Abstract
Transplant-associated thrombotic microangiopathy (TA-TMA) after hematopoietic stem cell transplantation (HSCT) is a severe complication associated with underlying endothelial damage. Secreted and released von Willebrand factor (vWF) correlates with endothelial cell injury, but its role in predicting prognosis for TA-TMA is unclear. In this prospective study, we evaluated the relationship between vWF and the incidence of TA-TMA after HSCT. A total of 79 consecutive patients undergoing HSCT from August 2016 to June 2018 were enrolled. Twenty-three (29%) patients met the established diagnostic criteria. Patients with TA-TMA had significantly higher nonrelapse mortality compared with those without TA-TMA (78.3% vs 8.9%; P < .001). Multivariable analysis demonstrated that vWF was a predictive biomarker for TA-TMA. The vWF value was higher for the TA-TMA group (mean ± standard deviation, 380.7% ± 78.8% vs 284.9% ± 104.5%; P < .001), and the area under the curve for vWF in the diagnosis of TA-TMA was 0.756. Furthermore, patients with ≥325% vWF had a higher 2-year cumulative hazard of TA-TMA (53.1% ± 8.2% vs 7.5% ± 4.2%; P < .001) and a lower 2-year survival (32.1% ± 9.1% vs 83.7% ± 6.2%; P < .001) compared with those with <325% vWF.
Conclusion:
von Willebrand factor is a useful predictor and prognostic measure for TA-TMA, which may help clinicians identify and manage this life-threatening disease earlier.
Keywords: transplant-associated thrombotic microangiopathy (TA-TMA), von Willebrand factor (vWF), ADAMTS13
Introduction
Hematopoietic stem cell transplantation (HSCT) is an important curative therapy for many hematopoietic disorders.1 but there are many significant severe complications.2 Transplantation-associated thrombotic microangiopathy (TA-TMA) is a serious complication that occurs after HSCT3 with a reported mortality rate ranging from 60% to 90%.4
The clinical manifestations of TA-TMA include microvascular hemolytic anemia (MAHA), thrombocytopenia, microvascular thrombosis, and multiple organ failure, and the emblematic pathological manifestations include arteriole endothelium injury and intra-arteriole thrombosis.5 Although the exact pathophysiology remains unclear, the endothelial dysfunction caused by chemoradiotherapy, immunosuppressive drugs, graft-versus-host disease (GVHD), infections, and complement dysregulation play an important role.6–8
von Willebrand factor is a multimeric glycoprotein present in blood plasma, the subendothelial matrix, storage granules in endothelial cells (Weibel-Palade bodies [WPBs]), and platelets (PLTs; α-granules).9 von Willebrand factor is released into the blood circulation and combines with PLTs to form a thrombosis when endothelium is damaged.10,11 Transplant-associated thrombotic microangiopathy is a type of endothelium damage disorder, but the diagnostic role of vWF has been controversial in different reports. This study was conducted to evaluate the relationship between vWF and TA-TMA incidence.
Materials and Methods
Patients
We enrolled patients who received HSCT between August 2016 and June 2018 in Guangdong Provincial People’s Hospital and who had been monitored serially for vWF levels. All patients provided informed consent.
Transplant-Associated Thrombotic Microangiopathy Definition
Transplant-associated thrombotic microangiopathy was diagnosed using the criteria proposed by Cho et al12: (1) lactate dehydrogenase (LDH) above the upper limit of normal, (2) thrombocytopenia (<50 000 U/L) or a 50% decrease in the PLT count, (3) de novo anemia with a hemoglobin (HBG) level below the lower limit of normal or anemia that requires transfusion, (4) microangiopathic changes, which are defined as schistocytes in peripheral blood or histologic evidence of microangiopathy on tissue specimens, and (5) a negative Coombs test and the absence of coagulopathy. All laboratory criteria had to be met and documented in at least 2 consecutive tests to be confirmed as positive. The TA-TMA diagnosis date was defined as the first date when all diagnostic criteria were met. If the diagnosis of thrombotic thrombocytopenia purpura (TTP) was suspected, then an ADAMTS13 test was conducted,13 and patients with Shiga toxin-producing bacteria infection within 2 weeks prior to the diagnosis of TA-TMA were removed to rule out Shiga toxin-associated hemolytic uremic syndrome.14
Laboratory and Clinical Monitoring
All of the HSCT recipients were closely monitored for 100 days post-HSCT. Routine blood detection was performed according to standard clinical practice. von Willebrand factor was regularly monitored weekly for all patients within the first 100 days after transplantation. When TA-TMA was suspected or diagnosed, monitoring was more frequent according to clinical needs and was continued regularly until recovery. For patients without TA-TMA, as the control group, vWF was only monitored during the regular follow-up and at day 100 or when GVHD, infection, or other indications warranted. Coagulation test, LDH, liver function, renal function, urinalyses, inflammatory markers, and viral detection were monitored once weekly within 100 days after the transplant, then every 2 weeks after 100 days post-HSCT. Cyclosporine A (CsA) trough levels were monitored twice weekly. Reticulocyte percentage analysis and the Coombs test were performed when hemolysis or thrombogenesis was suspected. If the diagnosis of TTP was suspected, an ADAMTS13 test was conducted.
The level of vWF antigen (vWF:Ag) was tested by an immune turbidimetric assay kit (STA Liatest, DIAGNOSTICA STAGO, Asnières-sur-Seine, France) on STA-R Evolution analyzer (DIAGNOSTICA STAGO, Asnières-sur-Seine, France), according to the kit instructions. At the beginning of examination, the specimens were tested with 8-fold dilution, and the test results of vWF:Ag range from 0% to 420%. The normal plasma VWF:Ag level in the adult population is usually in the range of 50% to 160% recommended by DIAGNOSTICA STAGO. The laboratory staff had selected 20 healthy people in Guangzhou for reference range verification, and one of them exceeded the upper limit value and met the standards of the national standardization committee (NCCLS). This reference range could be used in our laboratory. The standard deviation (SD) was 1.7% in the 20 times intra-assay repeatability experiment and 2.5% in the 10 times inter-assay repeatability experiment. This test was performed in the clinical laboratory of our hospital.
Acute GVHD was diagnosed and graded using established criteria.15 Liver function damage assessments were based on elevated liver enzymes and bilirubin. Fungal infection was based on clinical diagnoses.
Data Collection
Data were collected from case documentation, imaging reports, color Doppler ultrasounds, laboratory indicators, gastroenterological endoscopy, and telephone follow-up. The data gathered were confirmed by 2 independent researchers, and when the diagnosis of 2 researchers was inconsistent, a clinical hematologist of the transplant center, as the third expert, participated in the diagnosis. The indicators for the TA-TMA group were collected at onset and 100 days after transplantation for the control group. If TA-TMA was diagnosed, vWF and abnormal results would continue to be monitored until recovery to normal.
Outcome and Follow-Up
The primary study end point was diagnosis of TA-TMA. We compared TA-TMA patients with non-TA-TMA patients to investigate risk factors for TA-TMA. To study whether vWF was a risk factor for death, we considered all causes of death other than suicide as a secondary end point. All surviving patients were followed up until April 15, 2019.
Statistical Analyses
The median (range) and frequencies describe continuous and categorical variables. Categorical variables were analyzed using the χ2 or Fisher exact test, whereas continuous normal variables were assessed by the independent sample t test, and abnormal variables were tested by the Wilcoxon rank sum test. Conditional logistic regression was used to examine the correlation between risk factors and TA-TMA occurrence with respect to grades 2 to 4 acute GVHD (aGVHD), liver injury, inflammatory biomarkers, and vWF. The relationship between sensitivity and specificity of VWF to the diagnosis of TA-TMA was plotted with an receiver operating characteristic (ROC) curve. Survival and competing risk analyses were performed using the Kaplan-Meier method. Statistical analyses were performed with SPSS 20.0 (IBM, Chicago, Illinois) and MedCalc (MedCalc Software, Mariakerke, Belgium). P values less than .05 were considered statistically significant.
Results
Patient Clinical Characteristics
A total of 79 consecutive patients underwent HSCT from August 2016 and June 2018. The overall incidence of TA-TMA in our patient population was 29% (23/79). The median time of TA-TMA diagnosis was 76 days (range: 7-456 days) after HSCT. The date of last follow-up for all surviving patients was April 15, 2019. The median follow-up was 17 months (range: 0 to 32 months).
The patient characteristics and laboratory results are summarized in Table 1. Patients who had TA-TMA often had fever (P < .001), hypertension (P = .008), and/or pulmonary hypertension (P = .001). In addition, risk factors for TA-TMA included grades 2 to 4 aGVHD (P = .048), liver damage (P = .023), serious gastrointestinal bleeding (P = .001), and fungal infection (P = .002). However, no significant difference was found between the TA-TMA and non-TA-TMA groups with respect to age, recipient, donor sex, diagnosis of hematopoietic malignancies, risk stratification, pretreatment, GVHD prevention scheme, or grafts.
Table 1.
Clinical Characteristics and Laboratory Markers for the 79 HSCT Recipients.a
| Characteristics | Non TA-TMA (n = 56) | TA-TMA (n = 23) | P Value |
|---|---|---|---|
| Age, median, (IQR) | 28 (22-37) | 28 (21-37) | .255 |
| Sex | 1.000 | ||
| Female | 25 (44.6%) | 10 (43.5%) | |
| Male | 31 (55.4%) | 13 (56.5%) | |
| Disease | .359 | ||
| Acute lymphoblastic leukemia | 13 (23.2%) | 9 (39.1%) | |
| Acute myeloblastic leukemia | 33 (58.9%) | 9 (39.1%) | |
| Chronic myeloid leukemia | 2 (3.6%) | 1 (4.3%) | |
| Others | 8 (14.3%) | 4 (17.4%) | |
| HLA typing | .500 | ||
| Matched | 48 (85.7%) | 18 (78.3%) | |
| Mismatched | 8 (14.3%) | 5 (21.7%) | |
| aGVHD | .465 | ||
| Yes | 26 (46.4%) | 13 (56.5%) | |
| No | 30 (53.6%) | 10 (43.5%) | |
| Grades 2 to 4 aGVHD | 11 (19.6%) | 10 (43.5%) | .048 |
| Gastrointestinal aGVHD | 7 (12.5%) | 8 (34.8%) | .030 |
| Liver damage | 27 (48.2%) | 18 (78.3%) | .023 |
| Severe gastrointestinal bleeding | 18 (32.1%) | 17 (73.9%) | .001 |
| Severe infection | 19 (33.9%) | 19 (82.6%) | <.001 |
| Virus infection | 19 (33.9%) | 8 (34.8%) | 1.000 |
| Fungal infection | 3 (5.4%) | 8 (34.8%) | .002 |
| Fever | 13 (23.2%) | 16 (69.6%) | <.001 |
| Hypertension | 5 (8.9%) | 8 (34.8%) | .008 |
| Pulmonary hypertension | 4 (7.1%) | 9 (39.1%) | .001 |
| Death | 11 (19.6%) | 18 (78.3%) | <.001 |
| Laboratory markers | |||
| HGB, g/L, mean ± SD | 94.5 ± 27.3 | 68.2 ± 8.6 | <.001 |
| PLT, 109/L, mean ± SD | 107.2 ± 86.2 | 26.4 ± 17.8 | <.001 |
| LDH, U/L, median, (IQR) | 322.5 (235.2-423.0) | 516.0 (415.0-1078.0) | .001 |
| vWF, %, mean ± SD | 284.9 ± 104.5 | 380.7 ± 78.8 | <.001 |
| D-DI, ng/mL, median, (IQR) | 510 (290-1348) | 2450 (1030-15000) | .001 |
| Urine protein, g/L, median, (IQR) | 0.3 (0.0-0.3) | 1.0 (0.2-3.0) | .015 |
| CREA, μmol/L, mean ± SD | 83.5 ± 50.3 | 129.2 ± 74.1 | .002 |
| ALB, g/L, mean ± SD | 36.2 ± 5.8 | 32.3 ± 4.2 | .001 |
| CHE, U/L, mean ± SD | 5912.1 ± 2164.3 | 4447.1 ± 2103.1 | .007 |
| PCT, ng/mL, median, (IQR) | 0.12 (0.06-0.27) | 0.91 (0.17-2.44) | <.001 |
| CSA, ng/mL, mean ± SD | 211.6 ± 66.9 | 188.1 ± 87.2 | .206 |
| C3, mg/L, mean ± SD | 910.9 ± 247.2 | 844.2 ± 184.5 | .248 |
| C4, mg/L, mean ± SD | 226.7 ± 83.9 | 206.1 ± 45.6 | .272 |
Abbreviations: aGVHD, acute GVHD; ALB, albumin; CHE, cholinesterase; CREA, serum creatinine; CSA, cyclosporine A; D-DI, D dimer; GVHD, graft-versus-host disease; HGB, hemoglobin; HLA, human leukocyte antigen; HSCT, hematopoietic stem cell transplantation; LDH, lactate dehydrogenase; PCT, procalcitonin; PLT, platelet; SD, standard deviation; TA-TMA, transplantation-associated thrombotic microangiopathy; vWF, von Willebrand factor.
a Data with a normal distribution are represented as mean ± SD, and data with abnormal distributions are represented as median and (IQR).
There were also significant differences in laboratory indicators between the TA-TMA and non-TA-TMA groups. Patients with TA-TMA had lower levels of HGB (P < .001) and PLTs (P < .001). Unsurprisingly, the level of vWF was higher for those with TA-TMA (mean ± SD, 380.7% ± 78.8% vs 284.9% ± 104.5%; P < .001). Urinary protein (P = .015), serum creatinine (CREA) (P = .002), and procalcitonin (PCT) levels were higher in the TA-TMA group (P = .018). Cholinesterase (CHE; P = .007) and albumin (ALB; P = .001) levels were lower in the TA-TMA group compared with the control group. However, there were no statistically differences in complement C3, C4, and CSA.
Independent Early Sign and Outcome
von Willebrand factor was elevated at a median of 37 days (interquartile range [IQR]: 20-125) prior to TA-TMA diagnosis and 8 days (IQR: 2-22 days) before LDH increased. von Willebrand factor (odds ratio = 1.009; 95% confidence interval [CI]: 1.001-1.016; P = .027) was an independent early risk indication for TA-TMA occurrence in the binary logistic regression equation, which adjusted for GVHD (P = .346), fungal infection (P = .120), and PCT (P = .252).
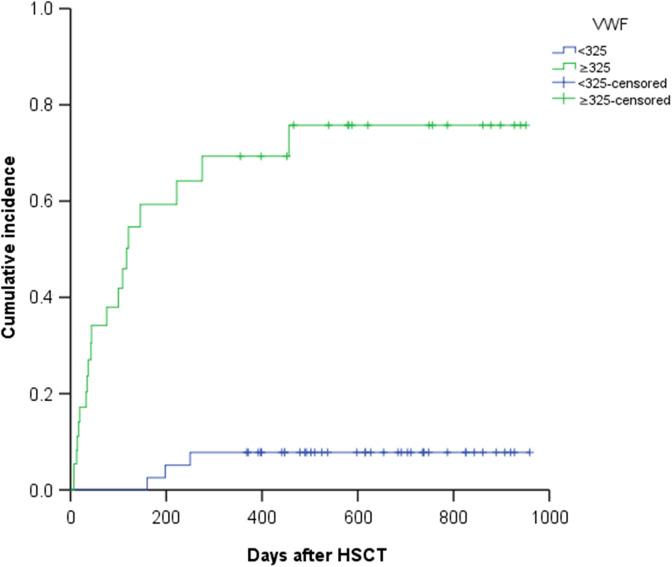
The ROC curve of vWF displays the relationship between the sensitivity and specificity of vWF in the diagnosis of TA-TMA, where the area under the curve (AUC) was 0.756 (95% CI: 0.636-0.874). The maximum Youden index calculated by MedCalc software was 0.542, and the associated criterion was greater than 324%, the corresponding sensitivity of vWF in the diagnosis of TA-TMA was 86.96% (95% CI: 66.4%-97.2%), and the specificity of vWF in the diagnosis of TA-TMA was 67.27% (95% CI: 53.3%-79.3%). The ROC curve of LDH displays the relationship between the sensitivity and specificity of LDH in the diagnosis of TA-TMA, where the AUC was 0.816. Although the AUC for LDH was greater than that for vWF, there was no statistically significant difference (P = .367) compared with vWF in the diagnostic efficacy for TA-TMA, which was obtained by Delong method in the MedCalc software. Figure 1 shows the relationship between the cumulative incidence of TA-TMA and different vWF levels. The Kaplan-Meier (K-M) curve was used to represent the difference in cumulative incidence between the ≥325% vWF group and the <325% vWF group. The outcome shows that the 2-year cumulative hazard rate for the ≥325% vWF group was significantly higher than that for the <325% group vWF (53.1% ± 8.2% vs 7.5% ± 4.2%; P < .001).
Figure 1.
The “<325” polyline represents the von Willebrand factor (vWF) < 325% group (40 patients), and the “≥325” polyline represents the vWF ≥ 325% group (38 patients). The “<325-censored” represents no transplantation-associated thrombotic microangiopathy (TA-TMA) event (37/40) was observed in the vWF < 325% group until the follow-up time, and the “≥325-censored” represents no TA-TMA event (18/38) was observed in the vWF ≥ 325% group until the follow-up time. Cumulative incidence comparison of the ≥325% vWF and <325% vWF groups. The Kaplan-Meier survival curve revealed a significantly higher cumulative hazard rate for TA-TMA in the ≥325 vWF group compared with the <325% vWF group (53.1% ± 8.2% vs 7.5% ± 4.2%; P < .001).
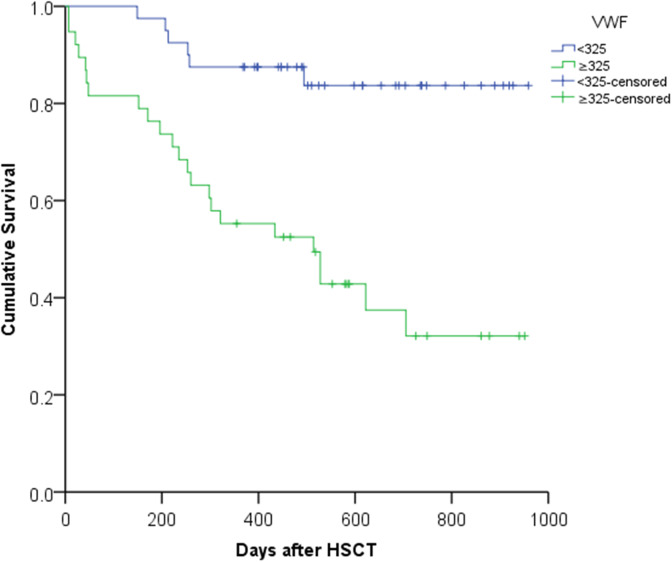
The mortality rate for patients with TA-TMA was much higher than that for those without TA-TMA (78% vs 19%; P < .001). The median survival was 101 days (range: 0-626 days) after TA-TMA diagnosis. The overall death rate was 36.7% (29/79), the recurrence death from leukemia accounted for 20.6% (6/29), and the TA-TMA-related mortality rate was 62.1% (18/29). Figure 2 shows the difference in 2-year cumulative survival after HSCT between the ≥325% vWF group and the vWF < 325% group in a K-M plot (32.1% ± 9.1% vs 83.7% ± 6.2%; P < .001).
Figure 2.
The “<325” polyline represents the von Willebrand factor (vWF) < 325% group (40 patients), and the “≥325” polyline represents the vWF ≥ 325% group (38 patients). The “<325-censored” represents no death event (34/40) was observed in the vWF < 325% group until the follow-up time, and the “≥325-censored” represents no death event (15/38) was observed in the vWF ≥ 325% group until the follow-up time. Cumulative survival curve comparing the ≥325% vWF group with the vWF <325% group. The cumulative survival rate of the VWF≥325 group was significantly lower than that of the vWF < 325% group after hematopoietic stem cell transplantation (HSCT; 32.1% ± 9.1% vs 83.7% ± 6.2%; P < .001).
Discussion
This was a 3-year case–control, prospective study to identify early predictors and prognostic factors for TA-TMA, which has high mortality after HSCT. This study established that a subset of laboratory and clinical markers provide significant predictive and prognostic value for the management of TA-TMA. These are a high level of vWF, gastrointestinal aGVHD, and fungal infection. In addition, this study helps clinicians decide on early intervention for this life-threatening complication by investigating the starting point of several early markers for TA-TMA.
von Willebrand factor plays an independent role in predicting the incidence of TA-TMA and patient outcome after HSCT. It has been demonstrated that an elevated level of vWF occurs earlier than LDH,16 and there is no statistically significant difference compared with vWF in the diagnostic efficacy for TA-TMA. von Willebrand factor is synthesized by endothelial cells and stored as ultra-large (UL) vWF multimers in WPBs.10,17 von Willebrand factor strings correspond to UL-vWF multimers that, after secretion from the endothelium, remain anchored to the endotheliocyte surface. In circulation, vWF multimers adopt a folded, globular conformation that shields the binding sites of the platelet glycoprotein Ib (GPIb) in the VWF A1 domain.18,19 However, immobilization of vWF at sites of vascular injury (via the vWF A3-collagen interaction) leads to vWF unfolding in response to the shear forces exerted by flowing blood.20 This event exposes vWF A1 domains that, in turn, reveal binding sites for the platelet GPIb receptor.21 This adhesion step, which is particularly relevant under high shear conditions, initiates platelet activation and aggregation, resulting in the formation of a platelet plug that seals the damaged vessel wall.10,22,23
In the TA-TMA group, we found that most of the patients (78.3%) had liver dysfunction before TA-TMA diagnosis, in particular, the production of ALB and CHE was reduced. Studies in rats and humans have shown that the ADAMTS13 activity can decrease 30% to 40% after a partial hepatectomy.13 ADAMTS13 (a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13) is predominantly synthesized in the hepatic stellate cells of liver,24,25 which is a vWF-cleaving protease, and degrades large vWF multimers.10,13,26 In addition, the liver synthesizes coagulation factors, including factor VIII, which enhances ADAMTS13 proteolytic activity by affecting the domain–domain interaction resulting from ADAMTS13 binding to vWF under shear forces.27,28 While severely deficient ADAMTS13 (<10%) activity is unique to TTP, there is ample evidence showing that ADAMTS13 is moderately decreased in other TMAs.29 In our clinical practice, TA-TMA patients had a normal or slightly decreased level of ADAMTS13, which is consistent with the literature. So, we suspect that the occurrence of TA-TMA is related to the imbalance of ADAMTS13/vWF. The ADAMTS13/vWF imbalance in TA-TMA may be affected by: (1) ADAMTS13 synthesized normally but with an explosive increase in vWF leading to relatively insufficient ADAMTS13 activity; (2) a decrease in ADAMTS13 synthesis due to liver function damage, although this is not a diagnosis criteria for TTP, which would lead to relatively insufficient activity for cleaving the increased vWF; (3) an ADAMTS13 inhibitor accompanying GVHD onset.
Grade 2 to 4 aGVHD is also a risk factor in patients with TA-TMA, and the incidence of gastrointestinal aGVHD is significantly increased in patients with TA-TMA. Acute GVHD and TA-TMA have been found to be closely related in a majority of studies.16,30 There are several other factors that promote TA-TMA, such as unrelated donors, exposure to calcineurin inhibitors, infections, and complement dysregulation.31 Fungal infection in TA-TMA occurrence had statistical significance, but the underlying mechanism remains unclear. The lack of positive results for calcineurin inhibitors in this study may be attributed to the strict control of the cyclosporine concentration in our center because many serious CSA complications were found in clinical application.
Transplant-associated thrombotic microangiopathy often leads to injuries to many organs, such as the kidney, liver, intestines, and lungs and often leads to hypertension, pulmonary hypertension, proteinuria, chronic kidney disease, pericardial effusion, and other symptoms.11,32,33 This study demonstrated these consequences in terms of hypertension, pulmonary hypertension, and kidney impairment.
There are some limitations of this study: this is a single-arm observational study, and the sample size is relatively small. We need to further expand the sample size to verify the effectiveness of vWF in the prediction, diagnosis, and prognosis of TA-TMA.
Supplementary Material
Acknowledgments
The authors thank the Department of Clinical Laboratory Medicine of Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, especially Xiaobin Fan. The authors also thank those who provided software support and language polishing in this work, especially Lei Jiang, Zehan Huang, and Han Yan.
Authors’ Note: Participants provided written informed consent approved by the Research Ethics Committee of Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences with the number NO.GDREC201414 7H(R1). Verbal informed consent was obtained from the patient(s) for their anonymized information to be published in this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Foundation of China (Nos. 81671585 and 81870121); Science and Technology Planning Project of Guangdong Province, China (Nos. 2015B020227003, 2015B020226001, and 2017B020230004); and Science and Technology Planning Project of Guangzhou, China (Nos. 201803040005 and 201803040011).
ORCID iD: Jianyu Weng  https://orcid.org/0000-0001-5446-292X
https://orcid.org/0000-0001-5446-292X
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Wang K, Lv M, Chang YJ, et al. Early myeloid-derived suppressor cells (HLA-DR-/lowCD33+CD16-) expanded by granulocyte colony-stimulating factor prevent acute graft-versus-host disease (GVHD) in humanized mouse and might contribute to lower GVHD in patients post allo-HSCT. J Hematol Oncol. 2019;12(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ruggeri A, Labopin M, Bacigalupo A, et al. Post-transplant cyclophosphamide for graft-versus-host disease prophylaxis in HLA matched sibling or matched unrelated donor transplant for patients with acute leukemia, on behalf of ALWP-EBMT. J Hematol Oncol. 2018;11(1):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fujiwara H, Maeda Y, Sando Y, et al. Treatment of thrombotic microangiopathy after hematopoietic stem cell transplantation with recombinant human soluble thrombomodulin. Transfusion. 2016;56(4):886–892. [DOI] [PubMed] [Google Scholar]
- 4. Stavrou E, Lazarus HM. Thrombotic microangiopathy in haematopoietic cell transplantation: an update. Mediterr J Hematol Infect Dis. 2010;2(3):e2010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Machida S, Onizuka M, Toyosaki M, et al. Danaparoid reduces the incidence of hematopoietic stem cell transplantation-associated thrombotic microangiopathy. Bone Marrow Transplant. 2017;52(2):307–309. [DOI] [PubMed] [Google Scholar]
- 6. Laskin BL, Goebel J, Davies SM, Jodele S. Small vessels, big trouble in the kidneys and beyond: hematopoietic stem cell transplantation-associated thrombotic microangiopathy. Blood. 2011;118(6):1452–1462. [DOI] [PubMed] [Google Scholar]
- 7. Uderzo C, Bonanomi S, Busca A, et al. Risk factors and severe outcome in thrombotic microangiopathy after allogeneic hematopoietic stem cell transplantation. Transplantation. 2006;82(5): 638–644. [DOI] [PubMed] [Google Scholar]
- 8. Jodele S, Fukuda T, Mizuno K, et al. Variable eculizumab clearance requires pharmacodynamic monitoring to optimize therapy for thrombotic microangiopathy after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2016;22(2):307–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen J, Chung DW. Inflammation, von Willebrand factor, and ADAMTS13. Blood. 2018;132(2):141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Noone DG, Riedl M, Licht C. The role of von Willebrand factor in thrombotic microangiopathy. Pediatr Nephrol. 2018;33(8):1297–1307. [DOI] [PubMed] [Google Scholar]
- 11. Jodele S, Laskin BL, Dandoy CE, et al. A new paradigm: diagnosis and management of HSCT-associated thrombotic microangiopathy as multi-system endothelial injury. Blood Rev. 2015;29(3):191–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cho BS, Yahng SA, Lee SE, et al. Validation of recently proposed consensus criteria for thrombotic microangiopathy after allogeneic hematopoietic stem-cell transplantation. Transplantation. 2010;90(8):918–926. [DOI] [PubMed] [Google Scholar]
- 13. Masias C, Cataland SR. The role of ADAMTS13 testing in the diagnosis and management of thrombotic microangiopathies and thrombosis. Blood. 2018;132(9):903–910. [DOI] [PubMed] [Google Scholar]
- 14. Keir LS, Saleem MA. Current evidence for the role of complement in the pathogenesis of Shiga toxin haemolytic uraemic syndrome. Pediatr Nephrol. 2014;29(10):1895–1902. [DOI] [PubMed] [Google Scholar]
- 15. Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15(6):825–828. [PubMed] [Google Scholar]
- 16. Ye Y, Zheng W, Wang J, et al. Risk and prognostic factors of transplantation-associated thrombotic microangiopathy in allogeneic haematopoietic stem cell transplantation: a nested case control study. Hematol Oncol. 2017;35(4):821–827. [DOI] [PubMed] [Google Scholar]
- 17. Haberichter SL, Jacobi P, Montgomery RR. Intersection of mechanisms of type 2A VWD through defects in VWF multimerization, secretion, ADAMTS-13 susceptibility, and regulated storage. Blood. 2003;101(4):1384–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shida Y, Swystun LL, Brown C, et al. Shear stress and platelet-induced tensile forces regulate ADAMTS13-localization within the platelet thrombus. Res Pract Thromb Haemost. 2019;3(2):254–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Emsley J. VWF (von Willebrand factor) comes in from the cold as a strategy to improve platelet storage. Arterioscler Thromb Vasc Biol. 2019;39(10):1893–1895. [DOI] [PubMed] [Google Scholar]
- 20. Zhao Y, Dong N, Shen F, et al. von Willebrand factor (VWF) propeptide binding to VWF D′D3 domain attenuates platelet activation and adhesion. J Thromb Haemost. 2007;5(9):1963–1970.17723136 [Google Scholar]
- 21. Butera D, Passam F, Ju L, et al. Autoregulation of von Willebrand factor function by a disulfide bond switch. Sci Adv. 2018;4(2): eaaq1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. De Ceunynck K, De Meyer SF, Vanhoorelbeke K. Unwinding the von Willebrand factor strings puzzle. Blood. 2013;121(2):270–277. [DOI] [PubMed] [Google Scholar]
- 23. Lasch M, Kleinert EC, Meister S, et al. Extracellular RNA released due to shear stress controls natural bypass growth by mediating mechanotransduction in mice. Blood. 2019;134(17):1469–1479. [DOI] [PubMed] [Google Scholar]
- 24. Uemura M, Tatsumi K, Matsumoto M, et al. Prophylactic fresh frozen plasma may prevent development of hepatic VOD after stem cell transplantation via ADAMTS13-mediated restoration of von Willebrand factor plasma levels. Blood. 2005;106(3):922–924. [DOI] [PubMed] [Google Scholar]
- 25. Levy GG, Nichols WC, Lian EC, et al. Mutations in a member of the ADAMTS gene family cause thrombotic thrombocytopenic purpura. Nature. 2001;413(6855):488–494. [DOI] [PubMed] [Google Scholar]
- 26. Turner N, Nolasco L, Dong JF, Moake J. . Von Willebrand factor cleaved from endothelial cells by ADAMTS13 remains ultra large in size. J Thromb Haemost. 2009;7(1):229–232. [DOI] [PubMed] [Google Scholar]
- 27. Cao W, Sabatino DE, Altynova E, et al. Light chain of factor VIII is sufficient for accelerating cleavage of von Willebrand factor by ADAMTS13 metalloprotease. J Biol Chem. 2012;287(39):32459–32466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bonazza K, Rottensteiner H, Schrenk G, et al. Shear-dependent interactions of von Willebrand factor with factor VIII and protease ADAMTS 13 demonstrated at a single molecule level by atomic force microscopy. Anal Chem. 2015;87(20):10299–10305. [DOI] [PubMed] [Google Scholar]
- 29. Martin K, Borgel D, Lerolle N, et al. Decreased ADAMTS-13 (A disintegrin-like and metalloprotease with thrombospondin type 1 repeats) is associated with a poor prognosis in sepsis-induced organ failure. Crit Care Med. 2007;35(10):2375–2382. [DOI] [PubMed] [Google Scholar]
- 30. Shayani S, Palmer J, Stiller T, et al. Thrombotic microangiopathy associated with sirolimus level after allogeneic hematopoietic cell transplantation with tacrolimus/sirolimus-based graft-versus-host disease prophylaxis. Biol Blood Marrow Transplant. 2013;19(2):298–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sahin U, Ataca Atilla P, Atilla E, Toprak SK, Demirer T. An overview of hematopoietic stem cell transplantation related thrombotic complications. Crit Rev Oncol Hematol. 2016;107:149–155. [DOI] [PubMed] [Google Scholar]
- 32. Sartain S, Shubert S, Wu MF, et al. Excellent outcomes of allogeneic hematopoietic stem cell transplantation in patients with paroxysmal nocturnal hemoglobinuria: a single-center study. Biol Blood Marrow Transplant. 2019;25(1):157–162. [DOI] [PubMed] [Google Scholar]
- 33. Cox K, Punn R, Weiskopf E, Pinsky BA, Kharbanda S. Pericardial effusion following hematopoietic cell transplantation in children and young adults is associated with increased risk of mortality. Biol Blood Marrow Transplant. 2017;23(7):1165–1169. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.