Abstract
In order to develop processing methods with high physiological activity for Protaetia brevitarsis larvae (PBL), subcritical water (SCW) extraction was applied. The dried powder (1 g) of PBL was extracted with 10 mL distilled water at 100, 200, and 300 °C for 30 min. The SCW treatment significantly (p < 0.05) increased some physiological activities of the PBL extracts. The SCW extract at 300 °C increased alcohol dehydrogenase, acetaldehyde dehydrogenase, and tyrosinase inhibitory activities from 192.3 ± 4.1% to 452.2 ± 0.5%, 125.4 ± 2.9% to 153.3 ± 0.4%, and − 7.0 ± 0.7% to 26.1 ± 1.4%, respectively, compared to the extract at 100 °C. Contrarily, the inhibition activity of angiotensin converting enzyme was the highest at 200 °C. These results suggest that SCW is a suitable method to extract and maintain the physiological activity of PBL.
Keywords: Protaetia brevitarsis larvae, Subcritical water, Alcohol metabolizing enzymes, ACE inhibitory activity, Tyrosinase inhibitory activity
Introduction
Insects are a group of invertebrates belonging to the Phylum Arthropoda. Their anatomy is divided into the head, thorax, and abdomen. There are around 1 million different species of insects and collectively, they account for over 90% of animals (van Huis et al., 2013). Insects inhabit most of the earth’s environment, are closely related to humans, and have traditionally been edible in some countries or regions; for example, the rice grasshopper and silkworm pupa are consumed in rural areas of Korea. Insects contain a variety of nutrients, including proteins, vitamins, and unsaturated fatty acids, and are highly prolific. Therefore, the Food and Agriculture Organization (FAO) of the United Nations is focusing on insects as a future food resource (van Huis et al., 2013). In line with this, the Korean Ministry of Food and Drug Safety has registered mealworm larvae, white-spotted flower chafer larvae, Korean rhinoceros larvae, and two-spotted cricket adult as novel food sources, as of 2014. Currently, seven species of insects, including rice grasshopper, silkworm pupa, and Bobbysis corpus, are legally permitted for manufacture and sale as food in Korea (Yun and Hwang, 2016). The white-spotted flower chafer (Protaetia brevitarsis, PB) is a large beetle, 17–22 mm long, with a color described as copper black and glossy. There are irregular white or yellow patterns of spots on the chest and blade of PB. PB is found in eastern Siberia and Asia, including the Korean peninsula.
PB larvae (PBL), known as ‘gumbengi’ in Korea, have been collected from straw roofs to be used in oriental medicine. PBL is a high-protein food source, with a total protein content of 54.13 g and 18.52 g of lipid per 100 g of dry weight (Korean Food Table, 2019). Due to the nutrient-rich nature of PBL, extensive research has been conducted to determine the physiological activity and food materialization of PBL (Chon et al., 2012; Choi et al., 2019; Kwon et al., 2013; Lee et al., 2019; Sung et al., 2016). To utilize PBL as a useful food source, it is necessary to develop a suitable processing method. Fermentation (Lee, 2018; Sim et al., 2018) and protein hydrolysis (Lee et al., 2017) are examples of processing methods that have been tested and reported. On the other hand, the extraction is the most basic process for commercialization of food materials. However, to the best of our knowledge, it’s difficult to find any researches for extraction of PBL. In this study, we aimed to manufacture PBL extracts with improved physiological activity using subcritical water (SCW).
Water and ethanol are generally used to extract useful substances used as food materials. The usefulness of water is limited by the fact that it mainly extracts hydrophilic substances. Contrarily, ethanol can be extracted with hydrophilic and hydrophobic substances. However, ethanol should be removed after extraction as an organic solvent. Further, the use of ethanol poses a risk to the environment. Water between 100 °C (boiling temperature) and 374 °C (critical temperature) under high pressure is referred to as SCW. Under SCW conditions, the polarity of water decreases, thereby mimicking properties of organic solvents. This allows for the extraction of various substances in a manner suitable for the environment (Carr et al., 2011). SCW can also break down covalent bonds such as ester, ether, and peptide bonds, making it advantageous for the extraction of useful materials (Kus, 2012). By using SCW, materials maintaining physiological activity can be extracted from sources such as watermelons (Kim et al., 2014a), oyster mushrooms (Jo et al., 2013), blue mussel (Han et al., 2018), and Styela clava (Jo et al., 2018). Therefore, in this study, we present the possibility of applying SCW to PBL by manufacturing the extract under three different temperatures and analyzing the physiological activity of alcohol metabolizing enzymatic activity, angiotensin converting enzyme (ACE) inhibition, and tyrosinase inhibition.
Materials and methods
Reagents
Acetaldehyde dehydrogenase (ALDH), alcohol dehydrogenase (ADH), arbutin, captopril, hippuryl-l-histidyl-l-leucine (HHL), NAD+, rabbit lung acetone powder, tyrosinase, and l-tyrosine were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). Hepos syrup (Choa Pharm. Co. Ltd., Seoul, Korea) was purchased from a pharmacy in Changwon city (Korea). Other reagents were purchased from Sigma-Aldrich Co.
Preparation of SCW extracts from PBL
PBL of third instar stage (90 days after birth) were provided by Gyeongsangnam-do Agricultural Research and Extension Services (Jinju, Korea) in March 2017. PBL were dried in a freeze dryer (FD 5512; Ilsin Lab Co., Seoul, Korea). Dried PBL were made into small particles with a grinder (HBL-3500S; Samyang Electronic Co., Gunpo, Korea), then passed through a 25 mesh sieve (Chunggyesanggongsa, Seoul, Korea). Dried PBL powder (0.1 g) was added with 10 mL of distilled water to a stainless steel tube (14 × 1 cm2) and sealed tightly with a cap, and left in a furnace (Daeil Engineering, Seoul, Korea). The extraction temperature was adjusted to 100, 200, or 300 °C for 30 min. The internal pressure of the stainless steel tube was 0.1–5 MPa. After extraction, the tubes were taken out of the furnace and cooled at room temperature for 30 min. SCW extracts were filtered with Whatman No. 3 filter paper (GE Healthcare UK Ltd., Buckinghamshire, UK); filtrates were used as specimens and stored at − 70 °C.
ADH activity
The effect of PBL extracted using SCW on ADH activity was measured by a modified Bergmeyer’s method (1974). After mixing 0.7 mL of distilled water, 0.38 mL of 1.0 M Tris–HCl buffer (pH 8.8), 0.15 mL of 20 mM NAD+ solution, 1.16% (v/v) ethanol, and 50 μL of the extract at 25 °C for 10 min, then 27.5 mL of ADH (5 unit/mL) was added to react. Absorbance of generated NADH was measured at 340 nm using a spectrophotometer (Optizen Pop, Mechasys Co., Ltd., Dajeon, Korea) to compare the relative activity to the control. Distilled water was used instead of samples in the control, and Hepos was used as a positive control. The ADH activity of sample was calculated by dividing the absorbance at the end of the reaction as a percentage of the absorbance of the control, in the following manner:
ALDH activity
The effect of SCW-extracted PBL on ALDH activity was measured by the method described by Koivula and Koivusalo (1975) with some modifications. That is, after mixing 1.05 mL of distilled water, 0.15 mL of 1.0 M Tris–HCl buffer (pH 8.0), 50 μL of 20 mM NAD+, 50 μL of 0.1 M acetaldehyde, 50 μL of 3.0 M KCl, and 50 μL of 0.33 M 2-mercaptoethanol, and 10 μL of sample, the mixture was allowed to stand for 10 min at 25 °C. The reaction was started by addition of 50 μL of ALDH (1 unit/mL). After 10 min of reaction at 25 °C, produced NADH was detected by measuring absorbance at 340 nm by a spectrophotometer (Optizen Pop, Merchasys Co., Ltd.) As in ADH activity measurements, distilled water was added instead of samples, and positive control was also used with Hepos syrup. ALDH activity of sample was calculated by the following equation.
ACE inhibitory activity
ACE inhibitory activity was measured using the method developed by Cushman and Cheung (1971). Firstly, 1 g of rabbit lung acetone powder was mixed with 20 mL of 50 mM sodium borate buffer (pH 8.3) and stirred for 24 h at 4 °C. The ACE solution was obtained as a supernatant after centrifugation for 10 min at 5000×g. ACE solution (50 μL) was added to 50 μL of SCW extract of PBL and was allowed to stand for 10 min at 37 °C. One hundred μL of 25 mM HHL was mixed and incubated at 37 °C for 60 min. To stop the enzymatic reaction, 250 μL of 1 M HCI was added and stirred for 30 s, followed by centrifugation at 5000×g for 10 min. The supernatant (200 μL) was completely dried at 80 °C for 30 min, and dissolved in 1 mL of distilled water. Absorbance was measured at 228 nm. Distilled water was used as a negative control, and captopril as a positive control. The ACE inhibitory activity was obtained using the following equation:
Tyrosinase inhibitory activity
Tyrosinase inhibitory activity was analyzed as described by Vanni et al. (1990). Each extract (100 μL) and 140 μL of 0.05 mM sodium phosphate buffer (pH 6.8) were added into a 96-well plate, and 40 μL of 1.5 mM l-tyrosine solution and 20 μL of mushroom tyrosinase (1500 U/mL) were added. After mixing, the solution was incubated at 37 °C for 15 min, absorbance was measured with a multiplate reader (Sunrise RC/TS/TS Color TC/TW/BC/6Filter, Techan Austria GmbH, Grödig, Austria) at 492 nm. Arbutin was used as a positive control.
Statistical analysis
All data are presented as means ± standard deviations (SDs). Statistical analysis was performed using SPSS software (SPSS Inc., Chicago, IL, USA). The significant differences between groups were assessed with one-way analysis of variance followed by Duncan’s multiple range test or Student’s t test. A p value < 0.05 was considered to be statistically significant.
Results and discussion
Effect of SCW extracts of PBL on the activity of alcohol metabolizing enzymes
Most of the alcohol absorbed into the body is metabolized by ADH and ALDH (Liber, 1991). The effect of SCW extracts of PBL on the activity of these alcohol metabolizing enzymes was investigated. The effects of threefold diluted SCW extracts on the activity of ADH are shown in Fig. 1. When the activity of ADH without the addition of the extract was 100%, the ADH activity was more than 100% after the addition of all diluted SCW extracts. There was a positive correlation between ADH activity and extraction temperature, as the ADH activity of the extract prepared at 300 °C showed 452.2 ± 0.5% activity. Hepos syrup, used as a control, is classified as a hepatic disease medicine used as a supplement to aid in liver dysfunction. It contains betaines as a medicinal ingredient and is widely used as a positive control in alcohol metabolizing enzyme studies. Undiluted and threefold diluted Hepos syrup showed 550.4 ± 3.2% and 249.1 ± 2.0% of ADH activity, respectively. Meanwhile, Kim et al. (2014b) showed that 10 mg/mL of the hot water extract of the domestic blue mussel, a well-known food in treating hangovers, resulted in 139.75% in ADH activity; however, the activity of ADH using a twofold dilution of Hepos was 195.49%. In this study, 0.1 g of PBL was extracted with 10 mL of water, and even if 100% of solids were completely extracted into SCW, the concentration of a threefold dilution extract was calculated to be a maximum of 3.3 mg/mL. When compared to the blue mussel, the SCW extract of PBL was more efficient since a lower concentration resulted in greater ADH activity.
Fig. 1.
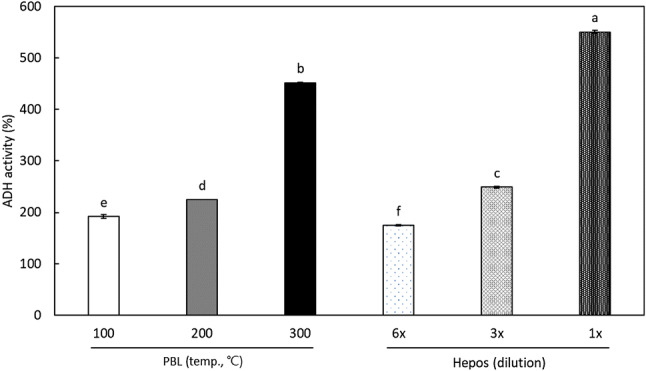
Effects of (A) threefold diluted SCW extracts from Protaetia brevitarsis larvae (PBL) and Hepos on alcohol dehydrogenase (ADH) activity. Each value represents mean ± SD. Values with different letters above the bars are significantly different by Duncan’s multiple range test at p < 0.05
In addition, the effects of threefold diluted SCW extracts of PBL on the activity of ALDH is indicated in Fig. 2. As noted above, the ALDH activity without any addition was 100%, which was further increased by the diluted PBL extracts. SCW extracts prepared at 100 °C and 200 °C showed similar activity, at nearly 125%, but the extract produced at 300 °C increased ALDH activity to 153.3 ± 0.3%. ALDH activity with undiluted and threefold diluted Hepos syrup, was 165.8 ± 3.2% and 128.1 ± 1.7%, respectively. The above results indicate that the SCW extract of PBL increases ALDH activity.
Fig. 2.
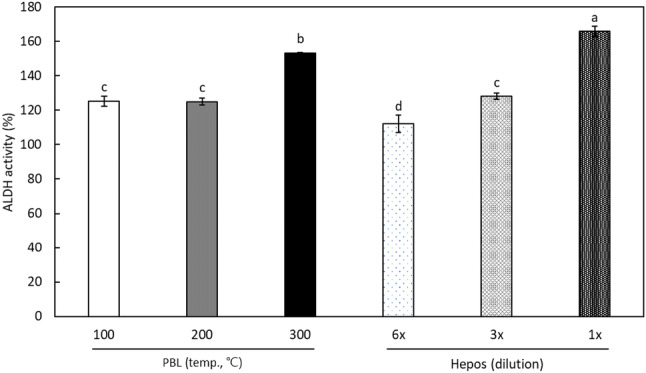
Effects of threefold diluted SCW extracts from Protaetia brevitarsis larvae (PBL) and Hepos on acetaldehyde dehydrogenase (ALDH) activity. Each value represents mean ± SD. Values with different letters above the bars are significantly different by Duncan’s multiple range test at p < 0.05
ADH and ALDH, the main alcohol metabolizing enzymes in the body, use NAD+ as a coenzyme. NAD+ is biosynthesized from niacin, which is absorbed by the body as a precursor and is regenerated from NADH by a malate-Asp shuttle to promote activation of alcohol metabolizing enzymes (Sugano et al., 1990). Raw and dried PBLs are reported to contain 1.62 and 3.97 mg/100 g of niacin, respectively (Korean Food Table, 2019). Aspartate (Asp) content of PBL was reported to be more than 4% of dry PBL weight (Chung et al., 2013). The niacin and Asp content of bean sprouts, another well-known food source for treating hangovers, is 0.7 mg/100 g and 1181 mg/100 g, respectively, and those of blue mussels are 2.5 mg/100 g and 677 mg/100 g, respectively (Korean Food Table, 2019). This suggests that PBL also contains elevated levels of niacin and Asp, which contribute to the production of NAD+ associated with alcohol metabolizing enzymes, compared with bean sprouts and blue mussels. Further studies of SCW extracts of PBL are necessary to determine the specific components required for the activation of alcohol metabolizing enzymes.
ACE inhibitory activity
Angiotensin II, a hormone involved in regulating hypertension through constriction of arterial vessels to raise blood pressure and by promoting the release of aldosterone in the adrenal glands, was produced by ACE. Therefore, measurement of inhibitory activity on ACE is a good indicator of the development of anti-hypertensive substances. The results of measuring the ACE inhibitory activity of PBL extracts are shown in Fig. 3. All the SCW extracts showed significant ACE inhibitory activity. The SCW extract prepared at 100 °C exhibited 34.0 ± 1.0% of ACE inhibitory activity, and the extract manufactured at 200 °C increased to 69.8 ± 1.6%. However, the ACE inhibitory activity of that extracted at 300 °C decreased to 53.0 ± 1.7%. This phenomenon can be explained by two factors. First, although SCW could extract active material(s) from PBL, the material or materials are potentially unstable at 300 °C. Second, it is possible that chemicals interfering with ACE inhibitory activity were also extracted at 300 °C. Contrarily, a positive control, captopril, showed 65.1 ± 3.7% ACE inhibitory activity at a concentration of 0.3 μg/mL.
Fig. 3.
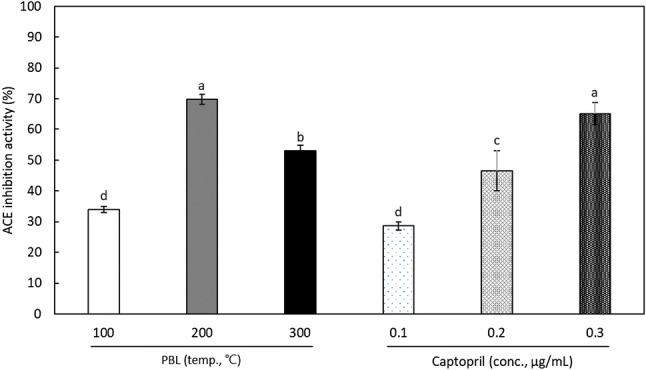
Angiotensin converting enzyme (ACE) inhibitory activity of SCW extracts from Protaetia brevitarsis larvae (PBL) and captopril. Each value represents mean ± SD. Values with different letters above the bars are significantly different by Duncan’s multiple range test at p < 0.05
The above results indicate that the appropriate conditions of SCW can efficiently extract substances with ACE inhibitory activity from PBL. ACE inhibitory activity in food sources is mainly caused by peptides (Lee and Hur, 2017) and has been reported to be highly effective when protein is hydrolyzed (Fujita et al., 2000). Therefore, it can be inferred that the ACE inhibitory activity is increased by the peptide produced by hydrolysis of PBL proteins under SCW condition, but further studies are required to confirm this notion.
Tyrosinase inhibitory activity
Tyrosinase convert tyrosine to 3,4-dihydroxyphenylalanine (DOPA) and DOPA-quinone, which help form components of the pigment in skin and hair. Melanin, a primary pigment, also acts as an important defense mechanism against ultraviolet radiation on skin, but excessive increases in epithelial melanin can produce freckles, wrinkles, and age spots (Kim and Uyama, 2005). Thus, materials with an inhibitory effect on tyrosinase activity are receiving increasing attention. As shown in Fig. 4, the SCW extracts of PBL prepared at 200 and 300 °C showed 15.6 ± 0.2 and 26.1 ± 1.4, respectively. Arbutin, used as a positive control, showed a tyrosinase inhibitory activity of 41.7 ± 0.5% at a concentration of 0.5 mg/mL. Although the tyrosinase inhibitory activity of the SCW extracts of PBL was significantly lesser than that of arbutin, this study is the first attempt, to our knowledge, to show the tyrosinase inhibitory potential of PBL.
Fig. 4.
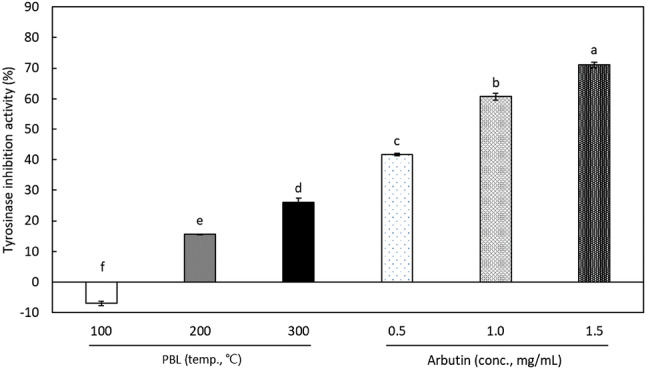
Tyrosinase inhibitory activity of SCW extracts from Protaetia brevitarsis larvae (PBL) and arbutin. Each value represents mean ± SD. Values with different letters above the bars are significantly different by Duncan’s multiple range test at p < 0.05
Correlation between ADH, ALDH, ACE inhibitory, and tyrosinase inhibitory activities
The correlations between ADH, ALDH, ACE inhibitory, and tyrosinase inhibitory activities of PBL extraction by SCW were analyzed, and the calculated coefficients of correlations are indicated in Table 1. ADH activity was positively correlated with ALDH activity (r = 0.982), however, ACE inhibitory activity was not correlated to either ADH (r = 0.151) or ALDH (r = 0.029) activity. These results suggest that additional factors, except those related to ADH and ALDH activities, might play on the ACE inhibitory activity of PBL extracts.
Table 1.
Correlation analysis in ADH, ALDH, ACE inhibitory activity (IA), and tyrosinase IA (TIA) of SWE of PBL
| ADH | ALDH | ACE IA | TIA | |
|---|---|---|---|---|
| ADH | 1 | 0.982** | 0.151 | 0.815** |
| ALDH | 1 | 0.029 | 0.733* | |
| ACE IA | 1 | 0.690* | ||
| TIA | 1 |
*Correlation is significant at p < 0.05
**Correlation is significant at p < 0.01
In this study, the SCW treatment effectively increases ADH, ALDH, ACE inhibitory, and tyrosinase inhibitory activities of PBL. Thus, this study provides further evidence that SCW treatment process could be a useful method to improve the health-promoting properties of PBL.
Acknowledgements
This work was supported by Kyungnam University Foundation Grant, 2019.
Compliance with ethical standards
Conflict of interest
The authors declared that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Sung-Mun Bae, Email: smbae@korea.kr.
Seung-Cheol Lee, Email: sclee@kyungnam.ac.kr.
References
- Bergmeyer HU. Alcohol dehydrogenase. In: Bergmeyer HU, editor. Methods of Enzymatic Analysis. New York, NY: Academic Press; 1974. pp. 428–429. [Google Scholar]
- Carr AG, Mammucari R, Foster NR. A review of subcritical water as a solvent and its utilisation for the processing of hydrophobic organic compounds. Chem. Eng. J. 2011;172(1):1–17. doi: 10.1016/j.cej.2011.06.007. [DOI] [Google Scholar]
- Choi IH, Yu R, Lim YJ, Choi GS, Choi SU, Hwang JI, Son JS, Chung TH. Antithrombotic efficacy of Protaetia brevitarsis extract. J. Enviorn. Sci. Int. 2019;28:639–643. doi: 10.5322/JESI.2019.28.7.639. [DOI] [Google Scholar]
- Chon JW, Kweon H, Jo YY, Yeo JH, Lee HS. Protective effects of extracts of Protaetia brevitarsis on carbon tetrachloride-induced hepatotoxicity in the mice. J. Seric. Entomol. Sci. 2012;50:93–100. [Google Scholar]
- Chung MY, Hwang JS, Goo TW, Yun EY. Analysis of general composition and harmful material of Protaelia brevitarsis. J. Life Sci. 2013;23:664–668. doi: 10.5352/JLS.2013.23.5.664. [DOI] [Google Scholar]
- Cushman DW, Cheung HS. Spectrophotometric assay and properties of the angiotensin-converting enzyme of rabbit lung. Biochem. Pharmacol. 1971;20:1637–1648. doi: 10.1016/0006-2952(71)90292-9. [DOI] [PubMed] [Google Scholar]
- Fujita H, Yokoyama K, Yoshikawa M. Classification and antihypertensive activity of angiotensin I-converting enzyme inhibitory peptides derived from food proteins. J. Food Sci. 2000;65:564–569. doi: 10.1111/j.1365-2621.2000.tb16049.x. [DOI] [Google Scholar]
- Han JK, Sung SC, Jo MJ, Lee SC. Antioxidant, ACE inhibitory, and acetylcholinesterase inhibitory activities of subcritical water extract of blue mussel. Food Sci. Biotenol. 2018;27:847–851. doi: 10.1007/s10068-018-0319-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo EK, Heo DJ, Kim JH, Lee YH, Ju YC, Lee SC. The effects of subcritical water treatment on antioxidant activity of golden oyster mushroom. Food Bioprocess Technol. 2013;6:2555–2561. doi: 10.1007/s11947-012-0793-x. [DOI] [Google Scholar]
- Jo MJ, Han JK, Sung SC, Lee SC. Preparation of subcritical water extract with improved physiological activity of Styela clava. J. Korean Soc. Food Sci. Nutr. 2018;47:1112–1117. doi: 10.3746/jkfn.2018.47.11.1112. [DOI] [Google Scholar]
- Kim SJ, Matsushita Y, Fukushima K, Aoki D, Yagami S, Yuk HG, Lee SC. Antioxidant activity of a hydrothermal extract from watermelons. LWT-Food Sci. Technol. 2014;59:361–368. doi: 10.1016/j.lwt.2014.04.041. [DOI] [Google Scholar]
- Kim SK, Ok DL, Park E, Lee SC. Effects of hot water extracts of domestic blue mussel and New Zealand green lipped mussel on alcohol metabolizing enzymatic, DPPH radical scavenging, and angiotensin converting enzyme inhibitory activities. J. Korean Soc. Food Sci. Nutr. 2014;43:1363–1368. doi: 10.3746/jkfn.2014.43.9.1363. [DOI] [Google Scholar]
- Kim YJ, Uyama H. Tyrosinase inhibitors from natural and synthetic sources: structure, inhibitory mechanism and perspective for the future. Cell Mol. Life Sci. 2005;62:1707–1723. doi: 10.1007/s00018-005-5054-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koivula T, Koivusalo M. Different forms of rat liver aldehyde dehydrogenase and their subcellular distribution. Biochim. Biophys. Acta. 1975;397:9–23. doi: 10.1016/0005-2744(75)90174-6. [DOI] [PubMed] [Google Scholar]
- Korean Food Table. Available from: http://koreanfood.rda.go.kr/kfi/fct/fctFoodSrch/list. Accessed Sept. 30, 2019.
- Kus NS. Organic reactions in subcritical and supercritical water. Tetrahedron. 2012;68:949–958. doi: 10.1016/j.tet.2011.10.070. [DOI] [Google Scholar]
- Kwon EY, Yoo J, Yoon YI, Hwang JS, Goo TW, Kim MA, Choi YC, Yu EY. Pre-treatment of the white-spotted flower chafer (Protaetia brevitarsis) as an ingredient for novel foods. J. Korean Soc. Food Sci. Nutr. 2013;42:397–402. doi: 10.3746/jkfn.2013.42.3.397. [DOI] [Google Scholar]
- Lee HJ, Seo M, Lee JH, Kim IW, Kim SY, Hwang JS, Kim MA. Inhibitory effect of Protaetia brevitarsis seulensis ethanol extract on neuroinflammation in LPS-stimulated BV-2 microglia. J. Life Sci. 2019;29:1096–1103. [Google Scholar]
- Lee HS, Ryu HJ, Song HJ, Lee SO. Enzymatic preparation and antioxidant activities of protein hydrolysates from Protaetia brevitarsis larvae. J. Korean Soc. Food Sci. Nutr. 2017;46:1164–1170. [Google Scholar]
- Lee SY, Hur SJ. Antihypertensive peptides from animal products, marine organisms, and plants. Food Chem. 2017;228:506–517. doi: 10.1016/j.foodchem.2017.02.039. [DOI] [PubMed] [Google Scholar]
- Lee YD. Properties of aqueous extract of Protaetia brevitarsis larva and mountain ginseng fermented by Lactobacillus brevis. J. Food Hyg. Saf. 2018;33:369–374. doi: 10.13103/JFHS.2018.33.5.369. [DOI] [Google Scholar]
- Liber CS. Hepatic metabolic and toxic effects of ethanol. Alcohol Clin. Exp. Res. 1991;15:573–592. doi: 10.1111/j.1530-0277.1991.tb00563.x. [DOI] [PubMed] [Google Scholar]
- Sim SY, Ahn HY, Seo KI, Cho YS. Physiological properties and biological activities of Protaetia brevitarsis seulensis larvae fermented by several kinds of micro-organisms. J. Life Sci. 2018;28:827–834. [Google Scholar]
- Sugano T, Handler JA, Yoshihara H, Kizaki Z, Thurman RG. Acute and chronic ethanol treatment in vivo increases malate-aspartate shuttle capacity in perfused rat liver. J. Biol. Chem. 1990;265:21549–21553. [PubMed] [Google Scholar]
- Sung GA, Kim MH, Park SN. Anti-inflammatory and whitening effects of Protaetia brevitarsis Seulensis extracts by oriental conversion methods. J. Soc. Cosmet. Sci. Korea. 2016;42:421–432. [Google Scholar]
- van Huis A, Itterbeeck JV, Klunder H, Mertens E, Halloran A, Muir G, Vantomme P. Edible insects: future prospects for food and feed security. Rome: Food and Agriculture Organization of the United Nations; 2013. [Google Scholar]
- Vanni A, Gastaldi D, Giunta G. Kinetic investigations on the double enzymic activity of the tyrosinase mushroom. Ann. Chim. Rome. 1990;80:35–60. [Google Scholar]
- Yun EY, Hwang JS. Status and prospect for development of insect foods. Food Sci. Ind. 2016;49:31–39. [Google Scholar]


