Abstract
This systematic review and meta-analysis aim to evaluate the association of wheat germ interventions and metabolic markers. An electronic search was performed by mid-May 2019 in the PubMed, Google Scholar, and Web of Science databases. Quality was evaluated using the risk of bias assessment tools. Thirty-three randomized controlled trials (RCTs) were identified, among which ten were suitable and systematically reviewed based on biomarkers (cholesterol, triglycerides, glucose, and oxidative stress). Three biomarkers in five eligible studies were investigated by meta-analysis. Total cholesterol showed non-significant results (p = 0.98), with standard mean difference (SMD) of − 0.01 (95% confidence interval; − 0.17, 0.16). The SMD was − 0.06 (95% CI − 0.41, 0.29, n = 4) for triglycerides and − 0.09 (95% CI − 0.62, 0.45, n = 2) for glucose. No biomarkers showed heterogeneity (0%). This review revealed non-significant association between wheat germ interventions and metabolic markers. Sensitive analysis with high-quality RCTs may be worth trying.
Keywords: Wheat germ, Metabolic markers, Cholesterol, Triglycerides, Glucose
Introduction
Metabolic syndrome (MetS) is an asymptomatic disorder that includes a cluster of metabolic abnormalities associated with obesity, hyperlipidemia, hypertension, and insulin resistance (Alberti et al., 2009). The causative factors of MetS are central obesity and insulin resistance, which lead to cardiovascular diseases (CVDs), diabetes, and stroke (Srikanthan et al., 2016). Oxidative stress and inflammation also contribute to the etiology of MetS (Soares and Costa, 2009). Metabolic markers such as triglyceride levels, high-density lipoprotein-cholesterol (HDL-C), low-density lipoprotein-cholesterol (LDL-C), hypertension, blood pressure, obesity, insulin, and oxidative stress are the criteria used to diagnose MetS. This non-communicable disease has become a significant major cause of mortality worldwide and increases the mortality rate of patients with type 2 diabetes and CVDs, coronary heart disease, and stroke (Ford, 2004). The American Heart Association reported that about 35% of adults and 50% of 60 years older in the US have MetS (Aguilar et al., 2015). The International Diabetes Federation stated that nearly 25% of the world’s population suffers from MetS (O’neill and O’driscoll, 2015). However, the prevalence varies by age, ethnicity, gender, and variation in the definition of MetS. Based on the International Diabetes Federation definition, the eastern country of Tunisia showed a MetS prevalence of 45.5%; in Iran, this value was 37.4% (Delavari et al., 2009).
Recently, many clinical studies have been conducted to evaluate the relationship between unhealthy dietary habits and chronic diseases such as CVD (Michas et al., 2014; Willett et al., 2002) and diabetes (Esposito et al., 2015; Hauner et al., 2012). The inclusion of functional components in diets plays an integral role in the public health sectors (De Jong et al., 2004; Vella et al., 2014). Refined grains are extracted from cereals by removing the bran and germ fractions. These fractions contained bioactive compounds such as phytochemicals, some essential micronutrients, vitamins, and dietary fiber. Many studies have demonstrated an association between CVD and whole grain and bran consumption (Aune et al., 2016; Charlton et al., 2012; Junejo et al., 2019; Zong et al., 2016). However, the results for germ are unclear (de Munter et al., 2007; Lupton et al., 1994).
Wheat (Triticum aestivum) is one of the most widely consumed edible whole grains worldwide and is used as a staple food in many countries. Wheat is comprised of nearly 80% endosperm, 15% bran, and 5% germ (Slavin, 2004). Wheat germ (the embryo) is a concentrated source of antioxidants such as polyphenols, carotenoids, and tocopherols (the most abundant natural source of vitamin E) (Vaher et al., 2010; Zhu et al., 2011). Wheat germ proteins are ample sources of amino acids, especially methionine, threonine, and lysine (Meriles et al., 2019). Wheat germ is typically discarded during the milling process but has been used to produce wheat germ oil. In the previous decade, numerous in vitro and in vivo studies have investigated the various health aspects of wheat germ, especially wheat germ oil (Arshad et al., 2013; Khedr, 2017) that can improve lipid metabolism (Khalil et al., 2010) and lower oxidative stress (Alessandri et al., 2006). Fermented wheat germ extract (FWGE) has been shown to have antimetastatic effects in cells and animals (Fajka-Boja et al., 2002; Heimbach et al., 2007; Hidvegi et al., 1998) including in colorectal (Farkas, 2005) and ovarian cancer (Koh et al., 2018). Many in vivo trials have been conducted to determine the preventive role of wheat germ on atherosclerosis, hypercholesterolemia (Rezq and Mahmoud, 2011), hyperlipidemia (Chadha et al., 2015), oxidative stress (El-Shorbagy, 2017), hepatotoxin (Akool, 2015) and insulin resistance (Iyer and Brown, 2011; Ojo et al., 2017). Some in vitro studies demonstrated the antioxidant and anti-inflammatory effects of wheat germ and wheat germ oil (Boros et al., 2001; Jeong et al., 2017; Park et al., 2015).
Hence, after reviewing numerous studies, this comprehensive systematic review aims to summarize the accessible scientific literature on wheat germ regarding its effectiveness with metabolic markers in humans.
Materials and methods
We carried out this systematic review and meta-analysis in accordance with the PRISMA statement (Moher et al., 2009) and Cochrane Collaboration (Higgins and Green, 2011) during all stages of execution and data reporting.
Literature search
A comprehensive search strategy was applied by using the medical and electronic databases Google Scholar, Medline (PubMed), and Web of Science without any restrictions on language or time to identify articles published by mid-May 2019. Research articles using “wheat germ” in the title and abstract were searched. To obtain more precise results, an advanced search was conducted with filters such as clinical trials, species (human) examined, and terms including “wheat germ” OR “randomized” OR “controlled trials”. To evaluate whether wheat germ is related to MetS, we identified the studies of wheat germ and metabolic markers using the terms cholesterol, glucose, oxidation, triglycerides, lipids, obesity, and blood pressure in combination with wheat germ. We screened additional review and systematic review studies to identify potentially related citations. Manual searching was performed to avoid the elimination of pertinent articles.
Study selection and eligibility criteria
This review was limited to randomized controlled trials (RCTs, either parallel or crossover) conducted solely in adult humans. PICOS (population, intervention, comparator, outcome, and study design) was established for the review. Eligibility criteria were based on the PICOS reporting tools (Methley et al., 2014). The study population included healthy persons or people who were at risk of disease occurrence such as pre-diabetes and impaired fasting glucose. Study interventions included wheat germ in the raw, extracted, powder, or oil forms that evaluated the effect of wheat germ in reducing the MetS by lowering its biomarkers like blood glucose, cholesterol, lipid contents, blood pressure, and overweight (obesity). The intervention was compared to control or placebo groups in a single or double-blinded manner. If any studies fulfilled these eligibility criteria, they were included in the systematic review regardless of the availability of analytical data for meta-analysis. The following studies were excluded from analysis: those in which participants had a disease, RCTs that did not report the effect of wheat germ on any metabolic markers, in vivo (non-human studies) and in vitro studies, papers with the abstract only, conference abstract, and observational, coherent, and case–control studies. In the selection process, all controversies and disagreements were resolved by discussion among the two investigators.
Data extraction
In the initial search, two researchers (HL and EJ) independently reviewed the title and abstracts of the articles under the PICOS framework. Next, descriptive data screened based on full-text articles were assessed for eligibility. A standard form included the following information from the selected articles: bibliographic details, study design, study origin, participants’ health status, age, sex, body mass index, groups description, a form of wheat germ, intervention period, washout period, dose amount, intake direction, physical and dietary intake details during an intervention, functionality of wheat germ, biomarker readings at baseline and post-intervention, outcomes measures, statistical results, compliance, and dropout rate.
There were insufficient data on dichotomous outcomes in the included studies. To utilize the available data in a meta-analysis, we included data for three metabolic markers (cholesterol, triglycerides, and glucose) in the meta-analysis as continuous outcomes.
Quality assessment
The quality of the selected trials was measured by Cochrane Collaboration’s tool to evaluate the risk of bias in the randomized trials (Higgins et al., 2011). The bias tools have the following respective domains: random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attribution bias), selective reporting (reporting bias), and other sources of bias. Each domain was rated as a low, high, and unclear risk. If at least one of the domains showed a high or unclear risk, we classified the overall result as a high or unclear risk, respectively. The overall evaluated result was considered as low risk if all domains showed a low risk in the respective study.
Statistical analysis
To conduct the meta-analysis, we used the review manager (RevMan) version 5.3 (Collaboration, 2016). Data in the included articles were continuous outcomes within the studies related to different metabolic markers. In the analytical method, we analyzed the random effects model by DerSimonian and Laird methods (DerSimonian and Laird, 1986). Follow-up from baseline in the experimental group was compared to that in the control group using the standard mean difference (SMD) as a primary effective measure. To identify the parametric relationship between the intervention group (wheat germ) and control group, we calculated the inverse of variance (IV) as the study weight in analysis and 95% confidence intervals (CIs) among the categories of metabolic markers. To more precisely examine the effect of cholesterol, we stratified cholesterol into subgroups: HDL-C and LDL-C.
Among the trials, some results were reported as the standard error, which was converted to standard deviation by multiplying the square root of the sample size.
Some values for triglycerides, cholesterol, and glucose were reported in mg/dL. We converted these values to units of mmol/L by dividing the values in mg/dL by a factor of 88.5, 38.6, and 18, respectively. To explore the heterogeneity in the results, l2 statistic was used for evaluation, which showed the total variation attributable to heterogeneity between studies. The results were considered significant when p < 0.05. Thresholds of heterogeneity of 0%, ≤ 25%, ≤ 50%, and ≤ 75% were considered as no, low, moderate, and high variations among the different outcomes.
Results and discussion
Studies included in the analysis
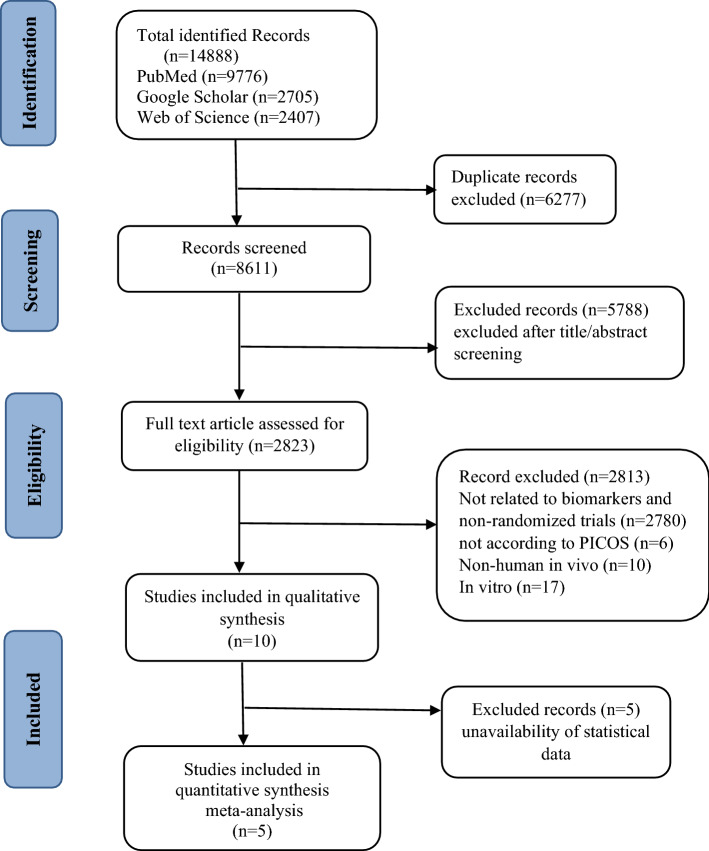
The detailed search strategy was performed, as shown in the PRISMA flow chart (Moher et al., 2009) (Fig. 1). We initially identified 14,888 studies in the three different databases, with 9776, 2705, and 2407 articles from PubMed, Google Scholar, and Web of Science, respectively. All references from these databases were imported to an Endnote library. After deleting duplicate references using EndNote x7, 8611 studies remained. Next, 2823 full-text articles remained after eliminating abstract, proceeding, and review papers. Forty-three articles were further reviewed after eliminating 2780 studies that failed to meet the inclusion criteria. In the preparatory mapping review, we tested many studies that demonstrated the health outcomes of wheat germ consumption. Most of these studies were dropped out because of unrelated functionality and study design.
Fig. 1.
PRISMA flow chart of study identification and selection for systematic review and meta-analysis
In the eligibility section, six articles were eliminated that did not evaluate whether the studies were non-randomized trials or described a diseased population (Haripriya and Premakumari, 2010; Zakaria et al., 2017). One study was also removed that did not report the functionality of wheat germ related to metabolic markers (Kobyliak et al., 2018). Finally, 10 in vivo (non-human studies) and 17 in vitro studies were eliminated. After critically reviewing the whole abstract and full-text of the articles, 10 potential studies were assessed by systematic review. However, the selection of included studies in this systematic review was challenging, because many were old studies (Cara et al., 1991; Cara et al., 1992b) with low quality RCTs (Rodionova et al., 2016). One of the studies did not report any analytical or statistical results. Still, it fulfilled the eligibility criteria of the systematic review, so it was included in the systematic review and excluded from the meta-analysis (Moreira-Rosário et al., 2016). The other four studies did not report statistical data related to metabolic markers that could be used in meta-analysis (Alessandri et al., 2006; Cara et al., 1992b; Ostlund Jr et al., 2003; Rodionova et al., 2016). Five out of ten pertinent studies describing the outcomes of metabolic markers could be used for meta-analysis (Cara et al., 1991; Cara et al., 1992b; Lin et al., 2004; Moreira-Rosário et al., 2019; Tripkovic et al., 2015). The remaining studies were excluded due to insufficient measurement of data.
Study characteristics
The characteristics of the included studies are summarized in Table 1. Studies were conducted in different countries between 1991 and 2019. The two most recent studies were performed in a southern European country, Portugal (Moreira-Rosário et al., 2016; Moreira-Rosário et al., 2019), whereas the three oldest were performed in France (Cara et al., 1992a; Cara et al., 1991; Cara et al., 1992b). The remaining five trials were carried out in five regions: Italy (Alessandri et al., 2006), Netherlands (Lin et al., 2004), Russia (Rodionova et al., 2016), United States (Ostlund Jr et al., 2003), and United Kingdom (Tripkovic et al., 2015). The included population were mostly community-based, and some subjects were from educational institutes. Approximately half of the trials described the selected participants as healthy and four trials included slightly hypercholesterolemic populations (Alessandri et al., 2006; Cara et al., 1991; Cara et al., 1992a). Only one trial reported that the participants had risk of CVD (Tripkovic et al., 2015). The ages of participants were 18–70 years and involved approximately 320 participants in these RCTs. Although all selected studies were RCTs, six studies used a cross-over design (Cara et al., 1991; Cara et al., 1992a; Moreira-Rosário et al., 2016; Moreira-Rosário et al., 2019; Tripkovic et al., 2015) and four used a parallel design (Alessandri et al., 2006; Cara et al., 1992b; Lin et al., 2004; Rodionova et al., 2016). Included studies used wheat germ in three forms: raw, powder, and oil. Four weeks was the average intervention period in nearly all trials. The number of participants varied from 6 (Cara et al., 1992b) to 60 subjects (Rodionova et al., 2016). The wheat germ dose ranged from 3 to 80 g/day at various intervals. The sample size of each selective study was the number of subjects who participated in this analysis from baseline to follow-up with data availability.
Table 1.
Summary of characteristics of eligible studies assessing the association between wheat germ and metabolic markers
| ID | Study design | Location | Health and age | Number and gender | Biomarkers | Group: intervention and control |
Dose and duration | Result |
|---|---|---|---|---|---|---|---|---|
| Moreira-Rosário (2019) | RCT1 (crossover) | Portugal |
Healthy people 34 year (mean age) |
55 both |
Cholesterol Triglycerides Glucose |
Inter3: WG4 enriched bread control: non-enriched bread |
6 g/day (in two snacks) 4 weeks |
× no effect |
| Moreira-Rosário (2016) | RCT (crossover) | Portugal |
Healthy people 18–60 years |
55 both |
Cholesterol Triglycerides Glucose |
Inter: WG enriched bread control: non-enriched bread |
6 g/day (in two snacks) 4 weeks |
× no effect |
| Rodionova et al. (2016) |
RCT (parallel) |
Russia |
Healthy people 16–65 years |
60 both |
Cholesterol Triglycerides |
Inter: 3.5 g/day WGO5 regardless of meal control: 3.5 g WGO in 50 g of meal, |
3.5 oil g/day (with or without meal) 30 days |
↓cholesterol ↓triglycerides |
| Tripkovic et al. (2015) | RCT (crossover) |
United Kingdom |
At risk of CVD2 35–55 years |
10 both |
Cholesterol Triglycerides Glucose |
G61: WG G2: inulin G3: refined grain (control) |
15 g/day (5 g/meal) 4 weeks |
↓ glucose × no effect on lipid profile |
| Alessandri et al. (2006) |
RCT (parallel) |
Ital |
Hypercholesterolemic 60.3 ± 6.1 years |
32 both | Oxidative stress |
G1: maize oil (vegetable oil) G2: WGO |
1 tbsp7/d for 8 weeks |
↓oxidative stress |
| Lin et al. (2004) |
RCT (parallel) |
Netherlands |
Normal to elevated Cholesterol 18–70 years |
60 both |
Cholesterol Triglycerides |
G1: chocolate with WG G2: chocolate without WG |
20 mg/day (4 chocolates/d) |
× no effect |
| Ostlund Jr et al. (2003) | RCT (crossover) | United States |
Healthy people 39 ± 12 years |
10 both | Cholesterol |
G1: WG G2: WGO G3: purified WGO |
80 g/day (1 muffin) |
↓cholesterol |
| Cara et al. (1992a) | RCT (crossover) | France |
Hypercholesterolemic and hyperglycemic 37–69 years |
19 both |
Cholesterol Triglycerides |
G1: raw WG G2: partially defatted WG |
20 g/day (incorporated in 3 times meal) 4 weeks |
↓ cholesterol ↓triglycerides |
| Cara et al. (1992b) |
RCT (parallel) |
France |
Healthy people 22–41 years |
6 males |
Cholesterol Triglycerides |
G1: oat bran G2: rice bran G3: wheat fiber G4: WG G5: control |
10 g/day total dietary fiber 2.8 g/day dietary fiber |
↓ cholesterol ↓ lipid profile |
| Cara et al. (1991) | RCT (crossover) | France |
Hypercholesterolemic and healthy subjects 35–68 years |
10 both |
Cholesterol Triglycerides |
G1: basal diet (as control) G2: basal diet + WG |
30 g/day (incorporated in meal) 4 weeks |
↓ cholesterol ↓triglycerides |
1RCT, randomized control trial; 2CVD, cardiovascular disease; 3inter, intervention; 4WG, wheat germ; 5WGO, wheat germ oil; 6G, group; 7tbsp, tablespoon
In this systematic review, many studies were older and included a very small study size with little information (Cara et al., 1991; Cara et al., 1992b). These studies did not report the mean difference, dropout compliance, and blinding (Rodionova et al., 2016).
Risk of bias within studies
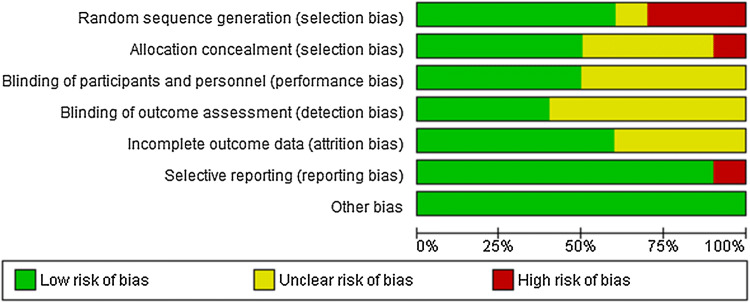
The biases in the clinical trials are described in Fig. 2. Among the ten randomized controlled trials, there was a low risk of selective reporting bias and other biases. However, performance and detection bias showed more than a 50% unclear risk because of the insufficient blinding of participants and outcome assessors. There was a high risk of selection bias compared to all other domains because of the inappropriate method of selection and incomplete knowledge in many trials. The detailed risk assessment results of bias in the clinical trials are shown in Table 2.
Fig. 2.
Risk of bias graph: review authors’ judgments regarding the risk of bias of each item presented as percentages across all included studies (n = 10)
Table 2.
Summary of the overall risk of bias in randomized controlled trials of prospective studies in the qualitative assessment
| Author (year) | Random sequence generation | Allocation concealment | Blinding of participants | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | Other biases | Overall bias |
|---|---|---|---|---|---|---|---|---|
| Cara et al. (1991) | High risk | Unclear | Unclear | Unclear | Unclear | Low risk | Low risk | High risk |
| Cara et al. (1992a) | High risk | Unclear | Unclear | Unclear | Unclear | Low risk | Low risk | High risk |
| Cara et al. (1992b) | Low risk | Unclear | Unclear | Unclear | Unclear | Low risk | Low risk | Unclear |
| Ostlund Jr et al. (2003) | Unclear | Unclear | Unclear | Unclear | Low risk | Low risk | Low risk | Unclear |
| Lin et al. (2004) | Low risk | Low risk | Low risk | Unclear | Low risk | High risk | Low risk | High risk |
| Alessandri et al. (2006) | Low risk | High risk | Low risk | Unclear | Low risk | Low risk | Low risk | High risk |
| Tripkovic et al. (2015) | Low risk | Low risk | Unclear | Low risk | Low risk | Low risk | Low risk | Unclear |
| Moreira-Rosário (2016) | Low risk | Low risk | Low risk | Low risk | Unclear | Low risk | Low risk | Unclear |
| Rodionova et al. (2016) | High risk | Low risk | Low risk | Unclear | Low risk | Low risk | Low risk | High risk |
| Moreira-Rosário (2019) | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
In a narrative literature review, the quality of selected studies was mostly unclear and high risk. These findings should be interpreted with caution, as the results showed very low certainty of the evidence for all health outcomes.
Effect of intervention on metabolic markers
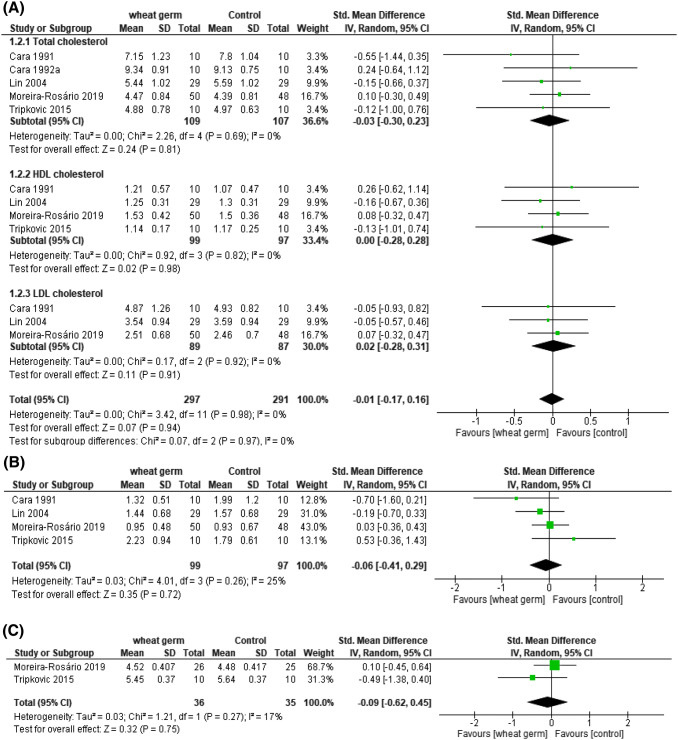
In the primary meta-analysis, five randomized controlled trials investigated the effect of total cholesterol in 109 participants in the intervention group and 107 subjects in the control group. The SMD of the wheat germ versus control groups was − 0.03 (95% CI − 0.30 to 0.23), revealing a non-significant reduction in cholesterol (p = 0.69) and negligible heterogeneity (I2 = 0%).
Cholesterol was evaluated as HDL-C and LDL-C; four trials used the HDL-C index to evaluate the SMD − 0.00 (95% CI − 0.28 to 0.28). The summary, in which the LDL-C index was used, found an SMD of − 0.02 (95% CI − 0.28 to 0.31) (Cara et al., 1991; Lin et al., 2004; Moreira-Rosário et al., 2019). Both the summaries of HDL-C and LDL-C showed the level of heterogeneity (I2 = 0%) with non-significant results (p = 0.82, 0.92, respectively). Thus, the overall results showed 0% heterogeneity with a non-significant effect (p = 0.98). Nearly all studies showed a non-significant effect of wheat germ intervention compared to the control group [Fig. 3(A)].
Fig. 3.
(A) Meta-analysis of wheat germ vs control group using the random-effect model and weighted by standard mean difference. Forest plot of outcome: effect of wheat germ on total cholesterol, HDL-C, and LDL-C. (B) Forest plot of wheat germ vs control: effect of wheat germ on triglycerides. (C) Forest plot of wheat germ vs control: effect of wheat germ on glucose
To determine the effect of wheat germ intervention on the triglycerides profile, four trials included 99 participants in the intervention group and 97 subjects in the control group. A non-significant (p = 0.26) reduction in triglycerides after consuming wheat germ was found, with an SMD of − 0.06 (95% CI − 0.41 to 0.29); no heterogeneity was detected (I2 = 0%) [Fig. 3(B)].
Two randomized controlled trials (Moreira-Rosário et al., 2019; Tripkovic et al., 2015) were added in the analysis to determine the effectiveness of wheat germ on lowering blood glucose levels. These trials included 36 participants in the intervention group and 35 in the control group. The pooled SMD was − 0.09 (95% CI − 0.62 to 0.45). The heterogeneity level measured by I2 was 0%, showing a non-significant result (p = 0.27). The meta-analysis results of these two trials were insufficient to determine whether wheat germ treatment reduces glucose levels [Fig. 3(C)]. Overall, the difference in the effects between wheat germ and control or placebo appeared to be clinically and statistically non-significant.
In summary of the evidence, our goal was to demonstrate the effect of one important part of staple food in a healthy or risk group population by systematized review of the previous literature. After performing the initial literature search on the broad topic “wheat germ” related to various health aspects, we could find only a few studies that reported the effect of wheat germ on multiple biomarkers like oxidative stress, blood cholesterol, triglycerides and glucose (that all are under the metabolic syndrome regime and more precisely, metabolic markers), and only a few studies were related to cancer, arthritis, and the immune system.
MetS is a broad term with many biomarkers, but we couldn’t find any other studies that might report on other biomarkers (obesity and blood pressure). Due to limited literature availability, our systematic review was limited to a few studies with some metabolic markers.
The strength of the current review is that only healthy and risk group individuals (slightly elevated blood lipids or glucose level) were included, rather than all diseased populations. The dietary impact varied with the usage of medications and other nutritional supplements. The metabolic rate and biomarker levels fluctuate under disease conditions; thus, evaluating only healthy participants will reveal more accurate and precise results. The data from RCTs were used rather than those obtained by other study designs. RCTs are more frequently performed in medical and clinical experiments and show low bias during testing.
Nevertheless, there were some limitations to this study. First, all eligible were studies conducted in either European countries or the United States. However, wheat is the dominant staple food in North Africa and West and Central Asia (Alexandratos and Bruinsma, 2012). Thus, the generalizability of these trials to other populations worldwide is limited. Second, there was heterogeneity in the dose, duration, and frequency of wheat germ intervention. An insufficient dose of wheat germ appeared to be among the major causes of non-significant results. A recent study proposed that a low level of wheat germ (6 g) intervention did not affect glucose and lipid metabolism (Moreira-Rosário et al., 2019). Thus, an increased dose and duration of wheat germ in the intervention group may show a preventive effect on the metabolic markers. At the last, the composition of wheat germ may be affected by factors such as the chemicals and preparation method used to treat the wheat germ. In the studies discussed here, several forms of wheat germ (raw, extracted, defatted, and oil) were added to different food commodities (chocolate pellets, bread rolls, and muffins).
In this comprehensive literature, the findings should be interpreted with caution based on the limited numbers of included studies. The review of individual studies revealed contradictory results. Some articles described the improvements in blood lipid levels (Cara et al., 1991; Cara et al., 1992a; Cara et al., 1992b; Rodionova et al., 2016) and glucose metabolism (Tripkovic et al., 2015), whereas others were unclear about the antihyperlipidemic and antihyperglycemic effect of wheat germ (Lin et al., 2004; Moreira-Rosário et al., 2016). The small intervention dose, short study duration, low study quality, some additional factors, and lack of blinding of the participants and outcomes by the assessors may have led to inconsistency among the observed results. Therefore, we could not reach the conclusion that would present any kind of quick suggestion about any significant improvement in metabolic markers with wheat germ intervention. Further relevant research is needed because these findings showed low certainty of the evidence for all health outcomes of wheat germ.
Best to our knowledge, this is the first systematic review and meta-analysis of the effects of wheat germ consumption on metabolic markers. In conclusion, there is little credible evidence for a relation between wheat germ intake and a reduced risk of metabolic markers. To evaluate the long-term effects of wheat germ consumption on the metabolic markers in humans, the well-designed randomized placebo-controlled trials with sufficient sample doses and optimal intervention duration may be worth trying.
Acknowledgements
This work was supported by Halim Scholarship Foundation and the Bio & Medical Technology Development Program of the National Research Foundation (NRF) funded by the Ministry of Science & ICT (2012M3A9C4048761).
Compliance with ethical standards
Conflict of interest
The authors declare that there is no conflict of interest.
Footnotes
The original version of this article was revised due to a retrospective Open Access order.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
7/8/2020
The article “Effect of wheat germ on metabolic markers: a systematic review and meta-analysis of randomized controlled trials”, written by Humna Liaqat, Eunseon Jeong, Kyeong Jin Kim and Ji Yeon Kim, was originally published Online First without Open Access.
Contributor Information
Humna Liaqat, Email: humnaliaqat710@gmail.com.
Eunseon Jeong, Email: todayjung@hanmail.net.
Kyeong Jin Kim, Email: jinnykim@seoultech.ac.kr.
Ji Yeon Kim, Email: jiyeonk@seoultech.ac.kr.
References
- Aguilar M, Bhuket T, Torres S, Liu B, Wong RJ. Prevalence of the metabolic syndrome in the United States, 2003-2012. J. Am. Med. Assoc. 2015;313:1973–1974. doi: 10.1001/jama.2015.4260. [DOI] [PubMed] [Google Scholar]
- Akool E-S. Molecular mechanisms of the protective role of wheat germ oil against cyclosporin A-induced hepatotoxicity in rats. Pharm. Biol. 2015;53:1311–1317. doi: 10.3109/13880209.2014.980584. [DOI] [PubMed] [Google Scholar]
- Alberti K, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart J-C, James WPT, Loria CM, Smith SC., Jr Harmonizing the metabolic syndrome: a joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; American heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- Alessandri C, Pignatelli P, Loffredo L, Lenti L, Del Ben M, Carnevale R, Perrone A, Ferro D, Angelico F, Violi F. Alpha-linolenic acid-rich wheat germ oil decreases oxidative stress and CD40 ligand in patients with mild hypercholesterolemia. Arterioscl. Throm. Vas. 2006;26:2577–2578. doi: 10.1161/01.ATV.0000242795.08322.fb. [DOI] [PubMed] [Google Scholar]
- Alexandratos N, Bruinsma J. World agriculture towards 2030/2050: the 2012 revision. ESA Working Paper No. 12-03, Rome. pp. 44-45 (2012)
- Arshad MS, Anjum FM, Khan MI, Shahid M. Wheat germ oil and α-lipoic acid predominantly improve the lipid profile of broiler meat. J. Agric. Food Chem. 2013;61:11158–11165. doi: 10.1021/jf4041029. [DOI] [PubMed] [Google Scholar]
- Aune D, Keum N, Giovannucci E, Fadnes LT, Boffetta P, Greenwood DC, Tonstad S, Vatten LJ, Riboli E, Norat T. Whole grain consumption and risk of cardiovascular disease, cancer, and all cause and cause specific mortality: systematic review and dose-response meta-analysis of prospective studies. Brit. Med. J. 2016;353:i2716. doi: 10.1136/bmj.i2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boros LG, Lapis K, Szende B, Tömösközi-Farkas R, Balogh Á, Boren J, Marin S, Cascante M, Hidvégi M. Wheat germ extract decreases glucose uptake and RNA ribose formation but increases fatty acid synthesis in MIA pancreatic adenocarcinoma cells. Pancreas. 2001;23:141–147. doi: 10.1097/00006676-200108000-00004. [DOI] [PubMed] [Google Scholar]
- Cara L, Armand M, Borel P, Senft M, Portugal H, Pauli A-M, Lafont H, Lairon D. Long-term wheat germ intake beneficially affects plasma lipids and lipoproteins in hypercholesterolemic human subjects. J. Nutr. 1992;122:317–326. doi: 10.1093/jn/122.2.317. [DOI] [PubMed] [Google Scholar]
- Cara L, Borel P, Armand M, Senft M, Lafont H, Portugal H, Pauli A-M, Boulze D, Lacombe C, Lairon D. Plasma lipid lowering effects of wheat germ in hypercholesterolemic subjects. Plant Foods Hum. Nutr. 1991;41:135–150. doi: 10.1007/BF02194082. [DOI] [PubMed] [Google Scholar]
- Cara L, Dubois C, Borel P, Armand M, Senft M, Portugal H, Pauli A, Bernard P, Lairon D. Effects of oat bran, rice bran, wheat fiber, and wheat germ on postprandial lipemia in healthy adults. Am. J. Clin. Nutr. 1992;55:81–88. doi: 10.1093/ajcn/55.1.81. [DOI] [PubMed] [Google Scholar]
- Chadha R, Ram H, Purohit A. Hypolipidemic effect of wheat germ oil in cholesterol fed Rabbits. Med. Drug Res. 2015;3:15–20. [Google Scholar]
- Charlton KE, Tapsell LC, Batterham MJ, O’Shea J, Thorne R, Beck E, Tosh SM. Effect of 6 weeks’ consumption of β-glucan-rich oat products on cholesterol levels in mildly hypercholesterolaemic overweight adults. Br. J. Nutr. 2012;107:1037–1047. doi: 10.1017/S0007114511003850. [DOI] [PubMed] [Google Scholar]
- De Jong N, Hoendervangers C, Bleeker J, Ocké M. The opinion of Dutch dietitians about functional foods. J. Hum. Nutr. Diet. 2004;17:55–62. doi: 10.1046/j.1365-277x.2003.00498.x. [DOI] [PubMed] [Google Scholar]
- de Munter JS, Hu FB, Spiegelman D, Franz M, van Dam RM. Whole grain, bran, and germ intake and risk of type 2 diabetes: a prospective cohort study and systematic review. PLoS Med. 2007;4:e261. doi: 10.1371/journal.pmed.0040261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delavari A, Forouzanfar MH, Alikhani S, Sharifian A, Kelishadi R. First nationwide study of the prevalence of the metabolic syndrome and optimal cutoff points of waist circumference in the Middle East: the national survey of risk factors for noncommunicable diseases of Iran. Diabetes Care. 2009;32:1092–1097. doi: 10.2337/dc08-1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control. Clin. Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- El-Shorbagy HM. Molecular and anti-oxidant effects of wheat germ oil on CCl4-induced renal injury in mice. J. Appl. Pharm. Sci. 2017;7:94–102. [Google Scholar]
- Esposito K, Maiorino MI, Bellastella G, Chiodini P, Panagiotakos D, Giugliano D. A journey into a Mediterranean diet and type 2 diabetes: a systematic review with meta-analyses. BMJ Open. 2015;5:e008222. doi: 10.1136/bmjopen-2015-008222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fajka-Boja R, Hidvegi M, Shoenfeld Y, Ion G, Demydenko D, Tomoskozi-Farkas R, Vizler C, Telekes A, Resetar A, Monostori E. Fermented wheat germ extract induces apoptosis and downregulation of major histocompatibility complex class I proteins in tumor T and B cell lines. Int. J. Oncol. 2002;20:563–570. [PubMed] [Google Scholar]
- Farkas E. Fermented wheat germ extract in the supportive therapy of colorectal cancer. Orvosi Hetilap. 2005;146:1925–1931. [PubMed] [Google Scholar]
- Ford ES. The metabolic syndrome and mortality from cardiovascular disease and all-causes: findings from the National Health and Nutrition Examination Survey II Mortality Study. Atherosclerosis. 2004;173:307–312. doi: 10.1016/j.atherosclerosis.2003.12.022. [DOI] [PubMed] [Google Scholar]
- Haripriya S, Premakumari S. Beta sistosterol of wheat germ reduces blood glucose in humans. J. Life Sci. 2010;2:87–92. [Google Scholar]
- Hauner H, Bechthold A, Boeing H, Brönstrup A, Buyken A, Leschik-Bonnet E, Linseisen J, Schulze M, Strohm D, Wolfram G. Evidence-based guideline of the German Nutrition Society: carbohydrate intake and prevention of nutrition-related diseases. Ann. Nutr. Metab. 2012;60:1–58. doi: 10.1159/000335326. [DOI] [PubMed] [Google Scholar]
- Heimbach JT, Sebestyen G, Semjen G, Kennepohl E. Safety studies regarding a standardized extract of fermented wheat germ. Int. J. Toxicol. 2007;26:253–259. doi: 10.1080/10915810701369709. [DOI] [PubMed] [Google Scholar]
- Hidvegi M, Raso E, Tomoskozi-Farkas R, Paku S, Lapis K, Szende B. Effect of Avemar and Avemar + vitamin C on tumor growth and metastasis in experimental animals. Anticancer Res. 1998;18:2353–2358. [PubMed] [Google Scholar]
- Higgins HJ, Green S. Cochrane handbook for systematic reviews of interventions version 5.1. 0 [updated March 2011]. The Cochrane Collaboration (2011)
- Higgins J, Altman D, Gøtzsche P, Jüni P, Moher D, Oxman A, Savovic J, Schulz K, Weeks L, Sterne J. Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. Brit. Med. J. 343: d5928 (2011) [DOI] [PMC free article] [PubMed]
- Iyer A, Brown L. Fermented wheat germ extract (Avemar) in the treatment of cardiac remodeling and metabolic symptoms in rats. Evid-Based Compl. Alt. 2011 (2011) [DOI] [PMC free article] [PubMed]
- Jeong H-Y, Choi Y-S, Lee J-K, Lee B-J, Kim W-K, Kang H. Anti-inflammatory activity of citric acid-treated wheat germ extract in lipopolysaccharide-stimulated macrophages. Nutrients. 2017;9:730. doi: 10.3390/nu9070730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junejo SA, Geng H, Li S, Kaka AK, Rashid A, Zhou Y. Superfine wheat bran improves the hyperglycemic and hyperlipidemic properties in a high-fat rat model. Food Sci. Biotechnol. 2019;29:559–567. doi: 10.1007/s10068-019-00684-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil F, Khalifa F, Barakat H, Hessin M. Ameliorative effect of wheat germ and/or grape seed oils on hematological, kidney functions and lipid profiles in rats co-administreted chlorpyrifos. Vet. Med. J. Giz. 2010;58:365–380. [Google Scholar]
- Khedr NF. Fish oil and wheat germ oil supplementation modulates brain injury in streptozotocin-induced diabetic rats. J. Diabetes. 2017;9:1012–1022. doi: 10.1111/1753-0407.12518. [DOI] [PubMed] [Google Scholar]
- Kobyliak N, Abenavoli L, Falalyeyeva T, Mykhalchyshyn G, Boccuto L, Kononenko L, Kyriienko D, Komisarenko I, Dynnyk O. Beneficial effects of probiotic combination with omega-3 fatty acids in NAFLD: a randomized clinical study. Minerva Med. 2018;109:418–428. doi: 10.23736/S0026-4806.18.05845-7. [DOI] [PubMed] [Google Scholar]
- Koh EM, Lee EK, Song J, Kim SJ, Song CH, Seo Y, Chae CH, Jung KJ. Anticancer activity and mechanism of action of fermented wheat germ extract against ovarian cancer. J. Food Biochem. 2018;42:e12688. [Google Scholar]
- Lin Y, Rudrum M, van der Wielen RP, Trautwein EA, McNeill G, Sierksma A, Meijer GW. Wheat germ policosanol failed to lower plasma cholesterol in subjects with normal to mildly elevated cholesterol concentrations. Metabolism. 2004;53:1309–1314. doi: 10.1016/j.metabol.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Lupton JR, Robinson MC, Morin JL. Cholesterol-lowering effect of barley bran flour and oil. J. Am. Diet. Assoc. 1994;94:65–70. doi: 10.1016/0002-8223(94)92044-3. [DOI] [PubMed] [Google Scholar]
- Meriles SP, Steffolani ME, León AE, Penci MC, Ribotta PD. Physico-chemical characterization of protein fraction from stabilized wheat germ. Food Sci. Biotechnol. 2019;28:1327–1335. doi: 10.1007/s10068-019-00594-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Methley AM, Campbell S, Chew-Graham C, McNally R, Cheraghi-Sohi S. PICO, PICOS and SPIDER: a comparison study of specificity and sensitivity in three search tools for qualitative systematic reviews. BMC Health Serv. Res. 2014;14:579. doi: 10.1186/s12913-014-0579-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michas G, Micha R, Zampelas A. Dietary fats and cardiovascular disease: putting together the pieces of a complicated puzzle. Atherosclerosis. 2014;234:320–328. doi: 10.1016/j.atherosclerosis.2014.03.013. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann. Intern. Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- Moreira-Rosário A, Pinheiro H, Calhau C, Azevedo LF. Can wheat germ have a beneficial effect on human health? A study protocol for a randomised crossover controlled trial to evaluate its health effects. BMJ Open. 2016;6:e013098. doi: 10.1136/bmjopen-2016-013098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira-Rosário A, Pinheiro H, Marques C, Teixeira JA, Calhau C, Azevedo LF. Does intake of bread supplemented with wheat germ have a preventive role on cardiovascular disease risk markers in healthy volunteers? A randomised, controlled, crossover trial. BMJ Open. 2019;9:e023662. doi: 10.1136/bmjopen-2018-023662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’neill S, O’driscoll L. Metabolic syndrome: a closer look at the growing epidemic and its associated pathologies. Obes. Rev. 2015;16:1–12. doi: 10.1111/obr.12229. [DOI] [PubMed] [Google Scholar]
- Ojo B, Simenson AJ, O’Hara C, Wu L, Gou X, Peterson SK, Lin D, Smith BJ, Lucas EA. Wheat germ supplementation alleviates insulin resistance and cardiac mitochondrial dysfunction in an animal model of diet-induced obesity. Brit. J. Nutr. 2017;118:241–249. doi: 10.1017/S0007114517002082. [DOI] [PubMed] [Google Scholar]
- Ostlund RE, Jr, Racette SB, Stenson WF. Inhibition of cholesterol absorption by phytosterol-replete wheat germ compared with phytosterol-depleted wheat germ. Am. J. Clin. Nutr. 2003;77:1385–1389. doi: 10.1093/ajcn/77.6.1385. [DOI] [PubMed] [Google Scholar]
- Park E, Kim HO, Kim G-N, Song J-H. Anti-oxidant and anti-adipogenic effects of ethanol extracts from wheat germ and wheat germ fermented with Aspergillus oryzae. Prev. Nutr. Food Sci. 2015;20:29. doi: 10.3746/pnf.2015.20.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezq AA, Mahmoud MY. Preventive effect of wheat germ on hypercholesteremic and atherosclerosis in rats fed cholesterol-containing diet. Pak. J. Nutr. 2011;10:424–432. [Google Scholar]
- Rodionova N, Isaev V, Vishnyakov A, Popov E, Safonova N, Srorublyovtsev S. Investigation of the effect of oil and flour from wheat germ meal on lipid metabolism of students and teachers of the university. Vopr. Pitan. 2016;85:57–63. [PubMed] [Google Scholar]
- Slavin J. Whole grains and human health. Nutr. Res. Rev. 2004;17:99–110. doi: 10.1079/NRR200374. [DOI] [PubMed] [Google Scholar]
- Soares R, Costa C. Oxidative stress, inflammation and angiogenesis in the metabolic syndrome. Netherlands: Springer; 2009. pp. 33–63. [Google Scholar]
- Srikanthan K, Feyh A, Visweshwar H, Shapiro JI, Sodhi K. Systematic review of metabolic syndrome biomarkers: a panel for early detection, management, and risk stratification in the West Virginian population. Int. J. Med. Sci. 2016;13:25. doi: 10.7150/ijms.13800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripkovic L, Muirhead NC, Hart KH, Frost GS, Lodge JK. The effects of a diet rich in inulin or wheat fibre on markers of cardiovascular disease in overweight male subjects. J. Hum. Nutr. Diet. 2015;28:476–485. doi: 10.1111/jhn.12251. [DOI] [PubMed] [Google Scholar]
- Vaher M, Matso K, Levandi T, Helmja K, Kaljurand M. Phenolic compounds and the antioxidant activity of the bran, flour and whole grain of different wheat varieties. Procedia Chem. 2010;2:76–82. [Google Scholar]
- Vella MN, Stratton LM, Sheeshka J, Duncan AM. Functional food awareness and perceptions in relation to information sources in older adults. Nutr. J. 2014;13:44. doi: 10.1186/1475-2891-13-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willett W, Manson J, Liu S. Glycemic index, glycemic load, and risk of type 2 diabetes. Am. J. Clin. Nutr. 2002;76:274S–280S. doi: 10.1093/ajcn/76/1.274S. [DOI] [PubMed] [Google Scholar]
- Zakaria H, Mostafa TM, El-Azab GA, El Wahab AMA, Elshahawy H, Sayed-Ahmed NA. The impact of fish oil and wheat germ oil combination on mineral-bone and inflammatory markers in maintenance hemodialysis patients: a randomized, double-blind, placebo-controlled clinical trial. Int. Urol. Nephrol. 2017;49:1851–1858. doi: 10.1007/s11255-017-1643-6. [DOI] [PubMed] [Google Scholar]
- Zhu K-X, Lian C-X, Guo X-N, Peng W, Zhou H-M. Antioxidant activities and total phenolic contents of various extracts from defatted wheat germ. Food Chem. 2011;126:1122–1126. [Google Scholar]
- Zong G, Gao A, Hu FB, Sun Q. Whole grain intake and mortality from all causes, cardiovascular disease, and cancer: a meta-analysis of prospective cohort studies. Circulation. 2016;133:2370–2380. doi: 10.1161/CIRCULATIONAHA.115.021101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Computer program
- Review Manager (RevMAn)[Computer program]. Version 5.3. 2014. Collaboration C. (2016)