Abstract
Recent studies have suggested that an increased peripheral monocyte count predicts a poor outcome in fibrosing interstitial lung disease (ILD). However, the association between an increased monocyte count and acute exacerbations (AEs) of fibrosing ILD remains to be elucidated. Our retrospective cohort study aimed to assess the impact of peripheral monocyte count on AEs of fibrosing ILD. We analyzed the electronic medical records of 122 consecutive patients with fibrosing ILD and no prior history of an AE, who were treated with anti-fibrotic agents from August 2015 to December 2018. We determined their peripheral monocyte counts at anti-fibrotic agent initiation and performed univariate and multivariate Cox regression analyses of time-to-first AE after anti-fibrotic agent initiation to assess the impact of monocyte count on AEs of fibrosing ILD. Twenty-six patients developed an AE during the follow-up period, and there was an increased monocyte count at anti-fibrotic agent initiation in these patients compared to those who did not develop an AE. There was also a significantly shorter time-to-first AE of fibrosing ILD in patients with a higher absolute monocyte count. Subgroup analyses indicated similar results regardless of the idiopathic pulmonary fibrosis diagnoses. This association was independently significant after adjusting for the severity of the fibrosing ILD. Using our results, we developed a simple scoring system consisting of two factors—monocyte count (<>380 µL−1) and ILD-gender, age, physiology score (<>4 points). Our findings suggest that the absolute monocyte count is an independent significant risk factor for AE in patients with fibrosing ILD. Our simple scoring system may be a predictor for AEs of fibrosing ILD, although further studies are needed to verify our findings.
Keywords: Acute exacerbation, fibrosing interstitial lung disease, idiopathic pulmonary fibrosis, monocyte
Background
Fibrosing interstitial lung disease (ILD) has a progressive phenotype characterized by declining lung function, worsening quality of life, and ultimately, early mortality.1,2 Idiopathic pulmonary fibrosis (IPF)is a prototype of progressive fibrosing ILDs (PF-ILDs)1 and is associated with substantially reduced health-related quality of life and survival. The disease course of IPF varies and is unpredictable; however, the median survival time from diagnosis has been reported to range from 3 years to 5 years.3–5
Annually, approximately 5–10% of patients with IPF experience acute respiratory worsening, that is, an acute exacerbation (AE).6,7 An AE is a major cause of morbidity and mortality among patients with IPF.4 It can also occur in ILDs other than IPF and is associated with a PF phenotype and significant morbidity.8,9 Although physiologically and functionally advanced disease has been recognized as a risk factor for AE,7 the prediction of its occurrence remains challenging. Several biomarkers have been reported to be predictors of AE. For example, baseline KL-6 has been reported to predict an increased risk for AE of IPF and is commonly measured in clinical settings in Japan.10 However, other studies have reported that KL-6 cannot reliably predict increased risk for AE.11,12 Therefore, there is a need to identify biomarkers that can easily and reliably predict an increased risk for AE.
Recently, Scott et al. reported that an elevated monocyte count is an independent predictor of poor prognosis in patients with IPF and other fibrotic disorders, including systemic sclerosis, myelofibrosis, and hypertrophic cardiomyopathy.13 We hypothesize that a higher absolute monocyte count is associated with an increased risk for AE of fibrosing ILD. To address this clinical question, we conducted a retrospective cohort study in a real-world setting.
Methods
This single-center retrospective study was approved by the Institutional Review Board of Saiseikai Kumamoto Hospital (IRB No. 502) and was conducted in accordance with the Declaration of Helsinki. Given that this was a retrospective study, the need for informed consent from participants was waived based on the “Ethical Guidelines for Medical and Health Research Involving Human Subjects” presented by the Ministry of Health, Labour, and Welfare.
Patients
We used electronic medical record data to identify consecutive patients with PF-ILD and without a history of AE who were treated with anti-fibrotic agents with treatment initiation between August 1, 2015, and December 31, 2018, at Saiseikai Kumamoto Hospital. All the patients received anti-fibrotic agents as standard of care. Study follow-up included an inpatient visit or a phone call by a research team member. The date of the last follow-up was identified as the date of death, last in-person visit, or last research phone call. The collected data were locked on June 30, 2019. Subjects were censored if they (1) had not experienced events by June 30, 2019, or (2) were lost to follow-up.
We also collected demographic, clinical, and pathological data from the electronic medical records. The clinical characteristics identified for all patients included age at anti-fibrotic agent initiation, gender, time from first visit to anti-fibrotic agent initiation (months), surgical lung biopsy (yes or no), clinical diagnosis of PF-ILD at anti-fibrotic agent initiation(IPF vs. others), serum KL-6 level, serum lactate dehydrogenase (LDH) level, ILD-gender, age, physiology (GAP) score,14 smoking history (never or ever smoker), and updated Charlson Comorbidity Index.15 We determined and analyzed the absolute monocyte count (µL−1), as part of a complete blood count, at the day (±2 days) of anti-fibrotic agent initiation. Further, we determined the baseline serum KL-6 and LDH levels on the day of or within 7 days before anti-fibrotic agent initiation. We calculated the ILD-GAP score from data obtained at anti-fibrotic agent initiation. We made the AE diagnosis of interstitial pneumonia based on the definition of AE of IPF proposed by Collard et al.7 from August 2016. Before August 2016, the clinical diagnosis of AE was made based on the Japanese Respiratory Society Criteria.16 These patients were subsequently re-evaluated and confirmed using the collard criteria. Moreover, we made the IPF diagnosis based on the IPF diagnostic criteria proposed by the American Thoracic Society/European Respiratory Society/Japanese Respiratory Society/Latin American Thoracic Association.6 Further, we included patients with features of possible usual interstitial pneumonia (UIP) and traction bronchiectasis on high-resolution computed tomography (HRCT) and no surgical lung biopsy, as previously described.17 The diagnosis of fibrosing ILD other than IPF was established after the discussion and integration of clinical, radiological, bronchoalveolar lavage, and pathological findings, as well as disease behavior and treatment response.
Statistical analyses
We summarized the patients’ baseline characteristics using percentages for categorical variables and medians and interquartile ranges (IQR) for continuous variables. We used the Mann–Whitney rank-sum test and Fisher’s exact test to perform between-group comparisons. We determined cutoff values using the receiver operating characteristic (ROC) curve by calculating the area under the curve (AUC) for the monocyte count and ILD-GAP scores. We used the Spearman’s rank correlation coefficient to assess the association between monocyte count and variables that could be potential risk factors for AE.
We defined time-to-event end points as the time from anti-fibrotic agent initiation to the first admission for AE. Further, we estimated time-to-end end points using the Kaplan–Meier method based on events, compared with Gray’s test. We used the Cox proportional hazards model to estimate the hazard ratio (HR) and its confidence interval (CI) in both univariate and multivariate analyses. To adjust for possible confounding variables, we used a multivariable Cox proportional-hazards model to determine the adjusted hazard ratio (aHR) and 95% CI using an a priori covariable of the ILD-GAP score. This covariable was used because we had previously reported that the ILD-GAP score could be a predictor of AE.12 We checked for the proportional hazards assumption using statistical tests. All tests were two-sided and performed at a significance level of 0.05. All statistical analyses were performed using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan),18 which is a graphical user interface for R Version 3.2.2 (The R Foundation for Statistical Computing, Vienna, Austria).
Results
We analyzed 122 consecutive patients with fibrosing ILD who were treated with anti-fibrotic agents without a previous history of AE. We excluded three patients who began anti-fibrotic agents after AE.
Table 1 presents the baseline demographic and clinical characteristics of the patients.
Table 1.
Baseline clinical characteristics of the patients.
| All | With AE | Without AE | p Value | |
|---|---|---|---|---|
| n = 122 | n = 26 | n = 96 | ||
| Age (years), median (IQR) | 68 (65, 73) | 66.5 (64, 68.75) | 69 (66, 74) | 0.084 |
| Male, n (%) | 89 (73.0) | 23 (88.5) | 66 (68.8) | 0.049 |
| Smoking history, yes, n (%) | 82 (67.8) | 17 (68.0) | 65 (67.7) | 1 |
| Comorbidity index, median (IQR) | 1 (1, 1) | 1 (1, 1) | 1 (1, 1) | 0.396 |
| Diagnosis of fibrosing ILD, n (%) | ||||
| IPF | 94 (77.0) | 17 (65.4) | 77 (80.2) | 0.12 |
| non-IPF | 28 (23.0) | 9 (34.6) | 19 (19.8) | |
| CT pattern, n (%) | ||||
| UIP | 85 (69.7) | 18 (69.2) | 67 (69.8) | 0.849 |
| Probable UIP | 28 (23.0) | 7 (26.9) | 21 (21.9) | |
| Indeterminate for UIP | 1 (0.8) | 0 (0.0) | 1 (1.0) | |
| Alternative diagnosis | 8 (6.6) | 1 (3.8) | 7 (7.3) | |
| Surgical lung biopsy, yes, n (%) | 36 (29.5) | 11 (42.3) | 25 (26.0) | 0.145 |
| ILD-GAP score, median (IQR) | 3 (2, 4) | 3.5 (3.0, 4.0) | 3.0 (2.0, 4.0) | 0.028 |
| ILD-GAP score, n (%) | 0.039 | |||
| 0–3 point | 76 (63.9) | 13 (50.0) | 65 (67.7) | |
| 4–5 point | 36 (29.5) | 8 (30.8) | 28 (29.2) | |
| 6–8 point | 8 (6.6) | 5 (19.2) | 3 (3.1) | |
| FVC % predicted, median (IQR) | 74.3 (61.3, 83.4) | 67.0 (55.1, 81.9) | 76.3 (62.8, 85.1) | 0.084 |
| DLCO % predicted, median (IQR) | 58.6 (47.2, 71.4) | 49.2 (38.2, 65.3) | 59.5 (49.5, 74.2) | 0.012 |
| KL-6 (U mL−1), median (IQR) | 1131 (793, 1912) | 1538 (988, 2198) | 1016 (773, 1730) | 0.055 |
| LDH (U mL−1), median (IQR) | 230 (202, 256) | 237 (207, 263) | 226 (200, 253) | 0.144 |
| Corticosteroid use, yes, n (%) | 27 (22.1) | 15 (57.7) | 12 (12.5) | <0.001 |
| Corticosteroid dose at baseline, median (IQR) | 0 (0, 0) | 7.5 (0, 15) | 0 (0, 0) | <0.001 |
| Absolutely monocyte count (µL−1), median (IQR) | 363 (307, 452) | 430 (328, 574) | 356 (306, 424) | 0.022 |
AE: acute exacerbation; ILD: interstitial lung disease; IPF: idiopathic pulmonary fibrosis; UIP: usual interstitial pneumonia; ILD: interstitial lung disease; GAP: gender, age, physiology; FVC: forced vital capacity; DLCO: diffusing capacity of the lungs for carbon monoxide; LDH: lactate dehydrogenase; IQR: interquartile range.
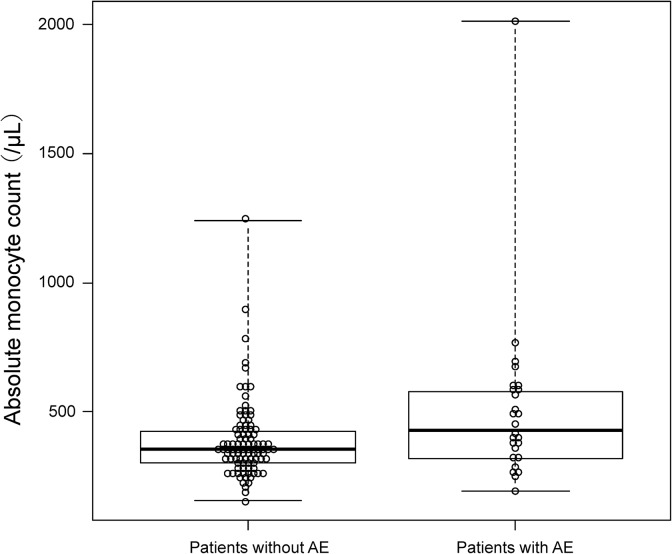
The study cohort included 89 (73%) males and the median (IQR) age of the patients was 68 (65–73). The median (IQR) follow-up time was 18.5 months (10.0–29.3). The median (IQR) time from the first visit to anti-fibrotic agent initiation was 12 months (6–36). Clinical diagnoses of fibrosing ILD at anti-fibrotic agent initiation were as follows: IPF (n = 94) and others (n = 28, chronic hypersensitivity pneumonitis = 20, collagen vascular disease-associated = 3, and unclassifiable = 5). The baseline median (IQR) values for percent predicted forced vital capacity and percent predicted diffusing capacity of the lungs for carbon monoxide (DLCO) were 70.8% (58.7–82.4) and 55.9% (45.1–68.4), respectively. Almost all the patients (n = 113: 92.7%) had UIP or probable UIP pattern on HRCT. The median (IQR) ILD-GAP score was 3 (2–4). There were significant between-group differences in sex, ILD-GAP score, and DLCO. The baseline absolute monocyte count was significantly higher in the patients who developed AE compared to those who did not. The median (IQR) monocyte count was 430 µL−1 (328–574) and 356 µL−1 (306–424) in patients who developed AE and those who did not, respectively. Figure 1 presents the distribution of the monocytes counts among our subjects.
Figure 1.
Comparison of the absolute monocyte count between patients who developed AE and those who did not. AE: acute exacerbation.
Table 2 presents the correlation analysis of the monocyte count and the patients’ backgrounds.
Table 2.
Correlation analysis of the monocyte count and patients’ baseline characteristics (Spearman’s rank correlation coefficients).a
| Monocyte counts | ||
|---|---|---|
| ρ | p Value | |
| Age | −0.0987 | 0.28 |
| FVC % predicted | 0.0327 | 0.721 |
| DLCO % predicted | −0.159 | 0.0823 |
| ILD-GAP score | 0.141 | 0.122 |
| KL-6 | 0.116 | 0.202 |
| LDH | −0.00502 | 0.956 |
| IPF diagnosis | 0.0861 | 0.346 |
| Time from first visit to anti-fibrotic agent initiation (month) | 0.0277 | 0.762 |
| Corticosteroid dose at baseline | −0.0168 | 0.854 |
ρ: Spearman’s rank correlation coefficients; FVC: forced vital capacity; DLCO: diffusing capacity of the lungs for carbon monoxide; ILD-GAP: interstitial lung disease-gender, age, physiology; LDH: lactate dehydrogenase; IPF: idiopathic pulmonary fibrosis.
a n = 122.
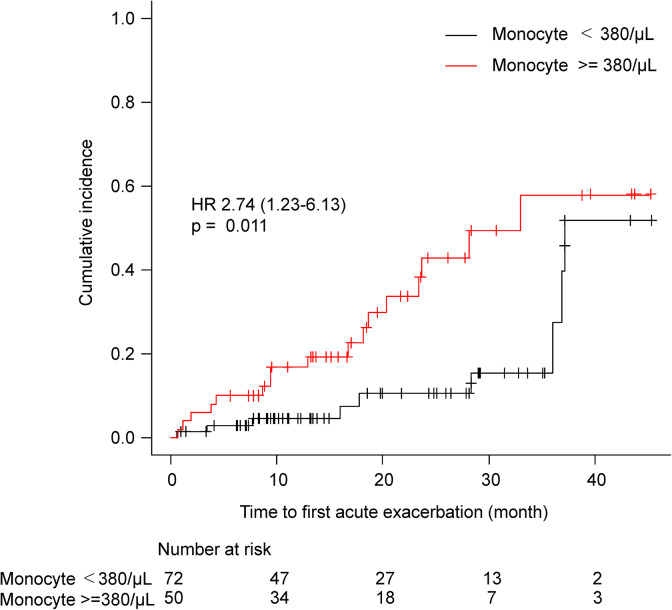
There were no significant differences between monocytes’ counts and lung functional impairments. Further, there were no associations between monocyte counts and possible variables other than pulmonary function (e.g. fibrosing ILD diagnosis, sex, corticosteroid use, and time from the first visit to anti-fibrotic agent initiation). The ROC curve analysis indicated that the cutoff level of monocyte count to predict for AE was 378 µL−1 (AUC: 0.647) (Online Supplementary Material, Figure 1), so we selected 380 µL−1 as the cutoff point. Figure 2 shows the time-to-first AE from anti-fibrotic agent initiation. The incidence of AE from anti-fibrotic agent initiation was significantly higher in patients with a high monocyte count compared to those with a low monocyte count (Gray’s test p = 0.011). A separate analysis of patients with IPF and those without revealed similar findings (Online Supplementary Material, Figures 2 and 3). Even among patients with non-IPF, higher monocytes counts were associated with a high risk of AE (p = 0.034).
Figure 2.
Cumulative incidence of AE of fibrosing ILD based on the monocyte count. AE: acute exacerbation; ILD: interstitial lung disease.
Table 3 presents the findings of univariate and multivariate Cox analyses of the association between the monocyte count and AE of fibrosing ILDs.
Table 3.
Univariate and multivariate analyses of Cox proportional hazard analysis.
| All patients n = 122 event = 26 | |||
|---|---|---|---|
| HR | 95% CI | p Value | |
| Univariate analysis | |||
| Monocyte count per 10 increase | 1.02 | 1.01–1.03 | <0.001 |
| Monocyte ≥380 µL−1 | 2.74 | 1.22–6.17 | 0.014 |
| Multivariate analysis | |||
| Model A | |||
| Monocyte count per 10 increase (continuous variables) | 1.02 | 1.01–1.03 | <0.001 |
| ILD-GAP score | 1.6 | 1.20–2.13 | 0.001 |
| Model B | |||
| Monocyte ≥380 µL−1 (categorical variable) | 2.83 | 1.25–6.44 | 0.013 |
| ILD-GAP score | 1.64 | 1.22–2.21 | <0.001 |
HR: hazard ratio; CI: confidence interval; ILD-GAP: interstitial lung disease-gender, age, physiology.
Univariate analysis indicated an association between increased monocyte counts (per 10 µL−1 increase) and shorter time to first AE (HR, 1.02; 95% CI: 1.01–1.03; p < 0.001). The univariate analysis also indicated an association between the monocyte count (380 µL−1 or over) as the categorical variable and a shorter time to first AE (HR, 2.74; 95% CI: 1.22–6.17; p = 0.014). Previously we showed that the ILD-GAP score could be an independent risk factor for AE. The association between the increased monocyte counts and a shorter time to the first AE remained similar after adjusting for the ILD-GAP score (monocyte count per 10 µL−1 increase as a continuous variable: aHR, 1.02; 95% CI: 1.01–1.03; p < 0.001; monocyte count ≥ 380 µL−1 as the categorized variable: aHR, 2.83; 95% CI: 1.25–6.44; p = 0.013; Table 3).
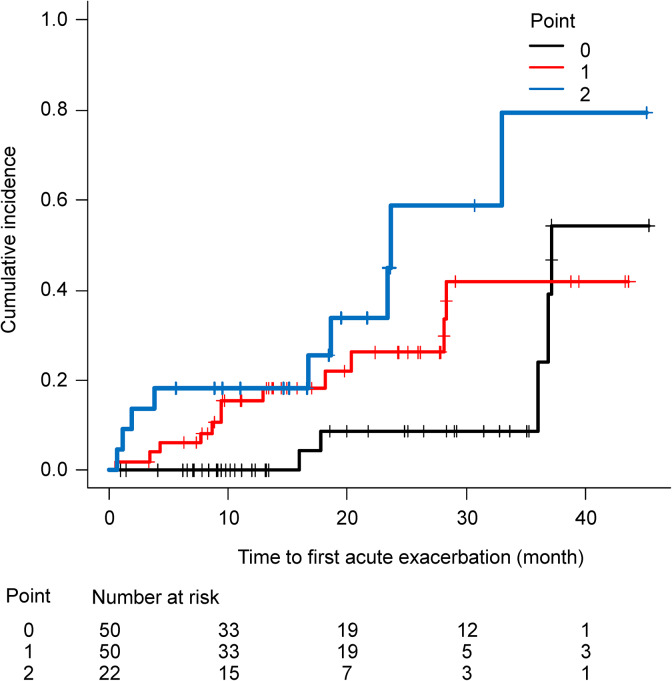
With these results, we built a simple prognostic scoring system (0–2 point) by combining the absolute monocyte count (1 point if ≥380 µL−1 and 0 point if <380 µL−1) and the ILD-GAP score (0 point for ILD-GAP 0–3 and 1 point for ILD-GAP 4–8). The C-statistic of this model was 0.703. The HR (per 1 point of this model) was 2.21 (95% CI: 1.31–3.74, p = 0.003). Figure 3 presents the Kaplan–Meier estimate of AE according to this index.
Figure 3.
Incidence of AE of fibrosing ILD according to the scoring system based on the monocyte count (≥380 µL−1 or not) and ILD-GAP score (ILD-GAP score ≥4 points or not). Risk factor “0” corresponds to an ILD-GAP score <4 and an absolute monocyte count <380 µL−1, risk factor “1” corresponds to only one of the two parameters being above the cutoff value, and risk factor “2” corresponds to both parameters being above the cutoff level. AE: acute exacerbation; ILD: interstitial lung disease; GAP: gender, age, physiology.
Discussion
Our findings demonstrate the possible use of absolute monocyte count as an independent significant risk factor for AE in patients with fibrosing ILDs. The simple scoring system, consisting of the ILD-GAP score and the monocyte count, may be a good predictor of AE-IPF.
Although anti-fibrotic agents have become a standard of care for patients with IPF and other fibrosing ILDs, they are still not widely used. Real-world data from Europe and the United States suggest that approximately 40% of patients with IPF are yet to receive an anti-fibrotic agent.19 This statistic may be attributed to physician perception of “mild” or “stable” disease that does not warrant therapy in patients.19 While AE cases have been reported more frequently in IPF patients with severe physiological impairment and comorbidities, AE can also occur in less advanced stages and even in the subclinical period.20 AE is a major cause of death associated with IPF and other fibrotic ILDs4 and AE occurrence has been reported to have a significant impact on the short-term and overall survival of patients with these disorders.9,11 Further, anti-fibrotic agents have been reported to reduce the risk of AE21 and hospitalization.22,23 Determining the risk for AE using the absolute peripheral monocyte count may help inform physician decisions about treatment with anti-fibrotic agents and the education of patients and their families.
Scott et al. reported that patients with a high monocyte count at diagnosis maintained their high count throughout the course of their disease.13 Our findings indicate an association of the monocyte count and pulmonary function impairment with time from the first visit to anti-fibrotic agent initiation. Furthermore, our findings indicate an independent association between a higher absolute monocyte count and an increased risk for AE of fibrosing ILDs, even after adjusting for ILD severity (ILD-GAP score). The GAP model is reported to accurately predict the risk of death in chronic ILD and mortality in major chronic ILD subtypes and all stages of the disease. We reported that the ILD-GAP score could be a risk factor of AE.12 Even after an adjusted ILD-GAP score that expresses disease severity and risk of AE, the association between a higher absolute monocyte count and an increased risk for AE of fibrosing ILDs remained significant. These findings are similar to those of Scott et al., indicating a similarity of the monocyte-derived risk profile throughout time. Further, the findings of these two studies suggest that the absolute monocyte count might be a reproducible risk factor for AE of fibrosing ILDs at any time in the disease course. Some studies have, however, reported a relationship between the monocyte count and decreased pulmonary function.24 The inconsistency of these results could be attributed to the timing of monocyte data collection and small sample size. However, there is a need for further studies to assess the association between an increased monocyte count and pulmonary function impairment.
Scott et al. defined a high monocyte count as that of ≥950 µL−1, which is a common cutoff value in studies on monocytosis. In our cohort, only 2 (1.6%) patients had a monocyte count of ≥950 µL−1. Therefore, we used a cutoff value of 380 µL−1 in this study. The different cutoff values could be attributed to the use of different endpoints (AE or overall survival [OS]). There remains a need for further research on the cutoff level of monocyte count in the management of patients with fibrosing ILDs.
We could not determine the underlying reason for the association between an increased monocyte count and the risk for AE. Monocytes have been reported to play important roles, not only in tissue repair but also in the mechanisms of fibrosis and tissue regeneration.25 One hypothesis is that the increased monocyte count expresses the disease activity of fibrosing ILDs and those patients with an increased monocyte count might simply be hypersensitive to AE triggers. An alternate hypothesis is based on the association between the microbiome and monocytes. Generally, an increased monocyte blood count occurs in response to chronic infections. Recent studies have assessed the relationships between chronic infection and disease progression of IPF26 and AE-IPF.27 A recent study demonstrated that the microbiome, monocytes, and other immune cells network in diseases associated with aging.28 Further studies will need to assess the relationship between an increased monocyte count, AE, and disease progression of fibrosing ILDs. These studies may even lead to the development of new therapeutic strategies for patients with fibrosing ILDs.
Medical therapies (including corticosteroids, immunosuppressants, and anti-fibrotic drugs) might have an unknown effect on the monocyte count and prognosis.
This study has several limitations. The biggest limitation of this study is its retrospective nature, which may have a number of associated biases. We included consecutive patients to minimize this bias; however, we could not exclude the possibility of the limitation remains. There were, however, no missing data regarding the baseline characteristics, which may strengthen its findings. Second, given the relatively small number of cohort cases, the lack of statistical significance may be due to insufficient power. Furthermore, given the relatively small number of AE cases, we could not perform a multivariate analysis with many factors. Third, our cohort did not include controls who were not taking anti-fibrotic agents. However, since anti-fibrotic agents are the standard care for patients with PF-ILDs,29 this limitation might be trivial. Fourth, while we generated a clinical model incorporating monocyte count, we could not validate it in this study, therefore, this model may only be taken as a hypothesis and should be evaluated using another cohort in the future.
Conclusions
In conclusion, this retrospective study demonstrated the absolute monocyte count as an independent significant risk factor for AE in patients with fibrosing ILDs. The monocyte count may be a useful and simple biomarker to assist clinical decisions for therapeutic interventions and follow-up in patients with fibrosing ILDs. Further prospective, large-scale, real-world data are needed to support and confirm the impact of increased monocytes on AE-fibrosing ILDs.
Supplemental material
Supplemental Material, eFigure1 for Monocyte count and the risk for acute exacerbation of fibrosing interstitial lung disease: A retrospective cohort study by Kodai Kawamura, Kazuya Ichikado, Keisuke Anan, Yuko Yasuda, Yuko Sekido, Moritaka Suga, Hidenori Ichiyasu and Takuro Sakagami in Chronic Respiratory Disease
Supplemental Material, eFigure2 for Monocyte count and the risk for acute exacerbation of fibrosing interstitial lung disease: A retrospective cohort study by Kodai Kawamura, Kazuya Ichikado, Keisuke Anan, Yuko Yasuda, Yuko Sekido, Moritaka Suga, Hidenori Ichiyasu and Takuro Sakagami in Chronic Respiratory Disease
Supplemental Material, eFigure3 for Monocyte count and the risk for acute exacerbation of fibrosing interstitial lung disease: A retrospective cohort study by Kodai Kawamura, Kazuya Ichikado, Keisuke Anan, Yuko Yasuda, Yuko Sekido, Moritaka Suga, Hidenori Ichiyasu and Takuro Sakagami in Chronic Respiratory Disease
Supplemental Material, e_Figure_legends for Monocyte count and the risk for acute exacerbation of fibrosing interstitial lung disease: A retrospective cohort study by Kodai Kawamura, Kazuya Ichikado, Keisuke Anan, Yuko Yasuda, Yuko Sekido, Moritaka Suga, Hidenori Ichiyasu and Takuro Sakagami in Chronic Respiratory Disease
Acknowledgments
We thank Drs Yoshihiko Sakata, Naoki Shingu, Jyunpei Hisanaga, Yoshitomo Eguchi, Tatuya Nitawaki, Miwa Iio, Kenta Nishiyama, and Kazunori Nakamura for their clinical assistance.
Author contributions: KK and KI planned the project; KK analyzed the data and wrote the manuscript; and KK, KI, KA, YY, YS, and HI collected the data. KI, MS, HI, and TS interpreted the results and helped to write the manuscript. All authors reviewed, revised, and approved the manuscript for submission. KK and KI are the guarantors of the article, taking responsibility for the integrity of the work as a whole.
Availability of data and materials: The datasets analyzed in the present study are available from the corresponding author upon reasonable request.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Kodai Kawamura  https://orcid.org/0000-0002-6981-0648
https://orcid.org/0000-0002-6981-0648
Supplemental material: Supplemental material for this article is available online.
References
- 1. Cottin V, Wollin L, Fischer A, et al. Fibrosing interstitial lung diseases: knowns and unknowns. Eur Respir Rev 2019; 28(151): 180100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kolb M, Vasakova M. The natural history of progressive fibrosing interstitial lung diseases. Respir Res 2019; 20(1): 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Raghu G, Chen SY, Yeh WS, et al. Idiopathic pulmonary fibrosis in US Medicare beneficiaries aged 65 years and older: incidence, prevalence, and survival, 2001–11. Lancet Respir Med 2014; 2(7): 566–572. [DOI] [PubMed] [Google Scholar]
- 4. Natsuizaka M, Chiba H, Kuronuma K, et al. Epidemiologic survey of Japanese patients with idiopathic pulmonary fibrosis and investigation of ethnic differences. Am J Respir Crit Care Med 2014; 190(7): 773–779. [DOI] [PubMed] [Google Scholar]
- 5. Richeldi L, Collard HR, Jones MG. Idiopathic pulmonary fibrosis. Lancet 2017; 389(10082): 1941–1952. [DOI] [PubMed] [Google Scholar]
- 6. Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011; 183(6): 788–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Collard HR, Ryerson CJ, Corte TJ, et al. Acute exacerbation of idiopathic pulmonary fibrosis. An international working group report. Am J Respir Crit Care Med 2016; 194(3): 265–275. [DOI] [PubMed] [Google Scholar]
- 8. Kolb M, Bondue B, Pesci A, et al. Acute exacerbations of progressive-fibrosing interstitial lung diseases. Eur Respir Rev 2018; 27(150): pii:180071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Suzuki A, Kondoh Y, Brown KK, et al. Acute exacerbations of fibrotic interstitial lung diseases. Respirology. Epub ahead of print 19 August 2019 DOI: 10.1111/resp.13682. [DOI] [PubMed] [Google Scholar]
- 10. Ohshimo S, Ishikawa N, Horimasu Y, et al. Baseline KL-6 predicts increased risk for acute exacerbation of idiopathic pulmonary fibrosis. Respir Med 2014; 108(7): 1031–1039. [DOI] [PubMed] [Google Scholar]
- 11. Kakugawa T, Sakamoto N, Sato S, et al. Risk factors for an acute exacerbation of idiopathic pulmonary fibrosis. Respir Res 2016; 17(1): 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kawamura K, Ichikado K, Ichiyasu H, et al. Acute exacerbation of chronic fibrosing interstitial pneumonia in patients receiving antifibrotic agents: incidence and risk factors from real-world experience. BMC Pulm Med 2019; 19(1): 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Scott MKD, Quinn K, Li Q, et al. Increased monocyte count as a cellular biomarker for poor outcomes in fibrotic diseases: a retrospective, multicentre cohort study. Lancet Respir Med 2019; 7(6): 497–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ryerson CJ, Vittinghoff E, Ley B, et al. Predicting survival across chronic interstitial lung disease: the ILD-GAP model. Chest 2014; 145(4): 723–728. [DOI] [PubMed] [Google Scholar]
- 15. Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol 2011; 173(6): 676–682. [DOI] [PubMed] [Google Scholar]
- 16. Azuma A, Nukiwa T, Tsuboi E, et al. Double-blind, placebo-controlled trial of pirfenidone in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2005; 171(9): 1040–1047. [DOI] [PubMed] [Google Scholar]
- 17. Raghu G, Wells AU, Nicholson AG, et al. Effect of nintedanib in subgroups of idiopathic pulmonary fibrosis by diagnostic criteria. Am J Respir Crit Care Med 2017; 195(1): 78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 2013; 48(3): 452–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Maher TM, Strek ME. Antifibrotic therapy for idiopathic pulmonary fibrosis: time to treat. Respir Res 2019; 20(1): 205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ley B, Collard HR, King TE., Jr Clinical course and prediction of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2011; 183(4): 431–440. [DOI] [PubMed] [Google Scholar]
- 21. Costabel U, Inoue Y, Richeldi L, et al. Efficacy of nintedanib in idiopathic pulmonary fibrosis across prespecified subgroups in INPULSIS. Am J Respir Crit Care Med 2016; 193(2): 178–185. [DOI] [PubMed] [Google Scholar]
- 22. Ley B, Swigris J, Day BM, et al. Pirfenidone reduces respiratory-related hospitalizations in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2017; 196(6): 756–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dempsey TM, Sangaralingham LR, Yao X, et al. Clinical effectiveness of antifibrotic medications for idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2019; 200(2): 168–174. [DOI] [PubMed] [Google Scholar]
- 24. Liu YZ, Saito S, Morris GF, et al. Proportions of resting memory T cells and monocytes in blood have prognostic significance in idiopathic pulmonary fibrosis. Genomics 2019; 111(6): 1343–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature 2013; 496(7446): 445–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Han MK, Zhou Y, Murray S, et al. Lung microbiome and disease progression in idiopathic pulmonary fibrosis: an analysis of the COMET study. Lancet Respir Med 2014; 2: 548–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Molyneaux PL, Cox MJ, Wells AU, et al. Changes in the respiratory microbiome during acute exacerbations of idiopathic pulmonary fibrosis. Respir Res 2017; 18(1): 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bodogai M, Moritoh K, Kim K, et al. Aging induced changes in the microbiome affect the crosstalk and activity of monocytes and B1a cells. J Immunol 2017; 198(1 Supplement): 154.5. [Google Scholar]
- 29. Flaherty KR, Wells AU, Cottin V, et al. Nintedanib in progressive fibrosing interstitial lung diseases. New England J Med 2019; 381(18): 1718–1727. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, eFigure1 for Monocyte count and the risk for acute exacerbation of fibrosing interstitial lung disease: A retrospective cohort study by Kodai Kawamura, Kazuya Ichikado, Keisuke Anan, Yuko Yasuda, Yuko Sekido, Moritaka Suga, Hidenori Ichiyasu and Takuro Sakagami in Chronic Respiratory Disease
Supplemental Material, eFigure2 for Monocyte count and the risk for acute exacerbation of fibrosing interstitial lung disease: A retrospective cohort study by Kodai Kawamura, Kazuya Ichikado, Keisuke Anan, Yuko Yasuda, Yuko Sekido, Moritaka Suga, Hidenori Ichiyasu and Takuro Sakagami in Chronic Respiratory Disease
Supplemental Material, eFigure3 for Monocyte count and the risk for acute exacerbation of fibrosing interstitial lung disease: A retrospective cohort study by Kodai Kawamura, Kazuya Ichikado, Keisuke Anan, Yuko Yasuda, Yuko Sekido, Moritaka Suga, Hidenori Ichiyasu and Takuro Sakagami in Chronic Respiratory Disease
Supplemental Material, e_Figure_legends for Monocyte count and the risk for acute exacerbation of fibrosing interstitial lung disease: A retrospective cohort study by Kodai Kawamura, Kazuya Ichikado, Keisuke Anan, Yuko Yasuda, Yuko Sekido, Moritaka Suga, Hidenori Ichiyasu and Takuro Sakagami in Chronic Respiratory Disease