Abstract
To investigate the early epidemic of COVID-19, a total of 176 confirmed COVID-19 cases in Shiyan city, Hubei province, China were surveyed. Our data indicated that the rate of emergence of early confirmed COVID-19 cases in Hubei province outside Wuhan was dependent on migration population, and the second-generation of patients were family clusters originating from Wuhan travelers. Epidemiological investigation indicated that the reproductive number (R0) under containment strategies was 1.81, and asymptomatic SARS-CoV-2 carriers were contagious with a transmission rate of 10.7%. Among the 176 patients, 53 were admitted to the Renmin Hospital of Hubei University of Medicine. The clinical characteristics of these 53 patients were collected and compared based on a positive RT-PCR test and presence of pneumonia. Clinical data showed that 47.2% (25/53) of COVID-19 patients were co-infected with Mycoplasma pneumoniae, and COVID-19 patients coinfected with M. pneumoniae had a higher percentage of monocytes (P < 0.0044) and a lower neutrophils percentage (P < 0.0264). Therefore, it is important to assess the transmissibility of infected asymptomatic individuals for SARS-CoV-2 transmission; moreover, clinicians should be alert to the high incidence of co-infection with M. pneumoniae in COVID-19 patients.
Keywords: SARS-CoV-2, asymptomatic infections, coinfection, COVID-19, Mycoplasma pneumonia
Introduction
By the end of 2019, just before the Chinese New Year, an outbreak of idiopathic pneumonia surfaced in Wuhan, China (Li Q. et al., 2020). Soon afterwards, the causative pathogen was identified as a novel coronavirus (Huang et al., 2020; Wang D. W. et al., 2020). With rapidly increasing clinical cases, person-to-person transmission was confirmed (Chan et al., 2020b). This novel coronavirus was later named by the International Committee on Taxonomy of Viruses as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (Coronaviridae Study Group of the International Committee on Taxonomy of Viruses, 2020). On January 30, 2020, a public health emergency of international concern was declared by the World Health Organization (WHO) (Public Health Emergency of International Concern declared by WHO, 2020). By March 30, 2020, a total of 82,545 coronavirus disease 2019 (COVID-19) cases were confirmed in China (China Center for Disease Control and Prevention, 2020) and more than 780,000 confirmed COVID-19 cases were identified globally (WHO Director-General's Opening Remarks at the Media Briefing on COVID-19-11 March, 2020). In Hubei Province, there were 67,801 confirmed cases (49,986 in Wuhan), including 7,984 severe cases (7,049 in Wuhan) and 3,187 deaths (2,430 in Wuhan) (China Center for Disease Control and Prevention, 2020). As previously reported, the most common symptoms at onset of COVID-19 were fever, cough, expectoration, headache, myalgia or fatigue, diarrhea, and hemoptysis, along with abnormal lesions on chest computed tomography (CT) (Shi et al., 2020; Wang C. et al., 2020; Wu and McGoogan, 2020). There was also evidence of lymphopenia in a proportion of patients (Chen H. J. et al., 2020).
The movement of millions of people with no effective protection measures is considered one of the main reasons for the spread of the epidemic; in particular, the massive population inflows from Wuhan to other hometowns before the Spring Festival fostered the outbreak of this disease to other regions. During the spread, interventional measures including lockdown of public places, cessation of highways and city traffic, wearing facial masks when outside, and refusion of social activities were taken to lower the transmissibility (Wuhan Novel Coronavirus Infection Pneumonia Epidemic Prevention Control Headquarters., 2020). Collection and analysis of the epidemiological and clinical characteristics of confirmed cases outside Wuhan helped to adopt and adapt strategies, resulting in prevention and control of the pandemic in these regions.
In this study, we summarized the dynamics and clinical features of the COVID-19 pandemic in Shiyan city in the Hubei province, a city 440 km from Wuhan city, based on the surveillance data up to February 24, 2020. It is very important to understand the epidemiological and clinical characteristics of COVID-19 in the surrounding cities of Wuhan. We hope that these data can provide positive suggestions for other cities to prevent the further spread of COVID-19.
Materials and Methods
Ethics
This study was conducted in accordance with the tenets of the Helsinki declaration. This study was approved by the institutional review board of Shiyan Renmin Hospital of Hubei University of Medicine, and the need for informed consent was waived. This study was designed as a retrospective case series, and no patients were directly involved in the study design, setting of research questions, or outcome measures. No patients were consulted for advice on interpretation or writing of results.
Epidemical Data Sources
Data of the 176 confirmed COVID-19 patients were collected from January 22, 2020 to February 6, 2020, and included seven children aged <14 years and 169 adults. COVID-19 was confirmed by two positive RT-PCR tests in hospitals. Asymptomatic carriers were quarantined at the hospital or hotels after having been discovered.
Per the guidelines on investigation and management of close contacts for COVID-19 patients issued by the Chinese Center for Disease Control, close contacts of suspected cases, clinically diagnosed cases, and confirmed cases 2 days before the onset of illness were required to meet the following criteria: family members living in the same room, medical workers without secondary protection, and sharing personal meals or communication in confined spaces. The contact traces of confirmed cases were informed by patients or family members, and the duration spans 14 days before onset.
Clinical Data Sources
Suspected cases were defined as meeting two of the following criteria: (1) fever, and/or respiratory symptoms; (2) presence of radiographic pneumonia; and (3) white blood cell (WBC) counts within upper limit of normal (ULN) or hypo-lymphocytosis during early course of the disease. Once the cases were identified, respiratory tract secretions and other samples were collected for real-time fluorescence reverse transcription-polymerase chain reaction (RT-PCR). In all, 176 patients who tested positive for SARS-CoV-2 nucleic acids were identified as confirmed cases and enrolled in the study.
Of these 176 confirmed patients, 53 (26 male and 27 female; mean age, 38 ± 17 years; age range, 6 months to 80 years) were admitted to the Department of Infectious Diseases, Department of Respiratory, Shiyan Renmin Hospital, Hubei University of Medicine. Data were collected and analyzed from the 53 patients from January 23, 2020 to February 24, 2020 (Table 1). The inclusion criteria were as follows: Suspected cases were screened according to the diagnosis and treatment protocol for COVID-19 pneumonia [Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (6rd Interim Edition), 2020].
Table 1.
Personal and clinical characteristics of 53 patients with COVID-19 in Shiyan city, Hubei province, China.
| Characteristics | All patients (n = 53) |
|---|---|
| Median (interquartile) age (in years) | 38 (28–47) |
| Age groups (in years) | |
| ≤ 14 | 6 (11.3%) |
| 15–30 | 12 (22.6%) |
| 31–59 | 29 (54.7%) |
| ≥60 | 6 (11.3%) |
| Sex | |
| Male | 26 (49.0%) |
| Female | 27 (50.9%) |
| Coexisting infection | |
| Mycoplasma pneumoniae | 25 (47.2%) |
| Other pathogens | 6 (11.3%) |
| Coexisting conditions | |
| Any | 26 (49.0%) |
| Hypertension | 3 (5.7%) |
| Diabetes | 1 (1.9%) |
| Chronic obstructive pulmonary disease | 8 (15.1%) |
| Cerebrovascular disease | 3 (5.7%) |
| Renal disease | 3 (5.7%) |
| Liver disease | 9 (17.0%) |
| Exposure history in Wuhan <2 weeks | |
| Yes | 6 (11.3%) |
| No | 47 (88.7%) |
| Familial cluster | 32 (60.4%) |
| Fever | 46 (86.8%) |
| Highest temperature (°C) | |
| <37.3 | 7 (13.2%) |
| 37.3–38.0 | 12 (22.6%) |
| 38.01–39.0 | 24 (45.3%) |
| >39.0 | 10 (18.9%) |
| Cough | 35 (66%) |
| Myalgia or fatigue | 17 (32.1%) |
| Expectoration | 32 (60.4%) |
| Hemoptysis | 1 (1.9%) |
| Headache | 14 (26.4%) |
| Diarrhea | 3 (5.7%) |
Values are medians (interquartile range) or numbers (percentage).
Sample Collection and Pathogen Identification
After admission to Shiyan Renmin Hospital, indirect immunofluorescent assay was performed to simultaneously detect IgM antibodies against the following main etiological agents of pneumonia: Legionella pneumophila, Mycoplasma pneumoniae, Coxiella burnetii, Chlamydia pneumoniae, adenovirus (AdV), influenza A virus (IAV), influenza B virus (IBV), parainfluenza virus type 1+2+3 (PIV 1+2+3), and respiratory syncytial virus (RSV). The Pneumoslide-M kit (Vircell IFA KIT) was used for testing according to the manufacturer's instructions. IgM antibody detections for Mycoplasma pneumoniae of the coinfection pneumonia patients were performed at least three times during the acute phase and recovery phase. IgM antibody for Mycoplasma pneumoniae was also quantified by Serodia-Myco II assay (Fujirebio Inc., Tokyo, Japan), and IgG antibody were tested by the mycoplasma EIA kit (EUROIMMUN Inc., German).
In addition, respiratory tract samples including sputum and nasopharyngeal swabs collected from the patients were tested for severe acute respiratory syndrome-associated coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV) by using Ag Path-ID One-Step RT-PCR Kit (Cat: AM1005, ABI) according to the manufacturer's instructions.
Respiratory tract samples were also used for real-time fluorescence RT-PCR to detect the presence of SARS-CoV-2 by using the SARS-CoV-2 (ORF1ab/N) nucleic acid detection kit (Cat: SJ-HX-009-2, Bio-germ, Shanghai, China) according to the manufacturer's instructions.
Antiviral Treatment
Interferon alpha (5 million U or equivalent dose per time for adults, 2 times a day for atomization inhalation), lopinavir (200 mg/pill for adults, 2 pills for each time, 2 times a day, the course of treatment was <10 days), ritonavir (50 mg/pill for adults, 2 pills for each time, 2 times a day, the course of treatment was <10 days), ribavirin (500 mg/pill for adults, 2–3 times a day for intravenous infusion, the course of treatment is not more than 10 days), and Abidol (200 mg for adults, 3 times a day, the course of treatment was not exceed 10 days) were used. Antiviral traditional Chinese medicine was used for adjuvant treatment.
Clinical Data Collection
Basic demographic and clinical data including age, sex, underlying diseases, and comorbidities were collected for each patient (Table 1). Laboratory findings of COVID-19 patients categorized by M. pneumoniae lgM antibody presence were recorded (Table 2). In addition, epidemiological histories were taken. Laboratory test results of standard blood counts (absolute white blood cells and lymphocytes); blood biochemistry (alanine transaminase, aspartate transaminase, creatine kinase, and creatinine); coagulation function; procalcitonin; C-reactive protein; erythrocyte sedimentation rate; and myocardial enzyme spectrum were compiled (Table 3). Additional data collected included medical imaging; treatment regimens (antiviral, antibacterial, systemic corticosteroid, immunoglobulin G, respiratory support); and prognosis (recovered and discharged, inpatient treatment, or death) (Table 4).
Table 2.
Laboratory findings of COVID-19 patients categorized by M. pneumoniae lgM antibody presence.
|
Mycoplasma lgM (–) |
Mycoplasma lgM (+) |
P-value | |
|---|---|---|---|
| Neutrophils (%) | 70.28 ± 2.558 | 59.64 ± 3.119 | 0.0264* |
| Lymphocytes (%) | 27.82 ± 3.389 | 34.41 ± 5.348 | 0.2904 |
| Monocytes (%) | 9.733 ± 1.615 | 18.18 ± 1.654 | 0.0044** |
| White blood cells (× 109/L) | 4.442 ± 0.399 | 5.046 ± 0.455 | 0.3242 |
| CRP (mg/L) | 15.04 ± 5.471 | 13.09 ± 4.005 | 0.7787 |
| LDH (U/L) | 254 ± 43.50 | 272 ± 57.25 | 0.8435 |
means a significant difference.
Table 3.
Laboratory findings in patients with COVID-19.
| Variables | All patients (n = 53) |
|---|---|
| White blood cell count (× 109/L) | 4.68 (3.32–5.08) |
| <4 | 21 (39.6%) |
| 4–10 | 32 (60.4%) |
| Neutrophil count (× 109/L) | 2.76 (1.96–3.81) |
| Lymphocyte count (× 109/L) | |
| <1.0 | 22 (41.5%) |
| ≥1.0 | 31 (58.5%) |
| Hemoglobin (g/L) | 123.0 (116.9–148.2) |
| Platelet count (× 109/L) | |
| <100 | 2 (3.8%) |
| ≥100 | 51 (96.2%) |
| C-reactive protein (mg/L) | |
| <5 | 31 (58.5%) |
| ≥5 | 22 (41.5%) |
| Aspartate aminotransferase (U/L) | |
| <40 | 42 (79.2%) |
| ≥40 | 11 (20.8%) |
| Potassium (mmol/L) | 3.2 (2.9–3.6) |
| Sodium (mmol/L) | 136 (128–142) |
| Creatinine (μmol/L) | |
| ≤ 133 | 51 (96.2%) |
| >133 | 2 (3.8%) |
| Creatine kinase (U/L) | |
| ≤ 185 | 48 (90.6%) |
| >185 | 7 (13.2%) |
| Lactate dehydrogenase (U/L) | |
| ≤ 245 | 46 (86.8%) |
| >245 | 7 (13.2%) |
| Procalcitonin (ng/mL) | |
| <0.1 | 42 (79.2%) |
| ≥0.1 | 11 (20.8%) |
| Pneumonia | 53 (100%) |
Values are medians (interquartile range) or numbers (percentage).
Table 4.
Treatment regimen and prognosis of patients with COVID-19.
| Treatment | n (percentage) |
|---|---|
| Antiviral | 53 (100%) |
| Antibacterial | 25 (47.2%) |
| Systemic corticosteroid | 12 (22.6%) |
| Human γ-immunoglobulin | 12 (22.6%) |
| Respiratory support | 48 (90.5%) |
| Nasal cannula | 12 (22.6%) |
| Non-invasive ventilation | 32 (60.4%) |
| Improved and discharged | 53 (100%) |
| Inpatient treatment | 53 (100%) |
| Death | 0 (0%) |
Values are numbers (percentage).
Statistical Analysis
Epidemiological and clinical data were collected and analyzed by Microsoft Office (version 2016) and GraphPad Prism (version 5.0), and the epidemiological figures were plotted using Microsoft Excel. Continuous clinical data were expressed as medians and ranges, and categorical data, as counts and percentages.
Results
Dynamics of the COVID-19 Epidemiology in Shiyan City
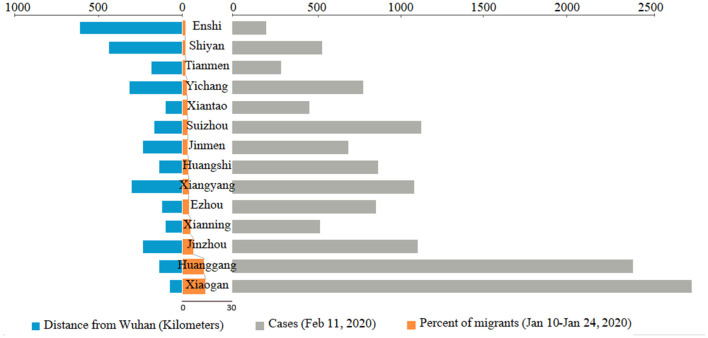
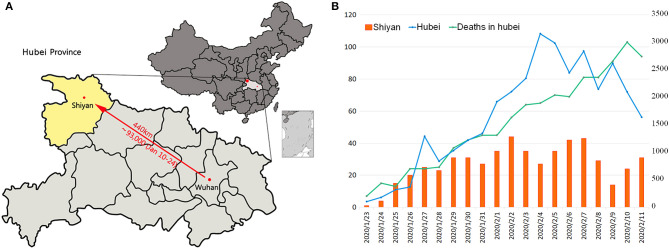
The resident population of Wuhan, the capital of Hubei province, was 11.081 million at the end of 2018, and the migrant population exceeded 5 million (National Health Commission of the PRC, 2020). At the beginning of “Chunyun” (migration during Spring Festival) from January 10, 2020 to January 24, 2020, most of the migrants from Wuhan travel to other counties and cities in Hubei province, accounting for about 69.4% of the total migrating population. They travel especially to Xiaogan (13.8%) and Huanggang (13.04%) (Data Came from Baidu Qianxi Map, 2020), which are adjacent to Wuhan (Figure 1). As of midnight of February 11, 2020, the confirmed COVID-19 cases in the cities outside Wuhan are migrate rate-dependent emerged (Figure 1). In Shiyan city, located in the southwest of Hubei province and 440 km from Wuhan city, the migrant population was 1.86% (about 93,000 people) which accounted for people who came back from Wuhan in the period between January 10 and January 24, 2020 (Figure 2A). Based on the incubation period of SARS-CoV-2, the epidemiological data of confirmed COVID-19 cases that emerged in Shiyan were collected from January 23, 2020. The number of confirmed COVID-19 cases onset in Hubei province showed a rapid increase before February 4, 2020, peaking at 3,156, and then showed a gradually decreasing trend (Figure 2B). The same trend of newly confirmed cases was also found in Shiyan city, with a peak of 44 cases that fluctuated between February 2 and February 7, 2020. As of midnight of February 23, there were a total of 669 confirmed cases in Shiyan, and only two related deaths. However, 374 patients were still under treatment in hospital including 20 with severe illness and 15 with critically severe illness. The overall case fatality rate (CFR) of COVID-19 patients in Hubei province was 3.82% from January 23 to February 11, 2020.
Figure 1.
The population migration and confirmed COVID-19 cases in Hubei province outside Wuhan. Flow of population migration from Wuhan to other cities in Hubei province between January 10 and January 24, 2020, during the “Chunyun” period. Data of COVID-19 cases were collected from the Chinacdc.com.
Figure 2.
Analysis of the population migration and trend of illness onset. (A) Geographical display of the distance of Shiyan from Wuhan. The migrant population is calculated using the percent of total migrated individuals. (B) The onset numbers of confirmed COVID-19 patients in Hubei province and Shiyan city. Deaths occurred up to February 11, 2020 in Hubei province were also counted.
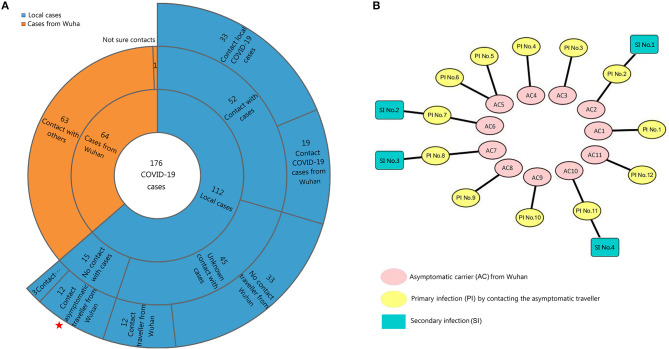
The 64 confirmed cases returning from Wuhan were surveyed. The 112 confirmed cases in local clusters without travel history to Wuhan implied that second-generation patients appeared in Shiyan through close contact; these included 52 local cases that had clear contact history with the COVID-19 patients from Wuhan or local citizens (Figure 3A). Fifteen of them with no close contact with COVID-19 patients, and 45 local cases with unknown sources had been infected with the virus. Notably, the onset of 12 cases were confirmed after close contact with 11 travelers from Wuhan who were asymptomatic carriers and showed no signs of illness after returning from Wuhan after nearly 1 month (Figure 3B). Another 12 cases caused four secondary infections in this period. The transmission rate caused by asymptomatic carriers was 10.7% (12/112).
Figure 3.
Contact history analysis of the 176 confirmed cases. (A) The contact history was obtained by patients or family members, and the duration spanned 14 days before symptom onset. Stars indicate the 12 cases after contact with infected asymptomatic carriers. (B) Twelve patients (primary infection, PI) infected by asymptomatic carriers (AC) from Wuhan; the secondary infections (SI) were surveyed. Eleven asymptomatic infections caused 12 primary infections and four secondary infections.
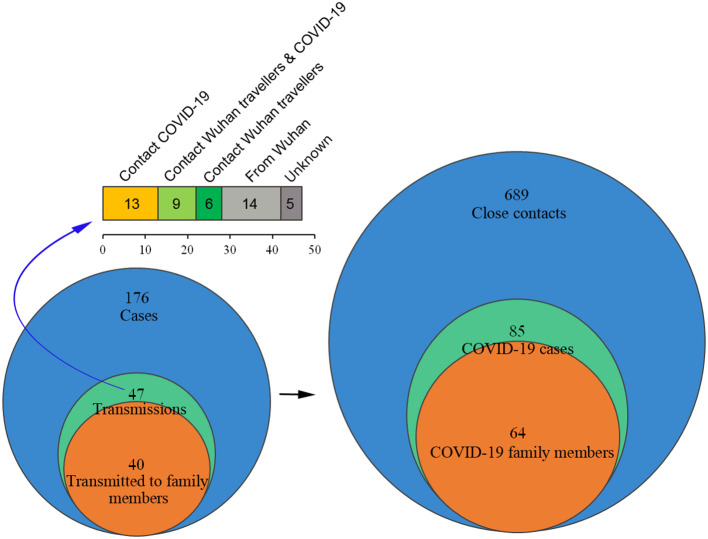
Among the 176 surveyed cases including Wuhan travelers and local citizens, 689 related close contacts were tracked (Figure 4). Forty-seven patients transmitted the virus and caused 85 confirmed cases, including 40 patients who transmitted the virus to 64 family members. The infection rate in our surveyed data was 12.34% (85/689), and the R0 was 1.81 (85/47), which is lower than the recent reports because of the family quarantine measures (Chen T. M. et al., 2020). Contact tracing of the 47 cases showed that 14 of them traveled back from Wuhan, 13 contacted COVID-19 patients, and nine came into contact with Wuhan travelers and confirmed COVID-19 cases. In addition, six patients had contact history with Wuhan travelers, and five showed unknown infection routes, which included three patients that transmitted the virus to their family members and colleagues and another two that spread the virus to their colleagues or friends.
Figure 4.
Survey of the close contacts of 176 confirmed cases. The close contacts mainly included family members, colleagues, or friends who lived together, shared meals, and/or physically communicated with the confirmed COVID-19 patients 2 days before the onset of illness. The close contacts were interviewed.
Clinical Characteristics of COVID-19 Cases in Shiyan
To better understand the clinical features of COVID-19 cases we tracked, up to February 23, 2020, the clinical data on 53 patients (26 male and 27 female) were collected in the Department of Infectious Diseases of Shiyan Renmin Hospital, Hubei Province, with laboratory-confirmed SARS-CoV-2 infection. Six (11.3%) of these patients were <14 years old, 12 (22.6%) were aged between 15 and 30 years, 29 (54.7%) were aged between 31 and 59 years, and six (11.3%) were ≥60 years. The median age was 38 years (interquartile range, 28–47 years) (Table 1). Interestingly, we noticed that 25 (47.2%) patients were co-infected with M. pneumoniae (Table 5), who had a lower neutrophils percentage (59.64 ± 3.119 vs. 70.28 ± 2.558, P < 0.0264) and higher monocytes percentage (18.18 ± 1.654 vs. 9.733 ± 1.615, P < 0.0044) compared with M. pneumoniae negative patients (Table 2). Six (11.3%) of the 53 COVID-19 patients were co-infected with other common respiratory pathogens, such as IAV, IBV, and RSV, respectively. Among the 53 COVID-19 patients, 26 (49.0%) had the following underlying diseases: three (5.7%) had hypertension, one (8%) had diabetes, eight (15.1%) had chronic obstructive pulmonary disease, three (5.7%) had cerebrovascular disease, three (5.7%) had renal disease, and nine (17.0%) had liver disease. Only six (11.3%) of the 53 patients had history of exposure in Wuhan. Twenty-two (60.4%) of the 53 patients were associated with familial clusters. The most common symptoms at illness onset were fever (46, 86.8%); cough (35, 66%); and expectoration (32, 60.4%). Other symptoms at illness onset were myalgia or fatigue (17, 32.1%); hemoptysis (1, 1.9%); headache (14, 26.4%); and diarrhea (3, 5.7%) (Table 1).
Table 5.
IgM antibody titers for the M. pneumoniae co-infection patients.
| IgM antibody titer | Number (n = 25) |
|---|---|
| 1:40 | 1 (4%) |
| 1:80 | 8 (32%) |
| 1:160 | 9 (36%) |
| 1:320 | 5 (20%) |
| 1:640 | 2 (8%) |
On admission, the blood counts of 21 (39.6%) of the 53 patients showed leucopenia (white blood cell count: <4 × 109/L) and 22 (41.5%) showed lymphopenia (lymphocyte count: <1.0 × 109/L) (Table 3). The levels of C-reactive protein (CRP) were elevated in 22 (41.5%) patients and the levels of aspartate aminotransferase (AST) were increased in 11 (20.8%) patients. Twenty-two (79.2%) patients had normal serum levels of procalcitonin (PCT) (<0.1 ng/mL). All patients had pneumonia and showed abnormalities on either chest CT or radiographs. Typical chest CT findings of infected patients on admission were bilateral or multiple lobular or subsegmental areas of consolidation or bilateral ground glass opacity (Figure 5). Of the 53 patients, only one patient (age, 40 years) was transferred to an intensive care unit for acute respiratory distress syndrome and received mechanical ventilation.
Figure 5.
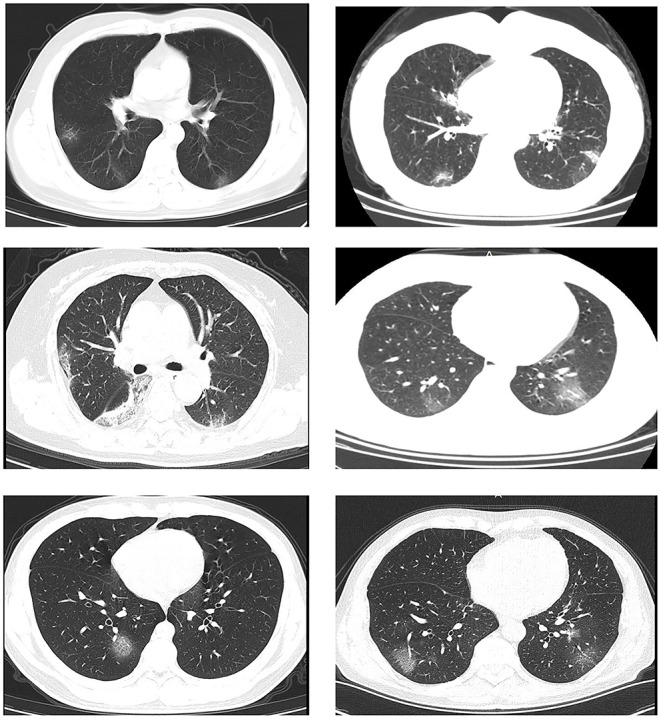
Transverse chest computed tomography images of patients with COVID-19. Transverse chest computed tomography of six patients with COVID-19 on admission showed bilateral or multiple lobular or subsegmental areas of consolidation or bilateral ground glass opacity.
All patients received antiviral treatment (Table 4). Among the 53 COVID-19 patients co-infected with M. pneumoniae, 25 (25/53, 47.2%) were given antibiotic (levofloxacin) treatment, and 12 (12/53, 22.6%) were given systematic corticosteroid and γ-immunoglobulin treatment. At the time of writing this paper, all 53 (100%) patients were discharged and there were no deaths. Fitness for discharge was based on subsiding of fever for at least 3 days, with improved evidence on chest radiography and viral clearance in samples from the lower respiratory tract.
Discussion
In Shiyan city, the first laboratory-confirmed case of COVID-19 was identified in January 23, 2020, and the epidemic has experienced an increasing trend before February 2. The growth phase of new cases is consistent with most other regions outside Wuhan in Hubei province. Because CT-based diagnosis of COVID-19 was considered a confirmatory criterion in Hubei province [Diagnosis Treatment Protocol for Novel Coronavirus Pneumonia (Trial Version 6, Revised), WHO China Office, 2020], more than 10,000 patients were treated in hospital on February 12, 2020 (Lu J. et al., 2020). The growth trend in Hubei province and Shiyan city was lower than the expected growth curve because of the strict quarantine measures (Peng et al., 2020; Roosa et al., 2020). During the Chinese traditional new year, most family members and relatives gather at home and share the festivities. Therefore, cluster cases occurred mainly among family members and originated either from Wuhan travelers or those that came into contacting with COVID-19 patients. The origin of the virus could not be confirmed in five patients, and 12 patients had made contact with passengers traveling from Wuhan who were asymptomatic carrier and not in the incubation period. Although asymptomatic and presymptomatic infection of SARS-CoV-2 has been reported recently, most of them subsequently developed symptoms (Arons et al., 2020; Bai et al., 2020; Rothe et al., 2020). There was no evidence that these 11 individuals from Wuhan had an incubation period of more than 1 month and transferred the virus to their family members during this period. Currently published research basically reported that confirmed cases in the presymptomatic stage can result in transmission (Liu et al., 2020; Yu et al., 2020), it is yet not clear the transmissibility and transmission rate by asymptomatic carriers. Therefore, it is necessary to assess the transmissibility of asymptomatic carriers, strengthening the management of asymptomatic patients and tracing the close contacts of asymptomatic individuals can close the loophole.
According to the statistics of clinical cases, children seem to be less infected. The infection is mainly concentrated in the age group of 31–59 years. Children and youth have been less infected, which may be due to other unknown reasons. According to previous reports, men are more likely to be infected with SARS-CoV-2 than women (Yang et al., 2020), but our study found no significant difference in the infection rate of men and women. Most importantly, 25 (47.2%) of the 53 COVID-19 patients were co-infected with M. pneumoniae. Common respiratory pathogens such as seasonal influenza viruses were not common in the 53 COVID-19 patients. Because monocytes increased after M. pneumoniae infection alone (Puljiz et al., 2006; Wang et al., 2019), indicating the involvement of monocyte-related mechanisms in the pathogenesis of M. pneumoniae co-infection in COVID-19 patients. This suggests that we should pay more attention to M. pneumoniae co-infection for COVID-19 patients during clinical testing and corresponding treatment. The existence of underlying diseases may promote the generation of SARS-CoV-2 infection to a certain extent. This is also one of the reasons for the higher mortality rate of the elderly COVID-19 patients (Ji et al., 2020). Only a few patients had been to Wuhan, while most of the other patients acquired local infections. This confirmed the strong infectivity of SARS-CoV-2; therefore, controlling local clusters is key to prevent outbreaks from imported cases. Fever, cough, and expectoration are the main clinical symptoms of COVID-19. However, it is particularly interesting to note that 13.2% patients in our study showed no fever symptom despite being infected. This suggests that the severity of SARS-CoV-2 infection could be graded by combining CRP levels with the patient's age (Lu H. et al., 2020). In this study, we showed that 41.5% of patients had abnormally high CRP levels (≥5 mg/L). On admission, decreased leukocyte and lymphocyte counts indicated that the immune function of patients was compromised, consistent with a previous report by Xu et al. (2020).
From the perspective of clinical treatment, antiviral treatment (including antiviral traditional Chinese medicine) played a better therapeutic role. In addition, early detection of infection and symptomatic treatment were essential to reduce mortality. However, RT-PCR test results had a false-negative rate (Chan et al., 2020a; Li Z. et al., 2020). At present, asymptomatic carriers have been identified (Guan et al., 2020), and patients discharged from hospitals may still be carriers of the virus (Lan et al., 2020). Therefore, it is very important to find more effective detection methods. According to our clinical observation, CT imaging can effectively detect SARS-CoV-2 infection. However, fever, cough, and other related symptoms cannot be used as absolute evidence of infection.
Controlling and stopping the outbreak of a new pathogen that is effectively transmitted from person to person remains extremely challenging for most countries, especially when the SARS-CoV-2 has become a global pandemic having spread to 114 countries (WHO Director-General's Opening Remarks at the Media Briefing on COVID-19-11 March, 2020). Therefore, vaccine research is crucial for effective treatment and control of viral transmission; however, animal experiments and clinical trials are time consuming and cannot produce immediate results. China has heavily invested in medical resources to treat the COVID-19 patients, especially elderly patients with severe and critical illness. Strict intervention measures adopted by the government should be referenced in other regions with heavy outbreaks. Moreover, high-quality epidemiological investigations can find close contacts and early asymptomatic infections, reducing the potential risk of transmission by asymptomatic infection could lead to the stabilized epidemic.
Data Availability Statement
All datasets presented in this study are included in the article/supplementary material.
Ethics Statement
This study was approved by the Shiyan Renmin Hospital of Hubei University of Medicine institutional review board and the need for informed consent was waived.
Author Contributions
LL, WL, JW, and ZL contributed to the design of experiments. LL, XL, XX, JY, JL, MJ, WD, HT, JZ, BL, and ZJ contributed to the conduction of experiments. LL, XL, XX, JY, WL, JW, and ZL contributed to the reagents. LL, XL, XX, JY, JL, MJ, WD, HT, JZ, BL, ZJ, WL, JW, and ZL contributed to the analyses of the data. LL, JW, and ZL contributed to the writing the paper. JW and ZL contributed to the editing the paper.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank the staff members at the Shiyan Centers for Disease Control and Prevention for their assistance in the epidemic data collection.
Footnotes
Funding. This work was supported by research grants from the National Natural Science Foundation of China (81902066, 81730061, and 81471942), the Natural Science Foundation of Hubei Provincial Department of Education (Q20192105 and Q20192104), the Hubei Provincial Natural Science Foundation (2018CFB185 and 2018CFB093), COVID-19 Epidemic Emergency Research Foundation (2020XGFYZR07 and 2020XGFYZR08), Cultivating Project for Young Scholar at Hubei University of Medicine (2017QDJZR08 and 2016QDJZR03), and National Undergraduate Training Program for Innovation and Entrepreneurship (201913249002). The funder had no role in the study design, data collection and analysis, data interpretation and writing.
References
- Arons M. M., Hatfield K. M., Reddy S. C., Kimball A., James A., Jacobs J.R., et al. (2020). Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. N. Engl. J. Med. 10.1056/NEJMoa2008457. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai Y., Yao L. S., Wei T., Tian F., Jin D. J., Chen L., et al. (2020). Presumed asymptomatic carrier transmission of COVID-19. JAMA 323, 1406–1407. 10.1001/jama.2020.2565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J. F., Yip C. C., To K. K., Tang T. H., Wong S. C., Leung K. H., et al. (2020a). Improved molecular diagnosis of COVID-19 by the novel, highly sensitive and specific COVID-19-RdRp/Hel real-time reverse transcription-polymerase chain reaction assay validated in vitro and with clinical specimens. J. Clin. Microbiol. 58:e00310-20. 10.1128/JCM.00310-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J. F., Yuan S. F., Kok K. H., To K. K., Chu H., Yang J., et al. (2020b). A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet 395, 514–523. 10.1016/S0140-6736(20)30154-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H. J., Guo J. J., Wang C., Luo F., Yu X., Zhang P. W., et al. (2020). Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet 395, 809–815. 10.1016/S0140-6736(20)30360-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T. M., Rui J., Wang Q. P., Zhao Z. Y., Cui J. A., Yin L. (2020). A mathematical model for simulating the phase-based transmissibility of a novel coronavirus. Infect. Dis. Poverty 9:24. 10.1186/s40249-020-00640-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- China Center for Disease Control Prevention (2020). Available online at: http://2019ncov.chinacdc.cn/2019-nCoV/ (accessed March 30, 2020).
- Coronaviridae Study Group of the International Committee on Taxonomy of Viruses (2020). The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 5, 536–544. 10.1038/s41564-020-0695-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Data Came from Baidu Qianxi Map (2020). Available online at: http://qianxi.baidu.com/ (accessed February 25, 2020).
- Diagnosis Treatment Protocol for Novel Coronavirus Pneumonia (6rd Interim Edition) (2020). China NHCOTPSRO. Available online at: http://www.gov.cn/zhengce/zhengceku/2020-02/19/content_5480948.htm (accessed February 25, 2020).
- Diagnosis Treatment Protocol for Novel Coronavirus Pneumonia (Trial Version 6 Revised), WHO China Office. (2020). Available online at: http://www.kankyokansen.org/uploads/uploads/files/jsipc/protocol_V6.pdf (accessed March 30, 2020).
- Guan W. J., Ni Z. Y., Hu Y., Liang W. H., Ou C. Q., He J. X., et al. (2020). Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 382, 1708–1720. 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C. L., Wang Y. M., Li X. W., Ren L., Zhao J., Hu Y., et al. (2020). Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395, 497–506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Y. P., Ma Z. R., Peppelenbosch M. P., Pan Q. (2020). Potential association between COVID-19 mortality and health-care resource availability. Lancet Glob. Health 8:e480. 10.1016/S2214-109X(20)30068-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan L., Xu D., Ye G. M., Xia C., Wang S., Li Y., et al. (2020). Positive RT-PCR test results in patients recovered from COVID-19. JAMA 323, 1502–1503. 10.1001/jama.2020.2783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Guan X. H., Wu P., Wang X., Zhou L., Tong Y., et al. (2020). Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N. Engl. J. Med. 382, 1199–1207. 10.1056/NEJMoa2001316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Yi Y., Luo X., Xiong N., Liu Y., Li S., et al. (2020). Development and clinical application of a rapid IgM-IgG combined antibody test for SARS-CoV-2 infection diagnosis. J. Med. Virol. 10.1002/jmv.25727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y. C., Liao C. H., Chang C. F., Chou C. C., Lin Y. R. (2020). A locally transmitted case of SARS-CoV-2 infection in Taiwan. N. Engl. J. Med. 382, 1070–1072. 10.1056/NEJMc2001573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H., Ai J., Shen Y., Li Y., Li T., Zhou X., et al. (2020). A descriptive study of the impact of diseases control and prevention on the epidemics dynamics and clinical features of SARS-CoV-2 outbreak in Shanghai, lessons learned for metropolis epidemics prevention. medRxiv. 10.1101/2020.02.19.20025031 [DOI] [Google Scholar]
- Lu J., Hu S., Fan R., Liu Z., Yin X., Wang Q., et al. (2020). ACP risk grade: a simple mortality index for patients with confirmed or suspected severe acute respiratory syndrome coronavirus 2 disease (COVID-19) during the early stage of outbreak in Wuhan, China. medRxiv. 10.1101/2020.02.20.20025510 [DOI] [Google Scholar]
- National Health Commission of the PRC (2020). Available online at: http://www.ldrk.org.cn/rsf/site/nmpw/index.html (accessed February 25, 2020).
- Peng L. R., Yang W. Y., Zhang D. Y., Zhuge C. J., Hong L. (2020). Epidemic analysis of COVID-19 in China by dynamical modeling. medRxiv. 10.1101/2020.02.16.2002346532129581 [DOI] [Google Scholar]
- Public Health Emergency of International Concern declared by WHO (2020). Available online at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen (accessed March 30, 2020).
- Puljiz I., Kuzman I., Dakovic O., Schönwald N., Mise B. (2006). Chlamydia pneumoniae and Mycoplasma pneumoniae pneumonia: comparison of clinical, epidemiological characteristics and laboratory profiles. Epidemiol. Infect. 134, 548–555. 10.1017/S0950268805005522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roosa K., Lee Y., Luo R., Kirpich A., Rothenberg R., Hyman J M., et al. (2020). Real-time forecasts of the COVID-19 epidemic in China from February 5th to February 24th, 2020. Infect. Dis. Model 5, 256–263. 10.1016/j.idm.2020.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothe C., Schunk M., Sothmann P., Bretzel G., Froeschl G., Wallrauch C., et al. (2020). Transmission of 2019-nCoV Infection from an asymptomatic contact in Germany. N. Engl. J. Med. 382, 970–971. 10.1056/NEJMc2001468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H. S., Han X. Y., Jiang N. C., Cao Y., Alwalid O., Gu J., et al. (2020). Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect. Dis. 20, 425–434. 10.1016/S1473-3099(20)30086-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Horby P. W., Hayden F. G., Gao G. F. (2020). A novel coronavirus outbreak of global health concern. Lancet 395, 470–473. 10.1016/S0140-6736(20)30185-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D. W., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. (2020). Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected Pneumonia in Wuhan, China. JAMA 323, 1061–1069. 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z. H., Yang L., Ye J., Wang Y. S, Liu Y. (2019). Monocyte subsets study in children with Mycoplasma pneumoniae pneumonia. Immunol. Res. 67, 373–381. 10.1007/s12026-019-09096-6 [DOI] [PubMed] [Google Scholar]
- WHO Director-General's Opening Remarks at the Media Briefing on COVID-19-11 March (2020) Available online at: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200331-sitrep-71-covid-19.pdf?sfvrsn=4360e92b_8 (accessed March 31, 2020).
- Wu Z., McGoogan J. M. (2020). Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 323, 1239–1242. 10.1001/jama.2020.2648 [DOI] [PubMed] [Google Scholar]
- Wuhan Novel Coronavirus Infection Pneumonia Epidemic Prevention Control Headquarters (2020). Available online at: http://www.hubei.gov.cn/zhuanti/2020/gzxxgzbd/zxtb/202001/t20200123_2014402.shtml (accessed March 12, 2020).
- Xu X. W., Wu X. X., Jiang X. G., Xu K. J., Ying L. J., Ma C. L., et al. (2020). Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ 368:m606 10.1136/bmj.m606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Lu Q., Liu M., Wang Y., Zhang A., Jalali N., et al. (2020). Epidemiological and clinical features of the 2019 novel coronavirus outbreak in China. medRxiv. 10.1101/2020.02.10.20021675 [DOI] [Google Scholar]
- Yu P., Zhu J., Zhang Z. D., Han Y. J. (2020). A familial cluster of infection associated with the 2019 novel coronavirus indicating potential person-to-person transmission during the incubation period. J. Infect. Dis. 221, 1757–1761. 10.1093/infdis/jiaa077 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All datasets presented in this study are included in the article/supplementary material.