Abstract
GC–MS metabolomics was used to discriminate the phytochemicals profile of Indonesian white, red, and black rice brans, and Japanese white rice brans. This technique was used for the first time to identify compounds in rice brans having cytotoxic activity against WiDr colon cancer cells. Orthogonal Projection to the Latent Structure (OPLS) analysis showed that protocatechuic acid (PA) was a discriminating factor found in black rice brans which strongly correlated with its cytotoxicity (IC50 8.53 ± 0.26 µM). Real time-PCR data demonstrated that PA cytotoxicity at different concentrations (1, 5, 10, 25 and 50 µg/mL) was mediated through different pathways. Bcl-2 expression was downregulated at all tested concentrations indicating apoptosis stimulation. At 1–10 ppm concentration, PA activated both intrinsic and extrinsic apoptosis pathways since the expression of p53, Bax, caspase-8, and caspase-9 were upregulated. At a higher dose (25 and 50 µg/mL), PA possibly involved in pyroptosis-mediated pro-inflammatory cell death by upregulating the expression of caspase-1 and caspase-7.
Electronic supplementary material
The online version of this article (10.1007/s10068-019-00725-2) contains supplementary material, which is available to authorized users.
Keywords: Rice brans, Cytotoxicity, Apoptosis, Metabolomics, Protocatechuic acid
Introduction
Cancer is the first leading cause of death in developed countries and the second in developing countries. Adoption of cancer-associated lifestyles such as smoking, physical inactivity, and ‘westernized’ diet, is one of the factors for increasing cancer occurrence in the last group of countries. Fifty-six percent of the cancer cases and 64% of cancer related-death occurred in the developing countries, most probably because of late-stage diagnosis and limited access to proper treatment. Colorectal cancer is one of the most common cancer including in Asia (Jemal et al., 2011). Living a healthy lifestyle including consuming healthy food is one of important preventive options to reduce cancer occurrence.
Rice bran is currently seen as underutilized waste-product of rice milling. Indonesia is among the eleven-top rice-growing countries in the world, contributing 8.8% of world rice production. The country’s rice bran production reaches 50.7 million tons/year and most of them are commonly used for animal feed. Recent researches showed that rice bran is a rich source of many potentially human health beneficial compounds. Various bioactivities of rice brans were reported including compounds responsible for the activity. Tocotrienol, tricin, triterpene alcohol, and sterol ferulates were considered as important active compounds in rice brans (Iqbal et al., 2005; Lerma-García et al., 2009), although more potential candidates still need to be elucidated. Factors such as rice varieties and geographical origin may affect phytochemical contents and bioactivities properties of rice brans (Laokuldilok et al., 2011). Rice brans ethanolic extracts obtained from 35 Srilankan rice varieties were reported to have in vitro α-amylase inhibitor and anti-glycation activities. The red rice brans were shown to have the higher activity as compared to the white ones (Premakumara et al., 2013). Protease-hydrolyzed rice brans extract was reported to reduce oxidative stress and atherosclerosis plaque formation in the aorta of ApoE –/– mice fed with a high-fat/cholesterol diet (HFD) after 21 weeks of intervention. The authors proposed that ferulic acid is the responsible compounds for the reported bioactivity (Perez-Ternero et al., 2017). In another study, a glycoprotein fraction of defatted rice brans was tested for immune-stimulatory activity in vitro. It was found that the glycoprotein was able to stimulate the secretion of TNF-α, IL-1β, and IL-6 in the mouse RAW 264.7 macrophage cell line (Park et al., 2013). The cytotoxicity of methanolic and dichloromethane extract of purple rice brans from three varieties of Thailand rice were also reported. All extracts showed good cytotoxicity against human hepatocellular carcinoma HepG2, prostate cancer LNCaP and murine normal fibroblast NIH3T3 cells. The authors suggested that γ-oryzanol and anthocyanins were the compounds important for the activity (Banjerdpongchai et al., 2014). However, the studies on cytotoxic compounds from Indonesian pigmented and unpigmented rice brans against colon cancer cell lines and their mechanism of action are still very scarce.
Metabolomics has been reported as a powerful tool to rapidly identify active compounds from a complex mixture such as plant extract (Yuliana et al., 2013). The method offers more benefits than classical bioassay-guided isolation where the target compounds must be isolated first before identification step. The technique can be coupled to different analytical platforms such as NMR (Yuliana et al., 2013), or GC–MS (Swamy et al., 2017). GC–MS is viewed as a highly efficient, sensitive, and reproducible analytical tool for small molecules analysis (Garcia and Barbas, 2011). LC–MS and GC–MS were used for quantitative analysis of phenolic acids content in several herbs (oregano, thyme, sage, and rosemary) and the results were compared. The authors reported that GC–MS was more stable and repeatable as compared to LC–MS. It also exhibited better selectivity and sensitivity. Compounds identification was also easier since this step is assisted by the use of built-in spectral library and fragmentation pattern (Kivilompolo et al., 2007). A combination of LC–MS and GC–MS based metabolomics was previously conducted to reveal phytochemicals profile of rice brans from three US rice brans varieties. The authors successfully identified 453 metabolites which consisted of various cofactors, vitamins, amino acids and several secondary metabolites (Zarei et al., 2017). A comprehensive rice brans metabolomics study which involved 17 rice cultivars from 7 countries was recently reported. The authors identified 34 metabolites which varied among different cultivars. Their health-related bioactivity was summarized from other previous reports, then their relationship with a number of genes responsible for various biosynthetic pathway in rice was determined. Such study is important as initial information when the bioactive metabolites content of the rice would be improved by rice breeding (Zarei et al., 2018). So far there is no report on the use of GC–MS based metabolomics which seek the correlation between rice brans’ metabolites and their bioactivities. Such correlation allows direct identification of the active metabolites from the crude or half-fractionated extracts. Once the activity is identified, a study to investigate the respective bioactivity mechanism can be conducted.
For the above-mentioned reasons, we chose to use GC–MS-based metabolomics to investigate compounds from rice brans which associate with cytotoxicity against WiDr human colon cancer cell. We used multivariate data analysis (PLS-DA and OPLS) to differentiate the chemical profile of different type of rice brans and to link their GC–MS profile to WiDr cytotoxic activity. The compounds which strongly correlate with the activity can be rapidly identified. Chemical validation was conducted by testing the cytotoxic activity of the pure identified active compound. This study reported for the first time, GC–MS metabolomics application to identify compounds in rice brans which has a strong correlation with the cytotoxic activity against human colon cancer lines. The cytotoxicity mechanism of the compound was studied further by quantifying the expression of several apoptosis-related genes such as caspases, p53, Bax, and Bcl-2 using the real time-PCR technique.
Materials and methods
Materials
Indonesian IR-64 white rice var. Ciherang (IWR), Indonesian red rice var. Cere (IRR), Indonesian black rice var. Cempoireng (IBR) was purchased from a local farmer in Bogor, West Java, Indonesia. The rice was immediately polished upon arrival according to the method described in the next section. Japanese white rice brans var. Japonica (JWR) was generously provided by Dr. Takuya Koseki (Yamagata University, Tsuruoka, Yamagata Perfecture, Japan). Methanol, N-methylN (trimethylsilyl) trifluoro acetamidepurum 97.0% (GC grade), methoxyamine hydrochloride (98%) were from Merck (Darmstadt, Germany). RPMI 1640 medium, DMEM (Dulbecco’s Modified Eagle Medium), trypsin, trypane blue, 3-(4,5-dimethylthiazol-2-yl)-2,2- diphenyltetrazolium bromide (MTT) were from Sigma Aldrich (Sigma Aldrich, St.Louis, MO). Methoxyl-amine-hydrochloride (98%) was from Fisher Scientific (Acros Organics, Geel, Belgium). All chemicals are of analytical grade. WiDr cells were obtained from CRRC University of Gadjah Mada, Yogyakarta, Indonesia. Chang cells were obtained from the Primate Studies Center, Bogor Agricultural University, Bogor, Indonesia.
Rice brans extract preparation
Rice grains were polished for 2 min., the obtained fresh rice brans were sieved (100 mesh), and stabilized using an autoclave (120 °C, 10 min). 50 g of rice brans was extracted using twice volume of methanol by 30 min sonication followed by filtration. The extraction was repeated three times. The filtrates were pooled, concentrated using rotary evaporator (< 40 °C), and put under nitrogen gas into dryness. Three to five replicates were prepared for each rice brans. The extracts were stored in the freezer prior to the analysis.
Cytotoxicity assay
Cytotoxicity assay was performed as described previously (Yulianto et al., 2016) with modification in the cell lines type. WiDr (ATCC CCL-218) and Chang (ATCC CCL-13) cells were seeded at a concentration of 2500 cells in 100 µL of DMEM growth medium containing 10% fetal bovine serum, 100 U/mL penicillin, and 100 µg/mL streptomycin 1%. Cells were incubated at 37 °C temperature and 5% CO2. Chang cells are non-tumor cells derived from the human hepatoma cell line. This cell was used to check the toxicity of the studied extract against normal cells (non-tumor cells). Different type of rice brans extract at different concentration was added after the cells reach 50% confluent (24 h). Cytotoxic activity was measured using a colorimetric MTT assay. MTT assay was performed on the fourth day, by adding tetrazolium salt (5 mg/mL) 10 µL per well and then incubated for 4 h (37 °C) to allow the formation of purple formazan crystals. The crystals were dissolved in 150 mL of ethanol, then the absorbance at 595 nm wavelength was read using a microplate reader (Bio-Rad, USA). The analysis was performed in triplicate. Significance of difference between means was calculated using one-way or two-way ANOVA followed by Tukey’s test (p < 0.05), while the statistical significance of the differences between cytotoxicity of protocatechuic acid with the 5-fluorouracyl was analyzed by Student’s t test (p < 0.05) using Microsoft Excel.
Gene expression analysis using real time-PCR
WiDr colon cancer cells were seeded in 12 wells plate containing RPMI-1640 and 10% PBS at 2 × 104 cells/well concentration. After that it was incubated for 24 h at 37° C. The media is then replaced with PCA at several concentrations. Cells without treatment were used as controls and the well without cells was used as a blank. After 48 h, the cells were harvested and stored at − 20 °C until the next step. RNA was extracted using Rneasy Mini Kit (Qiagen) according to the procedure described in the kit manual. The RNA concentration was calculated using Nano Drop. First strand cDNA is synthesized from RNA using Superscript III First Strand Synthesis System for RT-PCR. Gene expression was determined using real time-PCR with SSo Fast Evagreen Supermix after temperature optimization according to the procedure in the kit after temperature optimization. The genes tested were caspase 1, 7, 8, 9; Bax, Bcl-2, and p53 as well as Beta actin (ACTB) are used as housekeeping genes. The result was calculated using delta–delta cycle threshold (ΔΔCT) which was normalized using ACTB. The data was expressed as a mean value ± standard deviation (n = 3). One way ANOVA followed by Dunnett’s multiple comparison test was used to determine the significance of the PCA treatment with the control using Microsoft Excel and Graphpad InStat Demo version. A p-value of less than 0.05 was considered as statistically significant.
GC–MS analysis
Derivatization: 25 mg sample extract was put in a 2 mL centrifuge tube, immersed in 50 μL of pyridine and sonicated for 10 min (30 °C). The mixture was vortexed after the addition of 100 μL of methoxyamine-HCl (20 mg/mL in pyridine) and incubated for 2 h (60 °C). After the addition of 300 μL of MSTFA (N Methyl-N-(trimethylsilyl) trifluoroacetamide), the mixture was incubated for 30 min (60 °C), filtered, and covered with aluminum foil and left to stand overnight at room temperature (Javadi et al., 2014).
GC–MS analysis: 1 μL of the derivatized sample was injected in the splitless mode into the GC–MS system, which consisted of an Agilent 6890 gas chromatograph, an HP 5973 mass selective detector, and DB-5MS 5% phenyl methyl siloxane column (length 30 m, the inner diameter 250 μm, a film thickness 0.25 M μm). This column is solvent-rinseable, has high-temperature limit, and applicable for non-polar compounds as mentioned in the manual from the manufacturer. The initial oven temperature was set to 170 °C (5 min), and then increased to a target temperature of 315 °C (20 min) at a rate of 10 °C/min. Helium was used as the carrier gas at a flow rate 1 mL/min. The injector and ion source temperatures were set to 325 (250 °C). Mass spectra was acquired using a full scan and a monitoring mode with a mass scan range of 50 to 550 m/z after a solvent delay of 7 min. The spectra for each of the chromatogram peaks were compared with those in the NIST14 database library and the literature. The chromatogram and mass spectra were processed using an Agilent ChemStation, Automated Mass Spectral Deconvolution and Identification System (AMDIS) and Agilent’s Deconvoluted Reporting Software (DRS). A data preprocessing approach was employed after GC–MS to extract all of the related information from the raw data and to convert it into a data matrix.
Multivariate data analysis
Principal component analysis (PCA), partial least square discriminant analysis (PLS-DA) and Orthogonal Projection to the Latent Structure analysis (OPLS) were performed with SIMCA-P software (v. 14.0, Umetrics, Umeå, Sweden) using UV or Pareto scaling method (Yuliana et al., 2013).
Results and discussion
Cytotoxic activity profile of rice brans
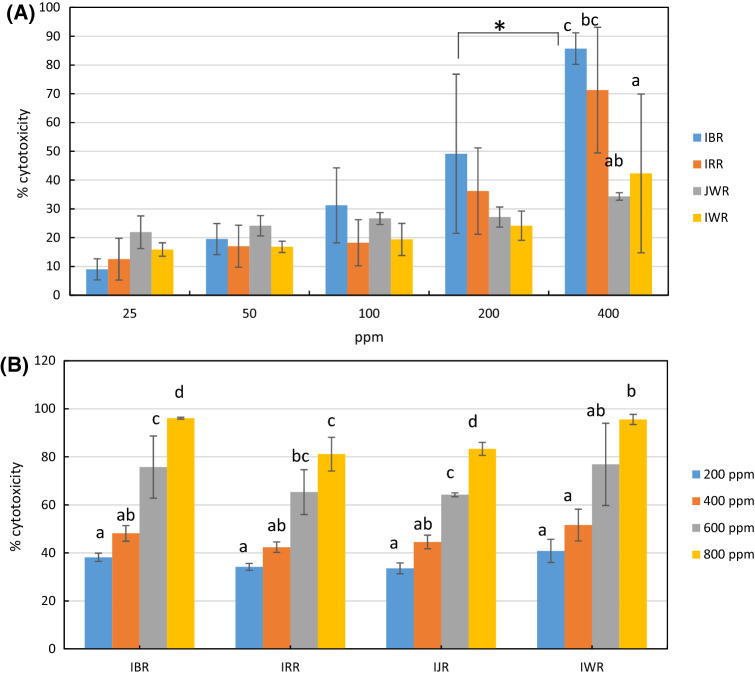
All rice brans sample showed significantly higher cytotoxic activity against WiDr cells at 400 ppm as compared to other concentrations. At 400 ppm, black rice brans had the highest cytotoxic activity against WiDr cells among other rice brans followed by red rice brans (86% and 71%, respectively). At this concentration, Indonesian and Japanese rice brans did not show noteworthy activity (22% and 34%, respectively). Black rice brans at 400 ppm also showed significantly higher activity than black rice brans at 200 ppm dose (Fig. 1A). All extracts showed cytotoxic activity against normal cells (Chang cells) in dose response manner but with no significant differences at 200 and 400 ppm dose (Fig. 1B). In case of black rice brans, at 400 ppm the cytotoxicity against normal cells was lower than cytotoxicity against WiDr cells. Thus, 400 ppm concentration was chosen to be used for the next metabolomics experiment.
Fig. 1.
Cytotoxic activity of different type of rice brans methanolic extracts at different concentration against normal cell lines (A) and WiDr cell lines (B). The values were the means of three to five replicates, each was analyzed in triplo. IBR = Indonesian black rice brans, IRR = Indonesian red rice brans, JWR = Japanese white rice brans, IWR = Indonesian white rice brans. Different letters or Asterix symbol shows significant differences in cytotoxicity at p < 0.05 (ANOVA, Tukey’s test)
Previous in vitro studies reported cytotoxic activity of rice brans against several colon cancer lines such as SW480, SW620, SGC-7901, HT-29, HCEC, CaCo-2, and HCT-116. Mechanisms involved in the cytotoxic activity are diverse; inducing apoptosis by elevating enzymes and proteins such as caspase-3 and caspase-8, inhibiting cells proliferation, and inhibiting cells growth by cell cycle arrests (Henderson et al., 2012). Different cancer cell lines might response differently against the given treatments. Here we reported for the first time cytotoxic activity of different type of rice brans against WiDr colon cancer lines. WiDr is a colon adenocarcinoma line derived from HT-29 (ATCC HTB-39). The cells expressed p53 antigen with a G to A mutation, resulting in Arg to His at position 273 (http://www.atcc.org/products).
Black rice brans were reported to have a higher anti-carcinogenic activity over non-pigmented rice brans against human leukemia HL-60, marmoset B lympho-blastoid B95-8, and Chinese hamster V79 lung cells (Nam et al., 2005). However, phytochemicals responsible for black rice brans superior performance were not reported in their study. Pigmented rice brans were rich in cyanidin-3-glucoside. Black rice brans contained higher gallic, hydroxybenzoic, and protocatechuic acids than red rice brans and normal rice brans (Laokuldilok et al., 2011). Confirmation whether any of the abovementioned compounds are responsible for the high cytotoxicity of black rice brans is required.
Rice brans GC–MS profile
Gas chromatography coupled to mass spectrometry (GC–MS) has been known as a gold standard in the field of analytical science. This instrument uses both retention time and mass spectral data to identify a compound of interest. Mass spectral data shows fragmentation pattern specific for a particular compound obtained after ionization by a fixed electron voltage. The data can be built into a database which can be shared among the GC–MS users (Garcia and Barbas, 2011). This is one of the reasons why GC–MS is widely used for metabolomics based work. Plenty of studies regarding the use of GC–MS for metabolomics were reported. Most of these studies focused on metabolites discrimination as results from different genotype/cultivar, climate, cultivation method, and post-harvest handling. As one of example, the metabolites differences between two genotypes of Salvia hispanica (chia seed) as a response to different levels of irrigation (irrigated and non-irrigated) was revealed by GC–MS based metabolomics (de Falco et al., 2018). It was found that the irrigated samples contained a higher amount of total phenolics, α-linolenic acid and other fatty acids, than the non-irrigated ones. The irrigated samples also showed higher antioxidant activity than the non-irrigated ones. However, which metabolites associated with the changes in antioxidant activity was not reported.
A different approach where GC–MS metabolomics was used to link changes in metabolites profile with the bioactivity of the treated was recently reported (Javadi et al., 2014). Metabolites variations in Malaysian herbal Cosmos caudatus extracts resulted from different extraction solvent was linked to their α-glucosidase inhibitory activity. As results, catechin, α-linolenic acid, and vitamin E were found to strongly associate with the activity. A similar approach was applied in this reported study. The representative chromatogram of each type of rice brans and putative compounds attributed to the peaks appearing in the chromatograms are presented in supplementary Figure (Fig. S1). Noticeably, peak at 7.8 min was only seen in IBR. The fragmentation pattern of the peak was then compared with those in the database library (NIST-14) and it was identified as protocatechuic acid. Other peaks which were more commonly found in other rice brans samples are attributed to glucose, fructose, and fatty acid. Next, several multivariate data analysis techniques such as PCA, PLS-DA, and OPLS were used to find the metabolites unique to each group in more detail and to assess their contributions to the cytotoxic activity.
PCA and PLS-DA Analysis
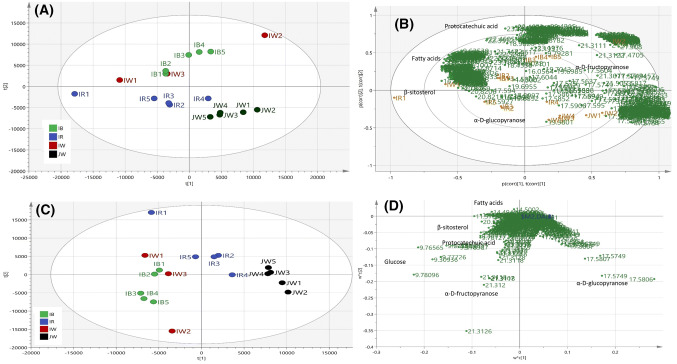
PCA is one of the most commonly used multivariate data analysis methods to observe the classification of samples by projecting the original multidimensional data into different principal components (PCs) so that variability of the samples can be evaluated in the simpler manner (Eriksson et al., 2013). Here PCA was used to get an overview of the classification pattern of different type of rice brans. The resulted PCA model had R2X cumulative of 97.6% and cumulative Q2 0.89 which means the model is reliable (Fig. 2A). It can be seen that Japanese white rice bran was separated from three other rice bran groups. Amongst Indonesian rice brans, red rice brans, and black rice brans clustered separately while Indonesian rice brans rather scattered between red and black rice brans. PCA loading bi-plot gave information on discriminating compounds for each group (Fig. 2B). Identification of these compounds was done by comparing with the library and also the data from previous studies. Relatively nonpolar compounds such as linoleic acid, octadecanoic acid, hexadecanoic acid, and beta-sitosterol were found to be higher in Indonesian rice brans. The presence of the fatty acids was marked by characteristics fragmentation pattern m/z 73, 117, 132, 145 and 313 (Carballeira and Lopez, 1989). Red rice brans and Japanese white rice brans were grouped close to each other and this group was marked by the higher amount of α-D-glucopyranoside. α-D-glucopyranoside was indicated by an intense relative intensity fragment at m/z 204 (da Silva et al., 2016). Protocatechuic acid was the discriminating compound in black rice brans. The mass fragmentation characteristic of protocatechuic acid TMS derivative was marked by the presence of fragment with m/z [M-177]+ 193, [M-89]+ 281, [M-15]+ 355, and parent ion M.+ 370 (Robbins, 2003). More distinct clustering was obtained by PLS-DA score plot (Fig. 2C), while PLS-DA loading plot gave similar information with those of PCA (Fig. 2D).
Fig. 2.
(A) PCA score plot and bi plot of rice brans’ GC–MS data explaining. (B) PCA loading bi-plot indicating several discriminating compounds for each cluster. (C) PLS-DA score plot shows more distinct classification than PCA, R2Y cumulative 0.845 Q2 cumulative = 0.582 D. PLS-DA loading plot indicating similar information about each group’s discriminating compounds. IBR = Indonesian black rice brans, IRR = Indonesian red rice brans, JWR = Japanese white rice brans, IWR = Indonesian white rice brans. Number 1 to 5 representing replications
OPLS analysis
OPLS is the recent modification of PLS (projection to the latent structure by mean least square) analysis. OPLS separates systematic variations in X (variables) into two parts, the first part that is related to Y and the other which is not related (orthogonal) to Y (Eriksson et al., 2013). Thus, OPLS improves the modeling power of PLS. In this research, GC–MS spectra of the rice brans extracts were used as X-matric, while the extracts cytotoxic activities against WiDr cells was used as Y-matric. As a result, the OPLS model explained 82.3% variation of the data, with the value of R2Y 0.771, and Q2 0.598. Permutation test conducted with 100 permutations resulting in value of R2 0.28 and Q2-1.13. All validation value indicating that rice brans GC–MS and WiDr cytotoxicity data correlation analysis were well modeled by OPLS (Eriksson et al., 2012).
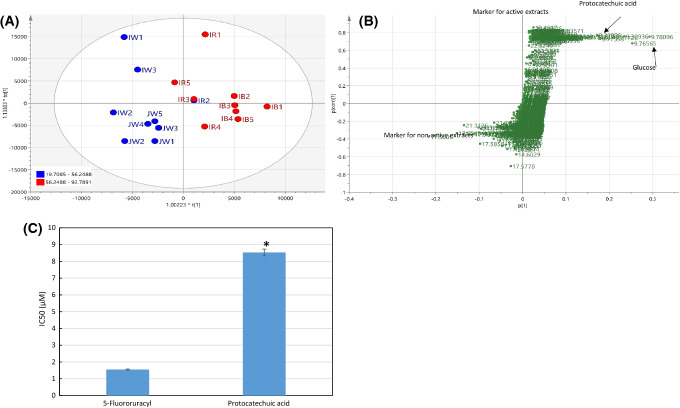
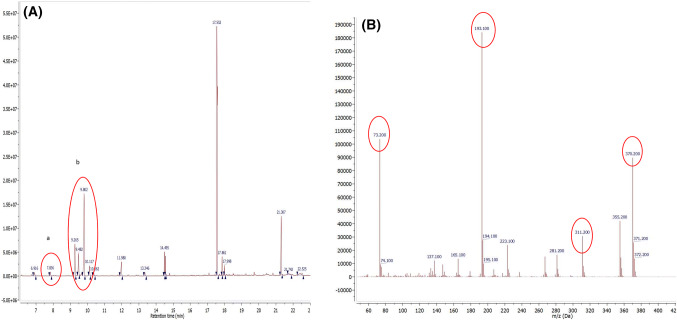
In the OPLS score plot, the more active rice brans were clustered differently from the less active ones (Fig. 3A). The OPLS S-plot was used to examine which compounds strongly associated with the two groups separation. TMS derivatives of d-glucose, d-fructose and protocatechuic acid, were found as the markers of the active groups (Fig. 3B) with variable of importance (VIP) value of d-glucose, d-fructose, and protocatechuic acid were 5.96, 4.76, and 1.18, respectively. The MS characteristics of fructose TMS derivative were observed from fragment with m/z and relative intensity 73 (100%), 217 (55%), and 147 (25%), while for glucose TMS derivative 204 (100%), 191(41%), 217(18%) (da Silva et al., 2016). MS characteristic for protocatechuic acid TMS derivative was explained previously while the chromatogram and the mass spectrum are presented in Fig. 4. All these compounds had positive Y-related coefficient value which means higher intensity of the respective compounds resulting in higher cytotoxic activity. Indeed, in their X-varian plot which shows information on compounds distribution over the fractions, the highest relative concentration of these three compounds were found in black rice samples (data not shown).
Fig. 3.
(A) OPLS score scater plot of rice brans’ GC–MS data. Red color representing samples with WiDr cytotoxic activity higher than 56.2% at 400 ppm while blue color for activity lower than 56%. (B) OPLS S-Plot of rice brans’ GC–MS data showing that protocatechuic acid, d-glucose and d-fructose had a strong correlation with WiDr cytotoxic activity. (C) Cytotoxic activity of protocatechuic acid is significantly lower than control drug 5-fluorouracyl (Student’s t test, p < 0.05) but still in acceptable micromolar range. The value is the mean of 3 replications
Fig. 4.
(A) Chromatogram of representative of black rice brans showing peaks at 7.8 min and 9.2–9.8 min which shown as strongly associated with cytotoxic activity as mentioned in the S-plot. (B) Mass spectrum of peak at 7.8 min which is attributed to protocatechuic acid
The strong correlation of d-glucose and d-fructose with rice brans cytotoxic activity needs further investigation. Antitumor activity of polysaccharides derived from rice brans was reported previously (Wang et al., 2016). However, whether the presence of these monosaccharides was originated from rice brans polysaccharides needs further confirmation. We focused to discuss on protocatechuic acid (PA), which also positively correlated with rice brans cytotoxic activity although weaker than the monosaccharides. PA was the major free phenolic compound found in Thai purple rice which the amount increased during cooking. The cooked rice containing the highest amount of PA demonstrated the highest anti-proliferative activity against human colon cancer cell Caco-2 (Chatthongpisut et al., 2015). PA is also present in other daily food such as in nuts, fruits, and vegetables. There is a number of studies discussing the toxicity of this compound in vitro using normal cells line (Lin et al., 2011; Yin et al., 2009) or in vivo using experimental animal (Tanaka et al., 2011).
Cytotoxic activity of PA
To confirm PA cytotoxic activity in WiDr cell lines, we tested PA reference compounds at different concentration. The IC50 was calculated and compared to the reference drug (5-fluorouracyl). PA was shown to have strong cytotoxic activity (IC50 8.53 ± 0.26 µM, or 1.3 µg/mL) although weaker than the drug (1.548 ± 0.038 µM), as shown in Fig. 3C. In other studies, PA anticancer related activity in experimental animals was reviewed elsewhere (Tanaka et al., 2011). It was reported that PA showed cancer preventive activity at the dose between 200 and 2000 ppm in the different type of cancers (oral cavity, liver, pancreatic, skin, and urinary bladder) in rodent. PA was reported as one of the major free phenolic acids in Thai purple rice cooked with a different method. PA cytotoxic effect at a concentration up to 8 µM on human breast cancer MCF7 cell, lung cancer A549 cell, HepG2 cell, cervix HeLa cell, and prostate cancer LNCaP cell were reported. The authors mentioned that this compound exerted their activity through apoptopic stimulation since it raised caspase-3 activity in the above-mentioned cancer cells and significantly increased caspase-8 activity at 2-8 μM concentration (Yin et al., 2009). However, PA cytotoxicity in human colon cancer cell WiDr and their mechanism are not reported yet.
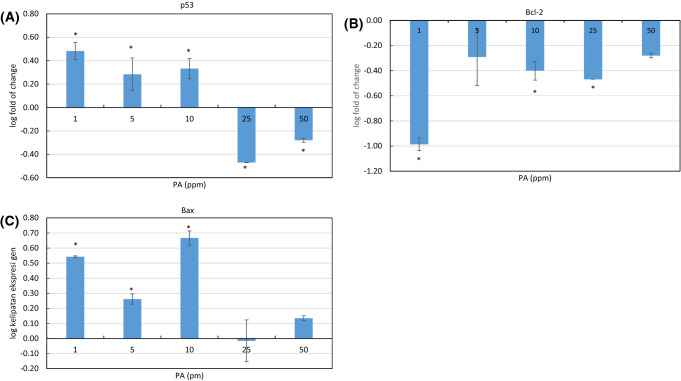
Observation of p53 gene expression was conducted to confirm PA apoptopic stimulation effect. P53 is the gene encoding tumor suppressor protein which responds to various cellular stress such as DNA damage or cell hypoxia. These stressors result in a rapid increment of p53 expression level which leads to various downstream responses such as intrinsic or extrinsic apoptosis. In the intrinsic pathway, p53 directly acts as the transcriptional activator of Bax gene. Upon its activation, Bax triggers mitochondria dysfunction which followed by caspases activation (Fulda et al., 1997; Levine, 1997). At 1–10 ppm concentration, PA stimulated p53 expression but at higher concentration (25 and 50 ppm), p53 was downregulated (Fig. 5A). Meanwhile, at all tested concentrations PA downregulated anti-apoptopic protein Bcl-2 with the highest decrement was at 1 ppm dose. At higher concentration the reduction still occurred but at a lower extent (Fig. 5B). Bcl-2 acts as an apoptosis inhibitor. It advantages cancer cells survival leading to their uncontrolled growth. Bcl-2 overexpression could halt p53 mediated apoptosis (Sen and D’Incalci, 1992). This might associate with p53 downregulation at 25 and 50 ppm concentration of PA. Oppositely, Bax, a pro-apoptopic protein binds to Bcl-2 but antagonizes its anti-apoptopic property, was upregulated at PA 1–10 ppm dose but with lower increment at a higher dose (Fig. 5C). PA was reported to upregulate Bax and caspase-3, but downregulated Bcl-2 in OVCAR-3 human ovarian cancer cells at the dose of 5, 10, 15, 20, and 25 µM (Xie et al., 2018). P53 was also initially upregulated, however, after 12 h. there was a slight reduction in p53 expression in the cell treated with 25 µM PA, almost similar situation with our study. In our study, WiDr cells were treated with PA for 48 h. Apparently, at the higher dose and longer incubation time, PA induced apoptosis in p53 independent pathway. In a previous study, cycloartenyl ferulate, a polyphenol isolated from rice bran, was reported to inhibit the growth of human colorectal adenocarcinoma SW480 in vitro. Beside interfering mitochondrial apoptosis pathway, the compound was also found to act through p53 dependent pathway, that is by elevating the expression of death receptor DR4 and DR5 (Kong et al., 2009).
Fig. 5.
The effect of PA at different concentration (1, 5, 10, 25, 50 ppm) to the expression of several apoptosis related-genes; p53 (A), Bcl-2 (B) and Bax (C), as measured by real time-PCR. Values are mean ± standard deviation (n = 3), *p < 0.05 versus control (ANOVA, Dunnett’s test)
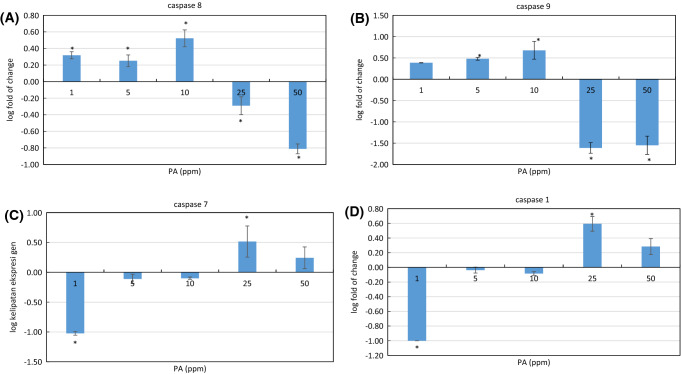
We further examined the effect of PA treatment to the expression of caspase-1, caspase-3, caspase-8, and caspase-9 in WiDr cells. Caspases family consists of proteolytic enzymes known for their importance in apoptosis and inflammation as reviewed elsewhere. Caspase-1 involved in inflammatory-mediated cell death or pyroptopic cell death, caspase-8 and caspase-9 are apoptosis initiator, while caspase-3 and caspase-7 are apoptosis executor. Caspase-9 activation in the intrinsic pathway is triggered by activation of p53 and pro-apoptosis protein such as Bax protein, while caspase-8 is activated separately via the extrinsic pathway through the death-inducing signaling complex (DISC). The activation of initiator caspases are subsequently followed by activation of effector caspase-3, caspase -6, and caspase -7 (Shalini et al., 2015). WiDr cells treated with PA at 1-10 ppm were shown to upregulate initiator caspase-8 and caspase-9, but the opposite effect was observed at the higher dose of 25 and 50 ppm (Fig. 6A, B). At 1–10 ppm dose, the effector caspase-7 was downregulated but then upregulated at the higher dose (Fig. 6C). Caspase-1 acts differently than other caspases which were discussed previously. Caspase-1 is activated by a different type of NOD- like receptors (NLRs) depends on the stimuli given. Caspase-1 is required to activate inflammatory cytokines which stimulate pyroptosis-mediated pro- inflammatory cell death (Shalini et al., 2015). In our study, PA at the lowest dose (1 µg/mL) was found to downregulate caspase-1, but the expression of this gen increased at the higher dose, particularly at 25 ppm (Fig. 6D), indicating other pathway related to inflammatory also involved in PA-induced programmed cell death. A similar effect was observed with caspase-7. At 1 µg/mL, PA downregulated caspase-7 but the opposite effect was seen at higher concentration. Caspase-7 was seen to be activated not only during apoptosis but also in inflammatory condition as reviewed elsewhere (Lamkanfi and Kanneganti, 2010). Activation of caspase-7 requires caspase-1 modulation, not caspase-8 and caspase-9. This activation is also different from the caspase-3 pathway (not measured in this experiment).
Fig. 6.
The effect of PA at different concentration (1, 5, 10, 25, 50 ppm) to the expression of several apoptosis related-genes; caspase-8 (A), caspase-9 (B) and caspase-1 (C), and caspase-7 (D), as measured by real time-PCR. Values are mean ± standard deviation (n = 3), *p < 0.05 versus control (ANOVA, Dunnett’s test)
The results of this study showed that rice brans of different variety and different geographical origins had different phytochemicals profile. This implies their in vitro cytotoxic effects on WiDr human colon cancer cells. Black rice brans were shown to have the highest cytotoxic activity followed by red rice brans. Protocatechuic acid (PA) was identified as the compound with strong correlation with the cytotoxic activity. This was validated by measuring cytotoxic effect of protocatechuic acid reference compound and indeed it showed good activity (IC50 8.53 ± 0.26 µM). The results of real time-PCR analysis revealed that protocatechuic acid promoted programmed cell death through different pathways depends on the dose. At a lower dose (1-10 µg/mL), the compound stimulated the intrinsic apoptosis pathway by upregulating p53, Bax, and caspase-9. At the same dose, it also activated the extrinsic pathway by modulating caspase-8. At the higher dose (25–50 µg/mL), protocatechuic acid upregulated caspase-1 and caspase-7 which indicates that the compound possibly involved in pyroptosis-mediated pro- inflammatory cell death.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1 Representative chromatograms of rice brans. Putative compound were numbered from 1 to 10 and determined by comparing with the compounds data base in NIST-14 Library which have the highest similarity percentage (1 = Protocatechuic acid, 2 = Fructose, 3 = Glucose, 4 = Hexanoic acid, 5 = Inositol, 6 = 9,12 - Octadecadienoic acid, 7 = Oleic acid, 8 = Octadecanoic acid, 9 = α-D-Glucopyranoside, 10 = β-sitosterol (PPTX 504 kb)
Acknowledgements
Ministry of Research and Higher Education Republic of Indonesia is gratefully acknowledged for partially funding this research through International Collaboration Research scheme with contract number 631/IT3.11/PL/2015.
Authors contribution
NDY: writing the manuscript, responsible for multivariate data analysis. MZT: responsible for sample extraction and in vitro experiment, proof read the manuscript. AK: responsible for GC–MS measurement, proof read the manuscript. FL: responsible for RT PCR experiment, proof read the manuscript. S: checking and read carefully the manuscript for any inappropriate content and misspelling.
Compliance with ethical standards
Conflict of interest
None.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Nancy Dewi Yuliana, Email: nancy_dewi@ipb.ac.id.
Mirna Zena Tuarita, Email: mirnatuarita@gmail.com.
Alfi Khatib, Email: alfikhatib@iium.edu.my.
Farida Laila, Email: flaila.safire@gmail.com.
Sukarno Sukarno, Email: dsukarno@gmail.com.
References
- Banjerdpongchai R, Wudtiwai B, Sringarm K. Cytotoxic and apoptotic-inducing effects of purple rice extracts and chemotherapeutic drugs on human cancer cell lines. Asian Pac. J. Cancer Prev. 2014;14:6541–6548. doi: 10.7314/APJCP.2013.14.11.6541. [DOI] [PubMed] [Google Scholar]
- Carballeira NM, Lopez MR. On the isolation of 2-hydroxydocosanoic and 2-hydroxytricosanoic acids from the marine spongeAmphimedon compressa. Lipids. 1989;24:89–91. doi: 10.1007/BF02535272. [DOI] [PubMed] [Google Scholar]
- Chatthongpisut R, Schwartz SJ, Yongsawatdigul J. Antioxidant activities and antiproliferative activity of Thai purple rice cooked by various methods on human colon cancer cells. Food Chem. 2015;188:99–105. doi: 10.1016/j.foodchem.2015.04.074. [DOI] [PubMed] [Google Scholar]
- Eriksson L, Rosén J, Johansson E, Trygg J. Orthogonal PLS (OPLS) modeling for improved analysis and interpretation in drug design. Mol. Inform. 2012;31(6–7):14–419. doi: 10.1002/minf.201200158. [DOI] [PubMed] [Google Scholar]
- de Falco B, Fiore A, Bochicchio R, Amato M, Lanzotti V. Metabolomic analysis by UAE-GC MS and antioxidant activity of Salvia hispanica (L.) seeds grown under different irrigation regimes. Ind. Crops Prod. 2018;112:584–592. doi: 10.1016/j.indcrop.2017.12.030. [DOI] [Google Scholar]
- Fulda S, Friesen C, Los M, Scaffidi C, Mier W, Benedict M, Nuñez G, Krammer PH, Peter ME, Debatin KM. Betulinic acid triggers CD95 (APO-1/Fas)- and p53-independent apoptosis via activation of caspases in neuroectodermal tumors. Cancer Res. 1997;57(21):4956–4964. [PubMed] [Google Scholar]
- Garcia A, Barbas C. Gas chromatography–mass spectrometry (GC–MS)-based metabolomics. pp.191-204. In: Methods in molecular biology. Metz TA (ed). Humana Press, Totowa, NJ, USA (2011) [DOI] [PubMed]
- Henderson AJ, Ollila CA, Kumar A, Borresen EC, Raina K, Agarwal R, Ryan EP. Chemopreventive properties of dietary rice bran: current status and future prospects. Adv. Nutr. Int. Rev. J. 2012;3:643–653. doi: 10.3945/an.112.002303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- http://www.atcc.org/products/all/CCL-218.aspx#characteristics
- Iqbal S, Bhanger MI, Anwar F. Antioxidant properties and components of some commercially available varieties of rice bran in Pakistan. Food Chem. 2005;93:265–272. doi: 10.1016/j.foodchem.2004.09.024. [DOI] [Google Scholar]
- Javadi N, Abas F, Hamid AA, Simoh S, Shaari K, Ismail IS, Mediani A, Khatib A. GC–MS-based metabolite profiling of Cosmos caudatus leaves possessing alpha-glucosidase inhibitory activity. J Food Sci. 2014;79(6):C1130–C1136. doi: 10.1111/1750-3841.12491. [DOI] [PubMed] [Google Scholar]
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J. Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- Kivilompolo M, Obůrka V, Hyötyläinen T. Comparison of GC–MS and LC–MS methods for the analysis of antioxidant phenolic acids in herbs. Anal. Bioanal. Chem. 2007;388:881–887. doi: 10.1007/s00216-007-1298-8. [DOI] [PubMed] [Google Scholar]
- Kong CKL, Lam WS, Chiu LCM, Ooi VEC, Sun SSM, Wong Y-S. A rice bran polyphenol, cycloartenyl ferulate, elicits apoptosis in human colorectal adenocarcinoma SW480 and sensitizes metastatic SW620 cells to TRAIL-induced apoptosis. Biochem. Pharmacol. 2009;77(9):1487–1496. doi: 10.1016/j.bcp.2009.02.008. [DOI] [PubMed] [Google Scholar]
- Eriksson L, Byrne T, Johansson E, Trygg J, Vikström C. Multi- and megavariate data analysis. Basic, Principle and Application. Third Revised Edition. Umetrics Academy Umeå, Sweden. pp 362-370 (2013)
- Lamkanfi M, Kanneganti T-D. Caspase-7: a protease involved in apoptosis and inflammation. Int J Biochem. Cell Biol. 2010;42:21–24. doi: 10.1016/j.biocel.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laokuldilok T, Shoemaker CF, Jongkaewwattana S, Tulyathan V. Antioxidants and antioxidant activity of several pigmented rice brans. J. Agric. Food Chem. 2011;59(1):193–199. doi: 10.1021/jf103649q. [DOI] [PubMed] [Google Scholar]
- Lerma-García MJ, Herrero-Martínez JM, Simó-Alfonso EF, Mendonça CRB, Ramis-Ramos G. Composition, industrial processing and applications of rice bran γ-oryzanol. Food Chem. 2009;115:389–404. doi: 10.1016/j.foodchem.2009.01.063. [DOI] [Google Scholar]
- Levine AJ. p53, the cellular gatekeeper for growth and division. Cell. 1997;88:323–331. doi: 10.1016/S0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- Lin HH, Chen JH, Chou FP, Wang CJ. Protocatechuic acid inhibits cancer cell metastasis involving the down-regulation of Ras/Akt/NF-κB pathway and MMP-2 production by targeting RhoB activation. Br. J. Pharmacol. 2011;162:237–254. doi: 10.1111/j.1476-5381.2010.01022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam SH, Choi SP, Kang MY, Kozukue N, Friedman M. Antioxidative, antimutagenic, and anticarcinogenic activities of rice bran extracts in chemical and cell assays. J. Agric. Food Chem. 2005;53:816–822. doi: 10.1021/jf0490293. [DOI] [PubMed] [Google Scholar]
- Park HY, Yu AR, Choi IW, Hong HD, Lee KW, Choi HD. Immunostimulatory effects and characterization of a glycoprotein fraction from rice bran. Int. Immunopharmacol. 2013;17(2):191–197. doi: 10.1016/j.intimp.2013.06.013. [DOI] [PubMed] [Google Scholar]
- Perez-Ternero C, Werner CM, Nickel AG, Herrera MD, Motilva MJ, Böhm M, de Sotomayor MA, Laufs U. Ferulic acid, a bioactive component of rice bran, improves oxidative stress and mitochondrial biogenesis and dynamics in mice and in human mononuclear cells. J. Nutr. Biochem. 2017;48:51–61. doi: 10.1016/j.jnutbio.2017.06.011. [DOI] [PubMed] [Google Scholar]
- Premakumara GAS, Abeysekera WKSM, Ratnasooriya WD, Chandrasekharan NV, Bentota AP. Antioxidant, anti-amylase and anti-glycation potential of brans of some Sri Lankan traditional and improved rice (Oryza sativa L.) varieties. J. Cereal Sci. 2013;58:451–456. doi: 10.1016/j.jcs.2013.09.004. [DOI] [Google Scholar]
- Robbins RJ. Phenolic acids in foods: an overview of analytical methodology. J. Agric. Food Chem. 2003;51:2866–2887. doi: 10.1021/jf026182t. [DOI] [PubMed] [Google Scholar]
- Sen S, D’Incalci M. Apoptosis biochemical events and relevance to cancer chemotherapy. FEBS Lett. 1992;307(1):122–127. doi: 10.1016/0014-5793(92)80914-3. [DOI] [PubMed] [Google Scholar]
- Shalini S, Dorstyn L, Dawar S, Kumar S. Old, new and emerging functions of caspases. Cell Death Differ. 2015;22:526–539. doi: 10.1038/cdd.2014.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva RAC, de Lemos TLG, Ferreira DA, Monte FJQ. Ximenia americana chemical and spectral studies of extracts of seeds: analysis of drimethylsilyl derivatives by gas chromatography and mass spectrometry. Am. J. Anal. Chem. 2016;07(02):192–202. doi: 10.4236/ajac.2016.72016. [DOI] [Google Scholar]
- Swamy MK, Arumugam G, Kaur R, Ghasemzadeh A, Yusoff MM, Sinniah UR. GC–MS-based metabolite profiling, antioxidant and antimicrobial properties of different solvent extracts of Malaysian Plectranthus amboinicus leaves. Evid. Based Complement. Altern. Med. 2017;2017:1–10. doi: 10.1155/2017/1517683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Tanaka T, Tanaka M. Potential cancer chemopreventive activity of protocatechuic acid. J. Exp. Clin. Med. 2011;3:27–33. doi: 10.1016/j.jecm.2010.12.005. [DOI] [Google Scholar]
- Wang L, Li Y, Zhu L, Yin R, Wang R, Luo X, Li Y, Li Y, Chen Z. Antitumor activities and immunomodulatory of rice bran polysaccharides and its sulfates in vitro. Int. J. Biol. Macromol. 2016;88:424–432. doi: 10.1016/j.ijbiomac.2016.04.016. [DOI] [PubMed] [Google Scholar]
- Xie Z, Guo Z, Wang Y, Lei J, Yu J. Protocatechuic acid inhibits the growth of ovarian cancer cells by inducing apoptosis and autophagy. Phyther. Res. 2018;32:2256–2263. doi: 10.1002/ptr.6163. [DOI] [PubMed] [Google Scholar]
- Yin M-C, Lin C-C, Wu H-C, Tsao S-M, Hsu C-K. Apoptotic effects of protocatechuic acid in human breast, lung, liver, cervix, and prostate cancer cells: potential mechanisms of action. J. Agric. Food Chem. 2009;57:6468–6473. doi: 10.1021/jf9004466. [DOI] [PubMed] [Google Scholar]
- Yuliana ND, Budijanto S, Verpoorte R, Choi YH. NMR metabolomics for identification of adenosine A1 receptor binding compounds from Boesenbergia rotunda rhizomes extract. J. Ethnopharmacol. 2013;150:95–99. doi: 10.1016/j.jep.2013.08.012. [DOI] [PubMed] [Google Scholar]
- Yulianto W, Andarwulan N, Giriwono PE, Pamungkas J. HPLC-based metabolomics to identify cytotoxic compounds from Plectranthus amboinicus (Lour.) Spreng against human breast cancer MCF-7 Cells. J. Chromatogr. B. 2016;1039:28–34. doi: 10.1016/j.jchromb.2016.10.024. [DOI] [PubMed] [Google Scholar]
- Zarei I, Brown DG, Nealon NJ, Ryan EP. Rice bran metabolome contains amino acids, vitamins & cofactors, and phytochemicals with medicinal and nutritional properties. Rice. 2017;10:24. doi: 10.1186/s12284-017-0157-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarei I, Luna E, Leach J, McClung A, Vilchez S, Koita O, Ryan E, Zarei I, Luna E, Leach JE, McClung A, Vilchez S, Koita O, Ryan EP. Comparative rice bran metabolomics across diverse cultivars and functional rice gene–bran metabolite relationships. Metabolites. 2018;8:63. doi: 10.3390/metabo8040063. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Representative chromatograms of rice brans. Putative compound were numbered from 1 to 10 and determined by comparing with the compounds data base in NIST-14 Library which have the highest similarity percentage (1 = Protocatechuic acid, 2 = Fructose, 3 = Glucose, 4 = Hexanoic acid, 5 = Inositol, 6 = 9,12 - Octadecadienoic acid, 7 = Oleic acid, 8 = Octadecanoic acid, 9 = α-D-Glucopyranoside, 10 = β-sitosterol (PPTX 504 kb)