Abstract
Particulate matter is a major contribution of air pollution and detrimental to human health. The in vitro antioxidant activities of a brown seaweed, Sargassum horneri ethanol extract (SHE) against particulate matter-induced oxidative stress were investigated by measuring 1,1-diphenyl-2-picrylhydrazyl (DPPH) free-radical scavenging activity, hydrogen peroxide (H2O2) scavenging activity, superoxide anion (O·−2) inhibition, hydroxyl radical (·OH) scavenging activity, reducing power, and the metal ion-chelating effect. All in vitro antioxidant activities were increased as the concentration of SHE increased (0–1000 μg/mL). When treated with particulate matter at 0–1000 μg/mL, the DPPH free radical, and H2O2 scavenging activities, reducing power, and metal ion-chelating abilities of SHE were significantly decreased (p < 0.05). These results indicate that Sargassum horneri, which is a rich source of bioactive compounds, can be used as a natural source of antioxidants in the food industries.
Keywords: Brown seaweed, Sargassum horneri ethanol extract, Urban particulate matter, Antioxidant activity, Natural antioxidant
Introduction
Urban particulate matter events have been generated by increased vehicular traffics and other combustion processes over the past two decades and can cause a severe environmental issue in East Asia especially, Korea and China (Fernando et al., 2018; Li et al., 2003). As reviewed by Raloff (2001), urban particulate matter includes carbon, copper, iron, sulfur, and other carcinogens. Because of the rapid surge in the level of urban particulate matter, cardiorespiratory related diseases have threated to human health (Li et al., 2003). The effect of urban particulate matter on human health have exhibited that reactive oxygen species generated by oxidative stress negatively act (Fernando et al., 2018; Li et al., 2003).
Reactive oxygen species (ROS), such as superoxide anion (O·−2), hydroxyl radical (·OH) and hydrogen peroxide (H2O2), are continuously generated through normal physiological processes from the oxygen we breath as well as external stimulations via tobacco smoke, certain pollutants, and ionizing radiation (Heo et al., 2005a). Because ROS are highly reactive and unstable, they can promote cell mutations or cell death (Kumar et al., 2008). These cell damages cause aging and severe diseases (Alho and Leinonen, 1999; Lemberkovices et al., 2002), which can be prevented or reduced by antioxidants.
Antioxidants are known to inhibit the generation of ROS (Jun et al., 2001). The most commercially available and currently used antioxidants in foods are synthetic antioxidants; however, growing concerns exist over their safety and toxicity and restriction for use (Heo et al., 2005b; Kumar et al., 2008). Thus, it is necessary to develop safe and inexpensive antioxidants from natural origins (Cho et al., 2011). Mostly, plants including fruits, vegetables, and herbs are known to the important sources for natural antioxidants (Larson, 1988). Seaweeds can also be the natural ingredients of potential antioxidant compounds for new medicinal or functional foods (Cho et al., 2011; Heo et al., 2005a). In particular, Alariaceae, Fucaceae, and Sargassaceae, which are brown seaweeds, have been reported to exhibit antioxidant activity (Wang et al., 2009). Phlorotannins that are polymers of phloroglucinol structurally contain phenol rings with their unique molecular skeleton function as electron traps (Wang et al., 2009; Xiaojun et al., 1996; Yan et al., 1999). Therefore, they are known to be more potent to scavenge free radicals than other polyphenols obtained from earthly plants (Ahn et al., 2007; Wang et al., 2009). Additionally, brown seaweeds contain several potential antioxidants, such as fucoxanthin, carotenoids, and tocopherols (Hosokawa et al., 2009; Miyashita and Takagi, 1987). Although many researchers have reported seaweeds to be a rich source of antioxidant compounds (Duan et al., 2006; Kuda et al., 2005; Lim et al., 2002; Park et al., 2004), no report has been described on the antioxidant activities of brown seaweed extracts against urban particulate matter-induced oxidative stress. In this study, the antioxidant activities of a brown seaweed, Sargassum horneri ethanol extract were investigated for effective suppression of the oxidative stress induced by urban particulate matter.
Materials and methods
Materials and chemicals
Urban particulate matter (CRM No. 28) collected from Beijing, China over 10 years (1996–2005) was purchased from the National Institute for Environmental Studies (NIES, Ibaraki, Japan). This environmentally certified reference material (CRM) was developed and certified by the NIES to determine multi-elements in aerosol particulate matter. Folin-Ciocalteu reagents, 1,1-diphenyl-2-picryl-hydrazyl (DPPH), 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS), peroxidase, iron (ΙΙ) sulfate heptahydrate (FeSO·47H2O), ethylenediaminetetraactic acid (EDTA), 2-deoxyribose, 2-thiobarbituric acid (TBA), 3-(2-pyridyl)-5,6-diphenyl-1,2,4-triazine-p,p′-disulfonic acid monosodium salt hydrate (ferrozine), iron (ΙΙ) chloride (FeCl2), potassium ferric cyanide, and ferric chloride (FeCl3) were obtained from Sigma-Aldrich (St. Louis, MO, USA). Trichloroacetic acid (TCA) was purchased from Samchun Co. Ltd. (Seoul, Korea). All chemicals were analytical grades.
Preparation of Sargassum horneri ethanol extract
Sargassum horneri was collected from Jeju Island, Korea. They were washed three times for 30 min and dried at 50 °C for 24 h by a hot air drying equipment until the moisture content was below 10%. After washing, they were ground into 40–50-mesh size using a pin mill (MF10; Ika-Werke GMBH & Co., Staufen, Germany). The S. horneri powder was mixed with 70% ethanol at a ratio of 1–1.5:8.5–9 and circulation extracted at 70 °C for 12 h. The extract was treated with white clay by stirring at 60 rpm for 2 h. After centrifugation at 8100 × g, the supernatant was collected and concentrated to one-fifth volume at 60 °C. The concentrates were suspended with 95% ethanol, and the suspension was centrifuged. The resulting supernatant (lot number: SJFC70180625) was then concentrated to 20% of the solid concentration and freeze dried to obtain S. horneri ethanol extract powder (SHE).
Preparation of urban particulate matter induced oxidation samples
The SHE (1 mg/1 mL) was dissolved in distilled water and the concentration was adjusted to 3.9–1000 µg/mL. For the urban particulate matter (PM)-induced oxidation, PM at 0 to 1000 μg/mL was combined with SHE at 62.5, 125, 250, and 500 μg/mL (SHE-PM).
Determination of the total phenolic content
The total phenolic content of SHE was determined by the Folin–Ciocalteu method of Wang et al. (2011) and Lee et al. (2009). Briefly, the SHE (100 μL) was mixed with 1.5 mL of distilled water and 100 μL of 2 N Folin-Ciocalteu reagent in a test tube. After 30 s, 300 μL of sodium carbonate (20% in distilled water) was added and the reaction mixture was mixed thoroughly. The mixtures were incubated at room temperature for 1 h in the dark. The absorbance was measured at 765 nm using a UV–vis spectrophotometer (OPTIZEN 2120UV; Mecasys, Daejeon, Korea). A standard curve with gallic acid solution (0.05–0.15 mg/mL) was used. The results were shown as mg of gallic acid equivalents (GAE) per 1 g of extract.
DPPH free radical scavenging activity
As one of in vitro antioxidant activities, the DPPH (1,1-diphenyl-2-picrylhydrazyl) free radical scavenging activity was measured by the method of Kim et al. (2006). The DPPH solution (1 mM in ethanol, 140 μL) was added to a 96-well plate containing 70 μL of SHE or SHE-PM and was mixed by pipetting. The mixtures reacted at room temperature for 30 min in the dark. The absorbance of the sample solution was measured at 517 nm using a microplate reader (Epoch™; BioTek Instruments Inc., Winooski, VT, USA). Distilled water was used as a blank. The activity was expressed as a percentage of the DPPH free radical scavenging activity relative to the blank using the following equation:
Hydrogen peroxide scavenging activity
Hydrogen peroxide scavenging activity was measured according to a slightly modified method of Heo et al. (2005a). Briefly, 100 μL of SHE or SHE-PM, 20 μL of hydrogen peroxide, and 100 μL of 0.1 M phosphate buffer (pH 7.4) were mixed in a 96-well plate and then were incubated at 37 °C for 5 min. ABTS (1.25 mM, 30 μL) and 30 μL of peroxidase (1 unit/mL; Sigma-Aldrich) were added to the mixture and incubated again at 37 °C for 10 min. The absorbance at 405 nm was measured using a microplate reader (Epoch™; BioTek Instruments Inc., Winooski, VT, USA). The hydrogen peroxide radical scavenging activity was determined using the following formula:
where Asample is the absorbance of the sample, Asample control is the absorbance of SHE itself at 3.9–1000 µg/mL, and Ablank is the absorbance of distilled water.
Superoxide anion scavenging activity
The superoxide anion scavenging activity was determined using the SOD Assay Kit-WST (Dojindo, Kumamoto, Japan) by the method of Heo et al. (2005a). Twenty microliter of SHE or SHE-PM was pipetted into each sample well and blank #2 well. Distilled water (20 μL) was used for blank #1 and blank #3 wells. Two hundred microliters of water-soluble tetrazolium salt (WST) working solution was added into each well and mixed by pipetting. Twenty microliters each of dilution buffer was added into blank #2 and blank #3 wells, 20 μL of enzyme working solution was added into each sample well and blank #1 well, and then the sample were mixed thoroughly. The reaction mixtures were incubated at 37 °C for 20 min, and the absorbances were read at 450 nm using a microplate reader (BioTek Instruments). The SOD inhibition rate (%) was calculated as [(Ablank #1 − Ablank #3) –(Asample − Ablank #2)]/(Ablank #1 − Ablank #3) 100, where Asample is the absorbance of the sample, Ablank #1 is the absorbance of the coloring without inhibitor, Ablank #2 is the absorbance of the sample blank, and Ablank #3 is the absorbance of the reagent blank.
Hydroxyl radical scavenging activity
The hydroxyl radical scavenging activity was measured according to a slightly modified method of Kim et al. (2009) and Kim et al. (2006). Hydroxyl radicals were generated by the Fenton reaction in the presence of FeSO4·7H2O. Briefly, 200 μL of SHE or SHE-PM was added to the test tube containing 200 μL of 10 mM FeSO4·7H2O, 200 μL of 1 mM EDTA, and 200 μL of 10 mM 2-deoxyribose. One milliliter of 0.1 M phosphate buffer (pH 7.4) was added into the mixture until the total volume reached 1.8 mL. Next, 200 μL of 10 mM H2O2 was added and incubated at 37 °C for 1 h. One mL of 2.8% TCA and 1 mL of 0.4% TBA were added to 1 mL of mixture, which was then placed in a boiling water bath for 10 min. After centrifugation at 395 × g for 5 min, the absorbance was measured at 532 nm using a microplate reader (Epoch™; BioTek Instruments Inc., Winooski, VT, USA). The hydroxyl radical scavenging activity (%) was calculated as follows:
where Asample is the absorbance of the sample and Ablank is the absorbance of distilled water.
Reducing power
The reducing power evaluates the reductive ability by measuring the amount of reductones, which can break the free radical chains and donate a hydrogen atom (Cho et al., 2011). The reducing power of SHE or SHE-PM was determined by the method of Sabeena Farvin and Jacobsen (2013). One milliliter of SHE or SHE-PM was mixed with 0.2 M phosphate buffer (pH 6.6, 1 mL) and 1% potassium ferricyanide (1 mL) and incubated at 50 °C for 20 min. One milliliter of 10% TCA was added and mixed well. An aliquot of the mixture (2 mL) was incubated with 2 mL of distilled water and 400 μL of 0.1% ferric chloride at room temperature for 10 min in the dark. The absorbance of the solution was measured at 700 nm using a UV–vis spectrophotometer (OPTIZEN 2120UV; Mecasys). Ascorbic acid (DC Chemical Co., Ltd., Seoul, Korea) was used as a positive control.
Metal ion chelation
The metal ion-chelating effect was measured according to the method of Lee et al. (2010) and Wang et al. (2009). Briefly, 100 μL of SHE or SHE-PM was mixed with 100 μL of FeCl2 in a 96-well plate. Next, 100 μL of 0.25 mM ferrozine was added and allowed to stand at room temperature for 10 min in the dark. After the reaction, the absorbance was determined at 562 nm using a microplate reader (Epoch™; BioTek Instruments Inc., Winooski, VT, USA). Distilled water instead of SHE or SHE-PM solution and 0.25 mM ferrozine were used as a control and blank, respectively. The percentage of the metal ion-chelating effect was determined as [(1 − (Asample − Ablank))/Acontrol] 100.
Statistical analysis
The data were presented as the mean ± standard deviation (SD) based on 3 independent experiments, and the results were analyzed by Student’s t test using Microsoft Excel 2010 (Microsoft Co.; Redmond, WA, USA). The symbols “*”, “**”, and “***” indicate the significance levels of p < 0.05, p < 0.005, and p < 0.0005, respectively.
Results and discussion
Phenolic compounds in SHE
The SHE at 1 mg/mL contained 67.58 mg of GAE/g of total phenolic compounds (Table 1). According to Luo et al. (2010), the ethyl acetate fractions of S. horneri contained 27.66 mg of GAE/g, which was lower than those in SHE. The methanolic extract at 70 °C and aqueous extract at 20 °C of S. coreanum contained 88.36 and 93.89 mg of GAE/g, respectively (Heo et al., 2005b). The amounts of total phenolics in brown seaweeds depended on an organic solvent for extraction. Phenolic compounds, which have many hydroxyl groups, are distributed extensively in the plants and show the ability to scavenge free radicals (Heo et al., 2005b; Shipeng et al., 2015). Previous studies have reported that the primary contributor in various seaweeds for antioxidant activity was phenolic compounds as well (Shipeng et al., 2015; Zhang et al., 2007).
Table 1.
Total polyphenol content of Sargassum horneri ethanol extract (SHE)
| SHE | |
|---|---|
| Total polyphenol contenta | 67.58 ± 1.31 |
aTotal phenolic content was expressed as mg gallic acid equivalents (GAE)/g SHE
In vitro antioxidant activities of SHE
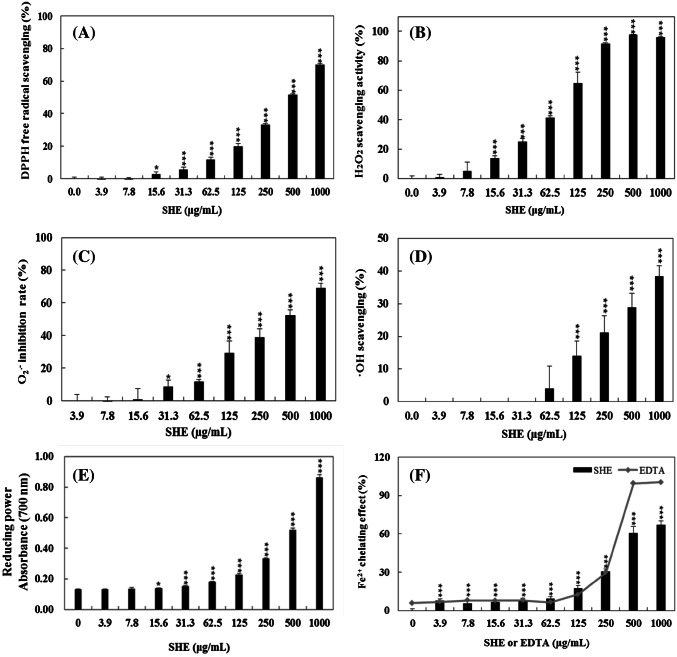
The in vitro antioxidant activities of SHE at 3.9–1000 μg/mL were shown as DPPH free radical scavenging activities, hydrogen peroxide (H2O2) scavenging activity, superoxide anion (O·−2) inhibition, hydroxyl radical (·OH) scavenging activity, reducing power, and metal ion-chelating effect (Fig. 1). As the concentration of SHE increased from 0 to 1000 μg/mL, the DPPH free radical scavenging activity increased (Fig. 1(A)). Particularly, the DPPH free radical scavenging activities of SHE at 500 and 1000 μg/mL were 51.5 and 69.9%, which showed the highest activity. Similar results were reported for other brown seaweed species (Heo et al., 2005b; Luo et al. 2010). For example, the enzymatic extract from E. cava showed approximately 70% of scavenging activities, which were greater than BHT (56.05%) (Heo et al., 2005b). Luo et al. (2010) reported the DPPH free radical scavenging activities of polyphenols extracted from different kinds of brown seaweeds; the methanol/chloroform extracts of S. kjellmanianum, S. horneri, S. thunbergii, S. pallidum, and S. fusiforme had a scavenging activity of 58.25, 43.82, 38.55, 29.42, and 24.20%, respectively.
Fig. 1.
In vitro antioxidant activity of Sargassum horneri ethanol extract (SHE) at different concentrations; (A) DPPH free radical scavenging activity. (B) Hydrogen peroxide (H2O2) scavenging activity. (C) Superoxide anion (O·−2) inhibition rate. (D) Hydroxyl radical (·OH) scavenging activity. (E) Reducing power. (F) Metal ion-chelating effect. Each bar represents the mean ± SD of three replicates. *(p < 0.05), **(p < 0.005), and ***(p < 0.0005) indicate statistical significance compared with the non-SHE-treated group
Figure 1(B) shows the hydrogen peroxide scavenging activity of SHE. The hydrogen peroxide scavenging activities of SHE at 250, 500 and 1000 μg/mL were the highest (91.71, 97.72, and 95.99%, respectively) among in vitro antioxidant activities. Heo et al. (2005b) also found that the hydrogen peroxide scavenging activity of enzymatic extracts from the brown seaweeds was in high. Particularly, Ishige okamurae protease-degrading extract showed the highest activity (96.27%), and carbohydrate-degrading and protease-degrading extracts of S. horneri indicated comparatively high hydrogen peroxide scavenging activities (92.69 and 88.09%, respectively). Moreover, all enzymatic extracts of E. cava showed 60–90% activities. These scavenging activities were greater than those of BHT, BHA, and α-tocopherol (approximately 50.32, 67.37, and 64.11%, respectively) (Heo et al., 2005b).
The superoxide anion inhibition rate of SHE at 31.3–1000 μg/mL was increased from 8.55 to 69.25% (Fig. 1(C)). Siriwardhana et al. (2003) recorded that the diethyl ether extract of a brown seaweed, H. fusiformis, had a high inhibition rate at 45%. Additionally, the methanol and chloroform extracts inhibited approximately 40% of superoxide anion. Heo et al. (2005a) reported that S. thunbergii methanol extract at 20 °C and 70 °C showed 94.14 and 97.41% of superoxide anion scavenging activity while water extract showed 93.10 and 87.98%, respectively. In addition, other brown seaweed species also inhibited 50% of superoxide anions (Heo et al., 2005a). Particularly, Sargassum spp. showed strong superoxide anion scavenging activity and could be used as an antioxidant source (Heo et al., 2005a). In this research, SHE at the concentration of 1000 μg/mL showed the highest superoxide anion inhibition rate. Interestingly, when SHE at 62.5 μg/mL was treated with PM at 0–1000 μg/mL, the superoxide anion inhibition rates were increased (data not shown). The cause might be that superoxide anion rapidly reacted with PM components. Because superoxide anion (O·−2) is a very reactive and immensely damaging free, it produces other types of free radicals and oxidizing agents (Lui and Ng, 2000; Tedesco et al., 2000). The damage caused by superoxide anion in cells is involved in the generation of cancer, aging and several metabolic diseases; thus, evaluating the superoxide anion scavenging activity was significant (Jia et al., 2014).
Figure 1(D) indicates the hydroxyl radical (·OH) scavenging activity of SHE at 3.9–1000 μg/mL. The hydroxyl radical scavenging activities of SHE at 62.5, 125, 250, 500, and 1000 μg/mL were 3.96, 14.12, 21.23, 28.88, and 38.48%, respectively, which increased with increasing concentration. However, the hydroxyl radical scavenging activity of SHE was relatively weak compared with those of the DPPH free radical, hydrogen peroxide, and superoxide anion discussed above. An earlier study by Nakai et al. (2006) showed that the hydroxyl radical scavenging activities of seven brown seaweeds such as S. confusum (4.4%), S. hemiphyllum (13.0%), S. yezoense (21.2%), S. micracanthum (25.3%), S. horneri (29.7%), S. patene (18.8%), and S. ringgoldianum (78.8%), were in their 50% ethanol extracts. Kim et al. (2006) also reported that the hydroxyl radical scavenging activities for methanolic extracts from S. horneri, Grateloupia filicina, Kjellmaniella crassifolia, Pyropia tenera, S. lomentaria, and Agarum cribrosum were 87.11, 35.10, 74.39. 59.20, 56.35, and 82.00%, respectively, which exhibited a positive effect more than SHE. Hydroxyl radical, which has a short half-life, is considered the strongest free radical. Therefore, it is the most reactive and damaging ROS (Devi et al., 2008; Koppenaol and Liebman, 1984). Additionally, hydroxyl radical is well-known to cause oxidative damage to DNA and protein as well as lipid peroxidation, which can extract hydrogen atoms from phospholipid membranes (Kitada et al., 1979; Namiki, 1990; Spencer et al., 1994). In the current study, SHE showed antioxidative activities at high concentrations (62.5–1000 μg/mL) and scavenged the hydroxyl radicals at the stages of the initiation and termination of peroxy radicals. When treated with particulate matter (PM) at 0–1000 μg/mL (SHE-PM), the hydroxyl radical scavenging activities of SHE at 62.5 and 125 μg/mL were decreased or increased depending on the concentration (data not shown). Similar to the superoxide anion, the cause might be that the hydroxyl radical reacted with the components very quickly. Because hydroxyl radical (·OH) has much too high standard reduction potential (2310 mV), it is not only a very strong oxidizing agent but also an electrophilic radical (Choe and Min, 2005).
The reducing power of SHE was evaluated by determining the amount of reductones that exhibit antioxidant activities by donating a hydrogen atom and destroying the free radical chains (Cho et al., 2011). Figure 1(E) shows the reducing power of SHE at different concentrations. As the concentration of SHE increased from 3.9 to 1000 μg/mL, there was an increase in the absorbance (0.18–0.86). A similar trend was reported by Sabeena Farvin and Jacobsen (2013) for the reducing power of the ethanol extracts of seaweeds from the Danish coast at 1000 μg/mL. It was observed that the water extracts of F. serratus showed high reducing power (2.5 ± 0.1) compared with ascorbic acid (2.28 ± 0.27 at 200 μg/mL), and the reducing power values of ethanol extracts from the brown seaweeds were ranged from 0.3 to 1.6 (Sabeena Farvin and Jacobsen, 2013). Additionally, the result of Luo et al. (2010) showed a higher reducing power of S. horneri in the methanol/chloroform (0.748), petroleum ether (0.723), and ethyl acetate (0.880) fractions than that of the positive controls from gallic acid (0.791) and ascorbic acid (0.706). Furthermore, Luo et al. (2010) reported that the reducing power of methanol/chloroform extracts from S. fusiforme, S. kjellmanianum, S. pallidum, S. thunbergii, and S. horneri increased with increasing concentration at 5–45 μg/mL. Particularly, SHE indicated a high reducing power at 250, 500, and 1000 μg/mL.
The metal ion-chelating effect of SHE at different concentrations is shown in Fig. 1(F) and was compared with a positive control, EDTA, which is an excellent chelator for ferrous ions. EDTA was well chelated for metal ions and its chelating effect was 100.38% at 1000 μg/mL. Metal ion chelation of SHE at 3.9–1000 μg/mL was 7.74–67.02%, which increased as the concentration of SHE increased. A similar result was reported that the metal ion-chelating effects of S. pallidum in the ethyl acetate fraction and n-butanol fraction were 48.25 and 51.34%, respectively, at the concentration of 2000 μg/mL (Ye et al., 2009). The ethanolic extracts from brown seaweeds, F. spiralis, showed the highest metal ion-chelating effect (half-maximal effective concentration, EC50: 242.9 ± 17.0 μg/mL) followed by F. distichus (350.0 ± 10.4 μg/mL), D. dichotoma (414.3 ± 14.0 μg/mL), and L. digitata (785.5 ± 18.0 μg/mL) (Sabeena Farvin and Jacobsen, 2013). Additionally, the metal ion-chelating effect of water extracts from the brown seaweeds F. serratus, F. vesiculosus, F. distichus, F. spiralis, S. muticum, S. latissimi, and L. digitata were more than 60% at 1000 μg/mL (Sabeena Farvin and Jacobsen, 2013).
Comparison of the in vitro antioxidant activities of SHE
For the comparison of the in vitro antioxidant activities of SHE, the half-maximal effective concentration (EC50) values of SHE were calculated and shown in Table 2. The EC50 of SHE for in vitro antioxidant activities were in the order of hydrogen peroxide scavenging activity (EC50 of 83.92 µg/mL) > reducing power (188.59 µg/mL) > metal ion-chelating effect (412.50 µg/mL) > superoxide anion inhibition rate (469.36 µg/mL) > DPPH free radical scavenging (550.23 µg/mL) > hydroxyl radical scavenging (1434.90 µg/mL). Particularly, hydrogen peroxide scavenging activity, which has the lowest EC50 value indicated the highest antioxidant activity of SHE. Hydroxyl radical (·OH) can be formed by the Fenton reaction of hydrogen peroxide (H2O2) in the presence of the iron and Harber–Weiss reaction in the presence of heat, UV light, or transition metals (Choe and Min, 2005; Nimse and Pal, 2015). When the enzymatic antioxidant, catalase is present in the peroxisome, H2O2 changes to water and oxygen (Nimse and Pal, 2015). Additionally, the nonenzymatic antioxidant, polyphenol interrupts free radical chain reactions (Nimse and Pal, 2015). Thus seaweeds, which possess nonenzymes (e.g. phenolic compounds) quickly react against hydrogen peroxide (H2O2) compared with other radicals. Moreover, SHE comprises several number of antioxidants (e.g., ascorbate, glutathione, phenolic compounds, tocopherols, and carotenoids). Phenolic compounds function as hydrogen atom donators (ROH → RO· + H·), and these cause the reduction of the ferric ion (Fe3+)-ferricyanide complex to the ferrous (Fe2+) form (Firuzi et al., 2005; Senevirathne et al., 2006; Srivastava et al., 2006). Therefore, SHE has the second highest reducing power in antioxidant activities. Among many metal ions, iron and copper ions cause oxidative damage by playing catalytic role because they are the most reactive and abundant in ROS formation (Lee et al., 2005). Particularly, copper ion consisting of tightly bound to chromatin can raise the intracellular level of free copper, catalyzing hydroxyl radical generation and released by initiated ROS (Lee et al., 2005). The EC50 of SHE in the metal ion-chelating effect was 412.50 µg/mL, which is the third highest antioxidant activity level. Seaweeds possess metal-binding capacities based on the components, such as carrageenan, agar, and alginate (Kumar et al., 2008). Superoxide anion and hydroxyl radical scavenging activities showed the lowest EC50 values among the in vitro antioxidant activities. Superoxide anion and hydroxyl radical have very high standard electron reduction potentials with the powerful oxidizing capabilities of the compounds at 940 mV and 2310 mV, respectively (Choe and Min, 2005). Therefore, the components in SHE could not quench superoxide anion and hydroxyl radical quickly and completely.
Table 2.
Comparison of the in vitro antioxidant activities (EC50, µg/mL) of the Sargassum horneri ethanol extract (SHE)
| In vitro antioxidant activities | EC50 (µg/mL) | Name of control | |
|---|---|---|---|
| SHE | Controlb | ||
| DPPH radical scavenging | 550.23 ± 7.26a | 6.90 ± 0.17 | Ascorbic acid |
| H2O2 scavenging | 83.92 ± 13.35 | 2.00 ± 0.12 | Ascorbic acid |
| O2·- inhibition rate | 469.36 ± 16.61 | 100.93 ± 1.21 | Ascorbic acid |
| ·OH scavenging | 1434.90 ± 17.45 | 2.08 ± 0.03 | BHT |
| Reducing power | 188.59 ± 0.24 | 63.74 ± 0.78 | Ascorbic acid |
| Fe2+ chelating | 412.50 ± 28.94 | 303.32 ± 3.52 | EDTA |
aValues are mean ± SD (n = 3). The EC50 value is defined as the amount of extract necessary to decrease the initial antioxidant activities by 50%
bAscorbic acid, BHT, and EDTA as a positive control were compared
In vitro antioxidant activities of SHE in oxidation induced by particulate matter
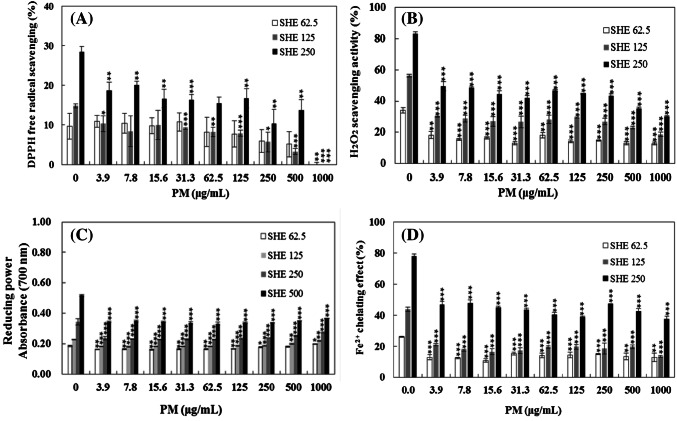
The in vitro antioxidant activities of SHE at 62.5, 125, and 250 μg/mL, when treated with particulate matter (PM) at 3.9–1000 μg/mL (SHE-PM), were reduced (Fig. 2). The DPPH free radical scavenging activities of PM at 500 μg/mL with SHE at 62.5, 125 and 250 μg/mL were 5.14, 3.31, and 13.7%, respectively, which were lower than those treated with SHE at 62.5, 125, and 250 μg/mL without PM. Following PM treatment at 1000 μg/mL, the DPPH scavenging activities of SHE disappeared, indicating that urban PM reduced the DPPH free radical scavenging activity of SHE and PM occurred in oxidation. The DPPH free radical, which is a stable form of free radical has been widely used to evaluate the free radical scavenging activity of antioxidants (Ye et al., 2009). The DPPH free radical scavenging activity was successfully used to verify the antioxidant activity of SHE in the oxidation induced by PM in the current study.
Fig. 2.
In vitro antioxidant activity of Sargassum horneri ethanol extract (SHE) at 62.5, 125, and 250 μg/mL induced by particulate matter (PM) at 0 to 1000 μg/mL (SHE-PM). (A) DPPH free radical scavenging activities. (B) Hydrogen peroxide (H2O2) scavenging activity. (C) Reducing power. (D) Metal ion-chelating effect. Each bar represents the mean ± SD of three replicates. ** (p < 0.005) and *** (p < 0.0005) indicate statistical significance compared with the S. horneri ethanol extract (SHE only)-treated group
Following PM treatment at 0–1000 μg/mL (SHE-PM), the hydrogen peroxide scavenging activities of SHE at 62.5, 125, and 250 μg/mL were decreased (Fig. 2(B)). The hydrogen peroxide scavenging activities following PM treatment at 3.9–1000 μg/mL with SHE at 62.5, 125, and 250 μg/mL were 18.20–12.82, 30.07–18.80, and 49.63–30.03%, respectively. These scavenging activities decreased with the increase in PM concentration compared with those for SHE only at 62.5 (34.09%), 125 (56.35%), and 250 μg/mL (83.27%), respectively. Hydrogen peroxide scavenging activity methods can measure the activity of the antioxidants effectively (Czochra and Widensk, 2002). Red and brown seaweeds such as Grateloupia filicina and Hizikia fusiformis were reported to have hydrogen peroxide scavenging ability (Athukorala et al., 2003; Siriwardhana et al., 2003). SHE, a brown seaweed, exhibited potential antioxidative activity to reduce hydrogen peroxide; however, the activity was decreased by PM.
The reducing power of SHE at 62.5, 125, 250, and 500 μg/mL was from 0.19, 0.23, 0.34, and 0.52 to 0.16–0.20, 0.18–0.22, 0.24–0.27, and 0.34–0.37, respectively, when treated with PM at 0–1000 μg/mL (Fig. 2(C)). Differences among the concentrations of PM were not significant. The reducing power and antioxidative activity are related (Kumar et al., 2008). The extracts including high levels of total phenolics can possibly reduce ferric iron, indicating that algal polyphenols may be the principal component responsible for these characteristic of the extracts (Sabeena Farvin and Jacobsen, 2013). Furthermore, the brown seaweed, Fucus was reported to possess high reducing power because of the existence of fucoidan (Ruperez et al., 2002). Therefore, reductants (i.e., antioxidants) has a major role to play to end of the free radical chain reaction because they inhibit lipid peroxidation by donating a hydrogen atom and the existence of reductants in SHE causes the reduction of the ferric ion (Fe3+) to the ferrous (Fe2+) form (Senevirathne et al., 2006; Srivastava et al., 2006).
When treated with PM at 3.9–1000 μg/mL, the metal ion-chelating effect of SHE at 62.5, 125, and 250 μg/mL was reduced from 13.0–12.9, 21.2–13.7, and 47.1–37.7%, respectively (Fig. 2(D)). Additionally, no significant differences were found in the metal ion-chelating effect among the concentrations of PM. The presence of PM at very low concentration reduced the metal ion-chelating effect. Chelating metal ions by an antioxidant molecule inhibits the generation of oxy radicals and the consequent oxidative damage (Kumar et al., 2008). Thus, the metal ion-chelating effect participates in the antioxidant mechanism because it reduces the concentration of the catalyzing transition metal (Kumar et al., 2008). Some studies have indicated that polyphenols of brown seaweeds are potent chelators which ability is dependent on the structure of phenolics, especially the location and number of the hydroxyl groups (Chew et al., 2008; Santoso et al., 2004; Senevirathne et al., 2006). Particularly, phlorotannins, which are usually present in brown seaweed, have a high capacity to chelate divalent metal ions, indicating that they are perhaps strong chelators of heavy metals (Chew et al., 2008; Toth and Pavia, 2000; Wang et al., 2009). Our study agrees in accordance with other studies that Sargassum horneri contains high total phenolic contents and shows a high metal ion-chelating effect with increasing concentration (Chew et al., 2008; Toth and Pavia, 2000; Wang et al., 2009). Furthermore, the methanol extract of P. antillarum with a high total polyphenol content (2430 ± 208 mg of GAE/100 g) as well as strong scavenging activity against DPPH (EC50 = 0.337 ± 0.025 mg/mL) exhibited the highest metal ion-chelating effect when the concentration was increased from 1 to 8 mg/mL (Chew et al., 2008). Senevirathne et al. (2006) also reported high total phenolic content, radical scavenging activities, and metal ion chelation for the 70% methanol extract of E. cava. Interestingly, others claimed that metal ion chelation played a minor role in the general antioxidative activities of polyphenols (Rice-Evans et al., 1996). Andjelkovic et al. (2006) showed that the iron-chelating ability of phenolic compounds was significantly lower than that of EDTA. Additionally, the high binding capacities in the ferrous absorption of seaweed dietary fibers, such as agar, algicantes, carrageenan, and fucoidan, have been observed (Sabeena Farvin and Jacobsen, 2013; Wang et al., 2009). Saiga et al. (2003) also reported some peptides and protein possessed the metal ion-chelating effect. Toth and Pavia (2000) reported that other compounds in the same with phytochelatins or polysaccharides (alginates and fucoidan) in A. nodosum of brown seaweed can chelate metal ions and detoxify to copper accumulation, thus these compounds were more effective than phlorotannins.
PM consists of carbon, sulfur, monoxide, heavy metals including arsenic, cadmium, copper, and zinc, and other carcinogens and exerts adverse health effects through the ability to generate ROS (Li et al., 2003). The generative of excessive ROS can lead to oxidative stress that is common in cancer cells (Li et al., 2003; Valko et al., 2006). Oxidative stress causes a cellular redox imbalance that has been found in diverse cancer cells comparison with normal cells (Valko et al., 2006). SHE exhibited in vitro antioxidant activities with scavenging various radicals and chelating metals with the increase in concentration in the current study. When treated with PM, the antioxidant activities of SHE decreased even at very low PM concentrations. Commonly, antioxidants are concerned with the direct conversion of ROS to less-reactive species, but antioxidant protection against free radicals should be used with caution. Because the antioxidant activities depended on the stage at which it is introduced (Valko et al., 2006), it is necessary to understand the antioxidative mechanism for functional components. To inhibit oxidative stress, the most critical action would reduce exposure to exogenous and endogenous sources of oxidative stress by the elimination of environmental carcinogens such as particulate matter (Valko et al., 2006).
In conclusion, SHE from brown seaweed suppressed the oxidative stress by scavenging free radicals and chelating metals. These were confirmed by the DPPH free radical scavenging ability, hydrogen peroxide scavenging ability, reducing power, and metal ion-chelating effect. The DPPH free radical and hydrogen peroxide scavenging activity of SHE increased as the concentration increased, and SHE at 1000 μg/mL showed the highest activity. Similarly, superoxide anion inhibition and hydroxyl radical scavenging increased with increasing concentration. Additionally, the reducing power and metal ion-chelating effect of SHE were concentration dependent. Particularly, metal ion chelation at 1000 μg/mL was high (approximately 70% chelated) compared with that using the positive standard EDTA. However, all antioxidant activities were drastically reduced following treatment with urban particulate matter (PM) at 3.9–1000 μg/mL (SHE-PM). The urban particulate matter induced a high oxidation level reducing the antioxidant activities of SHE. Nonetheless, these results demonstrate that SHE has high radical scavenging activities. Therefore, S. horneri, which is a rich source of bioactive compounds, can be used a natural source of antioxidants in the food and pharmaceutical industries.
Acknowledgements
This research was funded by the Ministry of Oceans and Fisheries, Republic of Korea, Project No. 2017-0377.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ju Hee Lee, Email: wngml9993@naver.com.
Hyo Jin Kim, Email: jyouding@gmail.com.
Youngheun Jee, Email: yhjee@jejunu.ac.kr.
You-Jin Jeon, Email: youjinj@jejunu.ac.kr.
Hyun Jung Kim, Email: hyunjkim@jejunu.ac.kr.
References
- Ahn GN, Kim KN, Cha SH, Song SCB, Lee J, Heo MS. Antioxidant activities of phlorotannins purified from Ecklonia cava on free radical scavenging using ESR and H2O2-mediated DNA damage. J. Eur. Food Res. Technol. 2007;226:71–79. [Google Scholar]
- Alho H, Leinonen J. Total antioxidant activity measured by chemiluminescence method. Method. Enzymol. 1999;299:3–15. doi: 10.1016/s0076-6879(99)99004-3. [DOI] [PubMed] [Google Scholar]
- Andjelkovic M, Camp JV, Meulenaer BD, Depaemelaere G, Socaciu C, Verloo M. Iron-chelation properties of phenolic acids bearing catechol and galloyl groups. Food Chem. 2006;98:23–31. [Google Scholar]
- Athukorala Y, Lee KW, Song CB, Ahn CB, Shin TS, Cha YJ, Shahidi F, Jeon YJ. Potential antioxidant activity of marine red alga Grateloupia filicina extracts. J. Food Lipids. 2003;10:251–265. [Google Scholar]
- Chew YL, Lim YY, Omar M, Khoo KS. Antioxidant activity of three edible seaweeds from two areas in South East Asia. LWT Food Sci. Technol. 2008;41:1067–1072. [Google Scholar]
- Cho ML, Lee HS, Kang IJ, Won MH, You SG. Antioxidant properties of extract and fractions from Enteromorpha prolifera, a type of green seaweed. Food Chem. 2011;127:999–1006. doi: 10.1016/j.foodchem.2011.01.072. [DOI] [PubMed] [Google Scholar]
- Choe E, Min DB. Chemistry and reactions of reactive oxygen species in foods. J. Food Sci. 2005;70:142–159. doi: 10.1080/10408390500455474. [DOI] [PubMed] [Google Scholar]
- Czochra MP, Widensk A. Spectrofluorimetric determination of hydrogen peroxide scavenging activity. Anal. Chem. 2002;452:177–184. [Google Scholar]
- Devi KP, Suganthy N, Kesika P, Pandian SK. Bioprotective properties of seaweeds: in vitro evaluation of antioxidant activity and antimicrobial activity against food borne bacteria in relation to polyphenolic content. BMC Complem. Altern. Med. 2008;8(1):1–11. doi: 10.1186/1472-6882-8-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan XJ, Zhang WW, Li XM, Wang BG. Evaluation of antioxidant property of extract and fractions obtained from a red alga, Polysiphonia urceolata. Food Chem. 2006;9:37–43. [Google Scholar]
- Fernando IPS, Jayawardena TU, Sanjeewa KKA, Wang L, Jeon YJ, Lee WW. Anti-inflammatory potential of alginic acid from Sargassum horneri against urban aerosol-induced inflammatory responses in keratinocytes and macrophages. Ecotox. Environ. Safe. 2018;160:24–31. doi: 10.1016/j.ecoenv.2018.05.024. [DOI] [PubMed] [Google Scholar]
- Firuzi O, Lacanna A, Petrucci R, Marrosu G, Saso L. Evaluation of the antioxidant activity of flavonoids by “ferric reducing antioxidant power” assay and cyclic voltammetry. Biochim. Biophys. Acta. 2005;1721:174–184. doi: 10.1016/j.bbagen.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Heo SJ, Cha SH, Lee KW, Cho SK, Jeon YJ. Antioxidant activities of chlorophyta and phaeophyta from Jeju island. J. Algae. 2005;20:251–260. [Google Scholar]
- Heo SJ, Park EJ, Lee KW, Jeon YJ. Antioxidant activities of enzymatic extracts from brown seaweeds. Bioresource Technol. 2005;96:1613–1623. doi: 10.1016/j.biortech.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Hosokawa M, Okada T, Mikami N, Konishi I, Miyashita K. Bio-functions of marine carotenoids. Food Sci. Biotechnol. 2009;18(1):1–11. [Google Scholar]
- Jia N, Li T, Diao X, Kong B. Protective effects of black currant (Ribes nigrum L.) extract on hydrogen peroxide-induced damage in lung fibroblast MRC-5 cells in relation to the antioxidant activity. J. Funct. Foods. 2014;11:142–151. [Google Scholar]
- Jun WJ, Han BK, Yu KW, Kim MS, Chang IS, Kin HY, Cho YH. Antioxidant effects of Origanum majorana L on superoxide anion radicals. Food Chem. 2001;75:439–444. [Google Scholar]
- Kim BM, Jun JY, Park YB, Jeong IH. Antioxidative activity of methanolic extracts from seaweeds. J. Korean Soc. Food Sci. Nutr. 2006;35:1097–1101. [Google Scholar]
- Kim YD, Mahinda S, Koh KS, Jeon YJ, Kim SH. Reactive oxygen species scavenging activity of jeju native citrus peel during maturation. J. Korean Soc. Food Sci. Nutr. 2009;38:462–469. [Google Scholar]
- Kitada M, Igarashi K, Hirose S, Kitagawa H. Inhibition by polyamines of lipid peroxidase formation in rat liver microsomes. Biochem. Biophys. Res. Comm. 1979;87:388–394. doi: 10.1016/0006-291x(79)91808-4. [DOI] [PubMed] [Google Scholar]
- Koppenaol WH, Liebman JF. The oxidizing nature of the hydroxyl radical. A comparison with the ferryl ion (FeO2+) J. Phys. Chem. 1984;88:99–101. [Google Scholar]
- Kuda T, Tsunekawa M, Goto H, Araki Y. Antioxidant properties of four edible algae harvested in the Noto Peninsula, Japan. J. Food Compos. Anal. 2005;18:625–633. [Google Scholar]
- Kumar KS, Ganesan K, Rao PVS. Antioxidant potential of solvent extracts of Kappaphycus alvarezii (Doty) doty—an edible seaweed. Food Chem. 2008;107:289–295. [Google Scholar]
- Larson RA. The antioxidants of higher plants. J. Phytochem. 1988;27:969–978. [Google Scholar]
- Lee JM, Hwang KT, Heo MS, Lee JH, Park KY. Resistance of Lactobacillus plantarum KCTC 3099 from kimchi to oxidative stress. J. Med. Food. 2005;8:299–804. doi: 10.1089/jmf.2005.8.299. [DOI] [PubMed] [Google Scholar]
- Lee BK, Shin HH, Jung JH, Hwang KT, Lee YS, Kim TY. Anthocyanins, polyphenols and antioxidant activities of black raspberry exudates. J. Korean Soc. Food Sci. Nutr. 2009;38:125–130. [Google Scholar]
- Lee KH, Senevirathne M, Ahn CB, Je JY. Biological compounds extracted from codium fragile by enzymatic hydrolysis and their biological activities. J. Korean Soc. Food Sci. Nutr. 2010;39:953–959. [Google Scholar]
- Lemberkovices E, Czinner E, Szentmihalyi K, Balazs A, Szoke E. Comparative evaluation of Helichrysi flos herbal extracts as dietary sources of plant polyphenols, and macro and microelements. Food Chem. 2002;78:119–127. [Google Scholar]
- Li N, Hao M, Phalen RF, Hinds WC, Nel AE. Particulate air pollutants and asthma: a paradigm for the role of oxidative stress in PM-induced adverse health effects. J. Clin. Immunol. 2003;109:250–265. doi: 10.1016/j.clim.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Lim SN, Cheung PCK, Ooi VEC, Ang PO. Evaluation of antioxidative activity of extracts from a brown seaweed, Sargassum siliquastrum. J. Agr. Food Chem. 2002;50:3862–3866. doi: 10.1021/jf020096b. [DOI] [PubMed] [Google Scholar]
- Lui F, Ng TB. Antioxidative and free radical scavenging activities of selected medicinal herbs. J. Life Sci. 2000;66:725–735. doi: 10.1016/s0024-3205(99)00643-8. [DOI] [PubMed] [Google Scholar]
- Luo HY, Wang B, Yu CG, Qu YI, Su CI. Evaluation of antioxidant activities of five selected brown seaweeds from China. J. Med. Plants Res. 2010;4:2557–2565. [Google Scholar]
- Miyashita K, Takagi T. Tocopherol content of Japanese algae and its seasonal variation. Agr. Biol. Chem. 1987;51:3115–3118. [Google Scholar]
- Nakai M, Kageyama N, Nakahara K, Miki W. Phlorotannins as radical scavengers from the extracts of Sargassum ringgoldianum. Mar. Biotechnol. 2006;8:409–414. doi: 10.1007/s10126-005-6168-9. [DOI] [PubMed] [Google Scholar]
- Namiki M. Antioxidants/antimutagens in foods. J. Food Sci. Nutr. 1990;29:273–300. doi: 10.1080/10408399009527528. [DOI] [PubMed] [Google Scholar]
- Nimse SB, Pal D. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 2015;5:27986–28006. [Google Scholar]
- Park PJ, Shahidi F, Jeon YJ. Antioxidant activities of enzymatic extracts from and edible seaweed Sargassum horneri using ESR spectroscopy. J. Food Lipids. 2004;11:15–27. [Google Scholar]
- Raloff J. III winds: dust storms ferry toxic agents between countries and even continents. Sci, News. 2001;160:218–220. [Google Scholar]
- Rice-Evans CA, Miller NJ, Paganga G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radical Biol. Med. 1996;20:933–956. doi: 10.1016/0891-5849(95)02227-9. [DOI] [PubMed] [Google Scholar]
- Ruperez P, Ahrazem O, Leal JA. Potential antioxidant capacity of sulfated polysaccharides from the edible marine brown seaweed Fucus versiculosus. J. Agric. Food Chem. 2002;50:840–845. doi: 10.1021/jf010908o. [DOI] [PubMed] [Google Scholar]
- Sabeena Farvin KH, Jacobsen C. Phenolic compounds and antioxidant activities of selected species of seaweeds from Danish coast. Food Chem. 2013;138:1670–1681. doi: 10.1016/j.foodchem.2012.10.078. [DOI] [PubMed] [Google Scholar]
- Saiga A, Tanabe S, Nishimura T. Antioxidant activity of peptides obtained from porcine myofibrillar proteins by protease treatment. J. Agric. Food Chem. 2003;51:3661–3667. doi: 10.1021/jf021156g. [DOI] [PubMed] [Google Scholar]
- Santoso J, Yoshie-Stark Y, Suzuki T. Anti-oxidant activity of methanol extracts from Indonesian seaweeds in an oil emulsion model. J. Fish. Sci. 2004;70:183–188. [Google Scholar]
- Senevirathne M, Kim SH, Siriwardhana N, Ha JH, Lee KW, Jeon YJ. Antioxidant potential of Ecklonia cava on reactive oxygen species scavenging, metal chelating, reducing power and lipid peroxidation inhibition. Food Sci. Technol. Int. 2006;12:27–38. [Google Scholar]
- Shipeng Y, Woo HC, Choi JH, Park YB, Chun BS. Measurement of antioxidant activities and phenolic and flavonoid contents of the brown seaweed Sargassum horneri: comparison of supercritical CO2 and various solvent extractions. J. Fish. Aqut. Sci. 2015;18:123–130. [Google Scholar]
- Siriwardhana N, Lee KW, Kim SH, Ha JW, Jeon YJ. Antioxidant activity of Hizikia fusiformis on reactive oxygen species scavenging and lipid peroxidation inhibition. Food Sci. Technol. Int. 2003;9:339–346. [Google Scholar]
- Spencer JPE, Jenner A, Aruoma OI, Evans PJ, Kaur H, Dexter DT, Jenner P, Lees AJ, Marsden DC, Halliwell B. Intensive oxidative DNA damage promoted by L-DOPA and its metabolites implications for neurodegenerative disease. FEBS J. 1994;353:246–250. doi: 10.1016/0014-5793(94)01056-0. [DOI] [PubMed] [Google Scholar]
- Srivastava A, Harish SR, Shivanandappa T. Antioxidant activity of the roots of Decalepis hamiltonii (Wight & Arn) LWT Food Sci. Technol. 2006;39:1059–1065. [Google Scholar]
- Tedesco I, Russo M, Russo P, Iacomino G, Russo GL, Carraturo A, Faruolo C, Moio L, Palumbo R. Antioxidant effect of red wine polyphenols on red blood cells. J. Nutr. Biochem. 2000;11:114–119. doi: 10.1016/s0955-2863(99)00080-7. [DOI] [PubMed] [Google Scholar]
- Toth G, Pavia H. Lack of phlorotannin induction in the brown seaweed Ascophyllum nodosum in response to increased copper concentrations. J. Marine Ecol. Prog. Ser. 2000;192:119–126. [Google Scholar]
- Valko M, Rhodes CJ, Moncol J, Lzakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem-Biol. Interact. 2006;160(1):1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Wang SM, Yu DJ, Song KB. Physicochemical characteristics of kohlrabi slices dehydrated by the addition of maltodextrin. J. Food Sci. Nutr. 2011;16:189–193. [Google Scholar]
- Wang T, Jonsdottir R, Olafsdottir G. Total phenolic compounds, radical scavenging and metal chelation of extracts from Icelandic seaweeds. Food Chem. 2009;116:240–248. [Google Scholar]
- Xiaojun Y, Xiancui L, Chengxu Z, Xiao F. Prevention of fish oil rancidity by phlorotannins from Sargassum kjellmanianum. J. Appl. Phycol. 1996;8:201–203. [Google Scholar]
- Yan XJ, Chuda Y, Suzuki M, Nagata T. Fucoxanthin as the mafor antioxidant in Hizikia fusiformis, a common edible seaweed. Biosci. Biotechnol. Biochem. 1999;63:605–607. doi: 10.1271/bbb.63.605. [DOI] [PubMed] [Google Scholar]
- Ye H, Zhou C, Sun Y, Zhan X, Liu J, Hu Q, Zeng X. Antioxidant activities in vitro of ethanol extract from brown seaweed Sargassum pallidum. Eur. J. Food Res. Rev. 2009;230:101–109. [Google Scholar]
- Zhang WW, Duan XJ, Huang HL, Zhang Y, Wang BG. Evaluation of 28 marine algae from the Qingdao coast for antioxidative capacity and determination of antioxidant efficiency and total phenolic content of fractions and sub fractions derived from Symphyocladia latiuscula (Rhodomelaceae) J. Appl. Phycol. 2007;19:97–108. [Google Scholar]