Abstract
The objective of this study was to track intentionally inoculated Leuconostoc mesenteroides (11251) and Lactobacillus brevis (B151) strains in kimchi using random amplified polymorphic DNA (RAPD), repetitive element palindromic PCR (rep-PCR), and comparative housekeeping gene sequences analysis. The 16S rRNA gene provided species-level information for 30 colonies randomly picked from kimchi inoculated with strains 11251 and B151. Out of 30 colonies, one colony was matched to strain 11251, and two colonies were found identical to strain B151 reference strain in inoculated kimchi. Notably, among the three tools, strain 11251 was best tracked by comparative gene sequence analysis, while strain B151 tracked by all three tools. Our results suggest that the gene sequence analysis is a more reliable tool for tracking of desired strains than RAPD and rep-PCR. Based on the findings, it is recommended that gene sequence analysis could be used to avoid misuse of industrially useful strains within the growing food industry.
Electronic supplementary material
The online version of this article (10.1007/s10068-019-00719-0) contains supplementary material, which is available to authorized users.
Keywords: Probiotic, Leuconostoc mesenteroides, Lactobacillus brevis, PCR
Introduction
Worldwide, bacterial strains present many challenges to the scientific community (Fournier et al., 2004), including increased virulence and transmissibility, antibiotic resistance, host range expansion, threats to human health, and genetic manipulation for bioterrorism (Fournier et al., 2004). Another recent concern is linked to the misuse of industrially useful bacterial strains. Thus, the identification and tracking of bacterial strains are vital aspects of modern biotechnology. For successful monitoring, targeted remedial efforts, including reliable strain typing approaches, are required. In general, a suitable typing method applies to all related isolates, amenable to computerized analysis, and has an excellent discriminatory power with reproducibility (Li et al., 2009). Generally, two methods, including phenotyping and genotyping, have been used for the typing of bacteria (Dan et al., 2014). Phenotyping includes conventional methods; however, it lacks sensitivity for the differentiation of bacterial strains, while molecular methods evaluate diversity and are sensitive enough to differentiate bacterial strains.
The genotypic tools offer a more robust phylogenetic classification and differentiation of lactic acid bacteria (LAB). The examples include restriction fragment length polymorphism (RFLP) (Park et al., 2012), amplified fragment length polymorphism (AFLP) (Karahan et al., 2010), pulsed-field gel electrophoresis (PFGE) (Singh et al., 2009), microarrays (Winkler and Kao, 2011), random amplified polymorphic DNA-PCR (RAPD-PCR) (Singh et al., 2009), and repetitive elements PCR (Rep-PCR) (Gevers et al., 2001), multilocus sequence typing (MLST) (Sabat et al., 2013) matrix-assisted laser desorption ionization-time of flight MS (MALDI-TOF MS) (Zeller-Péronnet et al., 2013).
A polyphasic approach (two or more typing techniques) may provide reliable information for the identification and characterization of the isolates (Felis and Dellaglio, 2007). Usually, the selection of the typing method depends on the research objectives, the availability of skilled personnel, and particularly the resources available in the laboratory.
Among all, three molecular typing namely RAPD-PCR, Rep-PCR, and comparative gene sequence analysis of the housekeeping loci were implemented in the present study to develop a simple tracking technology.
RAPD is a fast fingerprinting method using random primers that anneal to multiple locations generating required polymorphism (Singh et al., 2009). It is a rapid, simple, low-cost, and generally applicable technique that does not require any prior knowledge of the target sequence (Berthier and Ehrlich, 1999). On the other hand, Rep-PCR is a powerful, reproducible strain differentiation tool, used to amplify repetitive bacterial DNA elements with low cost and good discriminatory power (Gevers et al., 2001).
The comparative gene sequence analysis is based on MLST (Sabat et al., 2013). It utilizes nucleotide sequences of housekeeping loci to differentiate isolates of the microbial species (Bain et al., 2007). The tool has high discriminatory power and provides unambiguous results.
More than 400 species of LAB have been reported (Zhang et al., 2011). Leuconostoc species are epiphytic bacteria that are prevalent in the natural environment (Hemme and Foucaud-Scheunemann, 2004). They have been used as starters in many industrial and food fermentations (Wassie and Wassie, 2016) and also as a flavoring and texturizing agents (Dan et al., 2014). The fermentative capacity of the genus Leuconostoc has promoted its status as generally regarded as safe (GRAS) (Klaenhammer et al., 2005) and approved with the qualified presumption of safety (QPS) for food production and human consumption (Leuschner et al., 2010). Thus, Leuconostoc has been used to improve the safety and organoleptic attributes of various food products (Gemechu, 2015; Steele et al., 2013).
Another LAB strain, L. brevis, is a fermentative bacterium found in many ecological niches, such as fermented foods (sauerkraut and pickles), beverages, plants, and the human intestinal tract (Fusco et al., 2016). L. brevis has shown anti-microbial (Rushdy and Gomaa, 2013), anti-inflammatory (Riccia et al., 2007), and anti-influenza (Waki et al., 2014) effects and has been approved as GRAS (Rönkä et al., 2003).
The different traits of a LAB strain are often referred to as its fingerprint since they can be used to identify the same strain that is deliberately inoculated or suspected to be present in a food sample. The detection based on the fingerprint data provides evidence for the presence of a relevant isolate. Hence, the characterization of the bacterial isolates in suspected cases of misuse of LAB or any other bacterial strain can be performed with tracking technology that reliably matches the bacterial strain from a suspected product or site with that of the original strain. The present study aimed to utilize a multiphasic approach for the tracking of selected LAB strains deliberately inoculated in Korean traditional food kimchi (solid). The objective was accomplished by using various tools, such as 16S rRNA gene sequencing, RAPD, rep-PCR, and comparative sequence analysis of housekeeping loci.
Materials and methods
Viable cell counts
The seed culture was serially diluted to 10−6 in 0.88% saline, and 50 µL of this dilution was spread on De Man, Rogosa and Sharpe agar (MRS; BD Diagnostics, Sparks, MD, USA) plates to determine the number of viable cells. The colonies were counted, and the number of colony-forming units (CFU) was calculated with the following formula.
Bacterial strains, inoculation of kimchi and tracking
In the present study, two industrially relevant strains, Leuconostoc mesenteroides 11251 and Lactobacillus brevis B151 procured from the Korean Culture Collection of Probiotics (KCCP) were used as reference strains. The food sample kimchi (solid, homemade) with an unknown bacterial pool was selected for the study and was inoculated with Leu. mesenteroides and L. brevis, using a seed culture (at a 10th of the total volume of the sample). The seed culture (10−6) was poured on 3 g of kimchi (separate experiments for 11251 and B151 strains), followed by the addition of 25 mL of saline and mixed. The mixtures were spread on MRS agar plates and incubated at 37 °C overnight. The next day the number of viable cells was counted, and 30 colonies were randomly picked for analysis from each plate. For tracking the deliberately inoculated strains, 30 colonies were analyzed by RAPD, Rep-PCR, and comparative gene sequence analysis.
Primer synthesis, PCR and sequencing
All primers used in this study were synthesized by Macrogen sequencing service (Macrogen, Seoul, Korea). The amplification reactions were carried out in an automatic thermocycler (MyCycler™, BioRad, Hercules, CA, USA). Gel images were captured using the Bio-Rad Gel Doc XR + gel documentation system. Two DNA ladders, 100 bp (Bioneer, Daejeon, Korea) and 1 kb (Takara, Japan), were used as size markers. PCR products were purified from the gel with the Wizard SV Gel and PCR Clean-Up system kits (Promega, Madison, WI, USA) and sequencing was performed by Macrogen sequencing service (Seoul, Korea).
DNA isolation, 16S rRNA gene identification and RAPD analysis
Genomic DNA was isolated from 30 colonies from kimchi using the AccuPrep Genomic DNA Extraction kit (Bioneer). The strains were grown in MRS broth (BD Diagnostics) and incubated overnight at 37 °C. The procedure for DNA isolation, primers for 16S rRNA, and RAPD amplification (primers 239 and KAY3) were described previously (Kaur et al., 2017a).
Rep-PCR analysis using primers REP, ERIC, and (GTG)5
The Rep-PCR was performed using REP, ERIC, and (GTG)5 primers for the study of 30 individual colonies. The PCR procedure utilized has been described previously in a related publication (Kaur et al.,2017b).
Comparative gene sequence analysis
For comparative sequence analysis, the sequences of the housekeeping loci, atpA, groEL, gyrB, pheS, pyrG, rpoA, and uvrC from Leu. mesenteroides and dnaK, groEL, gyrB, pheS, recA, rpoA, and rpoB from L. brevis were compared with the gene sequences of controls to identify polymorphisms. Finally, the tracking was performed by comparative gene sequence analysis of the MLST, for which the forward and reverse sequences of each housekeeping gene were trimmed and analyzed with Bioedit Sequence Alignment Editor (v. 7.2.5) and aligned with ClustalX v. 1.83 (Hall, 1999). Colonies showing 1 or more single nucleotide polymorphisms (SNPs) were discarded. The information on genes, primers, sequence length, and products for 11251 and B151 have been described in supplementary Tables 1 and 2, respectively.
Results and discussion
Tracking of inoculated strains in kimchi
The monitoring of the inoculated strains was investigated using a homemade kimchi-a solid food source (a famous traditional Korean dish) with an unknown bacterial pool. The thirty randomly picked colonies were labelled as KM1-30 for Leu. mesenteroides and KB1-30 for L. brevis. The 16S rRNA gene sequencing results for Leu. mesenteroides showed that 15 colonies (KM1, KM2, KM5, KM6, KM7, KM14, KM15, KM20, KM21, KM22, KM24, KM25, KM26, KM29, and KM30) shared identity with Leu. mesenteroides, while 14 were identified as Lactobacillus sakei and 1 was identified as Lactobacillus curvatus (Table 1). On the other hand, the results for L. brevis-inoculated kimchi showed 2 colonies, KB5 and KB17 shared identity with L. brevis, and the remaining colonies were identified as Lactobacillus plantarum (Table 1).
Table 1.
16S rRNA gene sequencing results for Leu. mesenteroides (KM-11251) and L. brevis (KB-B151) inoculated in kimchi
| Sr. No. | Colony ID | BLAST result | Accession number | Colony ID | BLAST result | Accession number |
|---|---|---|---|---|---|---|
| 1. | KM1 | Leu. mesenteroides | MF541010 | KB1 | Lactobacillus plantarum | MF541040 |
| 2. | KM2 | Leu. mesenteroides | MF541011 | KB2 | Lactobacillus plantarum | MF541041 |
| 3. | KM3 | Lactobacillus sakei | MF541012 | KB3 | Lactobacillus plantarum | MF541042 |
| 4. | KM4 | Lactobacillus sakei | MF541013 | KB4 | Lactobacillus plantarum | MF541043 |
| 5. | KM5 | Leu. mesenteroides | MF541014 | KB5 | Lactobacillus brevis | MF541044 |
| 6. | KM6 | Leu. mesenteroides | MF541015 | KB6 | Lactobacillus plantarum | MF541045 |
| 7. | KM7 | Leu. mesenteroides | MF541016 | KB7 | Lactobacillus plantarum | MF541046 |
| 8. | KM8 | Lactobacillus sakei | MF541017 | KB8 | Lactobacillus plantarum | MF541047 |
| 9. | KM9 | Lactobacillus sakei | MF541018 | KB9 | Lactobacillus plantarum | MF541048 |
| 10. | KM10 | Lactobacillus sakei | MF541019 | KB10 | Lactobacillus plantarum | MF541049 |
| 11. | KM11 | Lactobacillus curvatus | MF541020 | KB11 | Lactobacillus plantarum | MF541050 |
| 12. | KM12 | Lactobacillus sakei | MF541021 | KB12 | Lactobacillus plantarum | MF541051 |
| 13. | KM13 | Lactobacillus sakei | MF541022 | KB13 | Lactobacillus plantarum | MF541052 |
| 14. | KM14 | Leu. mesenteroides | MF541023 | KB14 | Lactobacillus plantarum | MF541053 |
| 15. | KM15 | Leu. mesenteroides | MF541024 | KB15 | Lactobacillus plantarum | MF541054 |
| 16. | KM16 | Lactobacillus sakei | MF541025 | KB16 | Lactobacillus plantarum | MF541055 |
| 17. | KM17 | Lactobacillus sakei | MF541026 | KB17 | Lactobacillus brevis | MF541056 |
| 18. | KM18 | Lactobacillus sakei | MF541027 | KB18 | Lactobacillus plantarum | MF541057 |
| 19. | KM19 | Lactobacillus sakei | MF541028 | KB19 | Lactobacillus plantarum | MF541058 |
| 20. | KM20 | Leu. mesenteroides | MF541029 | KB20 | Lactobacillus plantarum | MF541059 |
| 21. | KM21 | Leu. mesenteroides | MF541030 | KB21 | Lactobacillus plantarum | MF541060 |
| 22. | KM22 | Leu. mesenteroides | MF541031 | KB22 | Lactobacillus plantarum | MF541061 |
| 23. | KM23 | Lactobacillus sakei | MF541032 | KB23 | Lactobacillus plantarum | MF541062 |
| 24. | KM24 | Leu. mesenteroides | MF541033 | KB24 | Lactobacillus plantarum | MF541063 |
| 25. | KM25 | Leu. mesenteroides | MF541034 | KB25 | Lactobacillus plantarum | MF541064 |
| 26. | KM26 | Leu. mesenteroides | MF541035 | KB26 | Lactobacillus plantarum | MF541065 |
| 27. | KM27 | Lactobacillus sakei | MF541036 | KB27 | Lactobacillus plantarum | MF541066 |
| 28. | KM28 | Lactobacillus sakei | MF541037 | KB28 | Lactobacillus plantarum | MF541067 |
| 29. | KM29 | Leu. mesenteroides | MF541038 | KB29 | Lactobacillus plantarum | MF541068 |
| 30. | KM30 | Leu. mesenteroides | MF541039 | KB30 | Lactobacillus plantarum | MF541069 |
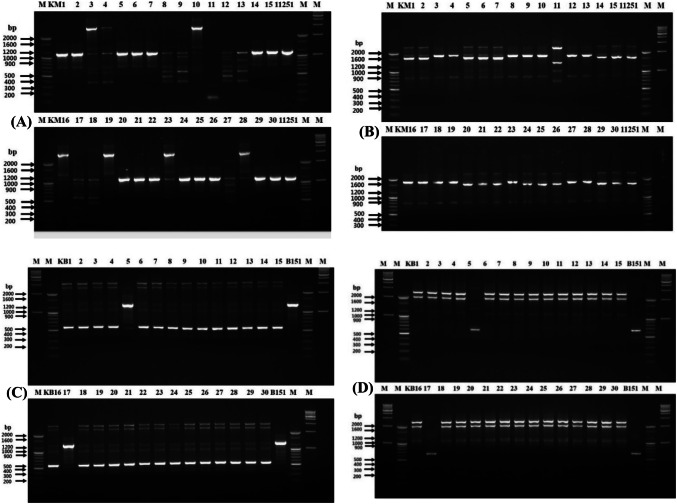
RAPD and rep-PCR were performed using 60 colonies to track the inoculated strains. As shown in Fig. 1, RAPD fingerprinting profiles showed 15 colonies out of 30 were found identical to the strain 11251, showing a bright band at 1100 bp with primer 239 (Fig. 1A) and a prominent band at 1600 bp along with two light bands with primer KAY3 (Fig. 1B). To get more clarity, these 15 colonies underwent longer electrophoresis for RAPD (Fig. S1A-B) and showed similar results. Therefore, it was assumed that these 15 colonies were likely to be identical to the strain 11251 inoculated initially.
Fig. 1.
RAPD-PCR analysis of 30 colonies picked from kimchi inoculated with L. mesenteroides 11251 using 239 primer (A) and KAY3 primer (B), Lane: 1–100 bp marker, Lane: 2–16 (colonies KM1-15), Lane: 17-strain 11251, Lane: 18–100 bp marker, Lane: 19–1 kb marker. Lower half of the Gel—Lane: 20–100 bp marker, Lane: 21–35 (colonies KM16-30), Lane: 36-strain 11251, Lane: 37–100 bp marker, Lane: 38–1 kb marker. From kimchi inoculated with L. brevis B151 using 239 primer (C), Lane: 1–1 kb marker, Lane: 2–100 bp marker, Lane: 3–17 (colonies KB1-15), Lane: 18-strain B151, Lane: 19–100 bp marker, Lane: 20–1 kb marker. Lower half of the Gel—Lane: 21-100 bp marker, Lane: 22–36 (colonies KB16-30), Lane: 37-strain B151, Lane: 38–100 bp marker, Lane: 39–1 kb marker and KAY3 primer (D), Lane: 1–1 kb marker, Lane: 2–100 bp marker, Lane: 3–17 (colonies KB1-15), Lane: 18-strain B151, Lane: 19–100 bp marker, Lane: 20–1 kb marker. Lower half of the Gel—Lane: 21–1 kb marker, Lane: 22–100 bp marker, Lane: 23–37 (colonies KB16-30), Lane: 38-strain B151, Lane: 39–100 bp marker, Lane: 40–1 kb marker. Positive controls-11251 and B151
Similarly, the L. brevis colonies from Kimchi were also typed, and only 2 colonies, strain KB5 and KB17, matched the original strain B151 fingerprint pattern using RAPD primers (239 and KAY3). The primer 239 showed a single band at 1200 bp (Fig. 1C), whereas primer KAY3 presented a prominent band at ~ 660 bp (Fig. 1D) in both (KB5 and KB17) similar to the reference strain. On a similar line to RAPD, all rep primers, (GTG)5, REP, and ERIC showed a banding pattern for the 15 colonies that were identical to that of strain 11251 in the KM culture (Fig. 2). The gel electrophoresis was also performed for an extended period using rep-PCR (Fig. S2A-C). With (GTG)5 primer for all colonies identical banding profile was observed with bands in a range from 430 to 1200 bp (Fig. 2A) (Fig. S2A). Rep primer generated the fingerprinting profile in the range of 300–5000 bp for 14 colonies out of 15 and was found to be identical to the inoculated strain. However, colony KM24 displayed a distinct banding profile with two additional bands of approximately 1200 and 1500 bp (Fig. 2B) (Fig. S2B). Similar to REP primer, 15 colonies except KM24 displayed identical profile to strain 11251 with bands at 900 and 1050 bp using ERIC primer, whereas an additional light band of 1600 bp was observed for colony KM24 (Fig. 2C) (Fig. S2C). Contrary to RAPD results, these findings suggested that the 14 colonies (Leu. mesenteroides) were matched strain 11251.
Fig. 2.
Rep-PCR analysis of 30 colonies picked from kimchi inoculated with L. mesenteroides 11251and L. brevis B151. From kimchi inoculated with L. mesenteroides 11251. Using (GTG)5 primer (A), Lane: 1–100 bp marker, Lane: 2–16 (colonies KM1-15), Lane: 17-strain 11251, Lane: 18–100 bp marker, Lower half of the Gel—Lane: 19–100 bp marker, Lane: 20–34 (colonies KM16-30), Lane: 35-strain 11251, Lane: 36–100 bp marker. Using REP primer (B), Lane: 1–100 bp marker, Lane: 2–16 (colonies KM1-15), Lane: 17-strain 11251, Lane: 18–100 bp marker, Lane: 19–1 kb marker, lower half of the Gel—Lane: 20–100 bp marker, Lane: 21–35 (colonies KM16-30), Lane: 36-strain 11251, Lane: 37–100 bp marker, Lane: 38–1 kb marker. Using ERIC primer (C), Lane: 1–100 bp marker, Lane: 2–16 (colonies KM1-15), Lane: 17-strain 11251, Lane: 18–100 bp marker, Lower half of the Gel—Lane: 19–100 bp marker, Lane: 20–34 (colonies KM16-30), Lane: 35-strain 11251, Lane: 36–100 bp marker. From kimchi inoculated with L. brevis B151. Using (GTG)5 primer (D), Lane: 1–100 bp marker, Lane: 2–16 (colonies KB1-15), Lane: 17-strain B151, Lane: 18–100 bp marker, Lower half of the Gel—Lane: 19–100 bp marker, Lane: 20–34 (colonies KB16-30), Lane: 35-strain B151, Lane: 36–100 bp marker. Using REP primer (E), Lane: 1–100 bp marker, Lane: 2–16 (colonies KB1-15), Lane: 17-strain B151, Lane: 18–100 bp marker, Lane: 19–1 kb marker, Lower half of the Gel—Lane: 20–100 bp marker, Lane: 21–35(colonies KB16-30), Lane: 36-strain B151, Lane: 37–100 bp marker, Lane: 38–1 kb marker. Using ERIC primer (F), Lane: 1–1 kb marker, Lane: 2–100 bp marker, Lane: 3–17 (colonies KB1-15), Lane: 18-strain B151, Lane: 19–100 bp marker, Lower half of the Gel—Lane: 20–1 kb marker, Lane: 21–100 bp marker, Lane: 22–36 (colonies KB16-30), Lane: 37-strain B151, Lane: 38–100 bp marker. Positive controls-11251 and B151
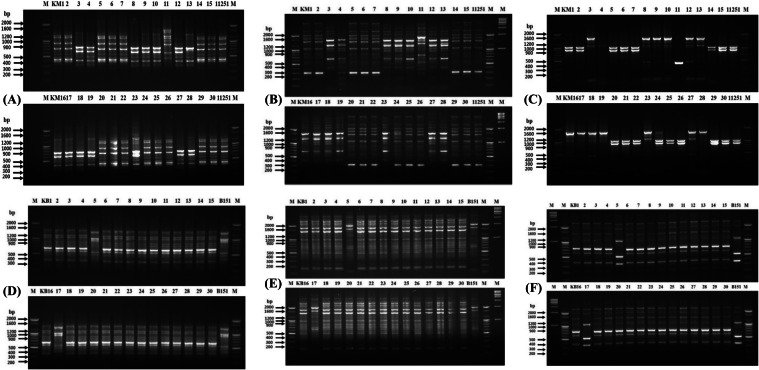
On the other hand, from KB culture, 2 colonies identified as L. brevis with RAPD displayed identical banding fingerprint with three rep primers (Fig. 2), which provided tracking information of strain B151. The bands were seen at 1000, 1100, and 1500 bp using a primer (GTG)5 (Fig. 2D). In the rep-PCR, bands at 1600 and 2000 bp were observed for KB5 and KB17 colonies, similar to the original strain (Fig. 2E). With ERIC primer, the 2 colonies showed bands at 350, 500, 1200, 2000, and 2200 bp, similar to strain B151 (Fig. 2F). Finally, the suspected bacterial colonies were tracked by comparative gene sequence analysis. Unexpectedly, the alignment results for the KM selected colonies showed SNPs in 5 out of 7 loci, i.e., atpA, groEL, gyrB, pheS, and uvrC (Fig. S3 A-H). The remaining 2 loci, pyrG, and rpoA had sequences identical to strain 11251. Thus, although there was similarity among the 16S rRNA gene sequences, RAPD, and rep-PCR (except for KM24 with primers REP and ERIC) matched to the reference strain, the SNPs in these five housekeeping loci could effectively discriminate the KM colonies from the reference strain. When the gene sequences were compared, the 14 colonies had a SNP in at least one gene. Results of SNPs analysis suggest that 14 out of 15 colonies were already present in the kimchi, and only KM22 had a sequence identical to the strain 11251 for the 7 loci. As all 7 loci sequences of colony KM22 were found identical to strain 11251, it was considered to be the deliberately inoculated reference strain.
Different observations were made with the KB culture. As expected, the two colonies did not show any SNP in any of the housekeeping genes (dnaK, groEL, gyrB, pheS, recA, rpoA, and rpoB) analyzed (Fig. S4A-G). The sequences of both colonies exactly matched the sequences of the original reference strain (B151), which suggested that KB5 and KB17 were strain B151, which was intentionally inoculated on kimchi. Our results indicate that the comparative sequence-based analysis best tracked the deliberately inoculated strains in kimchi compared to RAPD, rep-PCR, and 16S rRNA gene sequencing techniques. The results of the investigation with two LAB species in kimchi were encouraging. The results led to the conclusion that comparative gene sequences analysis based on the housekeeping loci was able to precisely differentiate and track the inoculated strains in the kimchi inoculated with Leu. mesenteroides, as some colonies were not accurately traced out by RAPD or rep-PCR, though showed an identical pattern to the reference strain.
Nucleotide sequence accession numbers
Gene sequences of housekeeping loci have been deposited in the GenBank under accession numbers dnaK-MF988118, MF988119, groEL-MF988129, MF988130, gyrB-MF988140, MF988141, pheS-MF988151, MF988152, recA-MF988162, MF988163; rpoA-MF988173, MF988174 and rpoB-MF988184, MF988185 for L. brevis and atpA-MG003174 to MG003188, pyrG-MG003196 to MG003210, uvrC-MG003218 to MG003232, pheS-MG003240 to MG003254, rpoA-MG003262 to MG003276, gyrB-MG003284 to MG003298 and groEL-MG003306 to MG003320 for Leu. mesenteroides.
The present manuscript describes the use of popular approaches for the differentiation and typing of LAB. The findings led to increasing our knowledge about how various molecular typing methods could be utilized to track desired bacterial strains in different food sources. The food industry is frequently developing new products to meet consumers’ demand under the stringent guidelines of regulatory bodies. A cutting-edge competition among food industries requires the development of starters with novel properties for the generation of value-added products to meet the needs of customers. Therefore, the selection of wild LAB strains, their identification, and characterization offer resources for the product development and improvement of existing commercial processes. However, the misuse of strains of business value cannot be denied. Therefore, the key uses of typing methods are not only to evaluate the phylogenetic relationships among microbial isolates but also to track and protect the commercially valuable strains.
The objective of the present study was demonstrated best while tracking Leu. mesenteroides 11251 in kimchi. It was assumed that the 15 colonies out of 30 identified as Leu. mesenteroides might be the inoculated strain, 11251. Fifteen was a surprisingly high number of colonies to be isolated from a 10−6 dilution. However, this could be because kimchi itself is a good source of many LAB, including Leu. mesenteroides and Leuconostoc citreum, during the initial and middle stages of kimchi fermentation (Chang and Chang, 2010). In the beginning, based on the 16S rRNA gene BLAST analysis, it was concluded that these colonies might be the original 11251 strain. Although 16S rRNA gene sequencing is well-documented method for the identification and phylogenetic analysis of LAB, however, technique often fails to discriminate between phylogenetically closely related species or subspecies of LAB (Fox et al., 1992; Temmerman et al., 2004), and two different strains that are highly similar may be misidentified due to incomplete sequencing of the 16S rRNA gene. Hence, the results are not 100 percent reliable (Björkroth et al., 2002). Genotypic fingerprinting methods, i.e., RAPD and rep-PCR, had similar discriminatory powers and found suitable for discrimination of a large number of microorganisms. The methods appear to be less reproducible and less comparable between different laboratories. As shown while tracking strain 11251 from kimchi, these methods showed low discriminatory power compared to gene sequences comparison. In support, it has been reviewed that the sequence analysis of the target gene loci provides adequate information for phylogenetic identification within species (Coppola et al., 2008). The chosen housekeeping gene loci must display higher variability in the target bacterial population than the 16S rRNA gene analysis (Eeom et al., 2018; Sarmiento-Rubiano et al., 2010; Sharma et al., 2018). However, for L. brevis B151 strain tracking from kimchi, except 2 colonies, all were identified as L. plantarum; the possible reason could be that L. plantarum becomes the prominent species during the later ripening stages of kimchi. In addition, the two colonies were ideally identified with all the three typing tools. These findings indicate that all tools like RAPD and rep-PCR could provide the initial monitoring information; however, comparative gene sequence analysis should be the choice. More precisely, it was inferred that the tracking study could not rely entirely on a single molecular tool. Incorporation of various molecular tools, as described in the introduction section for the identification of the target LAB, should be combined to have a better outcome.
In conclusion, a combined or polyphasic approach should be applied to acquire thorough and accurate information to identify the suspected target colonies in food products.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (DOCX 12195 kb)
Acknowledgements
This work was supported by the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry, and Fisheries (IPET) through the High Value-added Food Technology Development Program, funded by the Ministry of Agriculture, Food, and Rural Affairs (MAFRA; Grant No. 314073-03-2-HD040).
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Anshul Sharma, Email: anshul.silb18@gmail.com.
Jasmine Kaur, Email: jasskaur.0612@gmail.com.
Sulhee Lee, Email: sulhee2340@gmail.com.
Young-Seo Park, Email: ypark@gachon.ac.kr.
References
- Bain JM, Tavanti A, Davidson AD, Jacobsen MD, Shaw D, Gow NAR, Odds FC. Multilocus sequence typing of the pathogenic fungus Aspergillus fumigatus. J. Clinic. Microbiol. 2007;45:1469–1477. doi: 10.1128/JCM.00064-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthier F, Ehrlich SD. Genetic diversity within Lactobacillus sakei and Lactobacillus curvatus and design of PCR primers for its detection using randomly amplified polymorphic DNA. Int. J. Syst. Evol. Microbiol. 1999;49:997–1007. doi: 10.1099/00207713-49-3-997. [DOI] [PubMed] [Google Scholar]
- Björkroth KJ, Schillinger U, Geisen R, Weiss N, Hoste B, Holzapfel WH, Korkeala HJ, Vandamme P. Taxonomic study of Weissella confusa and description of Weissella cibaria sp. Nov., detected in food and clinical samples. Int. J. Syst. Evol. Microbiol. 52: 141-148 (2002) [DOI] [PubMed]
- Chang JY, Chang HC. Improvements in the Quality and Shelf Life of Kimchi by Fermentation with the Induced Bacteriocin-Producing Strain, Leuconostoc citreum GJ7 as a Starter. J. Food Sci. 2010;75:103–110. doi: 10.1111/j.1750-3841.2009.01486.x. [DOI] [PubMed] [Google Scholar]
- Coppola R, Blaiotta G, Ercolini D. Dairy products. pp. 31-90. In: Molecular techniques in the microbial ecology of fermented foods. Cocolin L, Ercolini D (eds). Springer, New York (2008)
- Dan T, Liu W, Sun Z, Lv Q, Xu H, Song Y, Zhang H. A novel multi-locus sequence typing (MLST) protocol for Leuconostoc lactis isolates from traditional dairy products in China and Mongolia. BMC Microbiol. 2014;14:150. doi: 10.1186/1471-2180-14-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eeom YJ, Son SY, Jung DH, Hur MS, Kim CM, Park SY, Shin WC, Lee SJ, Auh JH, Kim GW, Park CS. Diversity analysis of Saccharomyces cerevisiae isolated from natural sources by multilocus sequence typing (MLST) Food Sci. Biotechnol. 2018;27:1119–1127. doi: 10.1007/s10068-018-0335-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felis GE, Dellaglio F. Taxonomy of Lactobacilli and Bifidobacteria. Curr. Issues Intest. Microbiol. 2007;8:44–61. [PubMed] [Google Scholar]
- Fournier PE, Zhu Y, Ogata H, Raoult D. Use of highly variable intergenic spacer sequences for multispacer typing of Rickettsia conorii strains. J. Clinic. Microbiol. 2004;42:5757–5766. doi: 10.1128/JCM.42.12.5757-5766.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox GE, Wisotzkey JD, Jurtshunk P., Jr How close is close: 16S rRNA sequence identity may not be sufficient to guarantee species identity. Int. J. Syst. Evol. Bacteriol. 1992;42:166–170. doi: 10.1099/00207713-42-1-166. [DOI] [PubMed] [Google Scholar]
- Fusco V, Quero GM, Chieffi D, Franz CMAP. Identification of Lactobacillus brevis using a species-specific AFLP-derived marker. Int. J. Food Microbiol. 2016;232:90–94. doi: 10.1016/j.ijfoodmicro.2016.06.002. [DOI] [PubMed] [Google Scholar]
- Gemechu T. Review on lactic acid bacteria function in milk fermentation and preservation. Afr. J. Food Sci. 2015;9:170–175. doi: 10.5897/AJFS2015.1276. [DOI] [Google Scholar]
- Gevers D, Huys G, Swings J. Applicability of rep-PCR fingerprinting for identification Lactobacillus species. FEMS Microbiol. Lett. 2001;205:31–36. doi: 10.1111/j.1574-6968.2001.tb10921.x. [DOI] [PubMed] [Google Scholar]
- Hall TA. Bioedit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999;41:95–98. [Google Scholar]
- Hemme D, Foucaud-Scheunemann C. Leuconostoc, characteristics, use in dairy technology and prospects in functional foods. Int. Dairy J. 2004;14:467–494. doi: 10.1016/j.idairyj.2003.10.005. [DOI] [Google Scholar]
- Karahan AG, Başyiğit KG, Kart A, Sanlidere AH, Oner Z, Aydemir S, Erkuş O, Harsa S. Genotypic identification of some lactic acid bacteria by amplified fragment length polymorphism analysis and investigation of their potential usage as starter culture combinations in Beyaz cheese manufacture. J. Dairy Sci. 2010;93:1–11. doi: 10.3168/jds.2008-1801. [DOI] [PubMed] [Google Scholar]
- Kaur J, Lee S, Park YS, Sharma A. RAPD analysis of Leuconostoc mesenteroides strains associated with vegetables and food products from Korea. LWT Food Sci. Technol. 77: 383-388 (2017a)
- Kaur J, Lee S, Sharma A, Park YS. DNA profiling of Leuconostoc mesenteroides strains isolated from fermented foods and farm produce in Korea by repetitive-element PCR. Food Sci. Biotechnol. 26: 1667-1673 (2017b) [DOI] [PMC free article] [PubMed]
- Klaenhammer TR, Barrangou R, Buck BL, Azcarate-Peril MA, Altermann E. Genomic features of lactic acid bacteria effecting bioprocessing and health. FEMS Microbiol. Rev. 2005;29:393–409. doi: 10.1016/j.fmrre.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Leuschner RGK, Robinson TP, Hugas M, Cocconcelli PS, Richard-Forget F, Klein G, Licht TR, Nguyen-The C, Querol A, Richardson M, Suarez JE, Thrane U, Vlak JM, Von Wright A. Qualified presumption of safety (QPS): a generic risk assessment approach for biological agents notified to the European Food Safety Authority (EFSA) Trends Food Sci. Technol. 2010;21:425–435. doi: 10.1016/j.tifs.2010.07.003. [DOI] [Google Scholar]
- Li W, Raoult D, Fournier P-E. Bacterial strain typing in the genomic era. FEMS Microbiol. Rev. 2009;33:892–916. doi: 10.1111/j.1574-6976.2009.00182.x. [DOI] [PubMed] [Google Scholar]
- Park SH, Jung JH, Seo DH, Lee HL, Kim GW, Park SY, Shin WC, Hong S, Park CS. Differentiation of lactic acid bacteria based on RFLP analysis of the tuf gene. Food Sci. Biotechnol. 2012;21:911–915. doi: 10.1007/s10068-012-0119-9. [DOI] [Google Scholar]
- Riccia DD, Bizzini F, Perilli M, Polimeni A, Trinchieri V, Amicosante G, Cifone M. Anti-inflammatory effects of Lactobacillus brevis (CD2) on periodontal disease. Oral Dis. 2007;13:376–385. doi: 10.1111/j.1601-0825.2006.01291.x. [DOI] [PubMed] [Google Scholar]
- Rönkä E, Malinen E, Saarela M, Rinta-Koski M, Aarnikunnas J, Palva A. Probiotic and milk technological properties of Lactobacillus brevis. Int. J. Food Microbiol. 2003;83:63–74. doi: 10.1016/S0168-1605(02)00315-X. [DOI] [PubMed] [Google Scholar]
- Rushdy AA, Gomaa EZ. Antimicrobial compounds produced by probiotic Lactobacillus brevis isolated from dairy products. Ann. Microbiol. 2013;63:81–90. doi: 10.1007/s13213-012-0447-2. [DOI] [Google Scholar]
- Sabat AJ, Budimir A, Nashev D, Sá-Leão R, van Dijl J M, Laurent F, Grundmann H, Friedrich AW. On behalf of the ESCMID Study Group of Epidemiological Markers (ESGEM). Overview of molecular typing methods for outbreak detection and epidemiological surveillance. Euro Surveill. 18(4): 20380 (2013) [DOI] [PubMed]
- Sarmiento-Rubiano L, Berger B, Moine D, Zuniga M, Pérez-Martínez G. Yebra M Characterization of a novel Lactobacillus species closely related to Lactobacillus johnsonii using a combination of molecular and comparative genomics methods. BMC Genomics. 2010;11:504. doi: 10.1186/1471-2164-11-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S, Goswami P, Singh R, Heller KJ. Application of molecular identification tools for Lactobacillus, with a focus on discrimination between closely related species: A review. LWT Food Sci. Technol. 2009;42:448–457. doi: 10.1016/j.lwt.2008.05.019. [DOI] [Google Scholar]
- Sharma A, Kaur J, Lee S, Park YS. Analysis of Leuconostoc citreum strains using multilocus sequence typing. Food Sci. Biotechnol. 2018;27:1755–1760. doi: 10.1007/s10068-018-0417-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele J, Broadbent J, Kok J. Perspective on the Contribution of Lactic Acid Bacteria to Cheese Flavor Development. Curr. Opin. Biotechnol. 2013;24:135–141. doi: 10.1016/j.copbio.2012.12.001. [DOI] [PubMed] [Google Scholar]
- Temmerman R, Huys G, Swings J. Identification of lactic acid bacteria: culture dependent and culture-independent methods. Trends Food Sci. Technol. 2004;15:348–349. doi: 10.1016/j.tifs.2003.12.007. [DOI] [Google Scholar]
- Waki N, Matsumoto M, Fukui Y, Suganuma H. Effects of probiotic Lactobacillus brevis KB290 on incidence of influenza infection among schoolchildren: an open-label pilot study. Lett. Appl. Microbiol. 2014;59:565–571. doi: 10.1111/lam.12340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassie M, Wassie T. Isolation and identification of Lactic Acid Bacteria from raw cow milk. Int. J. Adv. Res. Biol. Sci. 2016;3:44–49. [Google Scholar]
- Winkler J, Kao KC. Transcriptional analysis of Lactobacillus brevis to n-butanol and ferulic acid stress responses. PLoS ONE. 2011;6:e21438. doi: 10.1371/journal.pone.0021438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeller-Péronnet V, Brockmann E, Pavlovic M, Timke M, Busch U, Huber I. Potential and limitations of MALDI-TOF MS for discrimination within the species Leuconostoc mesenteroides and Leuconostoc pseudomesenteroides. J. Verbr. Lebensm. 2013;8:205–214. doi: 10.1007/s00003-013-0826-z. [DOI] [Google Scholar]
- Zhang ZG, Ye ZQ, Yu L, Shi P. Phylogenomic reconstruction of lactic acid bacteria: an update. BMC Evol. Biol. 2011;11:1–12. doi: 10.1186/1471-2148-11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1 (DOCX 12195 kb)