Introduction
Cytomegalovirus infection and reactivation in immunocompromised patients can lead to serious systemic visceral involvement, such as pneumonitis, hepatitis, meningitis, and gastrointestinal disease.1 Cytomegalovirus disease conveys a high risk of morbidity and mortality.1 Cutaneous cytomegalovirus infections are not common, but they are of increasing clinical significance. To date, the most common presentation of cutaneous cytomegalovirus infection is anogenital ulcer.2 We present an atypical case of cutaneous cytomegalovirus infection with painful ulcers on the head.
Case report
A 62-year-old man presented with acute-onset painful ulcers on the scalp and face. Significant history included mycosis fungoides (granulomatous and folliculotropic subtype), diagnosed at aged 52 years, and he underwent radiation therapy with initial good response. He returned 1 year ago with increasing lesions over the head and neck. Repeated skin biopsy showed mycosis fungoides with large-cell transformation. He was treated with radiation therapy again, and interferon, with short-lived response. However, the disease progressed to involve extensive areas of skin. Imaging and extensive evaluation confirmed that disease was localized to the skin. He received gemcitabine; however, he developed pneumonitis. Respiratory evaluation with bronchoalveolar lavage did not isolate cytomegalovirus. Chemotherapy regimen was switched to brentuximab (he received 2 doses 3 weeks apart), with good response and resolution of scalp and face plaques and erosion.
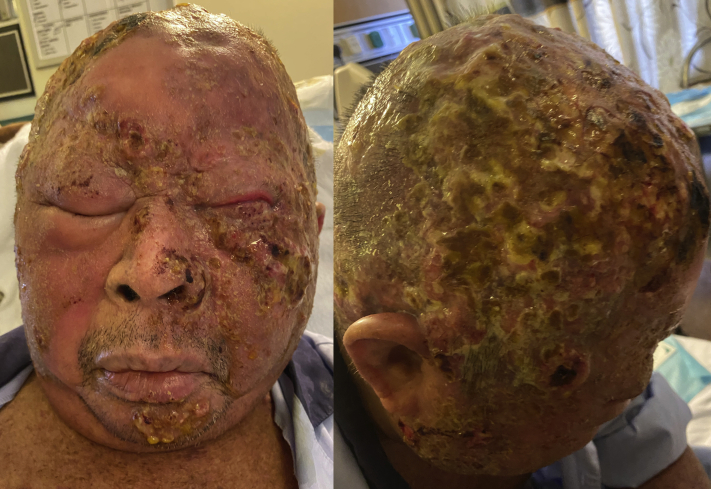
He presented 1 month later, with acutely worsening painful ulcers on the scalp and face for the past 2 weeks. On examination, there were multiple discrete ulcers with necrotic crusts and seropurulent discharge, some with scalloped edges, over the bilateral temporal areas and vertex (Fig 1). A swab of purulent exudate from the ulcers grew Stenotrophomonas, and he began receiving appropriate antibiotic according to sensitivity, with minimal improvement. His immunocompromised background raised suspicion of atypical viral infection, and we performed tests for herpes simplex virus, cytomegalovirus, and varicella zoster virus with a polymerase chain reaction swab. Cytomegalovirus polymerase chain reaction result was positive, whereas that for varicella zoster virus and herpes simplex virus was negative. Serum cytomegalovirus polymerase chain reaction was conducted to exclude systemic involvement, and the result was negative. Punch biopsy of the scalp was conducted, but the histology result from the ulcer was unyielding, showing only fibrinopurulent exudate with no evidence of cytomegalovirus (with negative cytomegalovirus stain result). Treatment with intravenous ganciclovir (5 mg/kg every 12 hours) was started, with rapid improvement in pain and drying up of lesions noted on day 2 of treatment (Fig 2). Intravenous ganciclovir was switched to oral valganciclovir 900 mg twice daily after 4 days because the patient wanted to be discharged, and he completed a total of 14 days of antiviral therapy, with further improvement to baseline (Fig 3). During this period, treatment for mycosis fungoides was withheld.
Fig 1.
Before initiation of ganciclovir.
Fig 2.
Day 2 of ganciclovir treatment.
Fig 3.
Day 14 of ganciclovir treatment.
Discussion
Cutaneous cytomegalovirus infection can have variable manifestations, including morbilliform rash, petechiae, purpura, plaques, nodules, papules, edema, vesicles, bullae, erosions, vasculitis, and pustules, the most common being ulcers.1 The painful ulcers can be varied in character or distribution. Anogenital ulcers are the most common manifestations (which could be due to preferential latency of cytomegalovirus in the gastrointestinal tract, leading to fecal shedding during reactivation2), followed by oral ulcers.1,3 To date, scalp and face involvement has been rarely reported. Review of the literature showed only 3 cases with involvement of the face and scalp: case 1- an infant presenting with multiple scalp ulcers, postulated to be due to pressure ulcer from prolonged periods of lying supine and becoming secondarily infected4; case 2- a renal transplant patient who presented with ulcerative lesions on the face, with cytomegalovirus DNA detected in tissue, along with cytomegalovirus antigenemia5; case 3- disseminated cutaneous cytomegalovirus manifestation with punched-out ulcers and pustules involving the head, trunk, and limbs after total-body electron beam irradiation for mycosis fungoides.6 Our patient shared the same predisposing risk factor as case 3 because radiation therapy may lead to local reactivation of the virus in the skin of patients with latent cytomegalovirus seropositivity. Another factor to consider in patients with cutaneous T-cell lymphoma is that there could be localized cytomegalovirus reactivation where there is active cutaneous T-cell lymphoma or locus minoris resistentiae.
Cytomegalovirus serology is not a routine screening test among patients with cutaneous or solid organ malignancy before initiation of chemotherapy; similarly, in our patient, it was not conducted previously. Cytomegalovirus reactivation is common during conventional chemotherapy, but it is probably self-limiting in many patients.7 However, cytomegalovirus seropositivity may play a role in the development or transformation of cutaneous T-cell lymphoma.8,9 A small pilot study also highlighted 2 subjects with Sézary syndrome at an advanced stage that required numerous treatments, both of whom were found to have cytomegalovirus infection of the skin without detectable cytomegalovirus DNA in the blood.10 This finding, similar to that in our patient, suggests a possible role of latent or cutaneous cytomegalovirus infection in complicating cutaneous T-cell lymphoma, especially in patients with aggressive disease or in those with lack of response to numerous lines of treatment for the lymphoma.
In summary, we present a case of atypical cutaneous cytomegalovirus infection in a patient with transformed mycosis fungoides, with excellent and rapid response to the first-line agent (intravenous ganciclovir followed by oral valganciclovir) despite the aggressive clinical presentation. Patients with cutaneous T-cell lymphoma may have a different pattern of cutaneous manifestations of cytomegalovirus infection compared with other immunocompromised patients, who commonly present with perianal or genital ulcers. Our patient presented with cutaneous cytomegalovirus ulcers on the face and scalp as the first and only manifestation, without any other organ involvement. It is important to recognize the skin as the first site of cytomegalovirus involvement and institute treatment early because it can precede a systemic or disseminated cytomegalovirus infection. It is also crucial to consider cytomegalovirus infection in the differential diagnosis of immunocompromised patients with skin ulcers that are refractory to antibiotic treatment.
Footnotes
Funding sources: None.
Conflicts of interest: None disclosed.
References
- 1.Drozd B., Andriescu E., Suarez A., de la Garza Bravo M.M. Cutaneous cytomegalovirus manifestations, diagnosis, and treatment: a review. Dermatol Online J. 2019;25(1):1–9. [PubMed] [Google Scholar]
- 2.Lei D., Saul K., Jackson J., Ishaq N.Y., Brodell R.T. Perianal ulceration in a young woman. Am J Dermatopathol. 2016;38(9):683–684. doi: 10.1097/DAD.0000000000000294. [DOI] [PubMed] [Google Scholar]
- 3.Choi Y.L., Kim J.A., Jang K.T. Characteristics of cutaneous cytomegalovirus infection in non-acquired immune deficiency syndrome, immunocompromised patients. Br J Dermatol. 2006;155:977–982. doi: 10.1111/j.1365-2133.2006.07456.x. [DOI] [PubMed] [Google Scholar]
- 4.Lal K., Dlova N., Mosam A., Khoza N. An infant presenting with multiple scalp ulcers. Pediatr Dermatol. 2014;31:729–730. doi: 10.1111/pde.12488. [DOI] [PubMed] [Google Scholar]
- 5.Ezzatzadegan Jahromi S., Lotfi Z., Torabi-Nezhad S., Aslani F.S., Pourabbas B. A potential role for cytomegalovirus in a facial ulcer in a renal transplant recipient. Transpl Infect Dis. 2016;18:457–460. doi: 10.1111/tid.12536. [DOI] [PubMed] [Google Scholar]
- 6.Kim J.K., Ahmad A., Selim M.A., Olsen E.A., Cardones A.R. Disseminated cutaneous cytomegalovirus infection following total body electron beam irradiation for mycosis fungoides. JAMA Dermatol. 2015;151(12):1380–1381. doi: 10.1001/jamadermatol.2015.2233. [DOI] [PubMed] [Google Scholar]
- 7.Kuo C.-P., Wu C.L., Ho H.T. Detection of cytomegalovirus reactivation in cancer patients receiving chemotherapy. Clin Microbiol Infect. 2020;14(3):221–227. doi: 10.1111/j.1469-0691.2007.01895.x. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt A.N., Robbins J.B., Greer J.P., Zic J.A. Conjugal transformed mycosis fungoides: the unknown role of viral infection and environmental exposures in the development of cutaneous T-cell lymphoma. J Am Acad Dermatol. 2006;54(5 suppl):S202–S205. doi: 10.1016/j.jaad.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 9.Herne K.L., Talpur R., Breuer-McHam J., Champlin R., Duvic M. Cytomegalovirus seropositivity is significantly associated with mycosis fungoides and Sézary syndrome. Blood. 2003;101(6):2132–2135. doi: 10.1182/blood-2002-07-2247. [DOI] [PubMed] [Google Scholar]
- 10.Ballanger F., Bressollette C., Volteau C., Planche L., Dreno B. Cytomegalovirus: its potential role in the development of cutaneous T-cell lymphoma. Exp Dermatol. 2009;18(6):574–576. doi: 10.1111/j.1600-0625.2008.00817.x. [DOI] [PubMed] [Google Scholar]