Abstract
Proton magnetic resonance spectroscopy (1H-MRS) has demonstrated that in vitro, lung cancer has higher lactate and choline signals than those of normal tissues. The detection of these metabolites in lung cancer in vivo by 1H-MRS would be useful for clinical diagnoses of lung cancer. We report the in vivo detection of lactate and choline in lung cancer by 1H-MRS in a 41-year-old Asian man who was diagnosed with pT4N0M0 ⅢA stage, right upper lobe lung adenocarcinoma. A lactate-lipid peak was observed near 1.33 ppm in the spectrum of lung cancer in vivo at TE = 30 ms, and it was inverted at TE = 135 ms, indicating that a lactate signal is contained in the lactate-lipid peak. A choline peak was also observed near 3.2 ppm in the spectrum with fat suppression at TE = 135 ms. An accumulation of similar cases will help determine the appropriate applications of 1H-MRS for lung cancer.
Keywords: Magnetic resonance spectroscopy, Lung cancer, Choline, Lactate
Introduction
Lung cancers examined in vitro by proton magnetic resonance spectroscopy (1H-MRS) have been shown to have higher lactate and total choline peaks compared to normal tissues [1,2]. The detection of these metabolites in vivo would be useful for the diagnosis of lung cancer. Here we report the detection of these metabolites by 1H-MRS in a patient with lung cancer.
Case Report
A 41-year-old Asian man was diagnosed with pT4N0M0 ⅢA stage, right upper lobe lung adenocarcinoma. Chest computed tomography (CT) showed a 98-mm solid tumor in the upper right lobe (Fig. 1A), and 18F-labeled fluorodeoxyglucose (18F-FDG) positron emission tomography/CT (18F-FDG PET/CT) showed a high maximum 18F-FDG standardized uptake value (SUVmax: 14.3) at the same site (Fig. 1B). On T2-weighted 2-dimensional half-Fourier acquisition single-shot turbo spin echo (2D HASTE), the tumor showed low- to iso-signal intensity with a high signal intensity inside (Fig. 1C). The patient was indicated for surgery for stage ⅢA lung cancer without mediastinal lymph node metastasis.
Fig. 1.
A: Contrast-enhanced chest CT shows a heterodense lobulated mass in the upper right lobe. B: In 18F-FDG PET/CT, the lobulated mass had high 18F-FDG accumulation (SUVmax = 14.3) with a sporadic reduction of 18F-FDG accumulation. C: Axial T2-weighted images of the lung cancer showed low- to iso-signal intensity with a high signal intensity inside. D: The high-intensity area corresponded to a hemorrhagic necrotic area. Hemorrhagic necrotic areas and necrotic areas were scattered within the tumor in macroscopic findings of formalin-fixed lung cancer. E: The overall image of the hematoxylin-eosin (HE)-stained pathological tissue in the square in panel D shows a geographic necrotic area in the tumor. F: The enlarged view (×10) of the square in panel E showing the adenocarcinoma with a necrotic region in the upper left.
At the preoperative examination, the patient's fasting blood sugar and HbA1c were 104 mg/dl and 5.6%, respectively. His arterial blood gas demonstrated a PaO2 = 100 kPa, pCO2 = 37.6 kPa, and pH = 7.49, BE = −0.2 mmol/L, and HCO3 = 23.9 mmol/L. His vital capacity and forced expiratory volume in 1 s (FEV1.0) were 4.45 L (predicted value, 3.85 L) and 3.37 L (predicted value 3.40 L), respectively. A right upper lobectomy and lymph node dissection (ND2a-2) was performed under thoracotomy. The solid tumor nodule in the excised upper right lobe consisted of adenocarcinoma with geographic necrosis (Fig. 1D-F).
We obtained the patient's written informed consent for his data and findings to be published before a series of MRI examinations was conducted. The MRI examination was performed on a whole-body 3 Tesla MR system (Magnetom Trio, A Tim System, Siemens Healthcare, Erlangen, Germany) using a body matrix coil. The first MRI examination involved a quick whole-lung survey with T2-weighted 2D HASTE, using the turbo spin echo technique in the axial, coronal, and sagittal orientations with a repetition time of 700 ms, echo time (TE) of 55 ms, turbo factor of 256, slice thickness of 5 mm, field of view (FOV) of 380 × 380 mm, and matrix of 320 × 256.
After the lesion identification on MR images, single-voxel 1H-MRS (SVS) was performed. Coronal, sagittal, and axial T2-weighted images of a quick whole-lung survey were used for voxel placement (Fig. 2A-C). After the automatic and manual shimming in the voxel, the full width at half maximum of the water peak became 43.7 Hz. 1H-MR spectra were obtained using a point-resolved spin-echo sequence with the following technical parameters: repetition time /TE = 2000/30 and 135 ms, and number of excitations = 120 for a voxel size of 2 × 2 × 2 cm3. The point-resolved spin-echo sequence was performed under spontaneous breathing without respiratory synchronization using regional saturation (RSat) in six directions to block magnetic resonance (MR) signals from other than the voxels and using a seven chemical shift-selective sequence for water suppression.
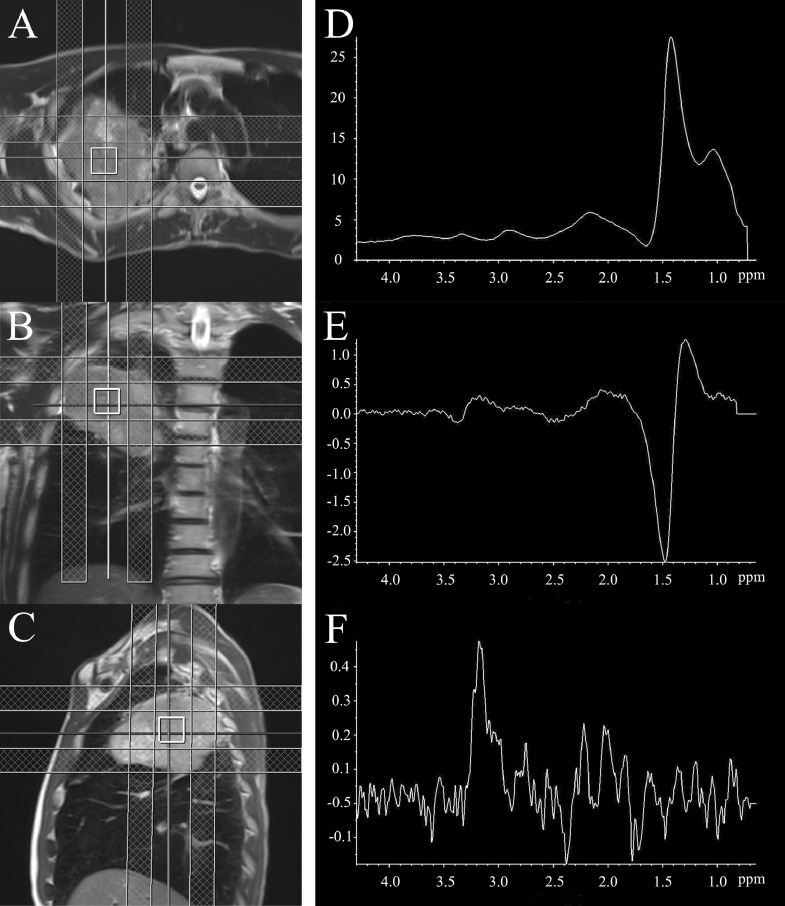
Fig. 2.
The voxel was set in three directions (axial; A, coronal; B, sagittal; C) of the T2-weighted image. The single-voxel 1H-MR spectrum of lung cancer was acquired under spontaneous breathing using PRESS (single voxel 2 × 2 × 2 cm, TR/TE = 2000/30 or 135 ms, NEX = 120) with six regional saturation (RSat) bands for outer volume suppression (checkered pattern). At TE = 30 ms, the bimodal peak at 1.0-1.5 ppm was upward (D), but at TE = 135 ms, the peak near 1.33 was inverted (E), indicating that the lactate peak was included in the lactate-lipid peak. At TE = 135 ms, when fat suppression was applied to the mobile lipid peak (1.3 ppm), a total choline peak was observed at 3.2 ppm (F).
The voxel hardly fluctuated in the tumor (fluctuation range: 2-3 mm). At TE = 135 ms, 1H-MR spectra were obtained with and without fat suppression. The acquisition time was 5 min 38 s. Focusing on the peaks near 1.33 ppm, the peak was upward at TE = 30 ms, but the peak was inverted at TE = 135 ms (Fig. 2D, E). In the spectrum at TE = 135 ms, when fat suppression was applied, a peak was detected at 3.2 ppm, which is the peak value of total choline (Fig. 2F).
Discussion
The use of MRI for examinations of in vivo lungs had been considered challenging due to low MR signal intensity and magnetic field instability due to respiratory variability in lumenal tissue. However, solid lesions such as lung cancer can be visualized by MRI, and the differentiation of lung cancer by diffusion-weighted MRI (DW-MRI) has been studied [3]. In vitro studies using 1H-MRS demonstrated that compared to normal tissues, lung cancer has higher choline and lactate peaks [1,2]. If these metabolites can be detected in vivo, their detection could be useful for lung cancer diagnoses.
Cancer tissues have enhanced glycolysis and higher lactate levels than normal tissues [4]. Thus, lactate can be a metabolite in the differential diagnosis of cancer, but it is actually elevated even in hypoxia, and it overlaps with mobile lipids in necrotic areas. These mobile lipids can also be an indicator of malignancy in a carcinoma [5]. However, in our patient the presence of a lactate peak within the lactate-lipid peak was confirmed. Although the 1H-MRS was performed for him without respiratory synchronization, it was conducted using RSat in 6 directions to prevent MR signal contamination from visceral fat other than lung cancer, while confirming with continuous MR images that other organs did not enter the voxel. The peak near 1.33 ppm at TE = 30 ms was inverted at TE = 135 ms. The spin echo signal repeats upright and inverted at a period of 1/spin coupling constant (J). Since the J value between CH3 and CH protons in lactate is 7.35 Hz, the lactate peak repeats inversion every TE = 1/J (135 ms). Therefore, the inversion of the peak near 1.33 ppm at TE = 135 ms revealed that the lactate-lipid peak contained the lactate peak.
In cancer cells, the choline peak rises for various reasons, including membrane metabolism [6], and the peak is a cancer marker in various types of cancer [7,8]. In our patient's case, the total choline peak could not be detected in the spectrum without fat suppression at TE = 135 ms. However, in the spectrum with fat suppression at TE = 135 ms, the lactate-lipid peak disappeared and the total choline peak appeared. That is, in lung cancer, since the total choline signal has a much weaker MR signal than the lactate-lipid signal, it has been difficult to detect the total choline signal in the spectrum under the same conditions as the lactate-lipid. In the future, in order to detect the choline peak in the lung cancer spectrum by 1H-MRS, it is necessary to suppress the lactate-lipid peak by using a long TE and fat suppression.
In conclusion, lactate and total choline peaks were detected in lung cancer in vivo by 1H-MRS. However, the detection of total choline required the suppression of the lactate-lipid signal because of the much weaker choline signal compared to the lactate-lipid signal.
Footnotes
Declarations of Interest: None.
References
- 1.Chen W., Zu Y., Huang Q., Chen F., Wang G., Lan W., Bai C., Lu S., Yue Y., Deng F. Study on metabonomic characteristics of human lung cancer using high resolution magic-angle spinning 1H NMR spectroscopy and multivariate data analysis. Magn Reson Med. 2011;66:1531–1540. doi: 10.1002/mrm.22957. [DOI] [PubMed] [Google Scholar]
- 2.Hanaoka H., Yoshioka Y., Ito I., Niitu K., Yasuda N. In vitro characterization of lung cancers by the use of 1H nuclear magnetic resonance spectroscopy of tissue extracts and discriminant factor analysis. Magn Reson Med. 1993;29:436–440. doi: 10.1002/mrm.1910290403. [DOI] [PubMed] [Google Scholar]
- 3.Chang N., Wang X.H., Cui L.B., Yin H., Jiang T., Chen F.L. Diagnostic performance of diffusion-weighted magnetic resonance imaging in pulmonary malignant lesions: a meta-analysis. Transl Lung Cancer Res. 2019;8:738–747. doi: 10.21037/tlcr.2019.10.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Warburg O., Wind F., Negelein E. The metabolism of tumors in the body. J Gen Physiol. 1927;8:519–530. doi: 10.1085/jgp.8.6.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Delikatny E.J.1, Russell P., Hunter J.C., Hancock R., Atkinson K.H., van Haaften-Day C., Mountford C.E. Proton MR and human cervical neoplasia: ex vivo spectroscopy allows distinction of invasive carcinoma of the cervix from carcinoma in situ and other preinvasive lesions. Radiology. 1993;188:791–796. doi: 10.1148/radiology.188.3.8351349. [DOI] [PubMed] [Google Scholar]
- 6.Glunde K., Bhujwalla Z.M., Ronen S.M. Choline metabolism in malignant transformation. Nat Rev Cancer. 2011;11:835–848. doi: 10.1038/nrc3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bartella L., Morris E.A., Dershaw D.D., Liberman L., Thakur S.B., Moskowitz C. Proton MR spectroscopy with choline peak as malignancy marker improves positive predictive value for breast cancer diagnosis: preliminary study. Radiology. 2006;239:686–692. doi: 10.1148/radiol.2393051046. [DOI] [PubMed] [Google Scholar]
- 8.Kurhanewicz J., Vigneron D.B., Hricak H., Narayan P., Carroll P., Nelson S.J. Three-dimensional H-1 MR spectroscopic imaging of the in situ human prostate with high (0.24–0.7-cm3) spatial resolution. Radiology. 1996;198:795–805. doi: 10.1148/radiology.198.3.8628874. [DOI] [PubMed] [Google Scholar]