Abstract
Background
Pediatric Blood and Marrow Transplant (PBMT) patients experience significant symptom distress. Mobile health (mHealth) technologies can be leveraged to improve understanding of the patient’s symptom experience by providing continuous, real-time, in-situ, patient-generated symptom data. This rich data stream can subsequently enhance symptom management strategies. However, limited research has been conducted in this area.
Objectives
This pilot study seeks to explore 1) the feasibility of integrating mHealth technologies to monitor symptom data for PBMT patients, and 2) to evaluate the study design, measures, and procedures.
Methods
An exploratory longitudinal design was employed to assess the feasibility of monitoring 10 PBMT patients’ symptoms using data from two mHealth technologies: 1) a smart phone mobile health application (app) to collect symptom data; and 2) a wearable tracking device (Apple watch) to collect physiologic data. Feasibility was measured as usability and acceptability. Monthly patient interviews and an end-of-study feasibility survey were employed and analyzed to further understand reasons for sustained interest in and attrition from the study.
Results
Overall usability of the wearable was 51% and app was 56%. Children reported devices were easy to use and acceptable. The study demonstrated acceptability with an enrollment rate of 83%, attrition rate of 30%, with 70% of the children remaining in the study for at least 40 days.
Discussion
This pilot study is among the first to explore the feasibility of using mobile technologies to longitudinally obtain patient-generated symptom data to enhance understanding of the PBMT symptom experience. In addition, it will improve our understanding of how these data present, interact, and cluster together throughout the post-transplant period.
Keywords: Pediatric blood and marrow transplant, mobile health, symptom science
Over 1,500 children undergo a pediatric blood and marrow transplant (PBMT) each year in the United States (D’Souza & Fretham, 2017). PBMT is an intense treatment and children undergoing the procedure experience significant symptom distress (Johnston et al., 2018). Symptom distress is characterized as “the degree of discomfort from specific symptom(s) as reported by the patient” (McCorkle & Young, 1978). The symptom distress PBMT patients experience is different from other chronic illnesses as it has multiple contributing factors and a lengthy trajectory. The underlying primary disease and its treatment, which often is relapsed cancer or a progressively debilitating disease, create bothersome symptoms (pain, fatigue, anxiety, depression) that exist before hospitalization for transplant (Anderson et al., 2007; Johnston et al., 2018). In addition, the aggressive pre-transplant conditioning treatment, typically a myeloablative regimen (high dose chemotherapy and total body irradiation), contributes to symptom distress (Rodgers, Krance, Street, & Hockenberry, 2013). These treatments have significant associated symptoms including risk for pain, fatigue, potential organ toxicity, infection, and bleeding (Parsons, Tighiouart, & Terrin, 2013; Rimkus, 2009). Finally, factors related to the transplant procedure contribute to symptom distress. During this phase, children are severely neutropenic, anemic, and thrombocytopenic putting them at risk for infection, debilitating fatigue, persistent pain, bleeding, and enteritis with nausea and vomiting (Rimkus, 2009; Vasquenza et al., 2015). Children typically experience multiple symptoms that persist for a prolonged period and report the distressing symptoms as the worst part of their illness and treatment (Rodgers, Krance, Street, & Hockenberry, 2014).
Accurate symptom assessment is crucial for optimal symptom management (Baggott et al., 2012; Hochstenbach, Zwakhalen, Courtens, van Kleef, & de Witte, 2016), however, it can be challenging for clinicians to obtain accurate symptom data during the post-transplant phase (Snaman et al., 2018; Vasquenza et al., 2015). Acquiring meaningful symptom data is complicated for many reasons, including the child’s developmental level and ability to articulate the symptom experience, and children are frequently too ill to accurately communicate their symptoms (Irwin et al., 2012). Moreover, research suggests parent caregiver and clinician reports (proxy reports), which are commonly used, frequently under-report both the prevalence and severity of children’s symptoms in acute diseases (Irwin et al., 2012; Pinheiro et al., 2018). This presents a significant threat to symptom management for this at-risk population.
Advances in mobile health (mHealth) technologies are reshaping chronic disease management (Kaplan et al., 2017). These technologies are popular, accessible, and can unobtrusively collect, monitor, and transmit near real-time patient-generated health data (Munos et al., 2016; Wesley & Fizur, 2015). Wearable devices such as the Apple Watch™ and Fitbit™, can passively collect real-time physiologic data (heart rate, (HR), step count) (Heintzman, 2015), while smart phone mobile applications (apps) can record and track real-time patient self-reported outcomes (Leahy, Feudtner, & Basch, 2018).
The literature shows using mHealth technology can improve disease self-management for people with asthma (Hui et al., 2017) and diabetes (Greenwood, Gee, Fatkin, & Peeples, 2017; Wu et al., 2017). In light of these findings, mHealth may offer new avenues to enhance symptom monitoring and management by capturing symptom data in novel ways. Dense streams of real-time patient-generated health data from these devices can illuminate symptom characteristics that may lead to improved understanding of symptom dynamics and subsequently support effective symptom management strategies (Heintzman, 2015; Jain, Powers, Hawkins, & Brownstein, 2015).
mHealth technology offers a possible solution to symptom management challenges for children undergoing a PMBT, however, research in this area is limited. While a few studies report feasibility results with mHealth symptom tools in pediatric cancer patients (Baggott et al., 2012; Macpherson et al., 2014), only one study by Rodgers, et al., (2013) explored the feasibility of using an app for eating-related issues in PBMT outpatients (Rodgers et al., 2013). Thus, our team designed a study using two mHealth devices, a wearable tracker and a smart phone app, to collect longitudinal symptom data (pre-transplant through the post-transplant period). We conducted a pilot study: 1) to examine the feasibility of using mHealth technologies to monitor, record, and transmit symptom data in children undergoing a PBMT patients 7–18 years of age; and 2) to identify and address design or procedural issues before conducting a larger study.
Theoretical Framework
The Theory of Unpleasant symptoms (TUS) guided our exploration and evaluation of the symptom experience and will guide future intervention development (Lenz, Pugh, Milligan, Gift, & Suppe, 1997). Mobile technologies offer a unique opportunity to collect real-time symptom data (timing, intensity, distress, and duration) for this exploration and evaluation.
Methods
This study used a single-site longitudinal exploratory design.
Participants and Setting
The study protocol was approved by the medical center Institutional Review Board. We recruited a convenience sample of ten children, 10–17 years of age at a major medical center in the southeastern United States. We chose a sample size of 10, which is supported by human factors testing that determined a minimum of five users are sufficient to assess usability benefits (Nielsen & Landauer, 1993). Our sample size was consistent with other studies testing the feasibility of mobile devices for adolescents with chronic disease (Baggott et al., 2012; Cafazzo, Casselman, Hamming, Katzman, & Palmert, 2012). Although children and adolescents differ developmentally, we chose to combine the age ranges for this pilot study due to the sample size and our feasibility objective. Eligible participants were children, aged 7–18 undergoing their first PBMT and able to read English. All children were enrolled prior to transplant and could remain in the study up to 120 days, which could include inpatient and outpatient days. A PBMT clinical team member initially approached the child and parent to elicit interest in the study. If interested, a study nurse obtained parental consent and child assent, enrolled the child in the study, and set up and explained the study devices.
Study Measures
Children were loaned two study devices; 1) a wearable tracker, initially the Microsoft Band II™, later changed to the series 1 Apple Watch™, and 2) an iPhone 6™ downloaded with an app, the Technology Recordings to better Understand PBMT (TRU-PBMT). This app was designed as a pediatric-friendly tool to collect symptom and health data (Vaughn, Jonassaint, Summers-Goeckerman, Shaw, & Shah, 2018). The app has a “symptom tracker” page where children can record symptoms, the intensity (using a visual analogue scale), the time they occur, and any interventions. The app also has a “Health” page where children can record daily care goals such as mouth care and bathing as well as a “Food Diary” page to track food intake and the Bristol stool chart to track stools. Children were asked to record in the app daily.
Feasibility data was collected using; 1) a wearable tracker to obtain daily physiologic data (HR, step count, sleep hours) as indicators of symptom distress; 2) a smart phone app to collect daily self-reported symptom data; 3) monthly structured interviews with children focused on their experiences and satisfaction with technology; and 4) a 23-question survey developed by the study team designed to evaluate feasibility. The survey was given at study completion and contained items addressing perceived technical capability, ease of use, satisfaction, and acceptability. Survey scores for technical capability ranged from 0 (problems using device) to 4 (no problems using device); ease of use ranged from 0 (very hard) to 4 (very easy); and satisfaction/acceptability ranged from 0 (very dissatisfied) to 4 (very satisfied).
Data Collection
At study enrollment, patient demographic and clinical characteristics were extracted from the electronic health record. Feasibility was assessed as mobile device acceptability and usability, and by an end-of-study feasibility survey.
Acceptability
Acceptability was measured by enrollment and attrition rates. Interview data was used to ascertain elements that led to sustained participation in or attrition from the study.
Usability
Wearable and app usability was measured as the proportion of missing device data and percentage of device use. Interview data regarding device use was also used to assess usability.
Data Analysis
SAS 9.4 (Cary, NC) statistical software was used for quantitative data analysis. The heat Map visualization was generated in RStudio (Version 3.5.0 – © 2009–2018). Descriptive statistics were used to summarize children’s characteristics, calculate the proportion of missing data for each device, calculate the percentage of device use throughout the study, and obtain enrollment and attrition rates. We also plotted the proportion of missing data with standard error across different time points to explore trends. All interview data was recorded in real time using written notes, then transcribed. We analyzed the data by organizing it according to each interview question and developing common themes within each question category.
Results
Children (N=10) were between 10–17 years of age (Mean = 14.4 years) and 60% female (Table 1).
Table 1.
Patient Demographics
| Participant | Gender | Race | Age | Days in Study |
|---|---|---|---|---|
| 1 | F | Black | 15 | 116 |
| 2 | F | White | 10 | 2 |
| 3 | F | Black | 12 | 92 |
| 4 | F | Black | 15 | 118 |
| 5 | F | Hispanic | 17 | 88 |
| 6 | M | White | 14 | 10 |
| 7 | M | Black | 15 | 40 |
| 8 | M | White | 17 | 77 |
| 9 | M | White | 17 | 2 |
| 10 | F | Black | 12 | 76 |
Acceptability
Of 12 children and their parents approached, 10 enrolled in the study (enrollment rate 83%). Seven (70%) remained in the study for at least 40 days. Two children withdrew from the study within 48 hours and a third withdrew on day 10 (attrition rate 30%). One child withdrew on day 40, the other six stayed in the study from 76–118 days. Acceptability was also evaluated using the structured interview data with 6 children who remained in the study for more than 40 days. All 6 who were interviewed responded (n = 6) that they were satisfied using the mobile devices.
Usability
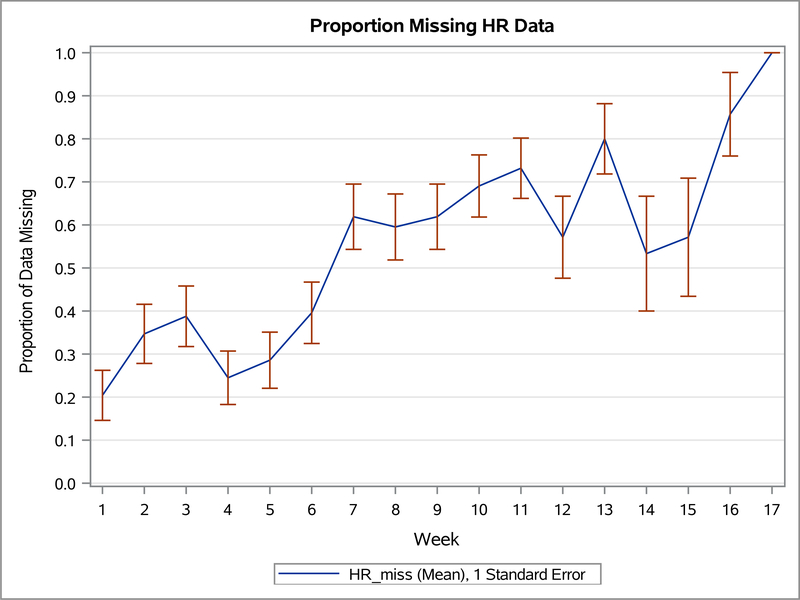
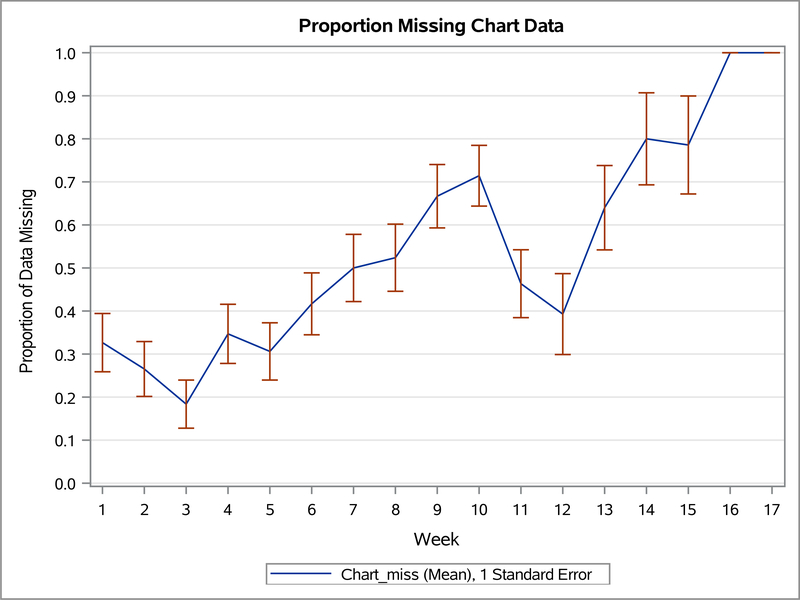
We evaluated usability for the seven children who used the devices (n=7). Usability was measured using wearable device and app usage and missing data from the wearable device and app. Figure 1 illustrates group mean empirical summary plots for proportion of weekly missing heart rate (wearable) and missing chart (app) data throughout the study weeks. The proportion of missing data increased overall for both devices during the study.
Figure 1.
Empirical Summary Plots
Figure 1.1. The empirical summary plot illustrates the trend of children’s weekly proportion of missing heart rate (wearable) data.
Figure 1.2. The empirical summary plot illustrates the trend of children’s weekly proportion of missing chart (app) data.
Table 2 shows the number of days each child (n=7) used the devices during the study and group mean of use for each device. Wearable usage was defined as using the wearable for more than 3 hours per day. Recording in the app usage was defined as charting in the app at least once per day. On average, children wore the wearable device 51% of their total days in the study and recorded data in the app an average of 56% of their days in the study.
Table 2.
Number of days children (n=7) wore or used mHealth tools while in study
| Pt 1 | Pt 3 | Pt 4 | Pt 5 | Pt 7 | Pt 8 | Pt 10 | Group Mean | |
|---|---|---|---|---|---|---|---|---|
| Days wearable was worn/Days in study | 70/116 | 69/92 | 40/118 | 42/88 | 21/40 | 46/77 | 19/76 | 51% |
| Days recorded in app/Days in study | 77/116 | 17/92 | 41/118 | 79/88 | 31/40 | 25/77 | 54/76 | 56% |
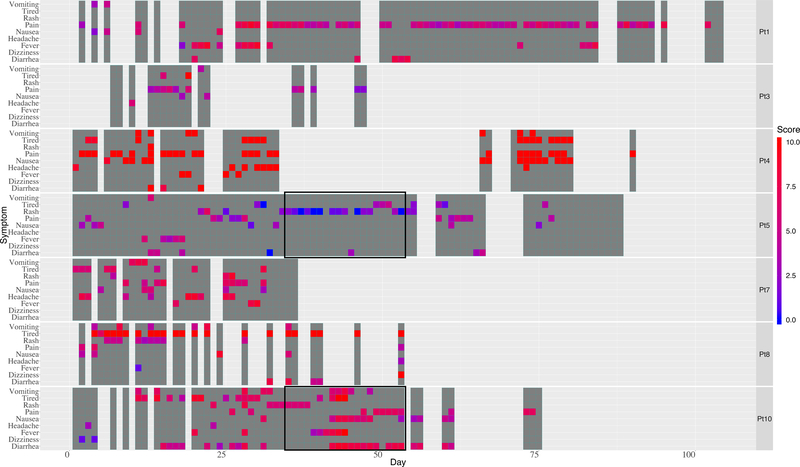
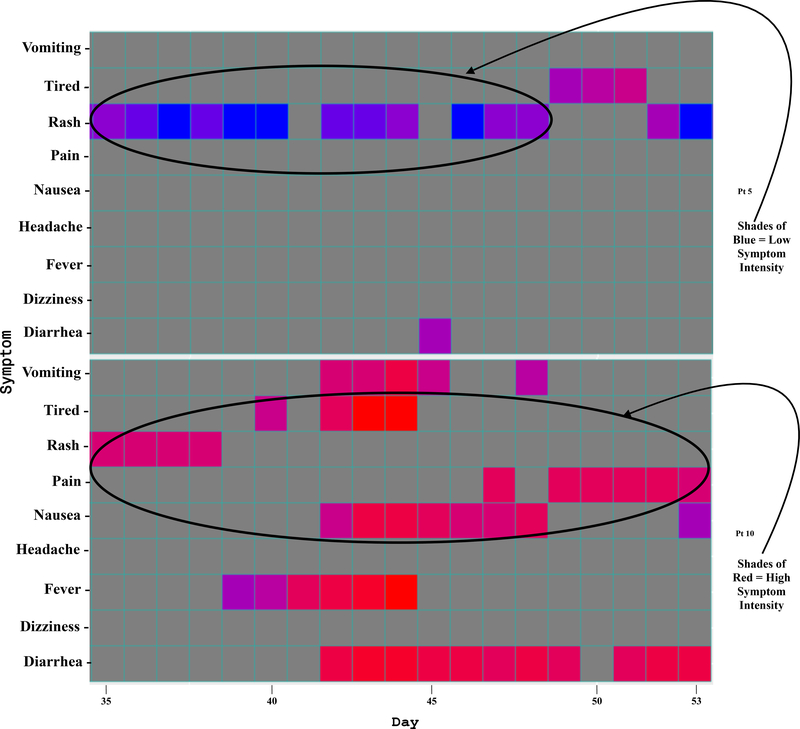
Another indicator of app usability is illustrated in the heat map (Figure 2). The dark gray shaded regions represent each day a child recorded in the app. The heat map also depicts symptom data recorded by the children (each child is listed on the right). It is a 2-dimensional chronological visualization of symptom occurrences and intensity. The x-axis shows the study day and the y-axis shows symptoms. The colors represent symptom intensity on a scale of 0–10 ranging from blue (low intensity) to red (high intensity).
Figure 2.
Heat Map Visualization
Figure 2.1. The heat map allows visualization of complex longitudinal data. The seven patients are listed at the right of each row. Dark gray represents a day the child engaged with the app. Symptom occurrence and intensity are depicted by bright colors ranging from blue (low intensity) to red (high intensity) correlating to a numeric scale from 0–10.
Figure 2.2. A zoom view of two segments of the heat map depicting symptom intensities with color.
Interview data
Children (n = 6) reported missing data from the wearable was due to forgetfulness (forgetting to wear and/or charge the device), feeling too ill to wear the device, or rashes or pain that prevented wear. For the app, children (n = 5) reported missing data was due to forgetfulness, pain, and fatigue. Children (n = 6) reported that overall the devices were easy to use and they encountered no technical difficulties. The three children who left the study within 10 days reported that it was too difficult to keep up with the device maintenance.
Feasibility survey results are summarized in Table 3. Scores ranged from 0–4. Children rated technical capability in terms of problems using the devices; scores ranged from 0 (problems using the device) to 4 (no problems using the device) with an average score of 3.18. They also rated ease of use in terms of 0 (very hard) to 4 (very easy) with an average score of 3.76. Children rated satisfaction/acceptability with scores between 0 (very dissatisfied) to 4 (very satisfied) with an average score of 2.52.
Table 3.
Feasibility Survey Average Scores
| Feasibility Survey Sections | Average Score |
|---|---|
| Technical Capability | 3.18 |
| Ease of Use | 3.76 |
| Satisfaction/Acceptability | 2.52 |
Design and Procedural issues
One design issue with the wearable tracker was identified by the first two children enrolled, who complained about the stiffness of the Microsoft Band II. In response we changed to the Apple Watch, which addressed the comfort issue, but did not collect sleep data. We added a sleep app to obtain this data. In addition, the Apple Watch had a shorter battery life (18–20 hours) than the Microsoft Band II (48 hours) and children reported difficulty in keeping up with charging the wearable. Of the four children who continued in the study as outpatients, three reported this as the main challenge of remaining in the study. Two children withdrew from the study once outpatient; one found device management too burdensome and the second lost the wearable. The third child did not wear the device due to a persistent rash and the fourth wore the device inconsistently until the 120-day timeline was reached.
Discussion
The use of mobile technology to collect continuous real-time patient data presents a unique opportunity to examine symptoms in novel ways. These real-time patient-generated health data may play an important role in understanding children’s symptom experiences and add to the body of symptom science knowledge. This study is the first to our knowledge to examine the feasibility of integrating two mHealth technologies to collect longitudinal PBMT patient symptom data.
Children, with the consent of their parents, were eager to enroll in the study and 70% stayed in the study for 40 days or more, indicating high levels of acceptability. Their rate of daily wearable use during the study averaged 51%, which we found encouraging when compared to other studies. Recent data from a study using a wearable tracker with children with juvenile idiopathic arthritis averaged 72% for logged activity over a 28-day period (Heale et al., 2018). Our findings were slightly lower, likely due to the children in our study being acutely ill, hospitalized for most of the study, and using the device over a longer period of time.
The heat map demonstrates app usability, and importantly, illustrates symptom intensities, patterns, and changes over time. Presenting data in this manner is a valuable approach that gives clinicians and researchers the opportunity to assess patient-reported symptoms each day and facilitate improved understanding of symptoms. The overall percentage of days children (n=7) used the app over the course of the study was 56%. This level of reporting is encouraging and suggests that mobile technology can be a useful method to collect patient-reported symptom data for children. App usage showed a decreasing trend over time and our app usage rates are similar to other studies. One study found a 27% app reporting rate for adult medication adherence over an 84 day time period (Becker et al., 2013) and a second study found a 45% app reporting rate for women reporting sleep disturbances over a 90 day time-period (Min et al., 2014).
The three children who withdrew within 10 days found the study demands too rigorous for how sick they were feeling. Two had already completed their pre-transplant conditioning and were experiencing significant symptom distress upon study enrollment. The other seven children began pre-transplant conditioning at the time of study enrollment, which may have provided an acclimation period to the technologies prior to the onset of intense symptoms thus improving device engagement. Six of these children reported a high level of satisfaction with the study and the devices, and reported that the devices were easy to use.
To ease participant burden, a study nurse assisted children with device maintenance (charging and software updates), suggested “scheduled” times to charge the wearable (i.e., during daily shower time and meal times), and encouraged parent caregivers to remind the children to use the devices. This level of device support, particularly the parent caregiver engagement, will be necessary for a larger study to optimize data collection and minimize participant burden. Other studies have had success with children with chronic diseases managing mobile devices more independently (Fortier, Chung, Martinez, Gago-Masague, & Sender, 2016), however, children in our study sample were acutely ill. We saw study engagement decrease once children were discharged to outpatient management. As outpatients, they came to the clinic each day for treatment, but had difficulty remembering to use the wearable and chart in the app. When asked, children reported the increased self-management responsibilities (frequent medications, central line care) superseded study involvement.
Future development will include a re-designed app that uses gamification and rewards to increase satisfaction and adherence to daily recording. The literature shows these features increase engagement and compliance with devices (Miller, Cafazzo, & Seto, 2016; Taylor, Ferguson, Peng, Schoeneich, & Picard, 2019). We may also incorporate a response button in the symptom tracking list for children to document “don’t feel well enough to chart”. This feature may improve understanding of missing data by identifying times when symptom distress prevents children from charting symptoms. We will also investigate a waterproof wearable that would decrease the number of times it is removed, thus decreasing the likelihood of children forgetting to put it back on after their daily shower.
Our approach had several strengths. This study demonstrated that it is feasible to collect patient-generated real-time longitudinal symptom data in acutely ill children using two mobile devices. Combining physiologic data from the wearable with child-reported symptom data from the app will enhance understanding of their symptom experience as it changes over time and contribute to the growing body of pediatric symptom science research.
Limitations of the study include that study engagement may have been influenced by the novelty of the mobile devices we provided, particularly if children did not already own these devices. We also measured feasibility based on device missing data, however, the limited battery life of the wearable plus the child’s level of health may underrepresent the child’s engagement. Final limitations were the limited sample size and use of a single institution for the study both of which may affect the generalizability of our findings.
Conclusion
Despite the proliferation of mHealth technologies, few studies have evaluated their role in symptom monitoring in children undergoing PBMT. Conducting a feasibility study with acutely ill children at the start of their hospitalization using mobile devices to obtain symptom data was an important first step in designing a larger study. Our ability to collect time-dense longitudinal symptom data from children and demonstrate acceptability of using mobile technology indicates that it is feasible. In addition, our findings uniquely highlight how mobile technologies can be used to obtain symptom data to enhance understanding and lead to symptom management strategies.
Acknowledgement
The Research reported in this publication is supported in part by the National Institute of Nursing Research of the National Institutes of Health under Award Number F31NR018100. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Additional funding is supported by a Duke Pediatric Chairs Award 2016. The authors would like to acknowledge Dr. Consuelo Arellano PhD, Research Associate Professor, for her mentorship in the statistical analysis.
Ethical Conduct of Research: The medical center Institutional Review Board approved the study protocol (Pro00068979). All authors complied with our institution’s ethical standards.
Footnotes
The authors have no conflicts of interest to report.
Clinical Trial Registration: Trial Number: NCT02895841 the trial was registered September 12, 2016, the first patient enrolled for this sub-study was October 25, 2017. Link: https://clinicaltrials.gov/ct2/show/NCT02895841
Contributor Information
Jacqueline Vaughn, Duke University School of Nursing, Durham, NC.
Siddharth Gollarahalli, Duke Health, Durham NC.
Ryan J. Shaw, Duke University School of Nursing, Durham, NC.
Sharron Docherty, Duke University School of Nursing, Durham, NC.
Qing Yang, Duke University School of Nursing, Durham, NC.
Chandni Malhotra, North Carolina State University, Raleigh, NC.
Erika Summers-Goeckerman, Duke Children’s Hospital, Durham, NC.
Nirmish Shah, Duke University Medical Center, Durham, NC.
References
- Anderson KO, Giralt SA, Mendoza TR, Brown JO, Neumann JL, Mobley GM, … Cleeland CS (2007). Symptom burden in patients undergoing autologous stem-cell transplantation. Bone Marrow Transplantation, 39(12), 759–766. doi: 10.1038/sj.bmt.1705664 [DOI] [PubMed] [Google Scholar]
- Baggott C, Gibson F, Coll B, Kletter R, Zeltzer P, & Miaskowski C (2012). Initial evaluation of an electronic symptom diary for adolescents with cancer. JMIR Res Protoc, 1(2), e23. doi: 10.2196/resprot.2175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker S, Kribben A, Meister S, Diamantidis CJ, Unger N, & Mitchell A (2013). User profiles of a smartphone application to support drug adherence--experiences from the iNephro project. PloS One, 8(10), e78547. doi: 10.1371/journal.pone.0078547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cafazzo J, Casselman M, Hamming N, Katzman D, & Palmert M (2012). Design of an mHealth app for the self-management of adolescent type 1 diabetes: a pilot study. Journal of Medical Internet Research, 14(3), e70. doi: 10.2196/jmir.2058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Souza A, & Fretham C (2017). Current Uses and Outcomes of Hematopoietic Cell Transplantation (HCT): CIBMTR Summary Slides. Retrieved from http://www.cibmtr.org
- Fortier MA, Chung WW, Martinez A, Gago-Masague S, & Sender L (2016). Pain buddy: A novel use of m-health in the management of children’s cancer pain. Computers in Biology and Medicine, 76, 202–214. doi: 10.1016/j.compbiomed.2016.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood DA, Gee PM, Fatkin KJ, & Peeples M (2017). A Systematic Review of Reviews Evaluating Technology-Enabled Diabetes Self-Management Education and Support. Journal of Diabetes Science and Technology, 11(5), 1015–1027. doi: 10.1177/1932296817713506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heale LD, Dover S, Goh YI, Maksymiuk VA, Wells GD, & Feldman BM (2018). A wearable activity tracker intervention for promoting physical activity in adolescents with juvenile idiopathic arthritis: a pilot study. Pediatric Rheumatology Online Journal, 16(1), 66. doi: 10.1186/s12969-018-0282-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heintzman ND (2015). A Digital Ecosystem of Diabetes Data and Technology: Services, Systems, and Tools Enabled by Wearables, Sensors, and Apps. Journal of Diabetes Science and Technology, 10(1), 35–41. doi: 10.1177/1932296815622453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochstenbach L, Zwakhalen S, Courtens A, van Kleef M, & de Witte L (2016). Feasibility of a mobile and web-based intervention to support self-management in outpatients with cancer pain. European Journal of Oncology Nursing, 23, 97–105. doi: 10.1016/j.ejon.2016.03.009 [DOI] [PubMed] [Google Scholar]
- Hui CY, Walton R, McKinstry B, Jackson T, Parker R, & Pinnock H (2017). The use of mobile applications to support self-management for people with asthma: a systematic review of controlled studies to identify features associated with clinical effectiveness and adherence. Journal of the American Medical Informatics Association, 24(3), 619–632. doi: 10.1093/jamia/ocw143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin DE, Gross HE, Stucky BD, Thissen D, DeWitt EM, Lai JS, … DeWalt DA(2012). Development of six PROMIS pediatrics proxy-report item banks. Health Qual Life Outcomes, 10, 22. doi: 10.1186/1477-7525-10-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain SH, Powers BW, Hawkins JB, & Brownstein JS (2015). The digital phenotype. Nature Biotechnology, 33(5), 462–463. doi: 10.1038/nbt.3223 [DOI] [PubMed] [Google Scholar]
- Johnston DL, Hyslop S, Tomlinson D, Baggott C, Gibson P, Orsey A, … Sung L (2018). Describing symptoms using the Symptom Screening in Pediatrics Tool in hospitalized children with cancer and hematopoietic stem cell transplant recipients. Cancer Med, 7(5), 1750–1755. doi: 10.1002/cam4.1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan HC, Thakkar SN, Burns L, Chini B, Dykes DM, McPhail GL, … Opipari-Arrigan L (2017). Protocol of a Pilot Study of Technology-Enabled Coproduction in Pediatric Chronic Illness Care. JMIR Res Protoc, 6(4), e71. doi: 10.2196/resprot.7074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leahy AB, Feudtner C, & Basch E (2018). Symptom Monitoring in Pediatric Oncology Using Patient-Reported Outcomes: Why, How, and Where Next. Patient, 11(2), 147–153. doi: 10.1007/s40271-017-0279-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz ER, Pugh LC, Milligan RA, Gift A, & Suppe F (1997). The middle-range theory of unpleasant symptoms: an update. ANS: Advances in Nursing Science, 19(3), 14–27. [DOI] [PubMed] [Google Scholar]
- Macpherson CF, Linder LA, Ameringer S, Erickson J, Stegenga K, & Woods NF (2014). Feasibility and acceptability of an iPad application to explore symptom clusters in adolescents and young adults with cancer. Pediatric Blood & Cancer, 61(11), 1996–2003. doi: 10.1002/pbc.25152 [DOI] [PubMed] [Google Scholar]
- McCorkle R, & Young K (1978). Development of a symptom distress scale. Cancer Nursing, 1(5), 373–378. [PubMed] [Google Scholar]
- Miller AS, Cafazzo JA, & Seto E (2016). A game plan: Gamification design principles in mHealth applications for chronic disease management. Health Informatics J, 22(2), 184–193. doi: 10.1177/1460458214537511 [DOI] [PubMed] [Google Scholar]
- Min YH, Lee JW, Shin YW, Jo MW, Sohn G, Lee JH, … Ahn SH (2014). Daily collection of self-reporting sleep disturbance data via a smartphone app in breast cancer patients receiving chemotherapy: a feasibility study. Journal of Medical Internet Research, 16(5), e135. doi: 10.2196/jmir.3421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munos B, Baker P, Bot B, Crouthamel M, de Vries G, Ferguson I, … Wang P (2016). Mobile health: the power of wearables, sensors, and apps to transform clinical trials. Annals of the New York Academy of Sciences, 1375(1), 3–18. doi: 10.1111/nyas.13117 [DOI] [PubMed] [Google Scholar]
- Nielsen J, & Landauer T (1993, April 24–29 1993). “A mathematical model of the finding of usability problems,” Paper presented at the Proceedings of ACM INTERCHI’93 Conference, Amsterdam, The Netherlands [Google Scholar]
- Parsons SK, Tighiouart H, & Terrin N (2013). Assessment of health-related quality of life in pediatric hematopoietic stem cell transplant recipients: progress, challenges and future directions. Expert Review of Pharmacoeconomics & Outcomes Research, 13(2), 217–225. doi: 10.1586/erp.13.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro LC, McFatrich M, Lucas N, Walker JS, Withycombe JS, Hinds PS, … Reeve BB (2018). Child and adolescent self-report symptom measurement in pediatric oncology research: a systematic literature review. Quality of life research : an international journal of quality of life aspects of treatment, care and rehabilitation, 27(2), 291–319. doi: 10.1007/s11136-017-1692-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimkus C (2009). Acute complications of stem cell transplant. Seminars in Oncology Nursing, 25(2), 129–138. doi: 10.1016/j.soncn.2009.03.007 [DOI] [PubMed] [Google Scholar]
- Rodgers CC, Krance R, Street RL, & Hockenberry MJ (2013). Feasibility of a symptom management intervention for adolescents recovering from a hematopoietic stem cell transplant. Cancer Nursing, 36(5), 394–399. doi: 10.1097/NCC.0b013e31829629b5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers CC, Krance R, Street RL, & Hockenberry MJ (2014). Symptom prevalence and physiologic biomarkers among adolescents using a mobile phone intervention following hematopoietic stem cell transplantation. Oncology Nursing Forum, 41(3), 229–236. doi: 10.1188/14.onf.229-236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snaman JM, Talleur AC, Lu J, Levine DR, Kaye EC, Sykes A, … Baker JN (2018). Treatment intensity and symptom burden in hospitalized adolescent and young adult hematopoietic cell transplant recipients at the end of life. Bone Marrow Transplantation, 53(1), 84–90. doi: 10.1038/bmt.2017.187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S, Ferguson C, Peng F, Schoeneich M, & Picard RW (2019). Use of In-Game Rewards to Motivate Daily Self-Report Compliance: Randomized Controlled Trial. Journal of Medical Internet Research, 21(1), e11683. doi: 10.2196/11683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasquenza K, Ruble K, Chen A, Billett C, Kozlowski L, Atwater S, & Kost-Byerly S (2015). Pain Management for Children during Bone Marrow and Stem Cell Transplantation. Pain Management Nursing, 16(3), 156–162. doi: 10.1016/j.pmn.2014.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughn J, Jonassaint J, Summers-Goeckerman E, Shaw RJ, & Shah N (2018). Customization of the TRU-PBMT App (Technology Recordings to better Understand Pediatric Blood and Marrow Transplant). Journal of Pediatric Nursing, 42, 86–91. doi: 10.1016/j.pedn.2018.07.007 [DOI] [PubMed] [Google Scholar]
- Wesley KM, & Fizur PJ (2015). A review of mobile applications to help adolescent and young adult cancer patients. Adolesc Health Med Ther, 6, 141–148. doi: 10.2147/ahmt.s69209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Yao X, Vespasiani G, Nicolucci A, Dong Y, Kwong J, … Li S (2017). Mobile App-Based Interventions to Support Diabetes Self-Management: A Systematic Review of Randomized Controlled Trials to Identify Functions Associated with Glycemic Efficacy. JMIR Mhealth Uhealth, 5(3), e35. doi: 10.2196/mhealth.6522 [DOI] [PMC free article] [PubMed] [Google Scholar]