Abstract
Based on epidemiological data provided by the World Health Organization (2018), cancer is the second most prevalent cause of death worldwide. Several factors are thought to contribute to the high mortality rate in cancer patients, including less-than-optimal diagnostic and therapeutic strategies. Thus, there is an urgent need to identify accurate biomarkers with diagnostic, prognostic, and potential therapeutic applications. In this regard, long noncoding RNAs (lncRNAs) hold immense potential due to their regulatory roles in cancer development and associated cancer hallmarks. Recently, CASC9 transcripts have attracted significant attention due to their altered expression during the pathogenesis of cancer and their apparent contributions to various cancer-associated phenotypes involving a broad spectrum of molecular mechanisms. Here, we have provided an in-depth review describing the known functions of the lncRNA CASC9 in cancer development and progression.
Introduction
Recent advances in cancer research have revealed several distinct cancer-associated “hallmarks” that allow tumor cells to migrate and invade secondary sites [1,2]. Recent studies have identified key molecules that, when dysregulated, can contribute to cancer development and progression. Of interest, recent studies have revealed that noncoding RNA molecules coordinate many biological functions, which correspond to ~98% of the human genome compared to ~2% of coding of RNA molecules [[3], [4], [5], [6]].
Additionally, mounting evidence suggests that an essential class of noncoding RNA molecules, long noncoding RNAs (lncRNAs), defined based on their size of greater than 200 nucleotides, regulate various cellular processes. These include transcription, splicing, translation, protein localization, epigenetics, cell structure integrity, cell cycle, heat shock responses, imprinting, stem cell pluripotency, reprogramming, embryogenesis, immune response regulation, cell differentiation, cell fate determination, cell proliferation, and cell migration [3,[7], [8], [9], [10], [11], [12], [13], [14], [15]]. Previous studies have detected alterations of lncRNAs in virtually all human cancers [3,4,6,16]. Further, lncRNAs have been implicated in modulating various cancer-related hallmarks such as sustained proliferative signaling [17], evasion of growth suppressors [18], replicative immortality [18], induction of angiogenesis, activation of invasion and metastasis [19,20], resistance to cell death [21], avoiding immune destruction [22], tumor-promoting inflammation [23], deregulation of cellular energetics [23], and genome instability [23]. For instance, the lncRNA H19 has been shown to regulate the proliferation of gastric cancer (GC) cells by binding with p53 [24], stimulation cell cycle progression through the G1/S transition in esophageal squamous cell carcinoma (ESCC) [25], and resistance of cell death in cholangiocarcinoma cells [26]. Similarly, the lncRNA HOTAIR has been implicated in promoting cellular proliferation, migration, and invasion in thyroid cancer cells [27]. Additionally, studies have demonstrated an association of lncRNA MALAT1 with cellular proliferation and apoptosis in colon cancer [28].
This review aims to discuss the recently discovered “cancer susceptibility candidate 9” (CASC9) lncRNA (Ensemble ID: ENSG00000249395) and its potential roles in the pathogenesis of cancer. Additionally, we discuss the potential utility of CASC9 as a prognostic marker and/or therapeutic target for cancer, as it was first identified in ESCC as an associated lncRNA having four transcriptional variants; [CASC9-201 (Ensemble ID: ENST00000504531.2), CASC9-202 (Ensembl ID: ENST00000521147.1), CACS9–203 (Ensembl ID: ENST00000522183.1), and CASC9-204 (Ensembl ID: ENST00000523313.1)]. Subsequently, these transcriptional variants were implicated in a variety of cancers, including breast, lung, and liver.
Here, we focus on the involvement of CASC9 in the progression of tumorigenesis and the advancement of cancers via dysregulating the defining hallmarks of cancer. For each of the cancer hallmarks, we have presented significant protein-coding and non-coding molecules that are regulated by CASC9. We have also discussed the potential prognostic and diagnostic applications of CASC9, and its potential as a therapeutic target.
Association of the long non‐coding RNA CASC9 with Various Hallmarks of Cancer
CASC9 in Sustaining Proliferative Cell Signaling
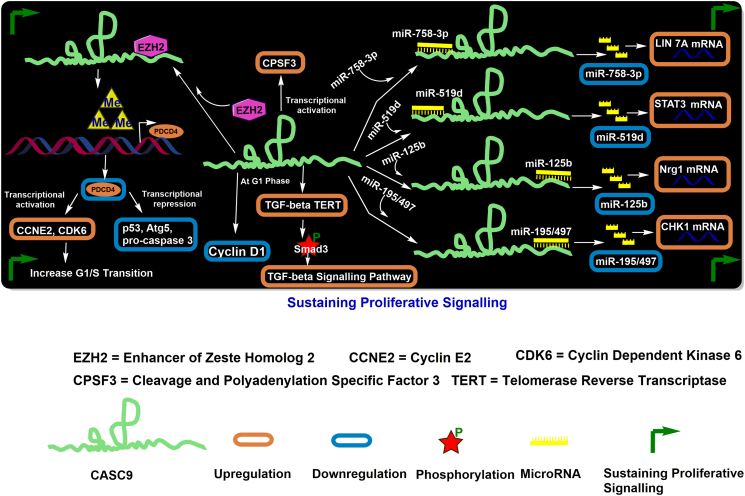
Cellular proliferation in healthy cells is tightly regulated by growth signals and cell cycle regulators to maintain cellular homeostasis. Cancer cells often contain disrupted and/or dysfunctional biological regulators enhancing uncontrolled cellular proliferation [23]. The ability of cancer cells to sustain continuous proliferative signals can be attained in several ways, i.e., excessive production of growth ligands, disruption of feedback mechanisms, and evasion of growth suppressors, etc. [1]. Among multiple factors associated with the uncontrolled proliferation of cells in various cancer types, CASC9 has been implicated as an essential factor for sustained cancer cell growth via distinct mechanisms. The deregulated expression of CASC9 was first identified in ESCC, where gain and loss of function assays revealed roles for CASC9 in promoting cell proliferation in vitro and tumor growth in vivo [29]. Subsequent studies found a direct correlation between CASC9 expression and cancer progression, where depletion of CASC9 reduced cell proliferation and colony formation of cancer cells compared to control cells, while overexpression had the reverse effect [[30], [31], [32], [33], [34]]. In biological systems, cellular proliferation and the cell cycle are tightly regulated by various checkpoints, tumor suppressor genes, oncogenes, and other downstream targets. In nasopharyngeal cancer (NPC), CASC9 was found to interact with hypoxia-inducible factor-1 alpha (HIF-1α), enhancing its stability and further promoting glycolysis in NPC, thereby increasing cellular proliferation [35]. In ESCC cells, CASC9 downregulated programmed cell death 4 (PDCD4) protein via reduction of associated mRNA levels. PDCD4 is a highly conserved gene involved in inhibiting the translational initiation of multiple genes, including tumor suppressor p53, apoptosis-related pro-caspase-3, and autophagy-related Atg5 (Figure 1) [36]. Studies on cell cycle regulators have identified a strong correlation between PDCD4 and CASC9, where PDCD4 rescued, in part, G1/S arrest caused by CASC9 knockdown. Mechanistically, knockdown of CASC9 reduced the expression of the S-phase cyclins cyclin E2 (CCNE2) and CDK6 while suppression of PDCD4 rescued their expression (Figure 1). Furthermore, a series of investigations using microarray analysis, chromatin immunoprecipitation assays, western blot analysis, etc., have confirmed a regulatory role of PDCD4 by recruiting enhancer like zeste homolog 2 (EZH2), which is associated with increased H3K27me3 methylation (Figure 1) [34]. Thus, these results suggested that CASC9 may play an intrinsic role in association with sustained cell cycle progression in a PDCD4-dependent manner in ESCC cells.
Figure 1.
Regulatory mechanisms of CASC9 in cancer cell proliferation.
Upregulation of CASC9 was also found to regulate cell cycle progression in the G1 phase via cyclin D1 in lung adenocarcinoma (LAD) (Figure 1) [30,[33], [34], [35],37]. Additionally, in vitro assays performed by Xi et al. (2020) demonstrated that the upregulation of CASC9 resulted in overexpression of the glucose transporter 1 (GLUT1) gene in TU212 laryngeal carcinoma cells, thus contributing to cellular proliferation [38]. Further, potential interactions between CASC9 and cleavage polyadenylation specificity factor subunit 3 (CPSF3) (Figure 1) were observed in colorectal cancers (CRCs) via RNA immunoprecipitation and RNA-protein pull-down assays [39].
Studies have reported that CASC9 modulated TGFβ2 mRNA stability and thus upregulated the expression levels of TGFβ and TERT, which in turn resulted in the increased phosphorylation of mothers against decapentaplegic homolog 3 (SMAD3) and ultimately activated the TGFβ signaling pathways and TERT complex function in CRC cells (Figure 1) [39]. Thus, it may be concluded that CASC9, in association with CPSF3 and TGFβ, contributed to an elevated proliferation of CRC cells [39]. Additionally, enzyme-linked immunosorbent assay analysis demonstrated increased expression levels of TGFβ in serum, tissues, and cells of cervical cancer [40].
Regulatory roles of miRNAs in cell proliferation via modulation of target genes have been uncovered in the recent past. For example, CASC9 has been shown to act as a competing endogenous RNA (ceRNA) by competitively binding to its target miRNAs at the 3′UTR region, inhibiting miRNA expression via a sponging effect [[41], [42], [43], [44]]. In glioma cancer cells, CASC9 was reported to exert a sponging effect on miR-519d, silencing its function and releasing its downstream target, the signal transducer and activator of transcription 3 (STAT3) transcription factor. The release of STAT3 further increased the transcription of CASC9, magnifying its oncogenic potential [43]. Furthermore, in MDA-MB-415 human breast cancer cells, CASC9 positively regulated checkpoint kinase1 (CHK1) by binding to the miR-195/147 cluster (Figure 1) [44]. Silencing of CASC9 resulted in increased levels of B-cell lymphoma 2 (BCL-2) (~2.0-fold), CCND1 (~2.5-fold), and CDK4 (~7.0-fold), as assessed by Western blot analysis, which facilitated breast cancer cell proliferation [44]. Suppression of CASC9 was also found to suppress the expression of miR-758-3p, which in turn released its target gene, LIN7A, increasing its expression in SKOV3 and A2780 human ovarian cancer cells that ultimately lead to ovarian cancer cell proliferation (Figure 1). Thus, a defined regulatory pathway, CASC9/miR-758-3p/LIN7A, was identified in ovarian cancer progression [41].
CASC9 was also found to have a disrupted expression in benign tumors such as hemangioma (HA) [42], where higher levels of CASC9 were detected in the tissues of proliferative-phase HA samples when compared to tissues in the involuting phase of HA and healthy tissue samples. Likewise, higher expression of CASC9 correlated with a higher level of cyclin D1 in HA cells (Figure 1). Further investigation revealed that miR-125b was a direct target of CASC9, where the expression of miR-125b negatively correlated with CASC9 expression in HA tissues. Additionally, neuregulin-1 (NRG1) was found to be a direct downstream target of miR-125b, which together regulated cancer cell proliferation (Figure 1). Neuregulin-1 is recognized as an oncogene in colon, lung, and gastric cancers, among others [45].
Taken together, the studies, as mentioned earlier, suggest that CASC9 participates as one of the factors responsible for sustained proliferative signals in cancer cells through targeting various cell cycle proteins, miRNAs, and transcription factors.
CASC9 in Invasion and Metastasis
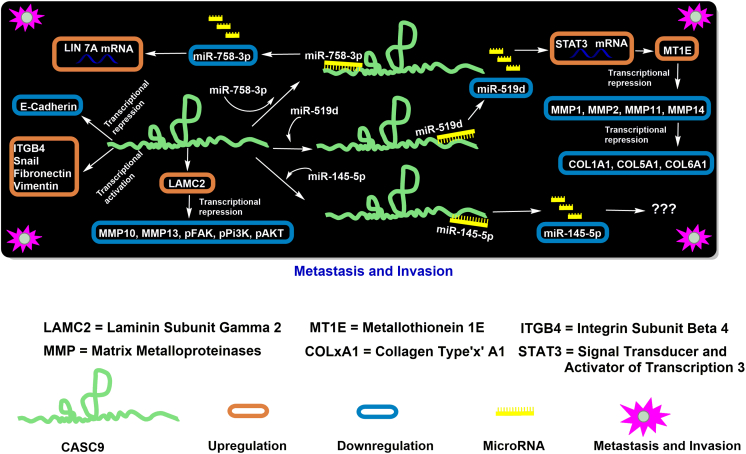
Invasion and metastasis are among several hallmarks of cancer that CASC9 has been demonstrated to impact. Invasion and metastasis involve a cascade of discrete steps involving cellular changes, viz., local invasion, intravasation via lymphatic and blood vessels, extravasation through the hematogenous lymphatic system into the parenchyma of distant tissues, and the formation of micrometastases and/or secondary tumors. This cascade is, in turn, broadly regulated by the epithelial-mesenchymal transition (EMT) process, where the cancer cells acquire multiple attributes that enable them to invade barriers, resist apoptosis, and disseminate.
Independent reports have revealed a correlation among CASC9 overexpression and migration and invasion of ESCC tumor cells [32,46]. Consistent with these reports, the siRNA-mediated knockdown of CASC9 decreased the migration and invasion potential of ESCC cells [32,46]. Besides, Western blot analysis confirmed that CASC9 exerted its function by regulating the expression of genes involved in EMT, extracellular matrix (ECM) interactions, and focal adhesion, where it suppressed the expression of the EMT markers vimentin, Snail, and fibronectin [32] and ECM-interacting proteins laminin subunit gamma 2 (LAMC2) and integrin subunit beta 4 (ITGB4) [46]. In contrast, the levels of E-cadherin increased (Figure 2) [32]. Further analyses confirmed that LAMC2 silencing attenuated the number of motile ESCC cells and inhibited the expression of matrix metallopeptidase (MMP) 10,13, focal adhesion kinase (pFAK), pPI3K, and pAKT (Figure 2). This indicated that LAMC2 was the primary downstream target of CASC9-induced metastasis, which was modulated via the FAK-PI3K/AKT signaling pathway [46].
Figure 2.
Regulatory mechanisms of CASC9 in cancer cell migration and invasion.
CASC9 has also been implicated in regulating the metastatic and invasive potential of tumor cells by sponging miRNAs. For example, CASC9 has shown to upregulate the expression of the MT1E gene encoding metallothionine 1E, which induced the inactivation of the matrix metallopeptidase 9 (MMP9) gene and downregulated the expression of other matrix metalloproteinase (MMP1, MMP2, MMP11, and MMP14) genes that break down the ECM. Further, it has been shown to regulate the genes encoding collagen subunits (COL1A1, COL5A1, and COL6A1) that constitute a part of the ECM, thereby collectively promoting tumorigenicity by increased invasion and metastasis (Figure 2) [47]. In glioma, miR-519d suppressed the expression of the STAT3 gene, which was rescued by the sponging activity of CASC9. Since CASC9 also harbors a binding site for STAT3 in its promoter region, increased STAT3 levels activated the transcriptional activity of CASC9 by a positive feedback loop via the STAT3-CASC9-miR-519-STAT3 axis [43]. Similarly, CASC9 upregulation resulted in increased migration and invasion of ovarian cancer cells (SKOV3 and A2780) using a transwell invasive assay [41]. CASC9 acted as a competing endogenous RNA in ovarian cancer cells, where it suppressed miR-785-3p and increased the expression of LIN7A, as shown in Figure 2. The specific role(s) of LIN7A has yet to be determined in ovarian cancer; however, Hu et al. (2019) found that overexpression of LIN7A rescued the suppressive effects of CASC9 silencing on cell migration and invasion [41].
In contrast, miR-145-5p was predicted as a putative target for CASC9 in hepatocellular carcinoma cells (HCC), but due to its inappreciable expression levels, it was rendered inadequate for further analyses [48]. Also, increased CASC9 expression in HCC cells resulted in no detectable difference in their proliferation rate, supporting the notion that lncRNAs were discordantly expressed in different tumor types and different subgroups of specific cancer types. Moreover, lncRNA CASC9 positively correlated with epithelial phenotypes in HCC due to increased expression of CDH1 (E-cadherin) and CDH2 (N-cadherin), while it decreased the expression of MMP9 and the migration potential of the cells [48]. However, Yu et al. (2017) demonstrated that CASC9 significantly promoted the invasion potential of the pancreatic cell lines SW190 and BxPC compared to control cells [49].
CASC9 promotes the invasion and metastasis through modulating the expression of miR-519, miR-145-5p, EMT-associated proteins, and matrix metallopeptidases in various cancers. Thus, CASC9 may be used as a diagnostic marker for the advanced stages of certain cancers.
CASC9 in Resisting Cell Death
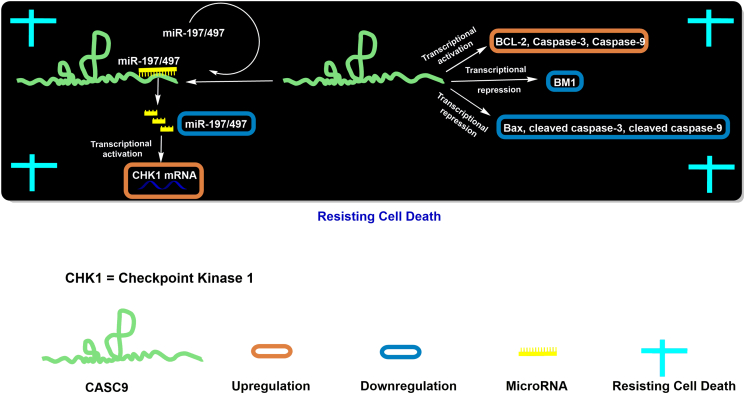
Apart from the regulatory role of CASC9, it is also associated with cellular proliferation, cellular invasion, and cellular metastasis, and it also plays an essential role in resisting cell death or apoptosis in various cancer cells. Moreover, it is well established that the ratio of BCL-2 and BCL-2-associated X protein (BAX) in the cell acts as an indicator for cellular apoptosis [50], where BCL-2 is antiapoptotic and BAX is proapoptotic. Cancer cells have the potential to tolerate both environmental and genomic stresses and thus exhibit resistance to apoptosis [23]. However, several studies have suggested that dysregulation of CASC9 expression may result in resistance to the death of cancer cells.
Recent evidence suggested that the upregulated expression of CASC9 in breast cancer tissues and cell lines correlated with various clinicopathological characteristics, as shown in Table 1 [44,51]. Silencing of CASC9 in MDA-MB-415 and MCF-7/DOX human breast cancer cells promoted their apoptotic potential [44,51], whereas silencing of CASC9 in MDA-MB-231 cells showed an opposite effect, as assessed by flow cytometry [44]. Moreover, investigation of apoptosis-associated proteins in the si-CASC9–treated MCF-7/DOX cells showed decreased levels of BCL-2, pro-caspase-3, and pro-caspase-9. On the other hand, BAX, cleaved caspase-3, and cleaved caspase-9 showed increased expression levels in breast cancer cells (Figure 3) [51]. However, another study suggested higher expression levels of BCL-2 and lower expression of caspase-3 in si-CASC9–treated MDA-MB-415 cell lines compared to untreated cells [44]. Shao et al. (2019) found a negative correlation of CASC9 expression with miR-197 and miR-497 expression in breast cancer tissues and a positive correlation with CHK1 levels, presumably by the sponging of miR-197 and miR-497 [44]. Another study has reported an upregulated expression of CASC9 in GC cells and tissues that correlated with various clinicopathological characteristics, as shown in Table 1 [52,53]. To investigate the role of CASC9 in apoptosis, two independent groups performed knockdown of CASC9 in the GC cell lines BGC823/DR, SGC7901/DR, SGC7901, and MKN-45 and showed increased apoptosis in these cells. Furthermore, Fang et al. (2019) reported that apoptosis in GC cells was regulated by downregulation of the B cell–specific Moloney murine leukemia virus integration site 1 (BMI1) protein in conjunction with other apoptosis-associated proteins (e.g., BCL-2) and cleaved caspase-3 [53]. Lo et al. (2019) showed an upregulated expression of CASC9 in CRC cell lines and tissues and found an association with various clinicopathological characteristics, as shown in Table 1. CASC9 possesses four transcript variants, and of these, CASC9-202 and CASC9-204 were the most abundant and dysregulated in HCT-116, SW620, and SW480 CRC cell lines. Knockdown of the dysregulated transcripts of CASC9 (CASC9-202, CASC9-204) led to a disruption of the cell cycle in the G2/M phase and thus increased the apoptotic potential of the cancer cells [41]. Another group reported that CASC9 was overexpressed in lung squamous cell carcinoma and correlated with various clinicopathological characteristics (as shown in Table 1). However, its expression was not associated with the apoptotic status of the cells [54]. Similarly, CASC9 was found to be dysregulated in hepatocellular carcinoma and ESCC cells [32,34,35,46,48], but no significant role in apoptosis in these cells has yet been established.
Table 1.
Implications of CASC9 with Various Hallmarks of Cancer Through Targeting Genes/Proteins/Pathways
| Hallmarks of Cancer | Cancer Types | Regulation of CASC9 | Clinicopathological Characteristics | Targets of CASC9 | References |
|---|---|---|---|---|---|
| Sustaining proliferative signaling | Esophageal squamous cell carcinoma | Up | Gender, age, tumor size, differentiation, tumor invasion, lymph node metastasis, TNM stage, smoking, drinking |
PDCD4, EZH2, Cyclin D1 CCNE2, CDK6, p53, |
[29,32,34,36,46] |
| Ovarian cancer | Up |
Age, tumor size, lymph node metastasis, FIGO stage |
Pro-caspase-3, Atg5 | [41] | |
| Breast cancer | Up | Age, tumor size, histological grade, lymph node metastasis, AJCC status, lymphovascular invasion, ER expression | LIN7A, miR-758-3p | [44,51] | |
| Lung cancer | Up | Gender, age, tissue type, tumor location, stage, distant metastasis, lymph node metastasis, primary tumor stage, anatomical classification | CHK1, miR-195/147, BCL-2, Cyclin D1, CDK4 | [37,54] | |
| Colorectal cancer | Up | Gender, age, tumor size, differentiation grade, tumor location, lymph node metastasis, TNM stage | CPSF3, TGFβ, TERT, p- SMAD3 | [39] | |
| Glioma | Up | - | miR-519d, STAT3 | [43] | |
| Hemangioma | Up | - | Cyclin D1, miR-125b, Nrg1 | [42] | |
| Laryngeal carcinoma | Up | - | GLUT-1 | [38] | |
| Nasopharyngeal carcinoma | Up | - | HIF-1α, Cyclin D1 | [35] | |
| Metastasis and invasion | Esophageal squamous cell carcinoma | Up | Gender, age, tumor size, differentiation, tumor invasion, lymph node metastasis, TNM stage, smoking, drinking | Vimentin, Snail, fibronectin, LAMC2, ITGB4, E-cadherin, MMP10, MMP13, pFAK, pPI3K, pAKT | [29,32,34,36,46] |
| Lung cancer | Up | Gender, age, tissue type, tumor location, stage, distant metastasis, lymph node metastasis, primary tumor stage, anatomical classification | - | [37,54] | |
| Hepatocellular carcinoma | Up | - | miR-145-5p, E-cadherin, N-cadherin, MMP9 | [48] | |
| Pancreatic ductal adenocarcinoma | Up | - | - | [49] | |
| Ovarian cancer | Up |
Age, tumor size, lymph node metastasis, FIGO stage |
miR-785-3p, LIN7A | [41] | |
| Breast cancer | Up | Age, tumor size, histological grade, lymph node metastasis, AJCC status, lymphovascular invasion, ER expression | - | [44,51] | |
| Glioma | Up | - | STAT3, MT1E, MMP9, |
[47] |
|
| Hemangioma | Up | - | MMP1, MMP2, MMP11, MMP14, COL1A1, COL5A1, COL6A1, miR- 519d | [42] | |
| Resisting cell death | Esophageal squamous cell carcinoma | Up | Gender, age, tumor size, differentiation, tumor invasion, lymph node metastasis, TNM stage, smoking, drinking | No significant role in apoptosis has been reported. | [29,32,34,36,46] |
| Hepatocellular carcinoma | Up | - | - | [48] | |
| Gastric cancer | Up | Gender, age, tumor size, histology, tumor stage, lymph node metastasis,, site, Bormann type, depth of invasion, infiltrating pattern, lymphatic/venous invasion | BMI1, BCL-2, cleaved caspase-3 | [52,53] | |
| Lung cancer | Up | Gender, age, tissue type, tumor location, stage, distant metastasis, lymph node metastasis, primary tumor stage, anatomical classification | - | [37,54] | |
| Breast cancer | Up | Age, histological grade, tumor size, lymph node metastasis, AJCC status, Lymphovascular invasion, ER expression | Bax, cleaved caspase-3, cleaved caspase-9, BCL-2, pro-caspase-3 and pro-caspase-9, miR-197, miR-497, CHK1 | [44,51] | |
| Colorectal cancer | Up | Gender, age, tumor size, differentiation grade, tumor location, lymph node metastasis, TNM stage | - | [39] | |
| Deregulation of cellular energetics | Nasopharyngeal cancer | Up | - | HIF-1α | [35] |
Figure 3.
Regulatory mechanisms of CASC9 in resisting cancer cell death.
Collectively, CASC9 hinders the apoptotic potential of the healthy cells, thus assisting in the transformation of healthy cells into a cancerous phenotype through regulating the expression of proapoptotic and antiapoptotic proteins and various miRNAs such as miR-197 and miR-497.
CASC9 in Cellular Energetics
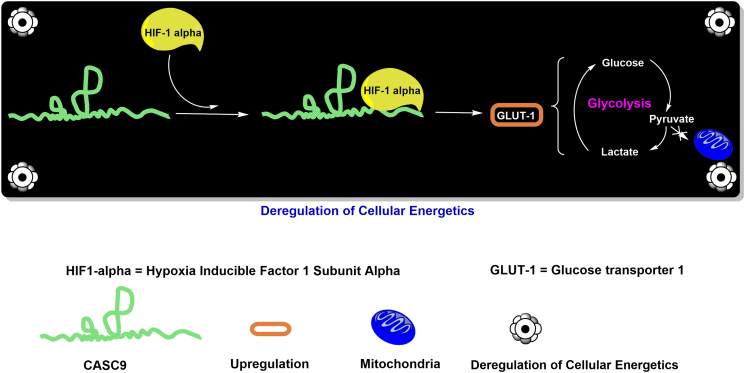
Past studies have documented the dependence of cancer cells on specific metabolic pathways. Metabolism in healthy cells often involves the conversion of glucose to pyruvate via a multistep process of glycolysis [23]. During aerobic respiration, pyruvate enters the mitochondria where it is oxidized to generate ATP to fulfill cellular energy demands. On the contrary, in cancerous cells, pyruvate is often directed away from the mitochondria to create lactate via the action of the enzyme lactate dehydrogenase (LDH/LDHA), which is characteristic of a hypoxic environment; this phenomenon is known as aerobic glycolysis or the “Warburg effect” [23].
Su et al. (2017) found that CASC9 was highly overexpressed (by ~12-fold) in the nasopharyngeal carcinoma (NPC) cell line CNE-1 when compared to a normal control cell line, NP-69. Additionally, similar results were observed in NPC tissues compared to their healthy counterparts [35]. The authors found that higher expression of CASC9 resulted in higher levels of HIF-1α in CNE-1 cells. They also demonstrated the binding of CASC9 to HIF-1α via immunoprecipitation assays [35]. HIF-1α is a known transcriptional regulator of oxygen homeostasis that regulates the expression of some critical genes such as glucose transporters (e.g., GLUT-1) that are responsible for controlling glycolysis in cancer cells. (Figure 4). Su et al. (2017) also performed a glucose uptake assay to validate the reprogramming of glycolysis by CASC9. The results demonstrated that the upregulation of CASC9 induced significantly higher glucose uptake and lactate production in CNE-1 cells compared to control cells [35].
Figure 4.
Regulatory mechanisms of CASC9 in cancer cell metabolism.
Furthermore, knockdown of CASC9 in CNE-1 cells led to a decreased level of lactate production compared to untreated cells, and its depletion in HIF-1α–deficient CNE-1 cells showed no significant increase in glucose uptake compared to control cells [35]. The results of this study suggested that CASC9 promoted glycolysis in cancer cells by increasing the levels of HIF-1α (Figure 4). Taken together, these results provided evidence that CASC9 was associated with HIF-1α reprogramming of glycolytic metabolism in cancer cells. Thus, CASC9 may prove to be a useful target to exploit for cancer therapy, though more studies are required on its mechanistic role(s) in NPC and other cancer types.
Closing Remarks and Future Directions
Despite recent advances involving novel diagnostic and prognostic strategies for cancer treatment, unfortunately, the global incidence of cancer is increasing worldwide with no significant decrease in cancer incidence and cancer-related mortality. To restrain cancer-related mortality, there is a critical need to develop novel prognostic and diagnostic strategies for cancer treatment. Recent studies involving new high-throughput RNA sequencing techniques have demonstrated the potential application of novel lncRNAs with potential applications as a biomarkers or therapeutic targets for personalized cancer treatment. In this review, we have discussed one such lncRNA, CASC9, and its potential roles in promoting various hallmarks of cancer. CASC9 can influence the expression of various transcription factors, oncogenes, and associated cancer signaling pathways involving cancer etiology and progression, such as metastasis and invasion, cell metabolism, resistance to cell death, and cellular proliferation.
Additionally, we have discussed the roles of CASC9 as a sponging RNA and its regulatory association with critical noncoding RNAs, i.e., miRNAs. Recently, several studies have focused on the roles of lncRNAs in cancer; however, studies focusing on lncRNA-based therapeutics and diagnostics are still in their infancy. We were not able to find relevant scientific data regarding the delivery-associated toxicity and potential stimulation of immune responses of lncRNA-based therapeutics.
Though many lncRNAs, including CASC9, have been discovered in the recent past, scientific knowledge stating the effects of lncRNA on targeted therapeutics is limited. Therefore, future investigation in regard to crucial cellular signaling molecules and specific pathways altered by specific lncRNAs in cancer may provide new insights toward the development of personalized biomarkers/targets for cancer diagnosis and prognosis. In conclusion, we are hopeful that this review will stimulate future research to expand our knowledge of cancer pathogenesis and assist in the discovery of new biological markers for personalized cancer therapies.
Acknowledgments
Acknowledgements
Funding
This work was supported by the Department of Science and Technology of India through the Indo-Russia grant (INT/RUS/RFBR/P-311) to A. J. and an NIH/NCI grant to K. M. V. (CA093279). A. J. is also thankful to ICMR for providing grants (5/13/81/2013-NCD-III). Uttam Sharma was supported by a DST INSPIRE fellowship grant (IF180680).
Author Contributions
U. S., V. A., S. T., and A. J. conceived the idea. U. S., T. S. B., V. A., and S. T. wrote the majority of the manuscript. U. S. composed the figures and table. K. M. V. and A. J. made critical revisions. All authors read and approved the final manuscript.
Compliance with Ethical Standards
Conflict of Interest
The authors declare no conflict of interest.
Ethical Approval
This review article does not contain studies with human participants or animals performed by any of the authors.
References
- 1.Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Rena O., Casadio C., Leo F., Giobbe R., Cianci R., Baldi S. Videothoracoscopic lung biopsy in the diagnosis of interstitial lung disease. Eur. J. Cardiothorac. Surg. 1999;16:624–627. doi: 10.1016/s1010-7940(99)00320-6. [DOI] [PubMed] [Google Scholar]
- 3.Khandelwal A., Bacolla A., Vasquez K.M., Jain A. Long non-coding RNA: a new paradigm for lung cancer. Mol. Carcinog. 2015;54:1235–1251. doi: 10.1002/mc.22362. [DOI] [PubMed] [Google Scholar]
- 4.Malhotra A., Jain M., Prakash H., Vasquez K.M., Jain A. The regulatory roles of long non-coding RNAs in the development of chemoresistance in breast cancer. Oncotarget. 2017;8:110671–110684. doi: 10.18632/oncotarget.22577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malhotra A., Sharma U., Puhan S., Chandra Bandari N., Kharb A., Arifa P.P. Stabilization of miRNAs in esophageal cancer contributes to radioresistance and limits efficacy of therapy. Biochimie. 2019;156:148–157. doi: 10.1016/j.biochi.2018.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tamang S., Acharya V., Roy D., Sharma R., Aryaa A., Sharma U. SNHG12: an LncRNA as a potential therapeutic target and biomarker for human cancer. Front. Oncol. 2019;9:901. doi: 10.3389/fonc.2019.00901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brannan C.I., Dees E.C., Ingram R.S., Tilghman S.M. The product of the H19 gene may function as an RNA. Mol. Cell. Biol. 1990;10:28–36. doi: 10.1128/mcb.10.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen B., Yu M., Chang Q., Lu Y., Thakur C., Ma D. Mdig de-represses H19 large intergenic non-coding RNA (lincRNA) by down-regulating H3K9me3 and heterochromatin. Oncotarget. 2013;4:1427–1437. doi: 10.18632/oncotarget.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fatica A., Bozzoni I. Long non-coding RNAs: new players in cell differentiation and development. Nat Rev Genet. 2014;15:7–21. doi: 10.1038/nrg3606. [DOI] [PubMed] [Google Scholar]
- 10.Grote P., Herrmann B.G. The long non-coding RNA Fendrr links epigenetic control mechanisms to gene regulatory networks in mammalian embryogenesis. RNA Biol. 2013;10:1579–1585. doi: 10.4161/rna.26165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gutschner T., Diederichs S. The hallmarks of cancer: a long non-coding RNA point of view. RNA Biol. 2012;9:703–719. doi: 10.4161/rna.20481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kino T., Hurt D.E., Ichijo T., Nader N., Chrousos G.P. Noncoding RNA gas5 is a growth arrest- and starvation-associated repressor of the glucocorticoid receptor. Sci Signal. 2010;3:ra8. doi: 10.1126/scisignal.2000568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Z., Rana T.M. Decoding the noncoding: prospective of lncRNA-mediated innate immune regulation. RNA Biol. 2014;11:979–985. doi: 10.4161/rna.29937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma L., Bajic V.B., Zhang Z. On the classification of long non-coding RNAs. RNA Biol. 2013;10:925–933. doi: 10.4161/rna.24604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wapinski O., Chang H.Y. Long noncoding RNAs and human disease. Trends Cell Biol. 2011;21:354–361. [Google Scholar]
- 16.Khandelwal A., Malhotra A., Jain M., Vasquez K.M., Jain A. The emerging role of long non-coding RNA in gallbladder cancer pathogenesis. Biochimie. 2017;132:152–160. doi: 10.1016/j.biochi.2016.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen J., Liu S., Hu X. Long non-coding RNAs: crucial regulators of gastrointestinal cancer cell proliferation. Cell Death Discov. 2018;4:50. doi: 10.1038/s41420-018-0051-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Oliveira J.C., Oliveira L.C., Mathias C., Pedroso G.A., Lemos D.S., Salviano-Silva A. Long non-coding RNAs in cancer: another layer of complexity. J Gene Med. 2019;21 doi: 10.1002/jgm.3065. [DOI] [PubMed] [Google Scholar]
- 19.Raveh E., Matouk I.J., Gilon M., Hochberg A. The H19 long non-coding RNA in cancer initiation, progression and metastasis — a proposed unifying theory. Mol. Cancer. 2015;14:184. doi: 10.1186/s12943-015-0458-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shen X.H., Qi P., Du X. Long non-coding RNAs in cancer invasion and metastasis. Mod. Pathol. 2015;28:4–13. doi: 10.1038/modpathol.2014.75. [DOI] [PubMed] [Google Scholar]
- 21.Rossi M.N., Antonangeli F. LncRNAs: new players in apoptosis control. Int J Cell Biol. 2014;2014:473857. doi: 10.1155/2014/473857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luo Y., Yang J., Yu J., Liu X., Yu C., Hu J. Long non-coding RNAs: emerging roles in the immunosuppressive tumor microenvironment. Front. Oncol. 2020;10:48. doi: 10.3389/fonc.2020.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lecerf C., Le Bourhis X., Adriaenssens E. The long non-coding RNA H19: an active player with multiple facets to sustain the hallmarks of cancer. Cell. Mol. Life Sci. 2019;76:4673–4687. doi: 10.1007/s00018-019-03240-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang F., Bi J., Xue X., Zheng L., Zhi K., Hua J. Up-regulated long non-coding RNA H19 contributes to proliferation of gastric cancer cells. FEBS J. 2012;279:3159–3165. doi: 10.1111/j.1742-4658.2012.08694.x. [DOI] [PubMed] [Google Scholar]
- 25.Tan D., Wu Y., Hu L., He P., Xiong G., Bai Y. Long noncoding RNA H19 is up-regulated in esophageal squamous cell carcinoma and promotes cell proliferation and metastasis. Dis. Esophagus. 2017;30:1–9. doi: 10.1111/dote.12481. [DOI] [PubMed] [Google Scholar]
- 26.Xu Y., Wang Z., Jiang X., Cui Y. Overexpression of long noncoding RNA H19 indicates a poor prognosis for cholangiocarcinoma and promotes cell migration and invasion by affecting epithelial-mesenchymal transition. Biomed. Pharmacother. 2017;92:17–23. doi: 10.1016/j.biopha.2017.05.061. [DOI] [PubMed] [Google Scholar]
- 27.Liu X., Liu G., Lu Y., Shi Y. Long non-coding RNA HOTAIR promotes cell viability, migration and invasion in thyroid cancer cells by sponging miR-17-5p. Neoplasma. 2019;67(2):229–237. doi: 10.4149/neo_2019_190310N208. [DOI] [PubMed] [Google Scholar]
- 28.Zhang J., Li Q., Xue B., He R. MALAT1 inhibits the Wnt/beta-catenin signaling pathway in colon cancer cells and affects cell proliferation and apoptosis. Bosn J Basic Med Sci. 2019;5(9):2442–2447. doi: 10.17305/bjbms.2019.4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pan Z., Mao W., Bao Y., Zhang M., Su X., Xu X. The long noncoding RNA CASC9 regulates migration and invasion in esophageal cancer. Cancer Med. 2016;5:2442–2447. doi: 10.1002/cam4.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang Y., Chen D., Liu H., Yang K. Increased expression of lncRNA CASC9 promotes tumor progression by suppressing autophagy-mediated cell apoptosis via the AKT/mTOR pathway in oral squamous cell carcinoma. Cell Death Dis. 2019;10:41. doi: 10.1038/s41419-018-1280-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klingenberg M., Gross M., Goyal A., Polycarpou-Schwarz M., Miersch T., Ernst A.S. The long noncoding RNA cancer susceptibility 9 and RNA binding protein heterogeneous nuclear ribonucleoprotein L form a complex and coregulate genes linked to AKT signaling. Hepatology. 2018;68:1817–1832. doi: 10.1002/hep.30102. [DOI] [PubMed] [Google Scholar]
- 32.Gao G.D., Liu X.Y., Lin Y., Liu H.F., Zhang G.J. LncRNA CASC9 promotes tumorigenesis by affecting EMT and predicts poor prognosis in esophageal squamous cell cancer. Eur. Rev. Med. Pharmacol. Sci. 2018;22:422–429. doi: 10.26355/eurrev_201801_14191. [DOI] [PubMed] [Google Scholar]
- 33.Zhou J., Xiao H., Yang X., Tian H., Xu Z., Zhong Y. Long noncoding RNA CASC9.5 promotes the proliferation and metastasis of lung adenocarcinoma. Sci Rep. 2018;8:37. doi: 10.1038/s41598-017-18280-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu Y., Hu L., Liang Y., Li J., Wang K., Chen X. Up-regulation of lncRNA CASC9 promotes esophageal squamous cell carcinoma growth by negatively regulating PDCD4 expression through EZH2. Mol. Cancer. 2017;16:150. doi: 10.1186/s12943-017-0715-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Su X., Li G., Liu W. The long noncoding RNA cancer susceptibility candidate 9 promotes nasopharyngeal carcinogenesis via stabilizing HIF1alpha. DNA Cell Biol. 2017;36:394–400. doi: 10.1089/dna.2016.3615. [DOI] [PubMed] [Google Scholar]
- 36.Jiang Y., Jia Y., Zhang L. Role of programmed cell death 4 in diseases: a double-edged sword. Cell. Mol. Immunol. 2017;14:884–886. doi: 10.1038/cmi.2017.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jin Y., Xie H., Duan L., Zhao D., Ding J., Jiang G. Long non-coding RNA CASC9 and HIF-1alpha form a positive feedback loop to facilitate cell proliferation and metastasis in lung cancer. Onco Targets Ther. 2019;12:9017–9027. doi: 10.2147/OTT.S226078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xi J., Wang Y., Liu H. GLUT-1 participates in the promotion of LncRNA CASC9 in proliferation and metastasis of laryngeal carcinoma cells. Gene. 2020;726:144194. doi: 10.1016/j.gene.2019.144194. [DOI] [PubMed] [Google Scholar]
- 39.Luo K., Geng J., Zhang Q., Xu Y., Zhou X., Huang Z. LncRNA CASC9 interacts with CPSF3 to regulate TGF-beta signaling in colorectal cancer. J. Exp. Clin. Cancer Res. 2019;38:249. doi: 10.1186/s13046-019-1263-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang J., Wang Q., Quan Z. Long non-coding RNA CASC9 enhances breast cancer progression by promoting metastasis through the meditation of miR-215/TWIST2 signaling associated with TGF-beta expression. Biochem. Biophys. Res. Commun. 2019;515:644–650. doi: 10.1016/j.bbrc.2019.05.080. [DOI] [PubMed] [Google Scholar]
- 41.Hu X., Li Y., Kong D., Hu L., Liu D., Wu J. Long noncoding RNA CASC9 promotes LIN7A expression via miR-758-3p to facilitate the malignancy of ovarian cancer. J. Cell. Physiol. 2019;234:10800–10808. doi: 10.1002/jcp.27903. [DOI] [PubMed] [Google Scholar]
- 42.Li X., Chen B., Chi D., Zhang Y., Jiang W. lncRNA CASC9 regulates cell migration and invasion in hemangioma endothelial cells by targeting miR-125a-3p/Nrg1. Onco Targets Ther. 2019;12:423–432. doi: 10.2147/OTT.S181914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu H., Li C., Yang J., Sun Y., Zhang S., Yang J. Long noncoding RNA CASC9/miR-519d/STAT3 positive feedback loop facilitate the glioma tumourigenesis. J. Cell. Mol. Med. 2018;22:6338–6344. doi: 10.1111/jcmm.13932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shao G., Wang M., Fan X., Zhong L., Wang Z., Zhang P. lncRNA CASC9 positively regulates CHK1 to promote breast cancer cell proliferation and survival through sponging the miR195/497 cluster. Int. J. Oncol. 2019;54:1665–1675. doi: 10.3892/ijo.2019.4734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chua Y.L., Ito Y., Pole J.C., Newman S., Chin S.F., Stein R.C. The NRG1 gene is frequently silenced by methylation in breast cancers and is a strong candidate for the 8p tumour suppressor gene. Oncogene. 2009;28:4041–4052. doi: 10.1038/onc.2009.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liang Y., Chen X., Wu Y., Li J., Zhang S., Wang K. LncRNA CASC9 promotes esophageal squamous cell carcinoma metastasis through upregulating LAMC2 expression by interacting with the CREB-binding protein. Cell Death Differ. 2018;25:1980–1995. doi: 10.1038/s41418-018-0084-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ganguly D., Fan M., Yang C.H., Zbytek B., Finkelstein D., Roussel M.F. The critical role that STAT3 plays in glioma-initiating cells: STAT3 addiction in glioma. Oncotarget. 2018;9:22095–22112. doi: 10.18632/oncotarget.25188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gramantieri L., Baglioni M., Fornari F., Laginestra M.A., Ferracin M., Indio V. LncRNAs as novel players in hepatocellular carcinoma recurrence. Oncotarget. 2018;9:35085–35099. doi: 10.18632/oncotarget.26202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yu X., Lin Y., Sui W., Zou Y., Lv Z. Analysis of distinct long noncoding RNA transcriptional fingerprints in pancreatic ductal adenocarcinoma. Cancer Med. 2017;6:673–680. doi: 10.1002/cam4.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Su M., Xiao Y., Ma J., Tang Y., Tian B., Zhang Y. Circular RNAs in cancer: emerging functions in hallmarks, stemness, resistance and roles as potential biomarkers. Mol. Cancer. 2019;18:90. doi: 10.1186/s12943-019-1002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jiang B., Li Y., Qu X., Zhu H., Tan Y., Fan Q. Long noncoding RNA cancer susceptibility candidate 9 promotes doxorubicinresistant breast cancer by binding to enhancer of zeste homolog 2. Int. J. Mol. Med. 2018;42:2801–2810. doi: 10.3892/ijmm.2018.3812. [DOI] [PubMed] [Google Scholar]
- 52.Shang C., Sun L., Zhang J., Zhao B., Chen X., Xu H. Silence of cancer susceptibility candidate 9 inhibits gastric cancer and reverses chemoresistance. Oncotarget. 2017;8:15393–15398. doi: 10.18632/oncotarget.14871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fang J., Chen W., Meng X.L. LncRNA CASC9 suppressed the apoptosis of gastric cancer cells through regulating BMI1. Pathol Oncol Res. 2019;26:475–482. doi: 10.1007/s12253-019-00703-3. [DOI] [PubMed] [Google Scholar]
- 54.Gao L., Guo Y.N., Zeng J.H., Ma F.C., Luo J., Zhu H.W. The expression, significance and function of cancer susceptibility candidate 9 in lung squamous cell carcinoma: a bioinformatics and in vitro investigation. Int. J. Oncol. 2019;54:1651–1664. doi: 10.3892/ijo.2019.4758. [DOI] [PMC free article] [PubMed] [Google Scholar]