample population could have influenced the slightly improved results in relation to our study. Vaughn et a.17 also observed that IBD patients under proactive TDM had a greater probability of remaining on infliximab after 3 years than those receiving infliximab doses at the discretion of the treating physician, this probability increasing in patients who achieved TSIC >5 μg mL−1.
The similarity among the survival curves shown in all the studies carried out in IBD patients is worth noting. During the first 2 years after the start of infliximab treatment, a pronounced slope is observed; however, after this period, the curves become flat, probably because the development of antibodies occurs mainly during these first months.36 In our study group, ATIs were only detected in this period.
Consistent with other studies,13, 16, 36 we found a low incidence of SIRs (2.5%) in patients subjected to mep‐TDM, whereas the percentage was significantly higher in the control group (10.4%). These results, in turn, could be related to the low proportion of patients with ATIs, since there is evidence that the development of antibodies may complement anaphilotoxin activation and production.36 However, as expected, the incidence of adverse reactions linked to the drug was similar in both groups. Our results support the association of immunogenicity and SIRs, suggesting a potential relationship between subtherapeutic TSICs and adverse effects, as well as the usefulness of mep‐TDM to improve the safety profile of infliximab therapy.
In the study group, a population TDM‐based Bayesian dose prediction was used, which provides a more efficient guide for dose adjustments.37 This strategy incorporates data from TSICs, demographics and other patient's characteristics to predict the TSICs evolution over the time. TDM‐based Bayesian dose prediction allowed TSICs within the therapeutic range to be reached in all new patients early after the induction phase. The high percentage of patients who yielded subtherapeutic TSICs at week 14 could indicate the convenience of conducting the first TDM during the induction phase, where higher TSICs are probably needed.38
Early proactive TDM is not yet a widespread practice, and reactive TDM in response to suboptimal disease control is emerging as the new standard of care for optimizing anti‐TNF therapy in IBD.29 By contrast, preliminary data that prove the usefulness of proactive TDM to improve therapeutic outcomes have been published and there are currently experts who believe that proactive TDM, which helps to optimize infliximab therapy before immunogenicity and/or LOR, should become standard clinical practice.16, 17, 39, 40, 41
At our centre, mep‐TDM has proved effective in reducing IBD‐related surgery and hospitalization, as well as in increasing the durability of infliximab treatment, preventing changes to second‐line treatments associated with higher direct costs. The analysis of 201 samples during a 3‐year period of mep‐TDM applied to infliximab dosing optimization in 81 patients showed that the potential benefits of proactive TDM outweigh the high cost of infliximab and ATIs determination and, consequently, mep‐TDM could be a cost‐effective strategy. A randomized prospective trial is yet to be carried out to confirm these results.
To ensure full clinical benefit of biological therapies, drug concentration measurements should be appropriately implemented and adequately interpreted. Many factors should be considered in the interpretation of TSIC, such as biological matrix, analytic variability, sampling time, variability pharmacokinetics, among others, which demand the involvement of experts with sufficient knowledge and training to adequately interpret and present TDM results.42
Multidisciplinary work is considered good practice in the healthcare system and the presence of multidisciplinary teams is well established in many healthcare institutions.43 This involves the coordinated efforts of specialists with expertise in their corresponding areas, the combination of their skills being a key aspect in helping to improve health outcomes. In our shared‐responsibility collaborative approach, doses were adapted to all the patients as required, and no patients needed additional visits or had wait for the next dose to adjust their treatment, unlike the experience reported in other studies.13, 44 Two recent surveys have yielded great heterogeneity in the use of TDM of biological agents in clinical practice, identifying the greatest barriers to their implementation.44, 45 Samaan et al.45 revealed that many clinicians lack confidence in their TDM knowledge and conclude that TDM results should be interpreted according to clinical context and, ideally, by a multidisciplinary team. While our study was not designed to assess the benefits of multidisciplinary team work, the involvement of experts with sufficient knowledge and training to adequately interpret the results obtained in the laboratory, present the TDM results and get involved in the decision‐making process has unquestionably contributed greatly towards the achievement of the clinical benefits expected from our mep‐TDM.
The available data that suggest the usefulness of proactive TDM are mainly the result of retrospective studies. Therefore, a strength of this study is that the data are largely prospective, coming from real‐life clinical practice and nonbiased with respect to patient selection, since the treatment management strategy used (mep‐TDM) was the same throughout the entire study.
However, among the limitations of the study are its nonrandomized nature and the use of historical population in the control group. Thus, the results should be confirmed by means of a prospective randomized controlled trial. Nonetheless, although this is considered the gold standard of study design, it is limited by several factors, such as resources, cost or even ethical standards. Another potential limitation is the use of a drug‐sensitivity assay, which means that ATIs cannot be measured in presence of the drug; however, different reports conclude that drug‐tolerant assays do not offer clinical benefits over drug‐sensitivity assays.16, 29, 42
In conclusion, empirical doses of infliximab in IBD patients result in a high percentage of subtherapeutic TSICs and ATI development. An early proactive‐TDM programme after the induction phase improves the long‐term outcomes of infliximab therapy, increasing durability of the drug, decreasing IBD‐related hospitalization and surgery and reducing ATIs and SIRs. Therefore, our preliminary results support the use of proactive TDM and, according to our experience, multidisciplinary care is a useful approach to personalize infliximab therapy for IBD patients.
COMPETING INTERESTS
There are no competing interests to declare.
CONTRIBUTORS
J.G.S.H., N.R. and M.V.C. conceived and designed the study. F.M. selected and had direct clinical responsibility for patients. J.G.S.H. analysed the data. J.G.S.H., N.R., M.V.C. and A.M.S. wrote the first draft of the manuscript. All authors reviewed and edited the manuscript and approved the final version of the manuscript.
ACKNOWLEDGEMENTS
The authors want to thank the support received from all the staff of our Pharmacokinetics Laboratory and the Pharmacy and Gastroenterology Services.
POPULATION PHARMACOKINETIC MODEL (POPPK)
| A. Patient characteristics | ||
|---|---|---|
| No. of patients | 108 | |
| No. of serum samples | 313 | |
| ATI, n (%) | 26 (8.3) | |
| Age (y), median (IQR) | 42 (18–79) | |
| Weight (kg), median (IQR) | 70 (55–82) | |
| Female, n (%) | 51 (48,6) | |
| IBD type | CD, n (%) | 84 (77.8) |
| UC, n (%) | 24 (22.2) | |
| Albumin (g dL−1), median (IQR) | 4.4 (4.1–4.9) | |
| C‐reactive protein (mg dL−1), median (IQR) | 0.40 (0,10–0.95) | |
| FCP (mg kg−1), median (IQR) | 125 (20–580) | |
| B. Pharmacokinetic parameters estimated and validation | ||
|---|---|---|
| Parameters |
Final Model Estimate (%RSE) |
Bootstrap (n = 1000) Mean (95% CI) |
| CL (L h−1) | 0.0158 (6%) | 0.0159 (0.0141–0.176) |
| Vc (L) | 4.8 (15%) | 4.9 (3.3–6.2) |
| Vp (L) | 4.13* | ‐‐ |
| Q (L h−1) | 0.30* | ‐‐ |
| ATI‐CL | 4.24 (7%) | 4.02 (1.71–7.56) |
| WGT‐CL | 0.177 (34%) | 0.182 (0.03–0.388) |
| FCP‐CL | 0.0175 (28%) | 0.0178 (0.0080–0.0269) |
| IIV‐CL (CV, %) | 22.8 (9%) | 22.6 (19.1–25.9) |
| RUV (CV, %) | 34.1 (11%) | 33.6 (30.7–37.0) |
CLi = CL * (WGT/70) WGT‐CL * (FCP/125) FCP‐CL * ATI‐CL ATI
If detectable anti‐infliximab antibodies, ATI‐CL = 1, else ATI‐CL = 0.
Fixed parameters according Fasanmade et al., 2009.
ATI: anti‐infliximab antibody; CD: Crohn's disease; CI: confidence interval; CL: clearance; CV: coefficient of variation; FCP: faecal calprotectin (mg kg−1); IBD: inflammatory bowel disease; IQR: interquartile range; IIV‐CL: interindividual variability on clearance; Q: intercompartment clearance; RSE: residual standard error; RUV: residual unexplained variability; UC: ulcerative colitis; Vc: central volume of distribution; Vp: peripheral volume of distribution; WGT: body weight (kg).
Sánchez‐Hernández JG, Rebollo N, Martin‐Suarez A, Calvo MV, Muñoz F. A 3‐year prospective study of a multidisciplinary early proactive therapeutic drug monitoring programme of infliximab treatments in inflammatory bowel disease. Br J Clin Pharmacol. 2020;86 1165–1175. 10.1111/bcp.14229
The authors confirm that the principal investigator for this paper is Fernando Muñoz and that he had direct clinical responsibility for the patients.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Magro F, Gionchetti P, Eliakim R, et al. Third European evidence‐based consensus on diagnosis and Management of Ulcerative Colitis. Part 1: definitions, diagnosis, extra‐intestinal manifestations, pregnancy, cancer surveillance, surgery, and Ileo‐anal pouch disorders. J Crohns Colitis. 2017;11(6):649‐670. [DOI] [PubMed] [Google Scholar]
- 2. Gomollon F, Dignass A, Annese V, et al. Third European evidence‐based consensus on the diagnosis and Management of Crohn's disease 2016: part 1: diagnosis and medical management. J Crohns Colitis. 2017;11(1):3‐25. [DOI] [PubMed] [Google Scholar]
- 3. Annese V, Duricova D, Gower‐Rousseau C, Jess T, Langholz E. Impact of new treatments on hospitalisation, surgery, infection, and mortality in IBD: a focus paper by the epidemiology committee of ECCO. J Crohns Colitis. 2016;10(2):216‐254. [DOI] [PubMed] [Google Scholar]
- 4. Sandborn WJ, Feagan BG, Hanauer SB, et al. A review of activity indices and efficacy endpoints for clinical trials of medical therapy in adults with Crohn's disease. Gastroenterology. 2002;122(2):512‐530. [DOI] [PubMed] [Google Scholar]
- 5. Vande Casteele N, Gils A. Pharmacokinetics of anti‐TNF monoclonal antibodies in inflammatory bowel disease: adding value to current practice. J Clin Pharmacol. 2015;55(Suppl 3):S39‐S50. [DOI] [PubMed] [Google Scholar]
- 6. González‐Fernández MÁ, Villamañán E, Jiménez‐Nácher I, Moreno F, Herrero A, Balsa A. Persistence of biological agents over an eight‐year period in rheumatoid arthritis and spondyloarthritis patients. Farm Hosp. 2019;43(1):24‐30. [DOI] [PubMed] [Google Scholar]
- 7. van Schie KA, Hart MH, de Groot ER, et al. The antibody response against human and chimeric anti‐TNF therapeutic antibodies primarily targets the TNF binding region. Ann Rheum Dis. 2015;74(1):311‐314. [DOI] [PubMed] [Google Scholar]
- 8. Maneiro JR, Salgado E, Gomez‐Reino JJ. Immunogenicity of monoclonal antibodies against tumor necrosis factor used in chronic immune‐mediated inflammatory conditions: systematic review and meta‐analysis. JAMA Intern Med. 2013;173(15):1416‐1428. [DOI] [PubMed] [Google Scholar]
- 9. Fasanmade AA, Adedokun OJ, Olson A, Strauss R, Davis HM. Serum albumin concentration: a predictive factor of infliximab pharmacokinetics and clinical response in patients with ulcerative colitis. Int J Clin Pharmacol Ther. 2010;48(5):297‐308. [DOI] [PubMed] [Google Scholar]
- 10. Fasanmade AA, Adedokun OJ, Ford J, et al. Population pharmacokinetic analysis of infliximab in patients with ulcerative colitis. Eur J Clin Pharmacol. 2009;65(12):1211‐1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Katsanos KH, Papamichael K, Feuerstein JD, Christodoulou DK, Cheifetz AS. Biological therapies in inflammatory bowel disease: beyond anti‐TNF therapies. Clin Immunol. 2019;206:9‐14. [DOI] [PubMed] [Google Scholar]
- 12. Sanchez‐Hernandez JG, Rebollo N, Munoz F, Martin‐Suarez A, Calvo MV. Therapeutic drug monitoring of tumour necrosis factor inhibitors in the management of chronic inflammatory diseases. Ann Clin Biochem. 2019;56(1):28‐41. [DOI] [PubMed] [Google Scholar]
- 13. Vande Casteele N, Ferrante M, Van Assche G, et al. Trough concentrations of infliximab guide dosing for patients with inflammatory bowel disease. Gastroenterology. 2015;148(7):1320‐1329. [DOI] [PubMed] [Google Scholar]
- 14. Vaughn BP, Sandborn WJ, Cheifetz AS. Biologic concentration testing in inflammatory bowel disease. Inflamm Bowel Dis. 2015;21(6):1435‐1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Verstockt B, Moors G, Bian S, et al. Influence of early adalimumab serum levels on immunogenicity and long‐term outcome of anti‐TNF naive Crohn's disease patients: the usefulness of rapid testing. Aliment Pharmacol Ther. 2018;48(7):731‐739. [DOI] [PubMed] [Google Scholar]
- 16. Papamichael K, Chachu KA, Vajravelu RK, et al. Improved long‐term outcomes of patients with inflammatory bowel disease receiving proactive compared with reactive monitoring of serum concentrations of infliximab. Clin Gastroenterol Hepatol. 2017;15(10):1580‐1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vaughn BP, Martinez‐Vazquez M, Patwardhan VR, Moss AC, Sandborn WJ, Cheifetz AS. Proactive therapeutic concentration monitoring of infliximab may improve outcomes for patients with inflammatory bowel disease: results from a pilot observational study. Inflamm Bowel Dis. 2014;20(11):1996‐2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lewis JD, Chuai S, Nessel L, Lichtenstein GR, Aberra FN, Ellenberg JH. Use of the noninvasive components of the Mayo score to assess clinical response in ulcerative colitis. Inflamm Bowel Dis. 2008;14(12):1660‐1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Daperno M, D'Haens G, Van Assche G, et al. Development and validation of a new, simplified endoscopic activity score for Crohn's disease: the SES‐CD. Gastrointest Endosc. 2004;60(4):505‐512. [DOI] [PubMed] [Google Scholar]
- 20. Harvey RF, Bradshaw JM. A simple index of Crohn's‐disease activity. Lancet. 1980;1(8167):514. [DOI] [PubMed] [Google Scholar]
- 21. Llinares‐Tello F, Rosas‐Gomez de Salazar J, Senabre‐Gallego JM, et al. Practical application of acid dissociation in monitoring patients treated with adalimumab. Rheumatol Int. 2014;34(12):1701‐1708. [DOI] [PubMed] [Google Scholar]
- 22. Rosen MJ, Minar P, Vinks AA. Applying pharmacokinetics to optimize dosing of anti‐TNF biologics in acute severe ulcerative colitis. Aliment Pharmacol Ther. 2015;41(11):1094‐1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yarur AJ, Kanagala V, Stein DJ, et al. Higher infliximab trough levels are associated with perianal fistula healing in patients with Crohn's disease. Aliment Pharmacol Ther. 2017;45(7):933‐940. [DOI] [PubMed] [Google Scholar]
- 24. Barlow NL, Mohammed P, Berg JD. Serum trough infliximab and anti‐infliximab antibodies in a cohort of gastroenterology and rheumatology patients' infliximab therapeutic drug monitoring. Ann Clin Biochem. 2016;53(Pt 4):477‐484. [DOI] [PubMed] [Google Scholar]
- 25. Bellisant E, Sébille V, Paintaud G. Methodological issues in pharmacokinetic‐pharmacodinamic modelling. Clin Pharm. 1998;35:151‐166. [DOI] [PubMed] [Google Scholar]
- 26. Harding SD, Sharman JL, Faccenda E, et al. The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Res. 2018; 46(D1):D1091–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Alexander SP, Christopoulos A, Davenport A. et al. THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: G protein‐coupled receptors. British Journal of Pharmacology, 2019; 176(S1):S21–S141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gross AS. Best practice in therapeutic drug monitoring. Br J Clin Pharmacol. 2001;52(Suppl 1):5S‐10S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Papamichael K, Cheifetz AS, Melmed GY, et al. Appropriate therapeutic drug monitoring of biologic agents for patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2019;17(9):1655‐1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Egan C, Doherty GA. Why do we need to improve monitoring of patients with inflammatory bowel disease (IBD) on biologic treatment? Expert Opin Biol Ther. 2019;19(9):907‐918. [DOI] [PubMed] [Google Scholar]
- 31. Ma C, Battat R, Jairath V, Vande CN. Advances in therapeutic drug monitoring for small‐molecule and biologic therapies in inflammatory bowel disease. Curr Treat Options Gastroent. 2019;17(1):127‐145. [DOI] [PubMed] [Google Scholar]
- 32. Vermeire S, Noman M, Van Assche G, Baert F, D'Haens G, Rutgeerts P. Effectiveness of concomitant immunosuppressive therapy in suppressing the formation of antibodies to infliximab in Crohn's disease. Gut. 2007;56(9):1226‐1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ben‐Horin S, Chowers Y. Review article: loss of response to anti‐TNF treatments in Crohn's disease. Aliment Pharmacol Ther. 2011;33(9):987‐995. [DOI] [PubMed] [Google Scholar]
- 34. Lee LYW, Sanderson JD, Irving PM. Anti‐infliximab antibodies in inflammatory bowel disease: prevalence, infusion reactions, immunosuppression and response, a meta‐analysis. Eur J Gastroenterol Hepatol. 2012;24(9):1078‐1085. [DOI] [PubMed] [Google Scholar]
- 35. Yanai H, Lichtenstein L, Assa A, et al. Levels of drug and antidrug antibodies are associated with outcome of interventions after loss of response to infliximab or adalimumab. Clin Gastroenterol Hepatol. 2015;13(3):522‐530, e2. [DOI] [PubMed] [Google Scholar]
- 36. Ungar B, Chowers Y, Yavzori M, et al. The temporal evolution of antidrug antibodies in patients with inflammatory bowel disease treated with infliximab. Gut. 2014;63(8):1258‐1264. [DOI] [PubMed] [Google Scholar]
- 37. Passot C, Pouw MF, Mulleman D, et al. Therapeutic drug monitoring of biopharmaceuticals may benefit from pharmacokinetic and pharmacokinetic‐pharmacodynamic modeling. Ther Drug Monit. 2017;39(4):322‐326. [DOI] [PubMed] [Google Scholar]
- 38. Papamichael K, Van Stappen T, Vande Casteele N, et al. Infliximab concentration thresholds during induction therapy are associated with short‐term mucosal healing in patients with ulcerative colitis. Clin Gastroenterol Hepatol. 2016;14(4):543‐549. [DOI] [PubMed] [Google Scholar]
- 39. Papamichael K, Osterman MT, Siegel CA, et al. Using proactive therapeutic drug monitoring of anti‐tumor necrosis factor therapy in inflammatory bowel disease: Froman old concept to a future standard of care? Gastroenterology. 2018;154(4):1201‐1202. [DOI] [PubMed] [Google Scholar]
- 40. Papamichael K, Vajravelu RK, Vaughn BP, Osterman MT, Cheifetz AS. Proactive infliximab monitoring following reactive testing is associated with better clinical outcomes than reactive testing alone in patients with inflammatory bowel disease. J Crohns Colitis. 2018;12(7):804‐810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Headlam J, Ford AC, Gracie DJ. Reactive versus proactive therapeutic drug monitoring in inflammatory bowel disease patients treated with infliximab: a self‐fulfilling prophecy. Clin Gastroenterol Hepatol. 2017;15(10):1638. [DOI] [PubMed] [Google Scholar]
- 42. Hoseyni H, Xu Y, Zhou H. Therapeutic drug monitoring of biologics for inflammatory bowel disease: an answer to optimized treatment? J Clin Pharmacol. 2018;58(7):864‐876. [DOI] [PubMed] [Google Scholar]
- 43. Marsilio M, Torbica A, Villa S. Health care multidisciplinary teams: the sociotechnical approach for an integrated system‐wide perspective. Health Care Manage Rev. 2017;42(4):303‐314. [DOI] [PubMed] [Google Scholar]
- 44. Grossberg LB, Papamichael K, Feuerstein JD, Siegel CA, Ullman TA, Cheifetz AS. A survey study of Gastroenterologists' attitudes and barriers toward therapeutic drug monitoring of anti‐TNF therapy in inflammatory bowel disease. Inflamm Bowel Dis. 2017;24(1):191‐197. [DOI] [PubMed] [Google Scholar]
- 45. Samaan MA, Arkir Z, Ahmad T, Irving PM. Wide variation in the use and understanding of therapeutic drug monitoring for anti‐TNF agents in inflammatory bowel disease: an inexact science? Expert Opin Biol Ther. 2018;18(12):1271‐1279. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


