Abstract
Thyroid metastasis revealing a primary lung cancer is an extremely rare condition. Only few cases have been reported in the literature. A multidisciplinary approach is essential for the diagnosis. The prognosis is generally poor. We report a case of a 50-year-old man presented with cervical nodules corresponding to a thyroid nodule and lymph nodes. The ultrasonography-guided fine-needle aspiration cytology of the thyroid nodule and a cervical lymphadenopathy concluded to a poorly differentiated adenocarcinoma. Cervical lymphadenopathy biopsy with immunohistochemistry and additional imaging explorations contributed to the diagnosis of a lung adenocarcinoma stage IVB. He died few days after the diagnosis.
Keywords: Adenocarcinoma of lung, Neoplasm metastasis, Thyroid neoplasms
1. Introduction
Despite a high blood supply of the thyroid, carcinomas rarely metastasize to this organ [1]. The clinical incidence of thyroid metastasis is less than 4% of all thyroid malignancies [2,3]. The main primary carcinomas metastasizing to the thyroid are renal cell (48.1%) and colorectal carcinomas (10.4%) [3]. A primary non-small cell lung cancer with metastasis to the thyroid gland represent an extremely rare condition [4,5]. Only few cases of primary lung adenocarcinoma metastasizing to the thyroid gland have been reported in literature [5,6]. We report a case of a thyroid metastasis revealing an unknown polymetastatic primary lung adenocarcinoma.
2. Case presentation
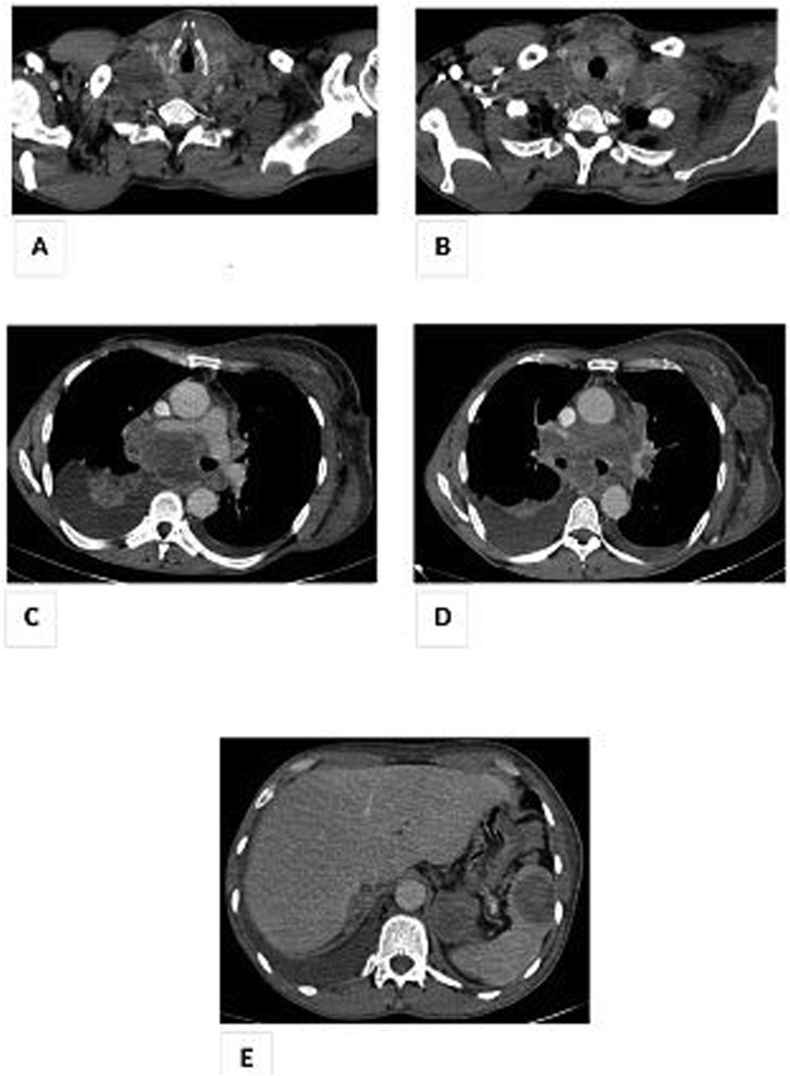
A 50-year-old man presented to the hospital with cervical nodules evolving 3 weeks before admission. He was an active smoker with 68 pack-year tobacco intoxication. He had an history of pulmonary tuberculosis treated 20 years ago. He was admitted first in Otorhinolaryngology (ENT) Department. Initial physical examination revealed a palpable thyroid nodule in the left lobe with bilateral cervical lymph nodes. The pulmonary examination was without particularities. Serum levels of thyroid stimulating hormone (TSH) and free thyroxine were within normal limits. Neck ultrasonography showed bilateral cervical lymph nodes and a 2.8 × 2.2 cm solid hypoechoic nodule of his left thyroid lobe. The nodule presented irregular borders with extra-thyroid extension. He underwent ultrasonography-guided fine-needle aspiration cytology (FNAC) of the thyroid nodule and a cervical lymphadenopathy. The two FNAC findings matched and showed clusters of tumor cells with basophilic cytoplasm and atypical nucleated nuclei in favor of a poorly differentiated adenocarcinoma (Fig. 1). Cervical lymphadenopathy biopsy under local anesthesia was performed. Pathological examination of the lymphadenopathy showed a massively infiltrated lymph node tissue by carcinomatous proliferation. The tumor cells were polygonal with eosinophilic cytoplasm and moderately atypical nuclei. Mitosis were numerous. Immunohistochemistry (IHC) showed positive tumor cells with cytokeratin (CK) 7 but negative with TTF1 (Fig. 1). Hence, further explorations were conducted. Chest X-ray showed a lung right sided suspicious opacity with a mediastinal widening. Contrast-enhanced Computed Tomography (CT) scan of the thorax showed a 11.4 × 10 × 9.7 cm right hilar mass presenting a mediastinal extension involving carina, pericardium, and esophagus with multiple hilar and mediastinal lymph nodes (Fig. 2. C + D). Cervical CT sections confirmed the ultrasound findings (Fig. 2. A + B). The abdominal CT scan showed multiple renal, adrenal, and splenic metastasis (Fig. 2. E). Bronchial fibroscopy was not performed due to a rapid deterioration of the lung function. After discussion of this case at the multidisciplinary discussion group meeting, the diagnosis of lung adenocarcinoma stage IVB was retained. The patient was planned for chemotherapy. Unfortunately, he presented a massive hemoptysis and succumbed to his disease despite a bronchial arterial embolization.
Fig. 1.
Fine-needle aspiration cytology (FNAC) of the thyroid nodule (A) and a cervical lymphadenopathy (B): clusters of tumor cells with basophilic cytoplasm and atypical nucleated nuclei.
Pathological examination of the lymphadenopathy (Biopsy):
(C) (HEx100): Lymph node tissue is massively infiltrated by carcinomatous proliferation
(D) (HEx400): Ganglion tumor cells are polygonal with eosinophilic cytoplasm and moderately atypical nuclei, mitosis are numerous. (E): Immunohistochemistry (IHC) testing for CK7: Tumor cells are positive for CK7.
Fig. 2.
Thoraco-abdomino-pelvic Computed Tomography (CT) scan in axial section and mediastinal window showing:
A + B: Hypodense left thyroid nodule.
C + D: Right mediastino-hilar tumor mass of 11 cm long axis with invasion of the carina, pericardium, esophagus with thrombosis of the right inferior pulmonary vein, of the right sub claviar vein as well as significant mass effect on the right internal jugular vein.
Multiple adenomegalies of secondary appearance mediastinal hilar bilateral and left axillary.
E: Bilateral renal, left adrenal and splenic secondary lesions.
3. Discussion
Despite an important vascular supply of the thyroid gland, the latter is rarely reported to be a metastatic site of non-thyroid tumors especially non-small cell lung cancer [7]. Thyroid metastasis represent only 1.9% of all thyroid fine-needle aspiration biopsies in a malignant context according to a multicenter study conducted in USA and Europe [1]. In our case, the thyroid lesion was the revealing sign of lung adenocarcinoma. To the best of our knowledge, only five cases of thyroid metastasis from lung adenocarcinoma have been reported in literature [6,8].
Such metastasis were seen in heavy smokers with an extensive metastatic stage [7]. When the primary cancer site is not known, distinguishing between metastasis and a primary thyroid cancer is challenging. In fact, there is no specific clinical or radiological features of the thyroid node to retain an accurate diagnosis [9]. In addition, The thyroid hormone balance is often within normal ranges [7]. Rarely thyroid dysfunction has been reported. The latter is due to gland destruction and infiltration by the tumor [10]. Hence, histopathological examination with IHC are necessary for the diagnosis. Different procedures are proposed for the pathological confirmation.
FNAC with ultrasound guidance is a widely used and a well established procedure [11]. In fact, abundant cellularity of metastasis associated to IHC often lead to a diagnosis [6,12,13]. But sometimes FNA may not afford enough tumor cells to retain a diagnosis and a biopsy is then required. Also, it may be difficult to make the difference between a primary thyroid cancer and a metastasis because of a dedifferentiation of the tumor or a lack of immunomarkers expression like TTF1 [13,14]. Some authors advocate surgery for thyroid metastasis depending on the type of the primary cancer if known, the site of the metastatic nodule, and the stage of the tumor (number of metastasis) [5]. In our case, the diagnosis of the thyroid metastasis was based on FNAC, which was further confirmed by the lymphadenopathy biopsy and IHC examination. The latter was not done for the FNAC samples because these aspirations were carried out in another structure before referring the patient to our hospital.
Treatment options for metastatic thyroid cancers depends on the primary neoplasm location, stage of the disease, and the Performance Status (PS) of the patient [7,10,12]. Surgery can be indicated in case of isolated metastasis to the thyroid [6]. For polymetastatic cancers, chemotherapy or targeted therapy are the recommended choices. Radiotherapy is a palliative procedure to treat symptoms caused by metastatic thyroid masses [15].
Survival of patients having a neoplasm with thyroid metastasis depends on the primary cancer site and the presence of other distant metastasis [6]. Thyroid metastasis are associated with a poor prognosis [7]. The mean survival rate is about 19 months [13]. Patients with a single thyroid metastatic lesion can benefit from surgery with a better prognosis [6,7,13,15]. Multiple metastases are associated to a worst evolution with a 5% survival rate at 5 years [16]. The pejorative prognosis of our case is in line with other published cases [10].
4. Conclusion
We reported a rare case of thyroid metastasis revealing a polymetastatic lung adenocarcinoma. Ultrasonography guided thyroid fine-needle aspiration cytology may help in the diagnostic approach. Metastases to the thyroid gland have been associated with poor outcomes. Even rarely seen, thyroid metastatic nodules related to nonthyroidal neoplasms must be evoked in the diagnostic approach of a thyroid lesion. The conjunction of clinical, radiological, cytological and histopathological features are necessary before any therapeutic decision.
Declaration of competing interest
None.
Acknowledgements
None.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.rmcr.2020.101065.
Contributor Information
Ahmed Ben Saad, Email: ahmedbensaad28@yahoo.fr.
Saousen Cheikh Mhamed, Email: saoussen.cheikh@gmail.com.
Asma Migaou, Email: migaou.asma@gmail.com.
Manel Njima, Email: manelnjima@yahoo.fr.
Asma Achour, Email: doc.asma.achour@hotmail.com.
Nesrine Fahem, Email: nesrinefahem@yahoo.fr.
Naceur Rouatbi, Email: naceur.rouatbi@rns.tn.
Samah Joobeur, Email: samah.joobeur@rns.tn.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Appendix A. Supplementary data
References
- 1.Pusztaszeri M., Wang H., Cibas E.S., Powers C.N., Bongiovanni M., Ali S. Fine-needle aspiration biopsy of secondary neoplasms of the thyroid gland: a multi-institutional study of 62 cases. Canc. Cytopathol. 2015 Jan;123(1):19–29. doi: 10.1002/cncy.21494. [DOI] [PubMed] [Google Scholar]
- 2.Lam K.Y., Lo C.Y. Metastatic tumors of the thyroid gland: a study of 79 cases in Chinese patients. Arch. Pathol. Lab Med. 1998;122(1):37–41. [PubMed] [Google Scholar]
- 3.Chung A.Y., Tran T.B., Brumund K.T., Weisman R.A., Bouvet M. Metastases to the thyroid: a review of the literature from the last decade. Thyroid. 2012 Mar;22(3):258–268. doi: 10.1089/thy.2010.0154. [DOI] [PubMed] [Google Scholar]
- 4.Narendra H., Ramana R.N., Revanth G., Rukmangadha N., Ananth P., Manickavasgam M. A case of synchronous isolated thyroid metastasis from a primary lung cancer presented as thyroid primary: diagnostic challenge! Lung India. 2016;33(3):326–329. doi: 10.4103/0970-2113.180912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dequanter D., Lothaire P., Larsimont D., De Saint-Aubain De Somerhausen N., Andry G. Métastases intra-thyroids: series of 11 cases. Ann. Endocrinol. 2004;65(3):205–208. doi: 10.1016/s0003-4266(04)95672-7. [DOI] [PubMed] [Google Scholar]
- 6.Khalil J., Elomrani F., Benoulaid M., Elkacemi H., Kebdani T., Errihani H., Benjaafar N. Isolated thyroid metastasis revealed an unknown lung adenocarcinoma: a case report. J. Med. Case Rep. 2015 Sep 27;9:221. doi: 10.1186/s13256-015-0663-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Can A.S., Köksal G. Thyroid metastasis from small cell lung carcinoma: a case report and review of the literature. J. Med. Case Rep. 2015 Oct 7;9:231. doi: 10.1186/s13256-015-0707-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao J., Yu Y.E., Li N.N., Wu Y.X., Shi J.N., Fang M.Y. Thyroid metastasis from non-small cell lung cancer. Int. J. Clin. Exp. Pathol. 2019 Aug 1;12(8):3013–3021. [PMC free article] [PubMed] [Google Scholar]
- 9.Albany C., Jain A., Ulbright T.M., Einhorn L.H. Lung cancer, thyroid cancer or both: an unusual case presentation. J. Thorac. Dis. 2011 Dec;3(4):271–273. doi: 10.3978/j.issn.2072-1439.2011.02.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dao A., Jabir H., Taleb A., Benchakroun N., Bouchbika Z., Nezha T. Lung adenocarcinoma with thyroid metastasis: a case report. BMC Res. Notes. 2017 Mar 21;10(1):130. doi: 10.1186/s13104-017-2449-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim T.Y., Kim W.B., Gong G., Hong S.J., Shong Y.K. Metastasis to the thyroid diagnosed by fine-needle aspiration biopsy. Clin. Endocrinol. (Oxf) 2005;62(2):236–241. doi: 10.1111/j.1365-2265.2005.02206.x. [DOI] [PubMed] [Google Scholar]
- 12.Prameela C.G., Ravind R., Sruthi K. Nonthyroidal metastatic lesion in thyroid: a missed diagnosis and a lesson learnt. J. Canc. Res. Therapeut. 2019;15(3):665–668. doi: 10.4103/jcrt.JCRT_1043_16. [DOI] [PubMed] [Google Scholar]
- 13.Basu A., Sen S., Das Chaudhuri A. Thyroid metastasis from lung cancer: a case series and review of the literature in Indian context. Hell. J'. Surg. 2016;88(3):176–180. doi: 10.1007/s13126-016-0311-0. [DOI] [Google Scholar]
- 14.Travis W.D. Pathology of lung cancer. Clin. Chest Med. 2002;23(1):65–81. doi: 10.1016/s0272-5231(03)00061-3. viii. [DOI] [PubMed] [Google Scholar]
- 15.Feldman E.R., Eagan R.T., Schaid D.J. Metastatic bronchoalveolar carcinoma and metastatic adenocarcinoma of the lung: comparison of clinical manifestations, chemotherapeutic responses and prognosis. Mayo Clin. Proc. 1992;67(1):27–32. doi: 10.1016/s0025-6196(12)60273-0. [DOI] [PubMed] [Google Scholar]
- 16.Heffess C.S., Wenig B.M., Thompson L.D. Metastatic renal cell carcinoma to the thyroid gland: a clinicopathologic study of 36 cases. Cancer. 2002;95(9):1869–1878. doi: 10.1002/cncr.10901. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.