Abstract
The aim of this study is to develop a computer-aided diagnosis method to help classify medical images of neck lymph nodes in head and neck cancer patients. According to the current practice guidelines, the classification of lymph node status is critical for patient stratification before treatment. Take extra-nodal extension (ENE) of metastatic neck lymph nodes, the status of ENE has been considered a single factor affecting the decision of whether systemic treatment with toxicity should be given to patients with otherwise non-advanced cancer status. Medical imaging prior to surgery serves as tools for clinical staging and determining the extent of neck lymph node dissection during the tumor resection surgery. The information contained in these images may also help determine the status of ENE and thus stratify patients for more precise treatment. In the current practice, there has been not a reliable computer-aided tool for this task. In this study, we used open-source software to investigate radiomic features that help distinguish malignant from benign and ENE from non-ENE lymph nodes. We have identified 89 features that can differentiate malignant from benign and 4 features that can differentiate ENE from non-ENE lymph nodes. Furthermore, we fed the significant features to a multilayer perceptron neural network to predict malignancy and ENE of lymph nodes and achieved 84% and 77% of accuracy in each task, respectively.
Keywords: Head and neck cancer, Magnetic resonance imaging, Radiomics, Lymph nodes
Introduction
Extra-nodal extension (ENE, previously called extracapsular spread or ECS) of metastatic neck lymph nodes has been known as a significant risk factor for distant metastasis and overall survival in head and neck squamous cell carcinoma (SCC) cancer patients and has been listed in the latest version of NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) for Head and Neck Cancers [1] as an adverse feature affecting the decision of whether systemic treatment should be given to patients with otherwise non-advanced cancer status. Medical imaging prior to surgery serves as tools for clinical staging and determining the extent of neck lymph node dissection during the tumor resection surgery. The information contained in these images may also help determine the status of ENE and thus stratify patients for more precise treatment.
The non-invasive classification between ENE and non-ENE lymph nodes will be especially essential for some head and neck SCC patients (such as oro- and hypopharyngeal cancers) whose definite pathological lymph node ENE status is usually not available since surgery is not the primary treatment option for this group of patients. Moreover, ENE status is not always accurately reported by radiologists who are not specialists in head and neck cancers. Developing a computer-aided method that helps identify ENE lymph nodes may provide oncologists more insights about patients’ lymph node status before treatment planning and thus improve patients’ outcome.
Some image features such as central necrosis and irregular border in metastatic lymph nodes with ENE have been known since the 1990s [2, 3]. One of the previous studies utilized these features and achieved 95% sensitivity with 85% specificity for ENE [4, 5]. However, the positive predictive value of this study was only 69%. The intensive human efforts involved in diagnosing ENE according to these qualitative features make it difficult to scale in daily radiology practice. That is one of the reasons why this previous study only analyzed 17 patients. In this project, we try to utilize a more automated way to identify features that can be explained quantitatively and also try to apply neural networks to help identify ENE without using any given image feature.
Material and Methods
Datasets
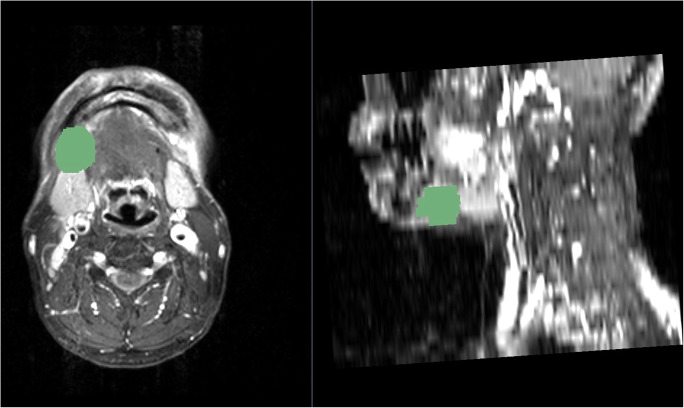
Pre-operative contrast-enhanced T1-weighted MRI images, surgical records, and post-operative histopathological results were reviewed before selecting lymph node for segmentation. All lymph node selected for analysis in the pre-operative staging contrast-enhanced T1-weighted MRI images had later undergone neck dissection and had histopathological diagnosis describing their lateralization, level, metastatic, and ENE status. After selecting lymph nodes of interest, an open source software 3D Slicer [6] and an extension Segmentation Wizard [7] were used for image reading and semiautomatic segmentation (Fig. 1) [8]. An experienced radiologist first correlated lymph nodes on MRI with corresponding pathological findings and then performed segmentation. The segmented lymph nodes were then categorized into three groups: (1) malignant with ENE, (2) malignant without ENE, and (3) benign. Pyradiomics is an open-source python package for easy and reproducible radiomic feature extraction from medical images [9]. The label of segmented lymph nodes and their corresponding dicom files were each saved as a respective individual file in NRRD format for further data analysis in pyradiomics. Another extension SlicerRadiomics that encapsulates pyradiomics library was also installed for quick feature extraction check purpose on the 3D Slicer viewer.
Fig. 1.
Lymph node segmentation with the 3D Slicer 4.10.0 with Segmentation Wizard extension
The dataset to be used comprises of 130 OCSCC patients treated at Chang Gung Memorial Hospital from 2010 to 2014. Pre-operative MRI images and post-operative pathological diagnoses of whether malignant metastasis and the ENE status of neck lymph nodes of interest with detailed localization are included. To the best of our knowledge, there are no public datasets of CT or MRI images of neck lymph nodes from squamous cell carcinoma patients with such detailed pathological reports available. One of the most relevant one is the TCIA CT lymph node dataset that collected mediastinal and abdominal lymph nodes from 176 patients [10]. This TCIA CT lymph node dataset aims for lymph node detection; therefore, only the information of lymph node sizes but no histopathological findings were included. The advantage of this dataset used in this study is that the access to the complete clinical records of these patients as institutional review board was approved. Other diagnostic images such as FDG PET/CT, the treatment patients received, and patients’ long-term prognosis are also available for future exploration.
Preprocessing of the Cropped Images
After segmenting 112 lymph nodes, only 68 volume of interests (VOI) from 25 patients were successfully analyzed. One of the reasons of this high failure rate is that most of the sizes of MRI scans performed before 2013 in our datasets were 256 × 256 instead of 512 × 512. It is possible to resize the image to 512 × 512 by resetting the pixel spacing with the Simple ITK tools. Since the difference in the size was due to the images taken by different MRI equipments, it may generate inconsistent pixel intensity distributions in our images and results in the noisy distribution in some of the radiomic features such as GLCM. Although this may possibly be solved by multi-domain learning techniques, this is not the focus of our main paper and will be left for our future work.
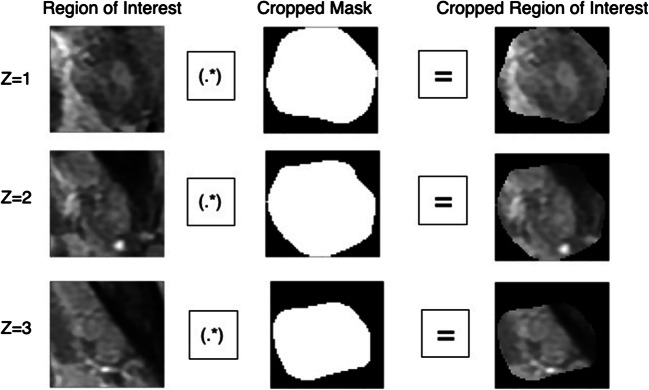
All images were normalized to mitigate the uncertainty of intensities from different scans. They were also resampled to three sets of pixel spacing, [1], [2], and [3]. Then we did matrix inner product of bounding box with cropped mask to get cropped ROI for analysis (Fig. 2).
Fig. 2.
Matrix inner product of the cropped mask with bounding box for ROI cropping
Statistical Analysis
Two sample t tests with equal and unequal variance were performed in 104 original radiomic features to identify features that are significantly different between malignant and benign lymph nodes, and ENE between non-ENE malignant lymph nodes. We also tried to feed the radiomic features into a multilayer perceptron neural network MLP classifier from scikit-learn to classify the lymph nodes. We used an MLP architecture with two hidden layers, where the first hidden layer has 5 nodes and 2 nodes for the second one. We trained the network with Adam optimizer where learning rate is set to be 2.5e-3 for 200 epochs. We used the binary cross-entropy loss as our loss function. The training loss is about 0.61 after 200 epochs of training.
Results
In the extracted radiomic features, there are 89 features that can differentiate malignant from benign lymph nodes (the top five features with lowest p values were listed in Table 1). The feature with the most significant discernibility is original_glcm_DifferenceAverage, which measures relationship between occurrences of pairs with similar intensity values and occurrences of pairs with differing intensity values. The top texture features associated with ENE are original_glcm_InverseVariance, original_glcm_DifferenceAverage, and original_glcm_Contrast (Table 2). Original_glcm_Contrast is the feature that is able to differentiate both lymph nodes with ENE from non-ENE and benign from malignant lymph nodes. Contrast is a measure of the local intensity variation, favoring values away from the diagonal (i = j). A larger value correlates with a greater disparity in intensity values among neighboring voxels. The accuracy for differentiating benign from malignant lymph nodes by MLP classifier (pixel spacing, [1]) was 75% if all 104 original features were fed into the neural network; the accuracy was improved to 84% if only features with p value < 0.05 were fed as inputs. The accuracy for differentiating ENE from non-ENE malignant lymph nodes was 77% with 80% of sensitivity, 75% of specificity, and 67% of positive predictive value if the most discerning 6 features were fed into the neural networks (Table 3).
Table 1.
Top 5 texture features discerning malignant from benign lymph nodes
| Feature class | Feature | p |
|---|---|---|
| GLCM | Difference average | < 0.0001 |
| GLCM | Contrast | < 0.0001 |
| GLCM | Id | < 0.0001 |
| GLCM | Difference variance | < 0.0001 |
| GLCM | Sum squares | < 0.0001 |
Table 2.
Top features discerning ENE from non-ENE malignant lymph nodes
| Feature class | Feature | p |
|---|---|---|
| Shape | Maximum 2D diameter slice | 0.030 |
| GLCM | Inverse variance | 0.036 |
| GLCM | Difference average | 0.038 |
| GLCM | Contrast | 0.048 |
Table 3.
Classification using multi-layer perceptron neural network using python scikit learn: MLP Classifier (solver=‘lbfgs’, alpha=1e-5, hidden_layer_sizes=(5, 2))
| Truth | Malignant | Benign |
| Predict | ||
| All 104 original features: Accuracy = 75% | ||
| Malignant | 31 | 9 |
| Benign | 8 | 20 |
| 89 features with p-value < 0.05: Accuracy = 83.8% | ||
| Malignant | 34 | 6 |
| Benign | 5 | 23 |
| Truth | ENE | Non_ENE |
| Predict | ||
| All 104 original features: Accuracy = 48.7% | ||
| ENE | 14 | 10 |
| Non_ENE | 10 | 5 |
| 6 features with p-value < 0.05: Accuracy = 76.9% | ||
| ENE | 18 | 6 |
| Non_ENE | 3 | 12 |
Discussion
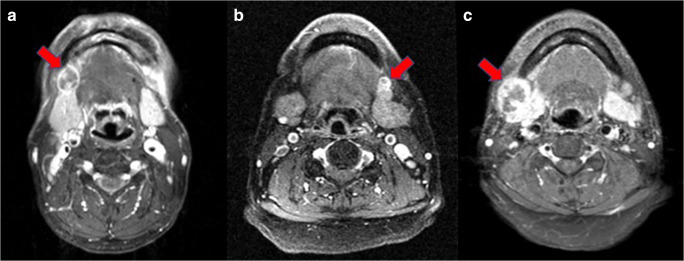
One of the advantages of radiomic feature analysis compared with deep learning is the relative interpretability. The most prominent feature that discerns ENE from non-ENE lymph nodes is the shape feature Maximum2D Diameter_Slice, which means the largest pairwise Euclidean distance between tumor surface voxels in the slice plane. This is a reasonable finding as the lymph nodes of ENE segmented tend to be larger. Another identified important texture feature, original_glcm_contrast, has a physical meaning that implies the heterogeneity among neighboring voxels. However, the Maximum2D Diameter_Slice feature may cause false negative and false positive prediction in smaller ENE and larger non-ENE malignant lymph nodes, respectively (Fig. 3).
Fig. 3.
a Successful prediction (true positive) of an ENE lymph node at the right neck; b false negative prediction of an ENE lymph node at the left neck; c false positive prediction of a non-ENE lymph node as an ENE one at the right neck
Pathology is the current gold standard for diagnosis of ENE lymph nodes. A computer-aided method that helps diagnose ENE status non-invasively has great value in patients who would not receive neck dissection to confirm the histopathological status of their lymph nodes. The first group is oropharyngeal and hypopharyngeal cancer patients in developed countries, where the incidence associated with HPV infection is increasing and organ preservation treatment such as chemoradiation may be preferred to maintain patients’ life quality. The second group is patients in less developed oral cavity cancer endemic countries where tumor resection surgery with lymph node dissection may not be accessible to every patient, so histopathological lymph node status cannot be evaluated. In this project, we have shown that MRI radiomic features with a neural network may help determine the ENE status that is essential in treatment planning when the histopathology is not available.
Future Works
Deep learning has been proved useful in various medical imaging tasks, including diabetic retinopathy [11], chest X-ray [12], and malignant lymph node detection in breast cancer. Lymph node classification is a problem that may be addressed through the latest frameworks of image recognition. In this project, we have proved that the value of identifying features was associated to malignancy and also extra-nodal extension. However, because of the limitation of sample size, we did not try to feed the images directly into neural networks but only the radiomic features. The next step to extend this research will be developing an automated lymph node detection method with text mining of pathology reports to collect lymph nodes with their pathological results more effectively. Automatic segmentation of lymph nodes in magnetic resonance images has been reported [13]. By combining automated lymph node detection and segmentation, it will be more feasible to incorporate the radiomic analysis into lymph node malignancy with ENE prediction in daily practice. In addition to contrast-enhanced T1 MRI images, other MRI sequences that provide useful visual information to radiologists are not segmented in this project. Using different sequences of MRI as a multi-modality approach for deep learning has been reported for classification of brain tumor. By applying an automated lymph node detection method, images from other MRI sequences of lymph nodes can be more easily obtained and used as inputs for multi-modality analysis.
Application of neural networks can further help classify lymph nodes. MRI is a relatively expensive imaging modality and may not be accessible for head and neck cancer patients of developing countries. In the following study, we will try to apply this technique to head and neck CT images that were collected years before MRI has become the standard staging tool in Chang Gung Memorial Hospital to identify image features that are specific to CT images. Furthermore, as the effect of ENE in metastatic lymph nodes has been reported in lung cancers [14], this classifier may be used in other types of cancers as well.
Conclusion
ENE is a lymph node pathological feature that has been proved as a significantly negative prognostic factor in oral cavity as well as other type of cancers. In this study, we demonstrated that we can use radiomic features extracted by an open source software and its extensions from pre-operative contrast-enhanced T1 MRI images with a five-layer neural network to classify lymph nodes into three groups: benign, malignant without ENE, and malignant with ENE. More promising results may be achieved if more samples can be obtained for training in more complicated neural networks.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Tsung-Ying Ho and Shy-Chyi Chin contributed equally to this work.
References
- 1.Colevas AD, Yom SS, Pfister DG, Spencer S, Adelstein D, Adkins D, Brizel DM, Burtness B, Busse PM, Caudell JJ, Cmelak AJ, Eisele DW, Fenton M, Foote RL, Gilbert J, Gillison ML, Haddad RI, Hicks WL, Hitchcock YJ, Jimeno A, Leizman D, Maghami E, Mell LK, Mittal BB, Pinto HA, Ridge JA, Rocco J, Rodriguez CP, Shah JP, Weber RS, Witek M, Worden F, Zhen W, Burns JL, Darlow SD. NCCN Guidelines Insights: Head and Neck Cancers, Version 1.2018. J Natl Compr Canc Netw. 2018;16:479–490. doi: 10.6004/jnccn.2018.0026. [DOI] [PubMed] [Google Scholar]
- 2.Pham TD, Watanabe Y, Higuchi M, Suzuki H. Texture Analysis and Synthesis of Malignant and Benign Mediastinal Lymph Nodes in Patients with Lung Cancer on Computed Tomography. Sci Rep. 2017;7:43209. doi: 10.1038/srep43209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Som PM. Detection of metastasis in cervical lymph nodes: CT and MR criteria and differential diagnosis. AJR Am J Roentgenol. 1992;158:961–969. doi: 10.2214/ajr.158.5.1566697. [DOI] [PubMed] [Google Scholar]
- 4.Zoumalan RA, Kleinberger AJ, Morris LG, Ranade A, Yee H, DeLacure M, Myssiorek D. Lymph node central necrosis on computed tomography as predictor of extracapsular spread in metastatic head and neck squamous cell carcinoma: pilot study. J Laryngol Otol. 2010;124:1284–1288. doi: 10.1017/S0022215110001453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aiken AH, Poliashenko S, Beitler JJ, Chen AY, Baugnon KL, Corey AS, Magliocca KR, Hudgins PA. Accuracy of Preoperative Imaging in Detecting Nodal Extracapsular Spread in Oral Cavity Squamous Cell Carcinoma. AJNR Am J Neuroradiol. 2015;36:1776–1781. doi: 10.3174/ajnr.A4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fedorov A, Beichel R, Kalpathy-Cramer J, Finet J, Fillion-Robin JC, Pujol S, Bauer C, Jennings D, Fennessy F, Sonka M, Buatti J, Aylward S, Miller JV, Pieper S, Kikinis R. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn Reson Imaging. 2012;30:1323–1341. doi: 10.1016/j.mri.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Github repository. Available at https://github.com/QTIM-Lab/SlicerSegmentationWizard.
- 8.Velazquez ER, et al. Volumetric CT-based segmentation of NSCLC using 3D-Slicer. Sci Rep. 2013;3:3529. doi: 10.1038/srep03529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Griethuysen JJM, Fedorov A, Parmar C, Hosny A, Aucoin N, Narayan V, Beets-Tan RGH, Fillion-Robin JC, Pieper S, Aerts HJWL. Computational Radiomics System to Decode the Radiographic Phenotype. Cancer Res. 2017;77:e104–e107. doi: 10.1158/0008-5472.CAN-17-0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roth HR, Lu L, Seff A, Cherry KM, Hoffman J, Wang S, Liu J, Turkbey E, Summers RM. A new 2.5D representation for lymph node detection using random sets of deep convolutional neural network observations. Med Image Comput Comput Assist Interv. 2014;17:520–527. doi: 10.1007/978-3-319-10404-1_65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gulshan V, Peng L, Coram M, Stumpe MC, Wu D, Narayanaswamy A, Venugopalan S, Widner K, Madams T, Cuadros J, Kim R, Raman R, Nelson PC, Mega JL, Webster DR. Development and Validation of a Deep Learning Algorithm for Detection of Diabetic Retinopathy in Retinal Fundus Photographs. JAMA. 2016;316:2402–2410. doi: 10.1001/jama.2016.17216. [DOI] [PubMed] [Google Scholar]
- 12.Lakhani P, Sundaram B. Deep Learning at Chest Radiography: Automated Classification of Pulmonary Tuberculosis by Using Convolutional Neural Networks. Radiology. 2017;284:574–582. doi: 10.1148/radiol.2017162326. [DOI] [PubMed] [Google Scholar]
- 13.Debats OA, Litjens GJ, Barentsz JO, Karssemeijer N, Huisman HJ. Automated 3-dimensional segmentation of pelvic lymph nodes in magnetic resonance images. Med Phys. 2011;38:6178–6187. doi: 10.1118/1.3654162. [DOI] [PubMed] [Google Scholar]
- 14.Lee Yung-Chie, Wu Chen-Tu, Kuo Shuenn-Wen, Tseng Yu-Ting, Chang Yih-Leong. Significance of Extranodal Extension of Regional Lymph Nodes in Surgically Resected Non-small Cell Lung Cancer. Chest. 2007;131(4):993–999. doi: 10.1378/chest.06-1810. [DOI] [PubMed] [Google Scholar]