Abstract
Talaromyces marneffei is an opportunistic fungal infection seen in immunocompromised patients including those with HIV/AIDS. It is usually seen in patients who live in or are from tropical Asia. In HIV patients, oropharyngeal and laryngeal lesions are usually part of disseminated infection. We describe a case of 63-year-old Vietnamese male with history of HIV/AIDS who presented with localized T. marneffei tonsillar infection without disseminated disease. Imaging studies showed a right tonsillar mass with right cervical lymphadenopathy which was initially thought to be malignancy. The patient underwent biopsy of the mass and histology showed noncaseating granulomas on hematoxylin and eosin stain as well as yeast on Grocott methenamine silver stain. Fungal culture of the biopsy specimen grew suede-like grayish-white colonies with diffuse underlying deep red color pigment which was identified as Talaromyces marneffei. The patient was treated with intravenous liposomal amphotericin B and achieved resolution of symptoms and tonsillar mass. In HIV/AIDS patients who are either from endemic regions or with history of travel to endemic areas particularly Southeast Asia and China, T. marneffei infection should be considered in differential diagnoses of a tonsillar mass.
Keywords: Talaromyces marneffei, HIV, Opportunistic infection, Tonsillar mass, Oral lesion
Introduction
Talaromyces marneffei (formerly Penicillium mareneffi) is an opportunistic fungal pathogen that causes infections in immunocompromised patients including those with HIV/AIDS. It is mostly seen in endemic regions (Southeast Asia, Northeastern India, Southern China, Hong Kong, and Taiwan) [1]. Most HIV/AIDS patients present with systemic signs and symptoms due to the disseminated nature of the infection and localized infections are uncommon [2]. The disseminated infection may result from reactivation of latent infection, reinfection, or primary infection [2]. A very low CD4 lymphocyte count has been reported among AIDS patients with disseminated infection and the disease can be another AIDS-defining illness [3,4]. We report a case of localized T.marneffei infection in a southeast Asian HIV/AIDS immigrant presenting as a tonsillar mass.
Case report
The patient was a 63-year-old Vietnamese man with history of HIV/AIDS who was brought to the emergency department by the family after he was found down in his home for an undetermined amount of time. Two years prior to presentation the patient was hospitalized with Pneumocystis jirovecii pneumonia. During that hospital course, he was diagnosed with HIV infection. His initial absolute CD4 cell count was 64 cells/μL and HIV RNA viral load was 830,000 copies/mL. He was started on antiretroviral therapy with emtricitabine/tenofovir alafenamide and darunavir/cobicistat. However, he discontinued all the medications and was lost to follow up within 2 months after discharge. He immigrated to Missouri in the United States as a tailor approximately 20–25 years prior to this encounter. He visited Vietnam last one year prior to presentation to meet with his family in Mekong Delta, the southernmost part of Vietnam, and has never traveled anywhere except Vietnam.
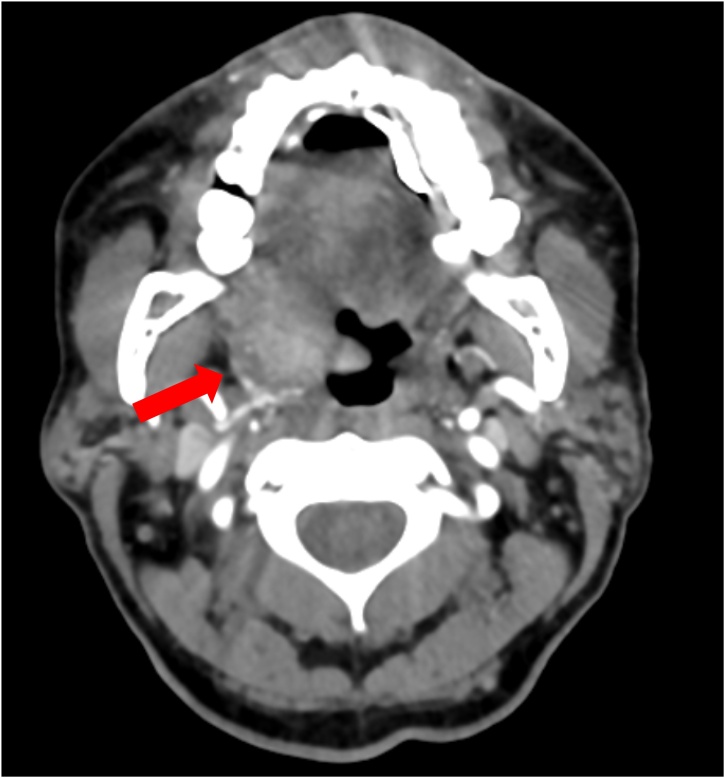
On presentation, he was febrile with a temperature 39 °C, respiratory rate 24 breaths per minute, and heart rate 115 beats per minute. Blood pressure was normal. Patient was alert, but oriented to person only and appeared confused. Physical examination showed normal heart, lung, abdominal, and neurological examinations. He had no skin lesions. Laboratory findings showed a platelet count 8,000 /μL and white blood cell count (WBC) 4,700 /μL. Lactic acid was elevated to 5.9 mmol/L (reference range: 0.5–2.2 mmol/L). Computed tomography (CT) of the head without contrast showed no acute intracranial findings. However, it incidentally revealed a right tonsillar mass with surrounding right cervical lymphadenopathy, and he admitted the presence of throat pain. CT angiogram of the neck was obtained which showed an ill-defined mass along the right lateral aspect of the hypopharynx involving the base of the tongue, right lingual tonsil, and right vallecula extending along the right palatine tonsil and into the pharyngeal space (Fig. 1). Magnetic resonance imaging of the brain showed findings consistent with sequela of HIV encephalopathy.
Fig. 1.
Computed tomography angiogram of the neck showed an ill-defined mass along the right lateral aspect of the hypopharynx involving the base of the tongue, right lingual tonsil, and right vallecula extending along the right palatine tonsil and into the pharyngeal space (red arrow).
Cerebrospinal fluid (CSF) study showed WBC 5 cells/μL, red blood cell count 305 cells/μL, protein 45 mg/dL, and glucose 45 mg/dL (serum glucose 90 mg/dL). CSF multiplex polymerase chain reaction testing was negative for E. coli K1, Haemophilus influenzae, Listeria monocytogenes, Neisseria meningitidis, Streptococcus agalactiae, Streptococcus pneumoniae, Enterovirus, Human simplex virus (HSV) 1, HSV 2, Cytomegalovirus (CMV), Varicella-Zoster virus, Human herpesvirus 6, Human parechovirus, and Cryptococcus neoformans/gattii. CSF Cryptococcal antigen (Ag) was negative. HIV RNA viral load was 1,620,000 copies/mL and absolute CD4 cell count was 46 cells/μL. Urine Histoplasma Ag, serum galactomannan assay, serum Cryptococcal Ag, viral hepatitis serologies, T-spot TB™ test and Toxoplasma IgM and IgG antibodies were all negative. Serum CMV DNA viral load was 13,680 IU/mL, and serum Epstein-Barr virus DNA viral load was 2,644 copies/mL. Bacterial, fungal, and acid-fast bacilli (AFB) blood cultures were all negative. He was started on intravenous ganciclovir 5 mg/kg every 12 h for CMV viremia and became afebrile.
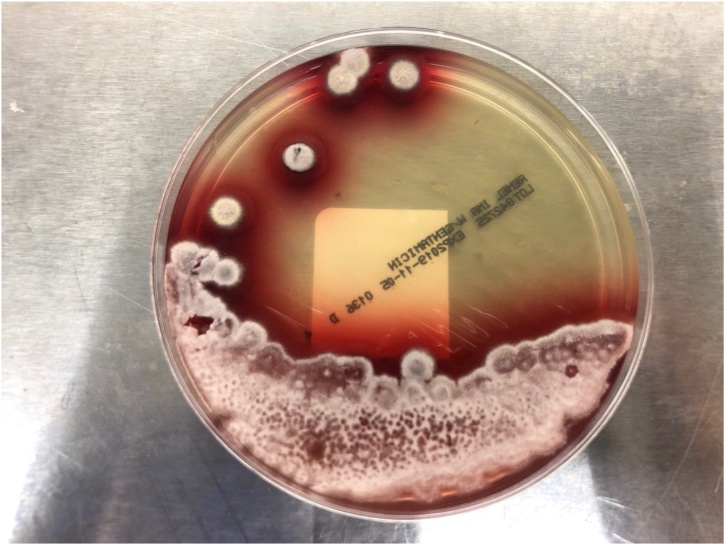
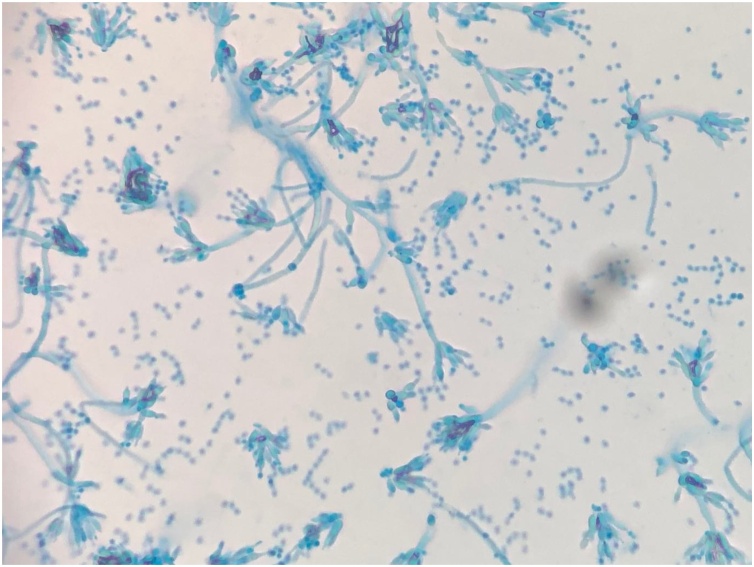
He underwent direct flexible laryngoscopy for the biopsy of the tonsillar mass and the base of the right tongue. An ulcerative and friable mass involving the right palatine tonsil wall was found and it appeared malignant. However, histology of the right tonsillar mass showed squamous epithelium with acute and chronic lympho-histiocytes, inflammation with crusting, and ulcer formation, and Grocott methenamine silver (GMS) stain revealed presence of yeast. The yeast cells were small (2−5 μm in diameter) and oval to ellipsoidal. The histology of the base of the right tongue showed noncaseating granulomas with intense chronic inflammation as well as yeast on GMS stain. AFB and Warthin-Starry stains were negative. Flow cytometry showed no evidence of lymphoproliferative disorder. Five days later fungal cultures incubated at 25 °C on Sabouraud dextrose agar grew suede-like grayish-white colonies with diffusible underlying deep red color pigment (Fig. 2). Microscopically, flask-shaped phialides in brush-like arrangement were seen on lactophenol cotton blue staining (Fig. 3). The culture isolate was sent to a reference laboratory and confirmed as Talaromyces marneffei.
Fig. 2.
Suede-like grayish-white colonies of Talaromyces marneffei with diffusible underlying deep red color pigment on Sabouraud dextrose agar (incubated at 25 °C).
Fig. 3.
A microscopic slide preparation of Talaromyces marneffei. Flask-shaped phialides in brush-like arrangement are seen on lactophenol cotton blue staining.
The patient was treated for T. marneffei infection with intravenous liposomal amphotericin B 4 mg/kg intravenous every 24 h for 2 weeks followed by oral itraconazole 200 mg twice a day. His throat pain resolved with significant decrease in the size of the tonsillar mass.
Discussion
We present a case of tonsillar mass that appeared as malignancy but turned out to be localized T. marneffei infection in an individual with HIV/AIDS. Oropharyngeal and laryngeal lesions are rare presentations in T. marneffei infection and they typically present as ulcerative lesions [5,6]. In HIV patients, oropharyngeal and laryngeal lesions were all reported as a part of T. marneffei disseminated infection [1,[5], [6], [7], [8], [9], [10], [11]]. Our case did not have cutaneous lesions, non-regional lymphadenopathy or other organ involvement, and fungal blood cultures were negative which suggests absence of disseminated disease. T. marneffei infection can be seen in patients who live in or are from tropical Asia, especially Thailand, northern India, China, Hong Kong, Vietnam and Taiwan [12]. Common clinical presentations include fever, weight loss, anemia, cough, skin lesions, hepatosplenomegaly and lymphadenopathy [1,13]. The incubation period varies from a few weeks to many years of exposure to the organism [2].
The differential diagnoses of oral lesions in HIV patients are broad and include infections, malignancy, and others such as immune-mediated and drug reactions [14]. Oral histoplasmosis typically appears as ulcerative lesions in oropharynx, gingiva, palate or tongue [15,16] although there was a case report of a malignancy-like oral mass lesion [17]. Cryptococcal infection could also present as oral ulcers although involvement of oral lesions in cryptococcosis is rare [18,19]. CMV-related oral ulcerations are infrequent, but can occur in HIV patients [20]. Oral lesions are common in HIV patients with syphilis and may present as chronic non-healing, deep, and solitary ulceration in mouth [14]. Bacillary epithelioid angiomatosis caused by Bartonella species may present as an erythematous, soft mass, and is clinically indistinguishable from oral Kaposi’s sarcoma [14]. Non-Hodgkin’s lymphoma presents as a rapidly enlarging mass on the palate or gingivae [14].
Conclusions
Even when radiographic and macroscopic findings of a mass lesion are most consistent with malignancy, it is important to send a biopsy specimen for cultures in case the etiology of the mass is not malignancy because identification of pathogens may not be possible by histopathological examination alone. In HIV patients, who are either from endemic regions or with history of travel to endemic areas particularly Southeast Asia and China, T. marneffei infection should be considered in differential diagnoses of a tonsillar mass.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial or not for profit sectors.
CRediT authorship contribution statement
Annapoorna Singh: Conceptualization, Writing - original draft. Sarah Atallah: Writing - review & editing. Ahmad Al-Shyoukh: Writing - review & editing. Matthew DaCunha: Writing - review & editing. Masako Mizusawa: Conceptualization, Writing - review & editing, Supervision.
Declaration of Competing Interest
The authors declare that there are no conflicts of interest.
Acknowledgement
We thank Microbiology Laboratory at Truman Medical Center for technical assistance.
References
- 1.Supparatpinyo K., Khamwan C., Baosoung V., Nelson K.E., Sirisanthana T. Disseminated Penicillium marneffei infection in southeast Asia. Lancet. 1994;344:110–113. doi: 10.1016/s0140-6736(94)91287-4. [DOI] [PubMed] [Google Scholar]
- 2.Nittayananta W. Penicilliosis marneffei: another AIDS defining illness in Southeast Asia. Oral Dis. 1999;5:286–293. doi: 10.1111/j.1601-0825.1999.tb00091.x. [DOI] [PubMed] [Google Scholar]
- 3.Tsang D.N., Li P.C., Tsui M.S., Lau Y.T., Ma K.F., Yeoh E.K. Penicillium marneffei: another pathogen to consider in patients infected with human immunodeficiency virus. Rev Infect Dis. 1991;13:766–767. doi: 10.1093/clinids/13.4.766-a. [DOI] [PubMed] [Google Scholar]
- 4.Supparatpinyo K., Chiewchanvit S., Hirunsri P., Uthammachai C., Nelson K.E., Sirisanthana T. Penicillium marneffei infection in patients infected with human immunodeficiency virus. Clin Infect Dis. 1992;14:871–874. doi: 10.1093/clinids/14.4.871. [DOI] [PubMed] [Google Scholar]
- 5.Nittayananta W., Chungpanich S. Oral lesions in a group of Thai people with AIDS. Oral Dis. 1997;3(Suppl 1):S41–45. doi: 10.1111/j.1601-0825.1997.tb00372.x. [DOI] [PubMed] [Google Scholar]
- 6.Kantipong P., Walsh D.S. Oral penicilliosis in a patient with human immunodeficiency virus in northern Thailand. Int J Dermatol. 2000;39:926–928. doi: 10.1046/j.1365-4362.2000.00988.x. [DOI] [PubMed] [Google Scholar]
- 7.Qiu Y., Tang Y., Zhang J., Yi X., Zhong X., Liu G. A retrospective analysis of seven patients with acquired immunodeficiency syndrome and pharyngeal and/or laryngeal Talaromyces marneffei infection. Clin Otolaryngol. 2017;42:1061–1066. doi: 10.1111/coa.12838. [DOI] [PubMed] [Google Scholar]
- 8.Tong A.C., Wong M., Smith N.J. Penicillium marneffei infection presenting as oral ulcerations in a patient infected with human immunodeficiency virus. J Oral Maxillofac Surg. 2001;59:953–956. doi: 10.1053/joms.2001.25881. [DOI] [PubMed] [Google Scholar]
- 9.Jones P.D., See J. Penicillium marneffei infection in patients infected with human immunodeficiency virus: late presentation in an area of nonendemicity. Clin Infect Dis. 1992;15:744. doi: 10.1093/clind/15.4.744. [DOI] [PubMed] [Google Scholar]
- 10.Duong T.A. Infection due to Penicillium marneffei, an emerging pathogen: review of 155 reported cases. Clin Infect Dis. 1996;23:125–130. doi: 10.1093/clinids/23.1.125. [DOI] [PubMed] [Google Scholar]
- 11.Borradori L., Schmit J.C., Stetzkowski M., Dussoix P., Saurat J.H., Filthuth I. Penicilliosis marneffei infection in AIDS. J Am Acad Dermatol. 1994;31:843–846. doi: 10.1016/s0190-9622(94)70242-x. [DOI] [PubMed] [Google Scholar]
- 12.Vanittanakom N., Cooper C.R., Fisher M.C., Sirisanthana T. Penicillium marneffei infection and recent advances in the epidemiology and molecular biology aspects. Clin Microbiol Rev. 2006;19:95–110. doi: 10.1128/CMR.19.1.95-110.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kawila R., Chaiwarith R., Supparatpinyo K. Clinical and laboratory characteristics of penicilliosis marneffei among patients with and without HIV infection in Northern Thailand: a retrospective study. BMC Infect Dis. 2013;13:464. doi: 10.1186/1471-2334-13-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bajpai S., Pazare A.R. Oral manifestations of HIV. Contemp Clin Dent. 2010;1:1–5. doi: 10.4103/0976-237X.62510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Antonello V.S., Zaltron V.F., Vial M., de Oliveira F.M., Severo L.C. Oropharyngeal histoplasmosis: report of eleven cases and review of the literature. Rev Soc Bras Med Trop. 2011;44:26–29. doi: 10.1590/s0037-86822011000100007. [DOI] [PubMed] [Google Scholar]
- 16.Hernández S.L., López De Blanc S.A., Sambuelli R.H. Oral histoplasmosis associated with HIV infection: a comparative study. J Oral Pathol Med. 2004;33:445–450. doi: 10.1111/j.1600-0714.2004.00183.x. [DOI] [PubMed] [Google Scholar]
- 17.Chroboczek T., Dufour J., Renaux A. Histoplasmosis: an oral malignancy-like clinical picture. Med Mycol Case Rep. 2018;19:45–48. doi: 10.1016/j.mmcr.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glick M., Cohen S.G., Cheney R.T., Crooks G.W., Greenberg M.S. Oral manifestations of disseminated Cryptococcus neoformans in a patient with acquired immunodeficiency syndrome. Oral Surg Oral Med Oral Pathol. 1987;64:454–459. doi: 10.1016/0030-4220(87)90152-6. [DOI] [PubMed] [Google Scholar]
- 19.Lynch D.P., Naftolin L.Z. Oral Cryptococcus neoformans infection in AIDS. Oral Surg Oral Med Oral Pathol. 1987;64:449–453. doi: 10.1016/0030-4220(87)90151-4. [DOI] [PubMed] [Google Scholar]
- 20.Heinic G.S., Greenspan D., Greenspan J.S. Oral CMV lesions and the HIV infected. Early recognition can help prevent morbidity. J Am Dent Assoc. 1993;124:99–105. doi: 10.14219/jada.archive.1993.0051. [DOI] [PubMed] [Google Scholar]