Abstract
The present study was conducted to investigate the ability of two probiotic strains, L. acidophilus PTCC 1643 and L. rhamnosus PTCC 1637, to bind aflatoxin B1 (AFB1, 20 ng/ml) in comparison with yogurt starter cultures, at equal bacterial count (~ 109 LogCFU/ml) during a 21-day storage period at 4 °C. All assessed treatments exhibited high percentages of AFB1-binding, ranged from 64.56 to 96.58%. However, the ability of probiotic bacteria was statistically higher than yogurt starter cultures. Aflatoxin binding ability of the selected lactic acid bacteria was dependent on both time and bacteria species. The highest and the lowest percentages of AFB1-removal was observed at 11th day of cold storage by L. rhamnosus (96.58 ± 3.97%) and at the first day of storage for yogurt starter cultures (64.56 ± 5.32%), respectively. The stability of bacterial cells-AFB1 complex was remarkable, since only 0.84–26.75% of bounded AFB1 was released from bacterial cells after 3 times washing during the storage period.
Keywords: Aflatoxin removal, Probiotic yogurt, Binding ability, Complex stability
Introduction
The human diet may contain a miscellaneous array of natural mutagenic or carcinogenic compounds, due to the pollution of raw materials or the formation of toxic metabolites throughout food processing, cooking or storage (Osowski et al., 2010; Wang et al., 2011). Generally, mycotoxins are naturally occurring poisonous secondary metabolites of filamentous fungi and mainly produced by Aspergillus, Penicillium, and Fusarium species (Priyanka et al., 2014; Venkataramana et al., 2014). These toxic compounds play an indubitable performance in the reduction of the marketable and hygienic quality of various products (Dalié et al., 2010). The FAO (Food and Agriculture Organization of the United Nations) assessed that more than 25% of the world agricultural production is polluted by mycotoxins (Marin et al., 2013). Mycotoxin contamination of the food chain has a drastic impact and uncountable economic costs. However, the United States Food and Drug Administration (FDA) based on computer modeling, evaluated that the potential economic costs of crop losses due to aflatoxins, fumonisins and trichothecenes in the USA are expected to be $932 million per year (Milićević et al., 2010). In the same way, the large world consumption of the most important foodstuffs, such as corn (1033.7 million tonnes/year), milk (876 million tonnes/year), wheat (757.9 million tonnes/year), soybean (336.7 million tonnes/year) and peanut (45.45 million tonnes/year), makes the presence of mycotoxins as a serious problem in these products.
Aflatoxins (AFs), are highly toxic mycotoxins produced by some Aspergillus species especially A. flavus, A. parasiticus and rarely A. nomius. Currently, there are 20 related compounds defined by the term of AF (Prandini et al., 2009). Among them, AFB1 and AFB2 are produced by A. flavus, but AFG1 and AFG2 along with AFB1 and AFB2 are produced by A. parasiticus (Bennett and Klich, 2003; Kumar et al., 2017). Besides, AFM1 and AFM2 are the hydroxylated metabolites of AFB1 and AFB2, respectively in lactating animals and humans (Hussain and Anwar, 2008). Intake of aflatoxin contaminated foods and feeds could lead to acute and chronic aflatoxicosis, including carcinogenic, mutagenic, teratogenic, neurotoxic, oestrogenic, and immune suppressive effects (Groopman et al., 2008; Ishikawa et al., 2017; Jiang et al., 2015; Milićević et al., 2010; Sellamani et al., 2016; Smith et al., 2017; Sun et al., 2018). International Agency for Research on Cancer (IARC) classified AFB1 as a group I, carcinogen for humans (IARC, 2016).
Due to the harmful aftermaths of mycotoxins, certain procedures have been established to inhibit the development of these compounds and as well, to detoxify human foodstuffs and animal feedstuffs (Hathout and Aly, 2014; Kabak et al., 2006). These methods include: (1) the inhibition of mycotoxin pollution, (2) the decontamination/detoxification of foods and feedstuffs contaminated with mycotoxins, and (3) prevention of absorption of mycotoxin content of consumed food into the digestive tract (Hathout and Aly, 2014). Several methods, including chemical, physical and biological control strategies have been proposed and implemented to decrease level of aflatoxins in foods and feeds with varying degrees of successes (Abdallah et al., 2015; Bozoğlu, 2009; Karlovsky et al., 2016; Shao et al., 2016; Zaki et al., 2012). Among these methods, chemical and physical procedures have some limitations, such as concerning safety issues, losses in the nutritional value, altered organoleptic characteristics of the products, limited efficacy and cost implication (Gowda et al., 2007; Guan et al., 2011; Méndez-Albores et al., 2005; Puzyr et al., 2010). Therefore, it is crucial to attain innovative toxin removal or detoxifying approaches, especially for elimination of aflatoxins, to promote food safety.
Probiotics are described as “living microorganisms which when ingested in adequate amounts, beneficially influence the health of the host by improving the composition of intestinal microbiota” (FAO/WHO, 2002). Probiotics have shown physiological function and promote the body’s immunity (Nooshkam et al., 2018; Shah, 2000a; Yerlikaya, 2014). Yogurt, the best carrier of probiotics, traditionally is produced using Streptococcus thermophilus and Lactobacillus delbrueckii ssp. bulgaricus as starter cultures. Nowadays, an adequate level of lactic acid bacteria (LAB), particularly probiotic strains are considered as a key elements of yogurt quality (Ziarno and Zaręba, 2019). Several researchers have tested the lactic acid bacteria, as Lactobacillus and Bifidobacterium species for their ability to bind AFB1 both in vitro and in vivo conditions, due to their GRAS (generally recognized as safe) status and use as probiotics (Liew et al., 2018; Zoghi et al., 2014). For example, Peltonen et al. (2001) revealed that a properly high efficiency (more than 50%) of binding of aflatoxin B1 from a buffered saline solution is displayed by strains of Lactobacillus amylovorus and Lactobacillus rhamnosus, while Lactococcus lactis spp. cremoris, Bifidobacterium animalis and Bifidobacterium lactis were slightly less effective (40–50%). Corassin et al. (2013) compared the AFB1 binding capacity of L. delbrueckii spp. bulgaricus, L. rhamnosus, and B. lactis in combination with heat-inactivated S. cerevisiae. This assimilation certified complete mycotoxin binding (100%).
The hot and humid climates and storage condition of many food products as milk powders, polluted and unhygienic environment, frequent opening of bags contain these products, using unclean cups or containers for measurement, as well as, unclean and unsterilized packaging materials may lead to the growth of Aspergillus species, particularly Aspergillus flavus and subsequently aflatoxins production. Among aflatoxins, AFB1 is the most toxic metabolite as compared to other mycotoxins. Therefore, the main objectives of this investigation were to: (1) determine the AFB1-binding ability of the selected probiotic bacteria in comparison with yogurt starter cultures and (2) to evaluate the stability of the complexes (bacterial cells-AFB1) during 21-days cold storage period.
Materials and methods
Chemicals and media
Aflatoxin B1 (AFB1, from Aspergillus flavus) in powder form was purchased from Sigma-Aldrich, Germany. All the solvents used include methanol, acetonitrile, benzen and n-hexane were obtained from Merck (Germany) and dichloromethane was purchased from Samchun (Korea). For bacterial cultivation and counting, de Man–Rogosa–Sharpe (MRS) broth and agar (Liofilchem, Italia), peptone water (Merck) and bile salt powder (Merck) were used. Commercial Direct Vat Set (DVS) lyophilized pouches of yogurt starter cultures contain a mixed culture of Streptococcus thermophilus and Lactobacillus bulgaricus were supplied by Chr. Hansen company (Denmark). The lyophilized cultures were maintained according to the manufacturer’s instructions, at − 20 °C. The probiotic lactic acid bacteria strains, Lactobacillus acidophilus PTCC 1643 and Lactobacillus rhamnosus PTCC 1637, as lyophilized ampoules were procured from Iranian Research Organization for Science and Technology (Persian Type Culture Collection, PTCC), Tehran, Iran.
Preparation of bacterial strains and growth conditions
L. acidophilus and L. rhamnosus were selected based on their use in various foodstuff, especially dairy products, and on their ability to bind different kinds of food contaminant, such as mycotoxins and heavy metals (Bhakta et al., 2012; Haskard et al., 2001; Zoghi et al., 2014). L. rhamnosus was inoculated directly into MRS broth, and L. acidophilus activated in MRS broth supplemented with 0.05% cysteine hydrochloride, then incubated (BINDER Gmbh, Model KB 23, Germany) without shaking at 37 °C for 48 h, in aerobic and anaerobic conditions, respectively. The evaluation of bacterial cells concentration in the cultures were determined by conventional agar plating technique using MRS agar and further incubation of cultured plates at 37 °C for at least 48 h. At the end of the incubation period, bacterial counts were expressed as colony forming unit (CFU) per milliliter of the media. In order to storage, bacterial cells were kept at − 80 °C in MRS broth having 20% (v/v) glycerol, as cryoprotectant. At the time of experiments, strains were recovered from media, grown in MRS broth again and incubated, as previously described. After incubation, cells were collected by centrifugation at 6000 g for 10 min at 10 °C. Finally, bacterial pellets removed from the supernatant under the sterile conditions and washed once by sterile deionized water prior to use (El-Nezami et al., 1998; Elsanhoty et al., 2014).
Preparation of AFB1 standard solution
AFB1 powder was suspended in a mixture of HPLC grade acetonitrile/benzene (3:97 v/v) to obtain the approximate concentration of 10 µg/ml. Then, the standard solution was achieved by diluting the mixture in phosphate buffer saline (PBS) solution. The solvents were evaporated using a water bath (Memmert, Model WNB 14, Germany) by heating at 80 °C for 10 min (Haskard et al., 2001). The concentration of the standard solution was finalized and computed by the Lamberte-Beer equation (A = εcl) using the absorbance (A) at 354 nm, a molar absorptivity ε354 = 19.950 per Mol.cm and the optical distance crossed by light in the medium (l) (Zinedine et al., 2005). The obtained solution was transferred to a dark glass bottle and kept in refrigerator until used. An aliquot of this standard solution was diluted in PBS (pH ~ 7) to final concentration of 20 ng/ml and to perform the AFB1 binding assay. For drawing of calibration curve, standard solutions of AFB1 with different concentrations were prepared by dilution in acetonitrile, water and methanol (20:50:30 v/v/v, respectively). Calibrations curve were obtained by plotting the peak area for each calibration solution against the concentration of AFB1 injected (Sarlak et al., 2017). The correlation coefficient R2 was 0.9997.
Preparation of yogurt
Set-type yogurt samples were made according to the method of performed by Elsanhoty et al. (2014) with some modifications. Reconstituted milk was prepared by diluting 10 g of skimmed milk powder into 100 ml sterile deionized water. The milk was then stirred for 5 min and heated at 90 °C for 15 min to both destroy pathogen microorganisms and raise the denaturation of whey proteins. Next, the milk cooled down to incubation temperature (45 °C) and inoculum was added. As the minimum concentration of 108 CFU/ml of LAB is required for adequate and rapid binding of aflatoxins to the bacterial cell wall (Kabak and Var, 2008), the LAB strains was added so that the number of bacteria reaches to CFU/g. Different batches from yogurt were produced as: negative control (NCON), milk without AFB1 and probiotic strain and only inoculated with 0.05% yogurt starter cultures ( CFU/g); positive control (PCON), milk with 20 ng/ml AFB1 and without probiotic strain inoculated with 0.05% yogurt starter cultures; L. acidophilus probiotic yogurt (LAPY), milk with 20 ng/ml AFB1 inoculated with 0.025% yogurt starter cultures ( CFU/g) and L. acidophilus probiotic strain ( CFU/g); L. rhamnosus probiotic yogurt (LRPY), milk with 20 ng/ml AFB1 inoculated with 0.025% yogurt starter cultures and L. rhamnosus probiotic strain ( CFU/g). Therefore, the initial viable cell counts of all the yogurts (probiotics and non-probiotics) were similar ( CFU/g). All batches were incubated at 42 °C until reaching pH approximately to 4.60, at which the fermentation was terminated. The fermentation time to reach pH 4.60 for yogurt samples was about 4 h. Then, samples were immediately cooled and stored at 4 °C for 21 days. All analyses of the yogurt samples were performed in triplicate after production, and 1, 11- and 21-days during storage at refrigeration temperature.
Evaluation of pH and titratable acidity
The pH of the samples was measured using a digital pH meter (Metrohm Company, model 827, Switzerland) by direct immersion of the electrode in samples at room temperature. Titratable acidity was assessed via blending 9 g of sample with 9 ml of distilled water and titrating with 0.1 N NaOH using phenolphthalein (1% (w/v) in ethanol) as an indicator, to an end point of stable faint pink color for 30 s (Jooyandeh et al., 2015). Titratable acidity was asserted as a percentage of lactic acid.
Evaluation of syneresis
Syneresis was obtained by slight modification of the technique stated by Akgun et al. (2018). To measure syneresis, 5 g of yogurt weighed in centrifuge tubes and then centrifuged (HERMLE Labrotechnik Gmbh, Model Z 206 A, Germany) at 2500 rpm for 10 min at 25 °C. The collected liquid from the sample that separated in the top of tube was then gently poured off, weighed and regarded as syneresis.
Microbiological analysis
After serial dilution, bacteria were counted by the surface culture method. MRS-bile agar medium was used for the selective enumeration of probiotic bacteria (Kabak and Var, 2008).
Preparation of samples for AFB1 binding assay
For each sample, after gentle mixing, 5 g yogurt was transferred in falcon tubes. Subsequently, 40 ml of dichloromethane was added, and suspension was agitated for 15 min and filtered. Thereafter, 10 ml of the filtrate was heated at 60 °C and after evaporation, the residual oily was re-suspended in a mixture of 0.5 ml PBS, 0.5 ml methanol and 1 ml of n-hexane. The suspension was centrifuged (Eppendorf, Model AG 22331, Germany) at 3500 g for 15 min at 10 °C. After removing n-hexane (upper layer), 100 µl of the aliquot were diluted with 400 µl of deionized water and 100 µl of the diluted samples was experimented for the AFB1 assessment (El Khoury et al., 2011; Elsanhoty et al., 2014).
AFB1 binding assay by HPLC
The HPLC technique used for the analysis of unbound AFB1 present in the supernatant was carried out according to Soares et al. (2010) with minor modifications. The HPLC system (KNAUER smartline, Germany) equipped with a programmable fluorescence detector (RF-10AXL) and a pump solvent delivery system (model 1000). Separation was achieved by using a Eurospher 100-5 C18 reversed phase column (4.6 × 150 mm, 5 μm particle size, KNAUER Smartline) at 40 °C with an injection volume of 100 μl. Water–methanol–acetonitrile (5:3:2 vol/vol/vol) was used as the mobile phase, with a flow rate of 1 ml/min. The excitation and emission wavelengths were set at 365 and 460 nm, respectively. Under these conditions, the retention time of AFB1 was around 12 min. The percentage of the bound AFB1 by the examined strains suspension was determined using the following equation:
Stability of the bacterial cells-AFB1 complex
The amounts of AFB1 released from the bacteria-AFB1 complexes were investigated after repetitive washes (three times) by HPLC. For determination of bacterial cells-AFB1 complex stability, 1 g yogurt sample was mixed with 1.5 ml PBS solution for 15 s. Afterward, the mixture was kept at 37 °C for 5 min. For removing the bacterial cell, the suspension was centrifuged (Eppendorf, Model AG 22331, Germany) at 2000 g for 10 min at 10 °C s. Amount of AFB1 in the supernatant (at the third washing stage) was determined using HPLC (Peltonen et al., 2001; Utami et al., 2017).
Statistical analysis
Data were analyzed as a completely randomized factorial design. The significance of the difference between means was characterized by Duncan’s multiple range test (p < 0.05) using SPSS statistical software (version 20, SPSS Inc., USA). All statistics were the averages of triplicate trials, and the values were shown as the mean values. All graphs were created using Microsoft Excel 2013 software (Version 6.2, Palisade Corporation, New York, USA).
Results and discussion
pH and titratable acidity
The changes in pH of the tested samples after completion of the yogurt fermentation and during the 21-day cold storage (4 °C) are shown in Fig. 1. The average pH values for all samples ranged from 4.07 to 4.67 during the storage period. The highest pH value (4.67 ± 0.05) was recorded for PCON just after yogurt production and the lowest pH value (4.07 ± 0.09) was recorded for LAPY sample at the end of storage. PCONT demonstrated greater pH value (4.54 ± 0.07) than other samples at the all storage periods. However, there was no significant difference (p >0.05) between the pH values of non-probiotic yogurts, i.e. PCONT and NCON samples. Furthermore, probiotic yogurts had noticeably lower pH values than positive and negative controls (p < 0.05). Among probiotic yogurts, LRPY yogurts had generally lower pH value than LAPY samples but these differences were not significant (p > 0.05).
Fig. 1.
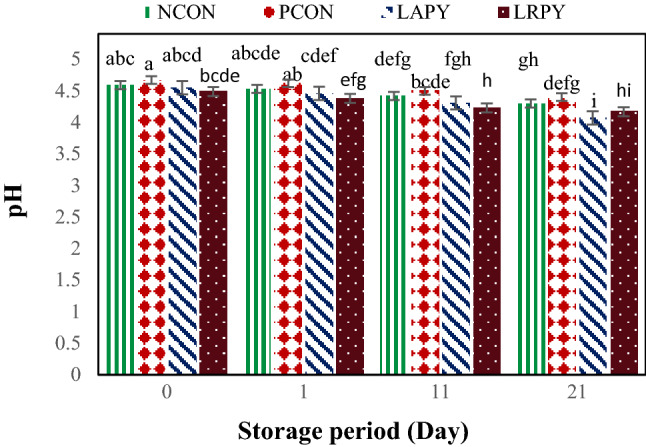
Changes in the pH levels of yogurt samples during the cold storage. Different lowercase letters indicate significant differences at p < 0.05
During storage period, the pH values of all yogurt samples declined significantly (p < 0.05). Nevertheless, pH values did not decrease lower than 4.0, which is usually considered unfavorable to the survival of probiotic bacteria (Yilmaz-Ersan and Kurdal, 2014). Beal et al. (1999) stated post acidification is the main reason of pH reduction during storage due to permanent metabolic activity of the starter cultures added to the product (mainly L. delbrueckii subsp. bulgaricus). This decrease in pH, is attributed to the use of residual carbohydrates by viable bacteria and production of several metabolites such as lactic acid, small amounts of CO2 and formic acid from lactose (Panesar and Shinde, 2012).
The pH reduction was slightly more in probiotic yogurts in comparison with control samples. The pH varied between 4.07 ± 0.09 and 4.55 ± 0.11 for the probiotic batches of yogurt and 4.30 ± 0.03 and 4.67 ± 0.05 for the control batches during the cold storage. These results were similar to the findings of Shaghaghi et al. (2013) who reported the lower pH for probiotic yogurts during the storage.
Like pH values, the titratable acidity (TA) values of yogurt samples during storage period were changed significantly (Fig. 2). The TA values of control and probiotic samples increased meaningfully (p < 0.05) throughout storage time and varied from 0.8 ± 0.07 to 1.77% ± 0.13. The increase in TA values of fermented milks during cold storage is a common phenomenon. In general, the higher TA were obtained with probiotics yogurt samples, i.e. LAPY and LRPY samples in comparison with controls. These finding were in agreement with Yilmaz-Ersan and Kurdal (2014) who reported the higher level of TA for probiotic yogurts but were in contrast with Güler-Akin and Akin (2007) who described the lower level of TA in probiotic yogurts due to growth inhibition of L. bulgaricus by probiotic bacteria. Many factors could influence the acidity/pH values of yogurts during storage period which from those, the type of starter cultures, manufacturing methods and storage conditions are the more important. However, it should be considered that yogurt starter cultures can only produce lactic acid while L. acidophilus and Bifidobacteria have capability to produce both lactic and acetic acids (Yilmaz-Ersan and Kurdal, 2014). As it is shown in Figs. 1 and 2, PCON yogurt samples contained 20 ng/ml AFB1 had slightly (p > 0.05) higher pH and lower TA in comparison with NCON. These findings indicate that addition of AFB1 to yogurt may have negative effect on starter cultures activity.
Fig. 2.
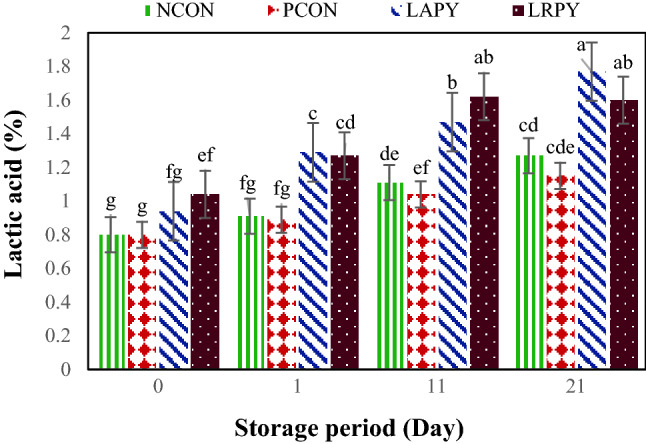
Changes in the titratable acidity percentages of yogurt samples during cold storage. Different lowercase letters indicate significant differences at p < 0.05
Syneresis
Syneresis, is one of the main problems of yogurt industry. This is the expulsion of whey from three‐dimensional casein networks, which turn out to be observable on the surface. Whey loss measures the level of collapsed gel and is an indicator for poor quality and stability. As it is shown in Fig. 3, control yogurt samples had lower syneresis in comparison with probiotic yogurts throughout the storage period. However, except at day 11, these differences were not significant (p > 0.05). The syneresis in the control samples during cold storage was in the range of 13.76 ± 0.12–26.57 ± 0.09%, while in the probiotic yogurts it was varied in the range of 16.29 ± 0.10–27.22 ± 0.11%. This may be due to the lower pH and the higher acidity in the probiotic yogurts in comparison with control samples, as a decrease in pH value in yogurt accelerates the syneresis (Athar et al., 2000). The highest syneresis was determined in LRPY sample (27.22%), while the lowest value was obtained in NCON sample (13.76%).
Fig. 3.
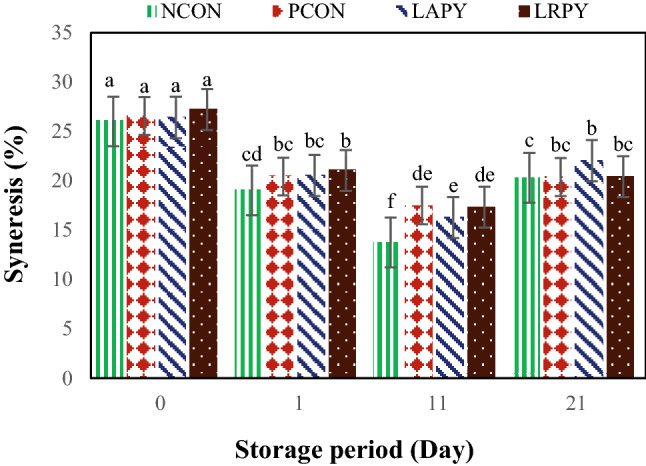
Changes in the syneresis values of yogurt samples during cold storage. Different lowercase letters indicate significant differences at p < 0.05
Furthermore, storage period had significant impact on the yogurt syneresis. The syneresis values of both controls and probiotic yogurts were meaningfully decreased (p < 0.05) until 11th day of the cold storage and thereafter, the amount of whey separation in yogurt samples increased noticeably. In agreement with our results, Tamjidi, et al. (2012) found that the separation of yogurt serum during storage had a decreasing trend from the first to 14th day of storage and thereafter it increased till the 21st day of storage. In contrary to our results, Yangilar and Çakmakçi (2017) and Güler-Akin and Akin (2007) reported a decrease in yogurt syneresis throughout cold storage period. As it is shown in Fig. 3, NCON samples had lower syneresis than PCON ones and this difference was significant at the 11th of storage period. The higher syneresis in PCON samples in comparison with NCON yogurts could be due to negative effect of added AFB1 on metabolic activity of yogurt starter cultures, which cause an increase in net pressure in protein network (Akın, 1998).
Viability of LAB strains
Numerous parameters may affect the viability of lactic acid bacteria in yogurt including type of bacterial strain, inoculation level, pH, presence of hydrogen peroxide and dissolved oxygen, extent of produced metabolites such as lactic acid and acetic acids, concentration of solutes (osmotic pressure), buffering capacity of the media, storage and incubation temperature, storage and fermentation time, availability of nutrients as well as growth promoters and inhibitors (Donkor et al., 2006). The changes of viable cell count of lactic acid bacteria, from the preparation time of yogurt samples up to the end of cold storage are shown in Fig. 4. There were significant differences (p < 0.05) among the viable counts of tested LAB strains; and in general, probiotics yogurt samples had higher bacterial counts than control samples. Although till the middle of storage period, LRPY yogurt samples had slightly the higher viable cell counts than LAPY samples, their number of viable cells at the end of storage were significantly reduced. As well, PCON samples had significantly lower bacterial counts than NCON and both probiotics yogurts throughout of storage period, indicated that AFB1 had negative effect on starter cultures viability.
Fig. 4.
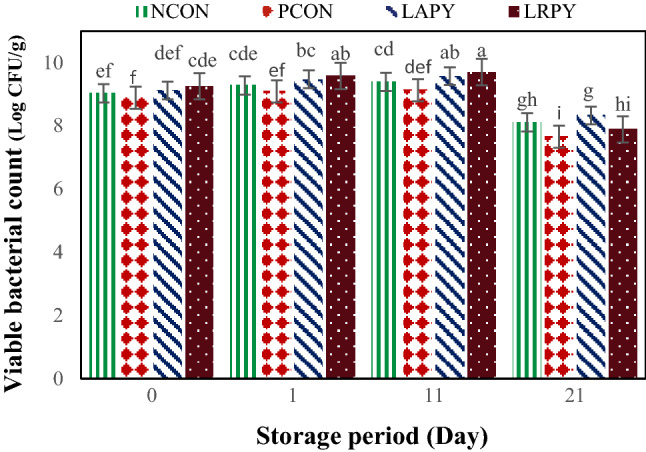
Changes of viable cell counts of lactic acid bacteria during cold storage. Different lowercase letters indicate significant differences at p < 0.05
The adverse effect of aflatoxin M1 on variability of LAB starter cultures have been previously demonstrated by Tajalli et al. (2014). Bacterial viability of all strains enhanced until 11th day of the storage and noticeably declined (p < 0.01) at the end of storage period. However, viable counts of both probiotic yogurts at the end of storage were above of standard limit (> 107 Log CFU/g). Similar findings were described by Birollo et al. (2000) and Güler-Akin and Akin (2007). However, in contrast with our results, Shaghaghi et al. (2013) and Shah (2000a, b) observed no significant changes of bacterial cell count in commercial yogurts containing L. acidophilus and B. bifidum throughout the storage period.
AFB1 binding ability of LAB strains
Generally, procedures to destroy the mycotoxins to the safe levels should have the following requirements: (1) deactivate or eliminate the toxin, (2) do not yield or release toxic remains in the foods and feeds, (3) sustain the nutritive values of the foods and feeds, (4) do not alter the sensory attributes and the quality characteristics of the product, and if feasible, (5) eliminate fungal spores (Park, 2002). Primary investigations have shown that the lactic acid bacteria can remove aflatoxins, in vitro and in vivo models. Nevertheless, it is complicated to compare results of aflatoxin binding levels from various investigations, owing to the possible effect of technical variations.
Results in Fig. 5 show that all the strains tested were able to absorb AFB1 efficiently, but at significant different levels (p < 0.01), which could be due to differences in the ability of their bacterial cell wall to absorb AFB1. The amount of bound AFB1 for all the yogurt treatments ranged from 64.56 ± 5.32 to 96.58 ± 3.97% during 21 days of the storage period. Elimination of AFB1 by all tested lactic acid bacteria was rapid, since at least 64% of AFB1 was removed after 1 day of storage. In agreement with our results, Tajalli et al. (2014) verified that more than 92% of AFM1 was removed by L. rhamnosus. Elgerbi et al. (2006) also in an investigation on the capability of strains of Lactobacillus spp., Lactococcus spp. and Bifidobacterium spp. to bind AFM1 declared that the extent of AFM1 bounded after 96 h by these strains ranged from 4.5 to 73.1%. Besides, El-Nezami et al. (1998) represented 67% reduction of AFB1 by L. case and Peltonen et al. (2001) reported 22.7–54.6% AFB1 reduction for three strains of L. rhamnosus.
Fig. 5.
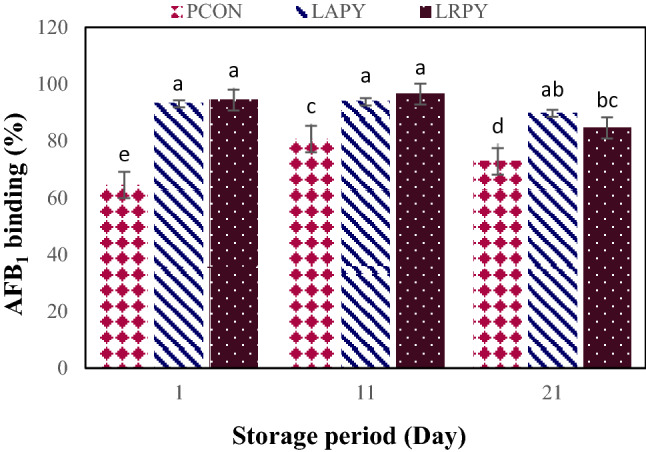
Changes in the AFB1 binding percentages by LAB in yogurt samples during cold storage. Different lowercase letters indicate significant differences at p < 0.05
Among tested bacterial strains, probiotic yogurts were able to remove the higher level of AFB1 in comparing with yogurt starter cultures, i.e. PCON sample throughout the storage period (p < 0.01). These findings were in accordance with the observations of Elsanhoty et al. (2014) who reported the higher AFM1 reduction in probiotic yogurt contained an equal mixture of yogurt starter cultures and L. plantrium as compared to yogurt sample. As it is shown in Fig. 5, until the middle of storage period, LRPY probiotic yogurts had a little more binding capability to AFB1, while at the end of storage, LAPY samples were able to eliminate slightly the higher percentages of AFB1 (p > 0.05).
Furthermore, the percentage of AFB1 absorption was time dependent and significant interaction between treatment and storage period was found (Fig. 5). In general, by increasing the time of storage up to 11th day, the amount of AFB1-binding ability by all yogurt samples significantly increased and reached to its maximum level but thereafter it noticeably decreased. The highest binding capacity of AFB1 for PCON, LAPY and LRPY were recorded as 80.72 ± 6.04%, 93.84 ± 3.15%, 96.58 ± 3.97%, respectively. As it demonstrated before (Fig. 4), the bacterial viability at the end of storage were significantly decreased by more than 1 log cycle and simultaneously substantial decrease of AFB1 absorption (p < 0.01) were found at this period (Fig. 5). However, the reduction of AFB1 binding in all the treatments throughout this storage interval were less than 10 percent. Similar results are reported by Abdelmotilib et al. (2018) and Tajalli et al. (2014). It is well confirmed that removal of AFB1 depends neither on bacterial viability and nor on metabolic alteration of the toxin by bacteria. Indeed, toxin removal occurs by absorption to bacterial cell wall constituents rather than covalent linkage or metabolic deterioration, and dead cells still show binding capability (Haskard et al., 2000, 2001). It is even indicated that the bacterial cell wall disruption due to the heat processing result in the bacteria surface becomes more reachable to form extra AFB1-bacteria linkage (Assaf et al., 2018; Liew et al., 2018). By bacterial absorption, toxin bioavailability is reduced, and consequently AFB1 uptake and its entrance to systemic circulation are also constricted (Solis-Cruz et al., 2018).
Stability of the bacteria-AFB1 complex during storage period
In addition to the kind of washing solution, the most important factors which affect the amount of complex stability (CS) of mycotoxins are the kind of bacteria strains and the level of toxin bound to bacterial cell. Bacteria strains differ due to the variations in their toxin-cell binding sites/cross-linked matrix that inhibits aflatoxin releasing (Utami et al., 2017). As well, the more aflatoxin adsorbed by bacterial cell, the longer time the adsorbed aflatoxin molecules would remain on the cell surface of bacteria (Lee et al., 2003).
The efficiency of selected LAB strains to bind AFB1 after continual washing with PBS (pH ~ 7) is presented in Fig. 6. In the CS assay, significant differences were found between yogurt treatments in regarding to release of AFB1 during 21 days of storage. The values of residual bound aflatoxin after three washes by bacteria during the storage time ranged from 73.25 ± 3.41–99.16 ± 3.13%. Related to complexes stability, all of the treatments were shown different behaviors through the storage time. Initially, the CS of PCON was low (73.25%), but gradually increased to 92.88 ± 3.59% on the eleventh day and at the end of storage reached to its maximum level (99.01 ± 3.16%). The stability of the L. acidophilus-AFB1 complex, at the beginning was 80.97 ± 3.20% and at 11th day of storage reached to its maximum level, i.e. 99.07 ± 3.07%, and then lessened to 84.06 ± 3.24% at the end of product shelf life. Although LRPY contained L. rhamnosus showed the highest CS among the other treatments during the 21-days storage, it exhibited the lowest CS at the end of cold storage. Its highest CS at the beginning (99.16%,) slightly decreased till 11th of storage day (98.78 ± 3.11%) and significantly reduced (77.31 ± 3.52%) after 21 days’ storage, indicating a minimum stability among the examined samples. Besides, considering the amount of bound AFB1 to the bacteria cells after washing (AFB1-AW), there were significant variations (p < 0.05) amongst yogurt treatments and the values for PCON, LAPY and LRPY samples at the end of storage were 72.16 ± 3.46%, 75.99 ± 3.57% and 65.44 ± 3.61%, respectively. However, results showed that LRPY samples had significantly the higher AFB1-AW than PCON (at 1st and 11th day of storage) and LAPY (at 1st day of storage) yogurts. Thus, in contrast to probiotic strains, yogurt starter cultures retained their AFB1-AW at the end of storage period. The obvious superiority of the yogurt starter cultures in toxin elimination at the end of storage may be due to its acid toleration which retains their cell integrity and prevents release of toxin from the bacterial cell wall.
Fig. 6.
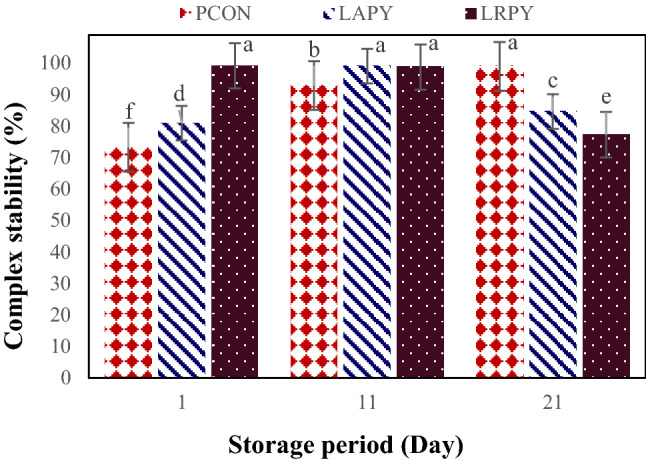
Changes in the stability of the bacteria-AFB1 complex in yogurt samples during cold storage. Different lowercase letters indicate significant differences at p < 0.05
The data for CS-AFB1 obtained at the initial and middle of storage are similar with those reported by Kabak and Var (2008) who reported 92.46–95.38% CS for AFM1 by Lactobacillus and Bifidobacterium strains and Topcu et al. (2010) who reported a considerable CS for AFB1 (77–83%) and patulin (75–81%) by Enterococcus faecium strains after three washes with PBS solution. Hernandez-Mendoza et al. (2009) also found that after PBS solution, approximately 60–70% of AFB1 binding to the bacterial cells was remained, proposing that the toxin is involved to the surface of bacteria. However, Elgerbi et al. (2006) contrariwise stated that after the initial rinsing of LAB strains with PBS, the amount of AFM1 liberated by bacterial cells was 85.7% (i.e. 14.3% CS) and after the third washing, nearly all adsorbed AFM1 was released by the selected bacteria. Similarly, Shah and Wu (1999) revealed that only 10–40% of the bound AFB1 was remained by probiotic strains when washed with water. Haskard et al. (2001) also reported a reversible binding of AFB1 after five washes.
It should be considered that the variations in results may be elucidated by the alterations in extraction techniques, inconsistency in milk composition, method of milk contamination, concentration of mycotoxin, time elapsed before analysis, storage temperature, and characteristics of LAB strains utilized for yogurt production (Motawee and Abd El-Ghany, 2011).
An innovative biological method to decrease the health risks of mycotoxins via binding the toxins is application of probiotic strains of lactic acid bacteria. This work demonstrated that lactic acid producing bacteria have a great capacity to adsorb AFB1 in yogurt. Our findings also revealed that binding of AFB1 was an irreversible progress because after three washes with PBS, all tested bacteria liberated a small amount of bound AFB1. Among tested LAB strains, probiotic yogurts particularly LRPY samples (yogurts containing L. rhamnosus) except at the end of storage had more AFB1 binding capacity and more complex stability. Based on statistical results related to AFB1-binding assay after washing the bacterial cells, no significant differences between PCON and LAPY samples at the end of storage were found, while LRPY samples (yogurts containing L. rhamnosus) had significantly lower AFB1-binding assay after bacterial cells washing.
Mycotoxin-adsorbing bacteria should be capable to bind the toxins sturdily and must retain their cell integrity without dissociating. It is well documented that the most important factors for the selection of LAB as probiotic strain are their ability to tolerate the harsh gastrointestinal tract conditions, i.e. the toxicity of bile salts and gastric acid conditions. On the other hand, the ability of probiotic strain to resist against mucins and even their utilization as substrate is one of the key factors to be considered in the probiotic selection criteria (Kirjavainen et al., 1998; Salminen et al., 1996). These abilities enable probiotic microorganisms to persist in digestive tract and resulted in proper bacteria colonization and mycotoxin reduction. Therefore, consumption of probiotic yogurt is safer than non-probiotic ones; particularly in the regions where milk is considerably contaminated to mycotoxins. However, further studies concerning the stability of the bacterial cells-AFB1 complex specially under gastrointestinal tract condition are required and more investigations are needed to understand the various potential mechanisms underlying probiotic action.
Acknowledgements
The authors acknowledge the financial support provided by Agricultural Sciences and Natural Resources University of Khuzestan.
Compliance with ethical standards
Conflict of interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Fatemeh Mosallaie, Email: sarehmosallaie2015@gmai.com.
Hossein Jooyandeh, Email: hosjooy@asnrukh.ac.ir.
Mohammad Hojjati, Email: hojjati@asnrukh.ac.ir.
Ali Fazlara, Email: a.fazlara@scu.ac.ir.
References
- Abdallah MF, Girgin G, Baydar T. Occurrence, prevention and limitation of mycotoxins in feeds. Anim. Nutr. Feed Technol. 2015;15:471–490. [Google Scholar]
- Abdelmotilib NM, Hamad GM, Elderea HB, Salem EG, El Sohaimy SA. Aflatoxin M1 reduction in milk by a novel combination of probiotic bacterial and yeast strains. Eur. J. Nutr. Food Saf. 2018;8:83–99. [Google Scholar]
- Akgun A, Yazici F, Gulec HA. The combined effect of probiotic cultures and incubation final pH on the quality of buffalo milk yogurt during cold storage. Food Sci. Nutr. 2018;6:492–502. doi: 10.1002/fsn3.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akın N. 1998. Water holding capacity index of concentrated yogurt made from cow and ewe milk. Congress and Exhibition of Food Engineering, 16-18 September, Gaziantep (1998)
- Assaf JC, Atoui A, El Khoury A, Chokr A, Louka N. A comparative study of procedures for binding of aflatoxin M1 to Lactobacillus rhamnosus GG. Braz. J. Microbiol. 2018;49:120–127. doi: 10.1016/j.bjm.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athar H, Shah MA, Khan U. Effect of various stabilizers on whey separation (syneresis) and quality of yogurt. Pak. J. Biol. Sci. 2000;3:1336–1339. [Google Scholar]
- Beal C, Skokanova J, Latrille E, Martin N, Corrieu G. Combined effects of culture conditions and storage time on acidification and viscosity of stirred yogurt. J. Dairy Sci. 1999;82:673–681. [Google Scholar]
- Bennett J, Klich M. Mycotoxins. Clin. Microbiol. Rev. 2003;16:497–516. doi: 10.1128/CMR.16.3.497-516.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakta JN, Ohnishi K, Munekage Y, Iwasaki K, Wei MQ. Characterization of lactic acid bacteria-based probiotics as potential heavy metal sorbents. J. Appl. Microbiol. 2012;112:1193–1206. doi: 10.1111/j.1365-2672.2012.05284.x. [DOI] [PubMed] [Google Scholar]
- Birollo GA, Reinheimer JA, Vinderola CG. Viability of lactic acid microflora in different types of yogurt. Food Res. Int. 2000;33:799–805. [Google Scholar]
- Bozoğlu F. Different mycotoxin inactivation applications and their inactivation mechanisms. Zb. Matice Srp. Prir. Nauke. 2009;117:27–35. [Google Scholar]
- Corassin CH, Bovo F, Rosim RE, Oliveira CAF. Efficiency of Saccharomyces cerevisiae and lactic acid bacteria strains to bind aflatoxin M1 in UHT skim milk. Food Control. 2013;31:80–83. [Google Scholar]
- Dalié DKD, Deschamps AM, Richard-Forget F. Lactic acid bacteria-potential for control of mould growth and mycotoxins: a review. Food Control. 2010;21:370–380. [Google Scholar]
- Donkor ON, Henriksson A, Vasiljevic T, Shah NP. Effect of acidification on the activity of probiotics in yogurt during cold storage. Int. Dairy J. 2006;16:1181–1189. [Google Scholar]
- Elgerbi AM, Aidoo KE, Candlish AAG, Williams AG. Effects of lactic acid bacteria and bifidobacteria on levels of aflatoxin M1 in milk and phosphate buffer. Milchwissenschaft. 2006;61:197–199. [Google Scholar]
- El Khoury A, Atoui A, Yaghi J. Analysis of aflatoxin M1 in milk and yogurt and AFM1 reduction by lactic acid bacteria used in Lebanese industry. Food Control. 2011;22:1695–1699. [Google Scholar]
- El-Nezami H, Kankaanpaa P, Salminen S, Ahokas J. Ability of dairy strains of lactic acid bacteria to bind a common food carcinogen, aflatoxin B1. Food Chem. Toxicol. 1998;36:321–326. doi: 10.1016/s0278-6915(97)00160-9. [DOI] [PubMed] [Google Scholar]
- Elsanhoty RM, Salam SA, Ramadan MF, Badr FH. Detoxification of aflatoxin M1 in yogurt using probiotics and lactic acid bacteria. Food Control. 2014;43:129–134. [Google Scholar]
- FAO/WHO. Guidelines for the evaluation of probiotics in food. Report of a joint FAO/WHO working group on drafting guidelines for the evaluation of probiotics in food, London Ontario, Canada, April 30 and May 1, 2002. Food and Agriculture Organization of the United Nations (FAO), Rome, Italy; World Health Organization (WHO), Geneva, Switzerland (2002)
- Gowda NKS, Suganthi RU, Malathi V, Raghavendra A. Efficacy of heat treatment and sun drying of aflatoxin-contaminated feed for reducing the harmful biological effects in sheep. Anim. Feed Sci. Tech. 2007;133:167–175. [Google Scholar]
- Groopman JD, Kensler TW, Wild CP. Protective interventions to prevent aflatoxin-induced carcinogenesis in developing countries. Annu. Rev. Public Health. 2008;29:187–203. doi: 10.1146/annurev.publhealth.29.020907.090859. [DOI] [PubMed] [Google Scholar]
- Guan S, Zhou T, Yin Y, Xie M, Ruan Z, Young J. Microbial strategies to control aflatoxins in food and feed. World Mycotoxin J. 2011;4:413–424. [Google Scholar]
- Güler-Akın MB, Akın MS. Effects of cysteine and different incubation temperatures on the microflora, chemical composition and sensory characteristics of bio-yogurt made from goat’s milk. Food Chem. 2007;100:788–793. [Google Scholar]
- Haskard C, Binnion C, Ahokas J. Factors affecting the sequestration of aflatoxin by Lactobacillus rhamnosus strain GG. Chem. Biol. Interact. 2000;128:39–49. doi: 10.1016/s0009-2797(00)00186-1. [DOI] [PubMed] [Google Scholar]
- Haskard CA, El-Nezami HS, Kankaanpää PE, Salminen S, Ahokas JT. Surface binding of aflatoxin B1 by lactic acid bacteria. Appl. Environ. Microbiol. 2001;67:3086–3091. doi: 10.1128/AEM.67.7.3086-3091.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hathout AS, Aly SE. Biological detoxification of mycotoxins: a review. Ann. Microbiol. 2014;64:905–919. [Google Scholar]
- Hernandez-Mendoza A, Garcia HS, Steele JL. Screening of Lactobacillus casei strains for their ability to bind aflatoxin B1. Food Chem. Toxicol. 2009;47:1064–1068. doi: 10.1016/j.fct.2009.01.042. [DOI] [PubMed] [Google Scholar]
- Hussain I, Anwar J. A study on contamination of aflatoxin M1 in raw milk in the Punjab province of Pakistan. Food Control. 2008;19:393–395. [Google Scholar]
- IARC (International Agency for Research on Cancer). Agents Classified by the IARC Monographs. vol. 1-116 Available from: http://monographs.iarc.fr/ENG/Classification/latest_classif.php (2016)
- Ishikawa AT, Hirooka EY, Alvares e Silva PL, Bracarense APFRL, Flaiban KKMDC, Akagi CY, Kawamura O, Costa MCD, Itano EN. Impact of a single oral acute dose of aflatoxin B1 on liver function/cytokines and the lymphoproliferative response in C57Bl/6 mice. Toxins. 2017;9:374. doi: 10.3390/toxins9110374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M, Peng X, Fang J, Cui H, Yu Z, Chen Z. Effects of aflatoxin B1 on T-cell subsets and mRNA expression of cytokines in the intestine of broilers. Int. J. Mol. Sci. 2015;16:6945–6959. doi: 10.3390/ijms16046945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jooyandeh H, Mortazavi A, Farhang P, Samavati V. Physicochemical properties of set-Style yoghurt as effected by microbial transglutaminase and milk solids contents. J. Appl. Environ. Biol. Sci. 2015;4:59–67. [Google Scholar]
- Kabak B, Dobson AD, Var IIL. Strategies to prevent mycotoxin contamination of food and animal feed: a review. Crit. Rev. Food Sci. Nutr. 2006;46:593–619. doi: 10.1080/10408390500436185. [DOI] [PubMed] [Google Scholar]
- Kabak B, Var IIL. Factors affecting the removal of aflatoxin M1 from food model by Lactobacillus and Bifidobacterium strains. J. Environ. Sci. Health, Part B. 2008;43:617–624. doi: 10.1080/03601230802234740. [DOI] [PubMed] [Google Scholar]
- Karlovsky P, Suman M, Berthiller F, De Meester J, Eisenbrand G, Perrin I, Oswald IP, Speijers G, Chiodini A, Recker T, Dussort P. Impact of food processing and detoxification treatments on mycotoxin contamination. Mycotoxin Res. 2016;32:179–205. doi: 10.1007/s12550-016-0257-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirjavainen PV, Ouwehand AC, Isolauri E, Salminen SJ. The ability of probiotic bacteria to bind to human intestinal mucus. FEMS Microbiol. Lett. 1998;167:185–189. doi: 10.1111/j.1574-6968.1998.tb13226.x. [DOI] [PubMed] [Google Scholar]
- Kumar P, Mahato DK, Kamle M, Mohanta TK, Kang SG. Aflatoxins: a global concern for food safety, human health and their management. Front. Microbiol. 2017;7:2170. doi: 10.3389/fmicb.2016.02170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YK, El-Nezami H, Haskard CA, Gratz S, Puong KY, Salminen S, Mykkänen H. Kinetics of adsorption and desorption of aflatoxin B1 by viable and nonviable bacteria. J. Food Prot. 2003;66:426–430. doi: 10.4315/0362-028x-66.3.426. [DOI] [PubMed] [Google Scholar]
- Liew WPP, Nurul-Adilah Z, Than LTL, Mohd-Redzwan S. The binding efficiency and interaction of Lactobacillus casei Shirota toward aflatoxin B1. Front. Microbiol. 2018;9:1–12. doi: 10.3389/fmicb.2018.01503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin S, Ramos AJ, Cano-Sancho G, Sanchis V. Mycotoxins: Occurrence, toxicology, and exposure assessment. Food Chem. Toxicol. 2013;60:218–237. doi: 10.1016/j.fct.2013.07.047. [DOI] [PubMed] [Google Scholar]
- Méndez-Albores A, Arambula-Villa G, Loarca-Piña MGF, Castano-Tostado E, Moreno-Martínez E. Safety and efficacy evaluation of aqueous citric acid to degrade B-aflatoxins in maize. Food Chem. Toxicol. 2005;43:233–238. doi: 10.1016/j.fct.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Milićević DR, Škrinjar M, Baltić T. Real and perceived risks for mycotoxin contamination in foods and feeds: challenges for food safety control. Toxins. 2010;2:572–592. doi: 10.3390/toxins2040572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motawee MM, Abd El-Ghany MA. Effect of some lactic acid bacteria strains on aflatoxins reduction in some dairy foods. In The 6th Arab and 3rd International Annual Scientific Conference on: Development of Higher Specific Education Programs in Egypt and the Arab World in the Light of Knowledge Era Requirements (2011)
- Nooshkam M, Babazadeh A, Jooyandeh H. Lactulose: Properties, techno-functional food applications, and food grade delivery system. Trends Food Sci. Technol. 2018;80:23–34. [Google Scholar]
- Osowski A, Pietrzak M, Wieczorek Z, Wieczorek J. Natural compounds in the human diet and their ability to bind mutagens prevents DNA–mutagen intercalation. J. Toxicol. Environ. Health, Part A. 2010;73:1141–1149. doi: 10.1080/15287394.2010.491044. [DOI] [PubMed] [Google Scholar]
- Panesar PS, Shinde C. Effect of storage on syneresis, pH, Lactobacillus acidophilus count, Bifidobacterium bifidum count of aloe vera fortified probiotic yogurt. Curr. Res. Dairy Sci. 2012;4:17–23. [Google Scholar]
- Park DL. Effect of processing on aflatoxin. In: Mycotoxins and food safety. Springer, Boston, M. A. pp. 173-179 (2002)
- Peltonen K, El-Nezami H, Haskard C, Ahokas J, Salminen S. Aflatoxin B1 binding by dairy strains of lactic acid bacteria and bifidobacteria. J. Dairy Sci. 2001;84:2152–2156. doi: 10.3168/jds.S0022-0302(01)74660-7. [DOI] [PubMed] [Google Scholar]
- Prandini A, Sigolo GTS, Filippi L, Laporta M, Piva G. On the occurrence of aflatoxin M1 in milk and dairy products. Food Chem. Toxicol. 2009;47:984–991. doi: 10.1016/j.fct.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Priyanka SR, Venkataramana M, Kumar GP, Rao VK, Murali HCS, Batra HV. Occurrence and molecular detection of toxigenic Aspergillus species in food grain samples from India. J. Sci. Food Agr. 2014;94:537–543. doi: 10.1002/jsfa.6289. [DOI] [PubMed] [Google Scholar]
- Puzyr AP, Burov AE, Bondar VS, Trusov YN. Neutralization of aflatoxin B1 by ozone treatment and adsorption by nanodiamonds. Nanotechnol. Russ. 2010;5:137–141. [Google Scholar]
- Salminen S, Laine M, von Wright A, Vuopio-Varkila J, Korhonen T, Mattila-Sandholm T. Development of selection criteria for probiotic strains to access their potential in functional food. A nordic and European approach. Biosci. Microflora. 1996;2:23–28. [Google Scholar]
- Sarlak Z, Rouhi M, Mohammadi R, Khaksar R, Mortazavian AM, Sohrabvandi S, Garavand F. Probiotic biological strategies to decontaminate aflatoxin M1 in a traditional Iranian fermented milk drink (Doogh) Food Control. 2017;71:152–159. [Google Scholar]
- Sellamani M, Kalagatur NK, Siddaiah C, Mudili V, Krishna K, Natarajan G, Putcha R, Venkata L. Antifungal and zearalenone inhibitory activity of Pediococcus pentosaceus isolated from dairy products on Fusarium graminearum. Front. Microbiol. 2016;7:1–12. doi: 10.3389/fmicb.2016.00890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaghaghi M, Pourahmad R, Adeli H. Synbiotic yogurt production by using prebiotic compounds and probiotic lactobacilli. Int. Res. J. App. Basic Sci. 2013;5:839–846. [Google Scholar]
- Shah NP. Some beneficial effects of probiotic bacteria. Biosci. Microflora. 2000;19:99–106. [Google Scholar]
- Shah NP. Probiotic bacteria: selective enumeration and survival in dairy foods. J. Dairy Sci. 2000;83:894–907. doi: 10.3168/jds.S0022-0302(00)74953-8. [DOI] [PubMed] [Google Scholar]
- Shah N, Wu X. Aflatoxin B1 binding abilities of probiotic bacteria. Biosci. Microflora. 1999;18:43–48. [Google Scholar]
- Shao Sh, Cai J, Du X, Wang Ch, Lin J, Dai J. Biotransformation and detoxification of aflatoxin B1 by extracellular extract of Cladosporium uredinicola. Food Sci. Biotechnol. 2016;25:1789–1794. doi: 10.1007/s10068-016-0272-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LE, Prendergast AJ, Turner PC, Humphrey JH, Stoltzfus RJ. Aflatoxin exposure during pregnancy, maternal anemia, and adverse birth outcomes. Am. J. Trop. Med. Hyg. 2017;96:770–776. doi: 10.4269/ajtmh.16-0730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares C, Rodrigues P, Freitas-Silva O, Abrunhosa L, Venâncio A. HPLC method for simultaneous detection of aflatoxins and cyclopiazonic acid. World Mycotoxin J. 2010;3:225–231. [Google Scholar]
- Solis-Cruz B, Hernandez-Patlan D, Hargis B, Téllez G. Control of aflatoxicosis in poultry using probiotics and polymers. pp. 149-169. In: Mycotoxins: Impact and Management Strategies. Njobeh PB, Stepman F. (eds), IntechOpen Limited, London, UK (2018)
- Sun Y, Dong G, Guangxin E, Liao M, Tao L, Lv J. The effects of low levels of aflatoxin B1 on health, growth performance and reproductivity in male rabbits. World Rabbit Sci. 2018;26:123–133. [Google Scholar]
- Tajalli F, Sarabi Jamab M, Adibpour N, Mehraban Sang Atash M, Karazhian R. Evaluation of Lactobacillus rhamnosus viability effect on reduction of aflatoxin M1 in probiotic yogurt. BioTechnol. An Indian J. 2014;10:16486–16491. [Google Scholar]
- Tamjidi F, Nasirpour A, Shahedi M. Physicochemical and sensory properties of yogurt enriched with microencapsulated fish oil. Food Sci. Technol. Int. 2012;18:381–390. doi: 10.1177/1082013211428212. [DOI] [PubMed] [Google Scholar]
- Topcu A, Bulat T, Wishah R, Boyacı IH. Detoxification of aflatoxin B1 and patulin by Enterococcus faecium strains. Int. J. Food Microbiol. 2010;139:202–205. doi: 10.1016/j.ijfoodmicro.2010.03.006. [DOI] [PubMed] [Google Scholar]
- Utami R, Utami T, Suparmo S, Rahayu ES. Binding of aflatoxin B1 to Lactobacillus paracasei SNP-2 and stability of bacteria-AFB1 Complex. Indonesian Food Nut. Prog. 2017;14:1–8. [Google Scholar]
- Venkataramana M, Nayaka SC, Anand T, Rajesh R, Aiyaz M, Divakara ST, Murali HS, Prakash HS, Rao PL. Zearalenone induced toxicity in SHSY-5Y cells: the role of oxidative stress evidenced by N-acetyl cysteine. Food Chem. Toxicol. 2014;65:335–342. doi: 10.1016/j.fct.2013.12.042. [DOI] [PubMed] [Google Scholar]
- Wang J, Ogata M, Hirai H, Kawagishi H. Detoxification of aflatoxin B1 by manganese peroxidase from the white-rot fungus Phanerochaete sordida YK-624. FEMS Microbiol. Lett. 2011;314:164–169. doi: 10.1111/j.1574-6968.2010.02158.x. [DOI] [PubMed] [Google Scholar]
- Yangilar F, Çakmakçi S. Probiotic shelf-life, mineral contents and others properties of probiotic yogurts supplemented with corn flour. Tarım Bilimleri Dergisi- J. Agr. Sci. 2017;23:472–481. [Google Scholar]
- Yerlikaya O. Starter cultures used in probiotic dairy product preparation and popular probiotic dairy drinks. Food Sci. Technol. 2014;34:221–229. [Google Scholar]
- Yilmaz-Ersan L, Kurdal E. The production of set-type-bio-yogurt with commercial probiotic culture. Int. J. Chem. Eng. Appl. 2014;5:402–408. [Google Scholar]
- Zaki MM, El-Midany SA, Shaheen HM, Rizzi L. Mycotoxins in animals: Occurrence, effects, prevention and management. J. Toxicol. Environ. Health Sci. 2012;4:13–28. [Google Scholar]
- Ziarno M, Zaręba D. The effect of the addition of microbial transglutaminase before the fermentation process on the quality characteristics of three types of yogurt. Food Sci. Biotechnol. 2019 doi: 10.1007/s10068-019-00640-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinedine A, Faid M, Benlemlih M. In vitro reduction of aflatoxin B1 by strains of lactic acid bacteria isolated from Moroccan sourdough bread. Int. J. Agric. Biol. 2005;7:67–70. [Google Scholar]
- Zoghi A, Khosravi-Darani K, Sohrabvandi S. Surface binding of toxins and heavy metals by probiotics. Mini-Rev. Med. Chem. 2014;14:84–98. doi: 10.2174/1389557513666131211105554. [DOI] [PubMed] [Google Scholar]


