Abstract
To identify potential therapeutic targets for pulmonary fibrosis induced by silica, we studied the effects of this disease on the expression of microRNAs (miRNAs) in the lung. Rattus norvegicus pulmonary silicosis models were used in conjunction with high-throughput screening of lung specimens to compare the expression of miRNAs in control and pulmonary silicosis tissues. A total of 70 miRNAs were found to be differentially expressed between control and pulmonary silicosis tissues. This included 41 miRNAs that were upregulated and 29 that were downregulated relative to controls. Among them, miR-292-5p, miR-155-3p, miR-1193-3p, miR-411-3p, miR-370-3p, and miR-409a-5p were found to be similarly altered in rat lung and transforming growth factor (TGF)-β1-induced cultured fibroblasts. Using miRNA mimics and inhibitors, we found that miR-1193-3p, miR-411-3p, and miR-370-3p exhibited potent anti-fibrotic effects, while miR-292-5p demonstrated pro-fibrotic effects in TGF-β1-stimulated lung fibroblasts. Moreover, we also found that miR-411-3p effectively reduced pulmonary silicosis in the mouse lung by regulating Mrtfa expression, as demonstrated using biochemical and histological assays. In conclusion, our findings indicate that miRNA expression is perturbed in pulmonary silicosis and suggest that therapeutic interventions targeting specific miRNAs might be effective in the treatment of this occupational disease.
Keywords: fibroblast, silicosis, microRNA, myocardin-related transcription factor, response serum factor, transforming growth factor, myofibroblast
Graphical Abstract
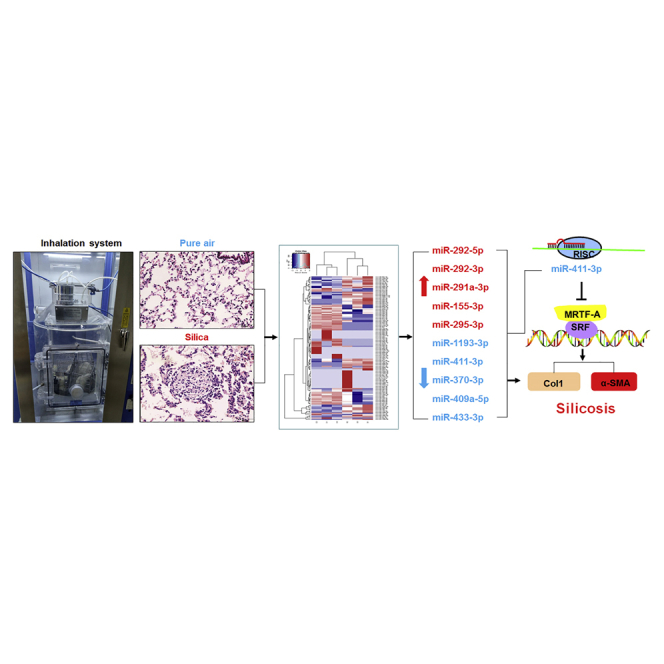
Using high-throughput screening, Gao et al. found that 70 miRNAs were differentially expressed after chronic exposure to silica dust in rats. Moreover, they also showed that miR-411-3p could significantly attenuate pulmonary silicosis by inhibiting the Mrtfa gene. These results suggest that therapeutic interventions targeting specific miRNAs might be effective in the treatment of this occupational disease.
Introduction
Occupational exposure to silica dust particles remains a major cause of pulmonary fibrosis.1 Although this form of pulmonary fibrosis can largely be prevented by implementing strict occupational regulations, it remains an important cause of respiratory morbidity and mortality in many countries throughout the world, such as China and other developing countries.2,3
MicroRNAs (miRNAs) are non-coding single-stranded RNA molecules with a length of 20–24 nt. They play a key role in the post-transcriptional regulation of a large number of genes.4 Indeed, it is estimated that 60% of all transcripts are regulated by miRNAs, illustrating the essential role that miRNAs play in almost all physiological and pathological processes.5 Because individual miRNAs tend to influence the expression of many functionally related genes, it has long been proposed that the targeting of specific miRNA species may be a useful strategy to ameliorate disease.6 For example, specific miRNAs have been shown to regulate entire sets of fibrosis-related genes in pulmonary fibrosis, and targeting these molecules has been shown to reduce the severity of this disease in experimental models.7
To date, very little is known about the role of miRNAs in the pathogenesis of pulmonary silicosis. However, studies have shown that certain miRNAs are altered in lung tissues in some silicosis model systems.8,9 For example, Yuan et al.10 have shown that miR-542-5p attenuates pulmonary silicosis by targeting integrin α6. And Yan et al.11 have shown that miR-503 has similar effects by reducing the epithelial-mesenchymal transition through a phosphatidylinositol 3-hydroxy kinase p85-dependent mechanism. Likewise, miR-19a-19b-20a has been shown to suppress transforming growth factor (TGF)-β1-induced activation of fibroblasts in both bleomycin- and silica-induced pulmonary fibrosis models.12 In this study, we sought to identify the full spectrum of miRNAs that are altered as a consequence of pulmonary silicosis and to explore the consequences of these changes on signaling pathways associated with this disease, with the aim of identifying new putative therapeutic targets.13 Using the inhalational model, we found that a large number of miRNAs were differentially expressed after chronic exposure to silica dust, including 41 miRNAs that were upregulated and 29 that were downregulated. Moreover, we found that 10 of the miRNAs demonstrating the most significantly altered expression in lung tissues following silica exposure also showed similar changes in primary lung fibroblasts induced with TGF-β1.
Activated lung myofibroblasts are a major contributor to the pathogenesis of pulmonary fibrosis and are characterized by positive expression of α-smooth muscle actin (α-SMA). The differentiation of fibroblasts to myofibroblasts can be induced by TGF-β1 and is associated with excessive extracellular matrix deposition.14,15 It has been well documented that serum response factor (SRF) and its transcription cofactor, myocardin-related transcription factor A (MRTF-A), can regulate the expression of α-SMA at the transcriptional level,16 and they have also been reported to promote the synthesis of collagen type I (Col I) and play a key role in pulmonary fibrosis.17 In the present study, we found that many of the miRNAs differentially expressed in pulmonary fibrosis had effects on myofibroblast differentiation and the production of extracellular matrix in vitro. Additionally, using bioinformatics prediction analysis, we identified the Mrtfa gene as a target of miR-411-3p. In further studies designed to examine the connection between miR-411-3p and Mrtfa, we showed that in vivo administration of miR-411-3p could significantly attenuate pulmonary silicosis in mice. Taken together, our findings indicate that pulmonary silicosis has marked effects on the expression of miRNAs in the lung and suggest that targeting specific miRNAs could be effective in reducing morbidity and mortality in this occupational disease.
Results
Silicosis Alters miRNA Expression in the Rat Lung
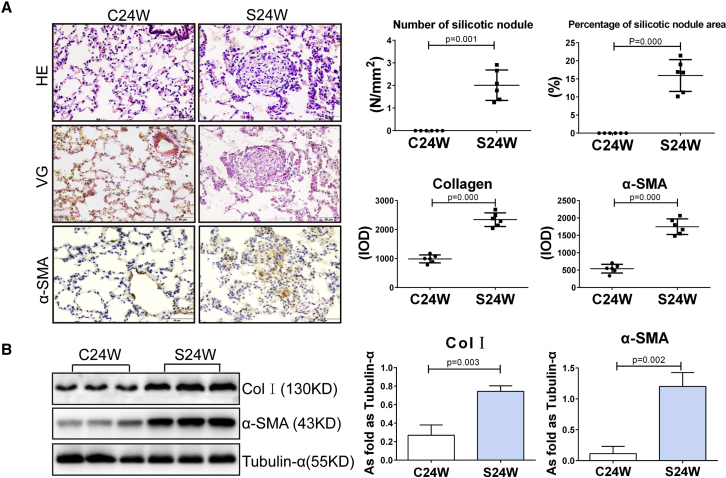
To determine the effects of pulmonary silicosis on the expression of miRNAs in the lung, we exposed rats to aerosolized silica dust particles daily for 24 weeks. This model system readily induced fibrotic remodeling in the lung typical of pulmonary silicosis, as has been reported previously.18 This included the accumulation of large numbers of silicotic lung nodules, extensive deposition of interstitial collagen, and increased numbers of α-SMA-positive cells (Figure 1A). We also found that Col I and α-SMA protein levels were significantly increased in the lungs of these animals relative to those in controls (p < 0.05; Figure 1B).
Figure 1.
Silicosis in Rats Induced by Inhalation of SiO2
(A) H&E staining, VG staining, and α-SMA immunohistochemical (IHC) staining in rat lung (scale bars, 50 μm). (B) The increasing levels of Col I and α-SMA in silicotic rats measured by western blot. (Data indicate mean ± SD; n = 6 independent experiments.)
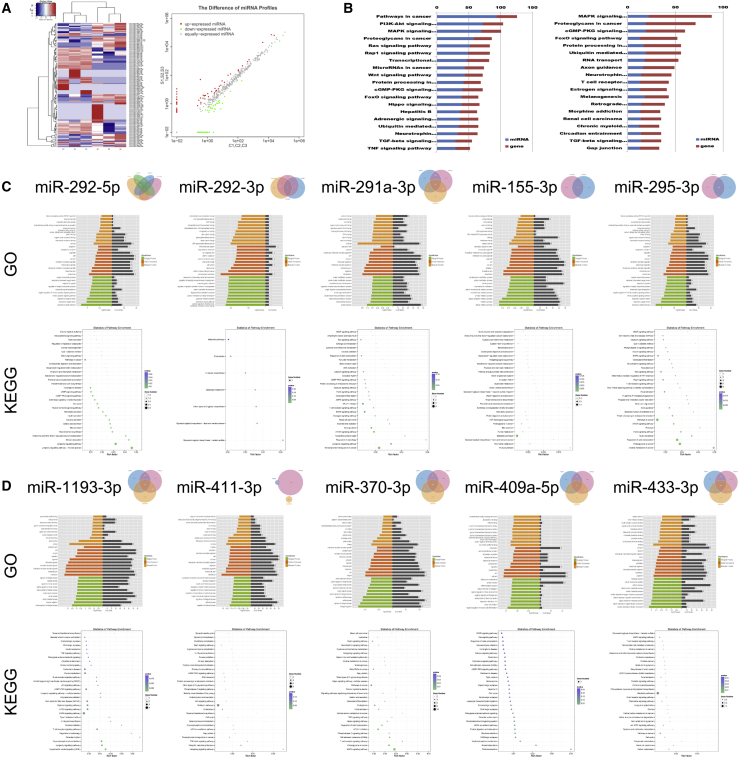
Having validated our model of pulmonary silicosis, we next examined the effects of silicosis on the expression of miRNAs in the lung. Selecting only those miRNAs whose expression significantly differed from that of control lung tissues (cutoff threshold of |log2(fold change)| ≥ 1 and p < 0.05), we identified 70 miRNAs that were differentially expressed in the silicotic lung. This included 41 miRNAs whose expression was increased and 29 whose expression was decreased. Clustering analysis and miRNA profiles are shown in Figure 2A and Table S1, respectively, and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis is shown in Figure 2B. Gene Ontology (GO) and KEGG pathway analyses for the top five upregulated and downregulated miRNAs are shown in Figures 2C and 2D.
Figure 2.
The Bioinformatics Analysis of Dyregulated miRNAs in Silicotic Rats
(A) The cluster analysis of miRNA profiles. (B) The KEGG pathway of upregulated miRNAs (left) and downregulated miRNAs (right). (C and D) The GO (C) and KEGG (D) pathway analyses of regulated mRNAs by 10 top changes of miRNAs.
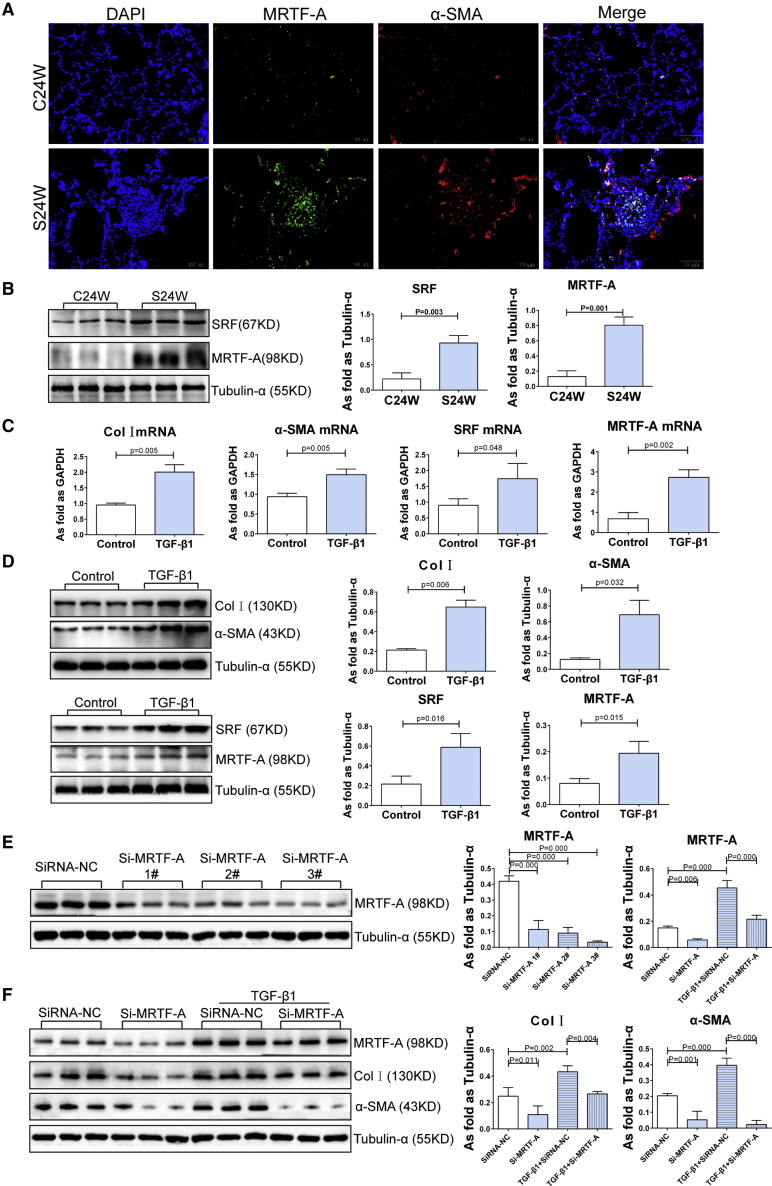
MRTF-A Participates in Myofibroblast Differentiation in Silicosis
Transcription of the contractile protein α-SMA has been reported to be mediated by the transcription factor SRF along with its co-activator, MRTF-A.19 In our previous study, we found that SRF levels were elevated in silicotic rats and also in TGF-β1-treated lung fibroblasts.14 As shown in Figure 3, in this study, we also observed co-expression of MRTF-A and α-SMA in silicotic lesions of rat lung tissue, and this was associated with increased MRTF-A and SRF protein levels in silicotic lungs. In addition, the expression of MRTF-A and SRF, as well as of Col I and α-SMA, were upregulated in lung fibroblasts induced by TGF-β1. Furthermore, knockdown of MRTF-A by small interfering RNA (siRNA) suppressed Col I and α-SMA levels in lung fibroblasts induced by TGF-β1. These results indicate that MRTF-A plays a role in pulmonary silicosis and that it contributes to myofibroblast differentiation induced by TGF-β1 by regulating the key effector proteins Col I and α-SMA.
Figure 3.
MRTF-A Participates in Myofibroblast Differentiation in Silicosis
(A) The co-expression of MRTF-A and α-SMA in lung of rats (scale bars, 100 μm). (B) Protein levels of SRF and MRTF-A were increased in silicotic rats. (C and D) The (C) mRNA levels and (D) protein levels of Col, α-SMA, SRF, and MRTF-A were increased in lung fibroblasts induced by TGF-β1. (E) The effective siRNA screened by western blotting. (F) siRNA-MRTF-A reduced the levels of MRTF-A, COL I, and α-SMA. (Data indicate mean ± SD; n = 3 independent experiments.)
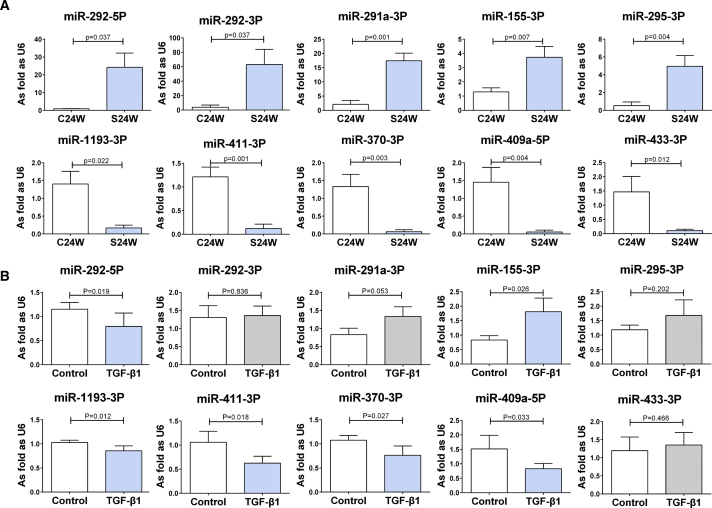
miRNA Expression Is Altered in the Silicotic Rat Lung and TGF-β1-Induced Fibroblasts
To validate the findings from the high-throughput miRNA screen, we used quantitative real-time PCR to examine the expression of the top five upregulated and downregulated miRNAs in silicotic rat lungs and TGF-β1-treated lung fibroblasts. As shown in Figure 4A, the quantitative real-time PCR analysis confirmed the changes in expression of these miRNAs in the lungs of silicotic rats, when compared with the control group. Similar directional changes in the expression of various miRNAs were identified in cultured lung fibroblasts induced by TGF-β1 (Figure 4B). This included a significant upregulation in miR-155-3p and a significant downregulation in miR-292-5p, miR-1193-3p, miR-411-3p, miR-370-3p, and miR-409a-5p (p < 0.05). Taken together, these results suggest that changes in the miRNA expression profile in pulmonary silicosis are, at least in part, downstream of TGF-β1 receptor activation.
Figure 4.
The Levels of miRNAs Identified in Rats or in Fibroblasts Measured by Fluorescent Quantitative Real-Time PCR
(A) The levels of miR-292-5p, miR-292-3p, miR-291a-3p, miR-155-3p, and miR-295-3p increased in silicotic rats accompanied with downregulation of miR-1193-3p, miR-411-3p, miR-370-3p, miR-409a-5p, and miR-433-3p. (B) The level of miR-155-3p was increased accompanied with reduced levels of miR-292-5p, miR-1193-3p, miR-411-3p, miR-370-3p, and miR-409a-5p measured by quantitative real-time PCR. (Data indicate mean ± SD; n = 3 independent experiments.)
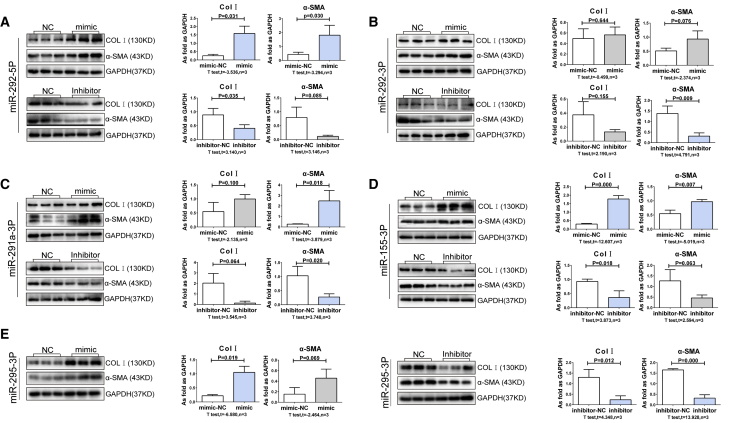
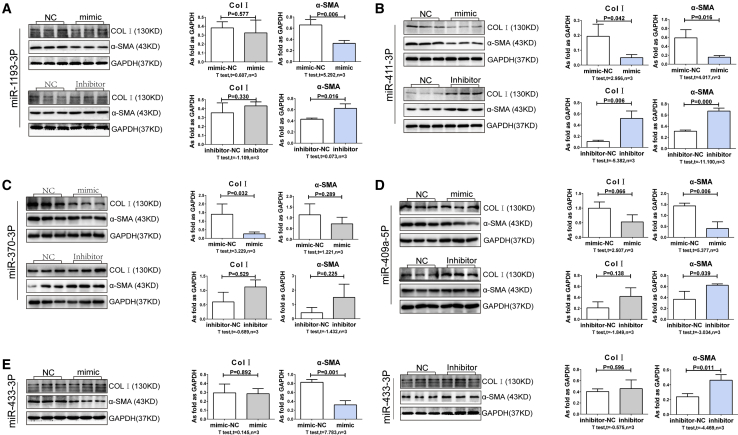
miRNAs Regulate Extracellular Matrix Production and Myofibroblast Formation
To assess whether the miRNAs altered in pulmonary silicosis impact on lung fibroblast behavior, we transfected cultured lung fibroblasts with different miRNA mimics and inhibitors. As showed in Figure 5 and Figure 6, mimics of miR-292-5p, miR-155-3p, and miR-295-3p markedly increased Col I expression, signifying an increase in extracellular matrix production. Moreover, we found that mimics of miR-292-5p, miR-291a-3p, and miR-155-3p promoted myofibroblast differentiation, as demonstrated by the increase in α-SMA expression. In contrast to these findings, we found that mimics of miR-411-3p and miR-370-3p attenuated Col I levels, while mimics of miR-1193-3p, miR-411-3p, miR-409a-5p, and miR-433-3p inhibited α-SMA expression (p < 0.05), indicating the antagonistic roles that miRNAs play, and the intricate balance in target gene expression they must maintain, in lung fibroblasts.
Figure 5.
The Mimics and Inhibitors of Upregulated miRNAs Regulate Col I and α-SMA in Lung Fibroblasts in FBS-free Medium
(A–E) The expressions of Col I and α-SMA in lung fibroblasts in FBS-free medium and treated with mimics and inhibitors of (A) miR-292-5p, (B) miR-292-3p, (C) miR-291a-3p, (D) miR-155-3p, and (E) miR-295-3p. (Data indicate mean ± SD; n = 3 independent experiments.)
Figure 6.
The Mimics and Inhibitors of Downregulated miRNAs Regulate Col I and α-SMA in Lung Fibroblasts in FBS-free Medium
(A–E) The expressions of Col I and α-SMA in lung fibroblasts in FBS-free medium and treated with mimics and inhibitors of (A) miR-1193-3p, (B) miR-411-3p, (C) miR-370-3p, (D) miR-409a-5p, and (E) miR-433-3p. (Data are mean ± SD; n = 3 independent experiments.)
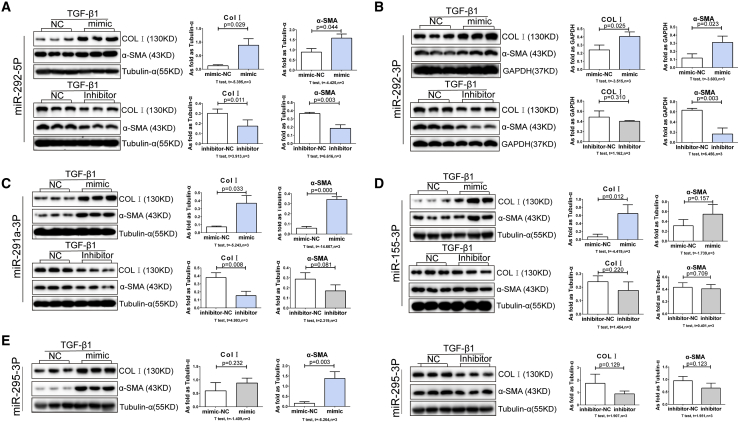
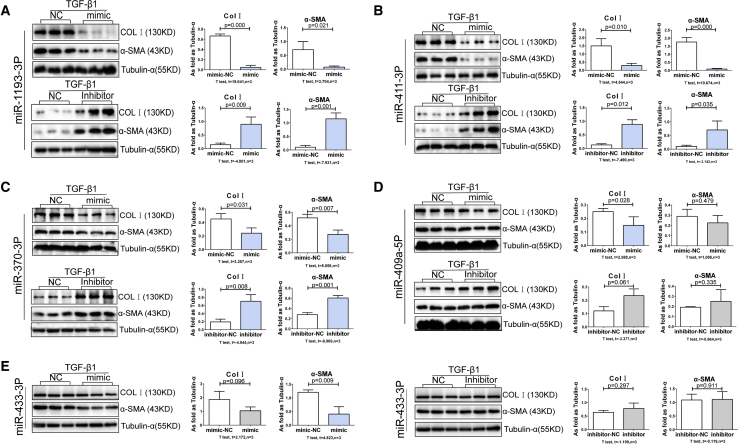
Next, we studied the effects of each miRNA on lung fibroblasts cultured in the presence of TGF-β1. Consistent with findings in unstimulated cells, we found that mimics of miR-292-5p, miR-292-3p, miR-291a-3p, and miR-155-3p increased Col I expression, whereas mimics of miR-292-5p, miR-292-3p, miR-291a-3p, and miR-295-3p promoted α-SMA expression in TGF-β1-treated lung fibroblasts (Figure 7). Likewise, mimics of miR-1193-3p, miR-411-3p, miR-370-3p, and miR-409a-5p attenuated Col I expression, while mimics of miR-1193-3p, miR-411-3p, miR-370-3p, and miR-433-3p inhibited TGF-β1-induced α-SMA expression (p < 0.05; Figure 8).
Figure 7.
The Mimics and Inhibitors of Upregulated miRNA Regulates Col I and α-SMA in TGF-β1-Induced Lung Fibroblasts
(A–E) The expressions of Col I and α-SMA in lung TGF-β1-treated fibroblasts by transfecting with mimics and inhibitors of (A) miR-292-5p, (B) miR-292-3p, (C) miR-291a-3p, (D) miR-155-3p, and (E) miR-295-3p. (Data indicate mean ± SD; n = 3 independent experiments.)
Figure 8.
The Mimics and Inhibitors of Downregulated miRNA Regulates Col I and α-SMA in TGF-β1-Induced Lung Fibroblasts
(A–E) The expressions of Col I and α-SMA in lung TGF-β1-treated fibroblasts by transfecting with mimics and inhibitors of (A) miR-1193-3p, (B) miR-411-3p, (C) miR-370-3p, (D) miR-409a-5p, and (E) miR-433-3p. (Data indicate mean ± SD; n = 3 independent experiments.)
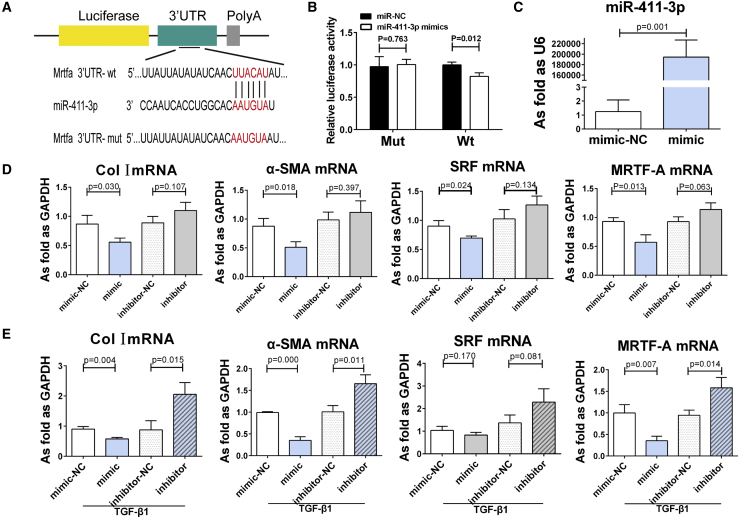
miR-411-3p Inhibits TGF-β1-Induced Myofibroblast Differentiation by Regulating Mrtfa
Given the aforementioned findings, we next searched for putative targets of miR-411-3p using the TargetScan online prediction tool (http://www.targetscan.org/vert_72/). TargetScan analysis identified Mrtfa as a potential target gene of miR-411-3p (Figure 9A). To confirm that Mrtfa was, indeed, a target of miR-411-3p, we performed luciferase reporter assays using the 3′ UTR of Mrfta, which harbors the putative miR-411-3p target site. As shown in Figure 9B, cells co-transfected with the Mrtfa-3′ UTR-WT (wild-type) reporter plasmid and miR-411-3p mimic demonstrated significantly reduced luciferase expression when compared with cells transfected with this reporter and the miR-411-3p negative control (p < 0.05). These findings indicate that MATF-A is a direct target of miR-411-3p.
Figure 9.
miR-411-3p Inhibited Mrtfa in Lung Fibroblasts Activated by TGF-β1
(A) The schematic diagrams of the luciferase reporter construction. WT and mutant (Mut) 3′ UTR of Mrtfa containing the binding site with miR-411-3p were inserted into pmir-GLO vector. (B) 293T cells were transfected with miR-411-3p mimics/miR-NC and the plasmid of Mrtfa-3′ UTR Mut/WT, and the relative luciferase activity was significantly suppressed by miR-411-3p mimics in the Mrtfa-3′ UTR WT group. (C) The increasing level of miR-411-3p by miR-411-3p mimics. (D and E) The mRNA levels of Col I, α-SMA, SRF, and MRTF-A in (D) lung fibroblasts and (E) TGF-β1-induced lung fibroblasts transfected with miR-411-3p mimic/inhibitor. (Data indicate mean ± SD; n = 3 independent experiments.)
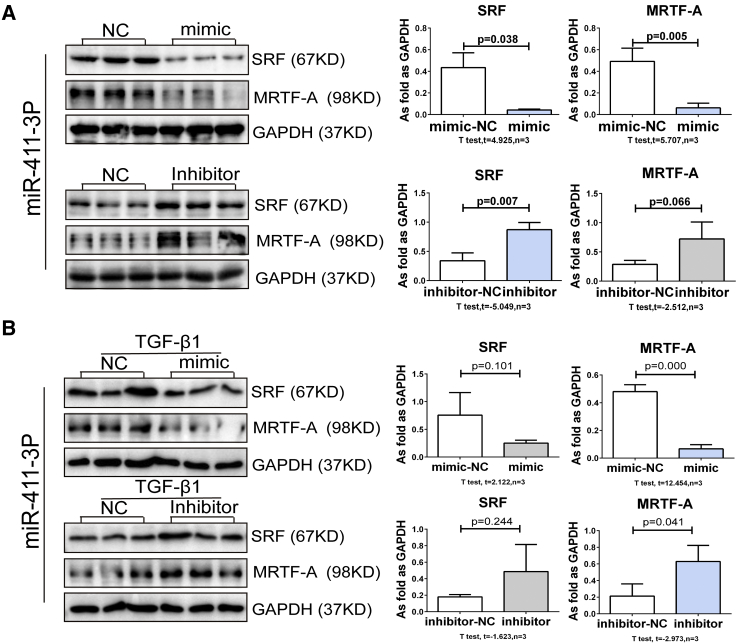
In addition to confirming Mrtfa as a target of miR-411-3p in reporter assays, we also found that treatment with miR-411-3p mimic suppressed Col I, α-SMA, and MRTF-A mRNA levels in fibroblasts cultured with or without TGF-β1 (Figures 9C–9E). Furthermore, MRTF-A protein expression was also reduced in these cells (Figures 10A and 10B). mRNA and protein levels for SRF were also reduced in unstimulated fibroblasts following treatment with miR-411-3p mimic (Figures 9D and 10A). Conversely, treatment with an miR-411-3p inhibitor resulted in an increase in mRNA levels for Col I, α-SMA, and MRTF-A in fibroblasts induced by TGF-β1 (Figure 9E) and an increase in the protein expression of MRTF-A and SRF in these cells, regardless of TGF-β1 treatment (Figures 10A and 10B).
Figure 10.
miRNA-411-3p Inhibited MRTF-A in Lung Fibroblasts Activated by TGF-β1
(A and B) The protein levels of SRF and MRTF-A in (A) lung fibroblasts and (B) TGF-β1-induced lung fibroblasts transfected with miR-411-3p mimic/inhibitor. (Data indicate mean ± SD; n = 3 independent experiments.)
miR-411-3p Attenuates Silica-Induced Pulmonary Fibrosis In Vivo
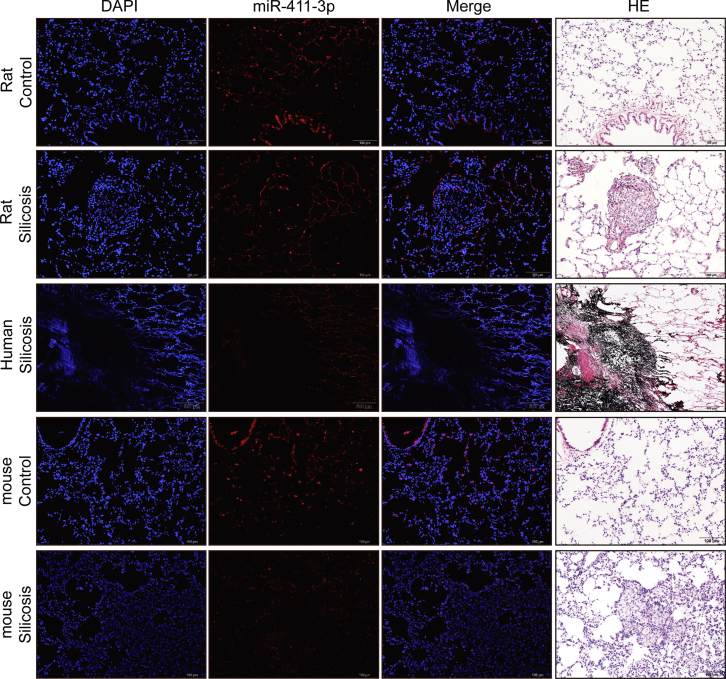
Because miR-411-3p inhibited the expression of pro-fibrotic factors in vitro, we next examined whether administering this miRNA could ameliorate pulmonary silicosis in an experimental model of this disease. First, we confirmed by in situ hybridization that miR-411-3p levels were decreased in silicotic lesions of rat lung tissues and also verified similar reductions in mouse and human silicotic tissues using normal lung tissues as a control (Figure 11).
Figure 11.
The Location of miR-411-3p in Lung Tissue Observed with In Situ Hybridization
Nuclear was stained by DAPI (blue), and miR-411-3p was marked by Cy3 (red) in immunofluorescence staining. H&E staining indicated the morphological change at the same location of immunofluorescence staining (scale bars: rat control, 100 μm; rat silicosis, 100 μm; human silicosis, 500 μm; mouse control, 100 μm; mouse silicosis, 100 μm).
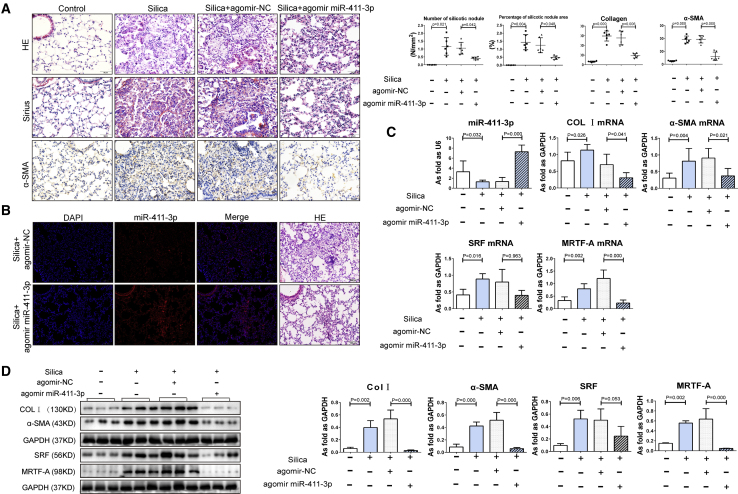
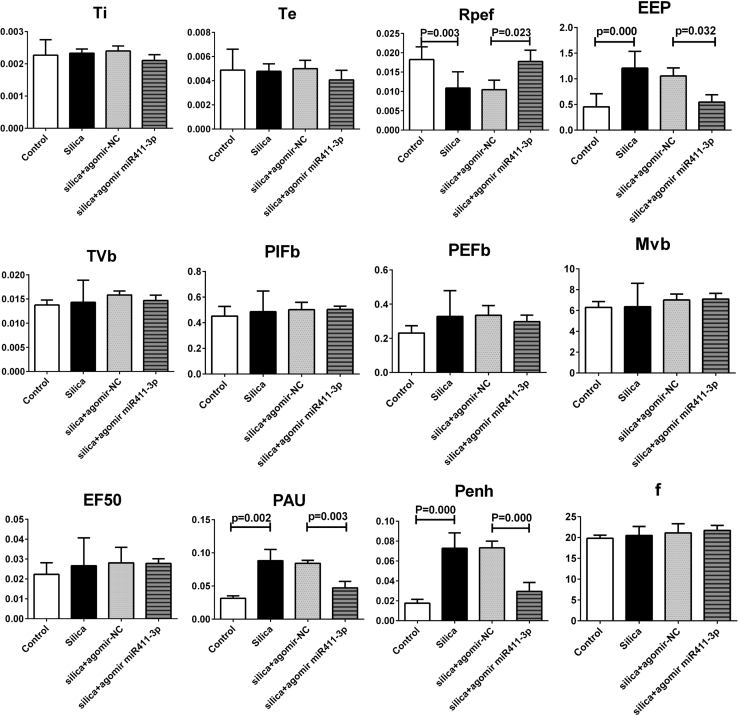
Next, to assess the therapeutic potential of miR-411-3p in pulmonary silicosis, we administered a miR-411-3p agomir to mice 1 week after oropharyngeal instillation of silica dust. As showed in Figure 12A, histological analysis revealed that lung fibrosis was attenuated by miR-411-3p agomir treatment at day 14 after silica administration. In situ hybridization also revealed a concomitant increase in miR-411-3p levels in the lung tissue of mice that were treated with agomir (Figure 12B). Moreover, we found that administration of miR-411-3p agomir to mice markedly reduced mRNA and protein levels for MRTF-A as well as α-SMA and Col I (Figures 12C and 12D). In addition, miR-411-3p agomir treatment improved the lung function of mice exposed to silica (Figure 13). These results indicate that miR-411-3p can also regulate MRTF-A in vivo and can exert potent anti-fibrotic effects in the mouse lung.
Figure 12.
Agomir of miR-411-3p Inhibited Silicosis in Mice Exposed to Silica
(A) H&E, Sirius red staining, and positive expression of α-SMA showed silicotic lesions induced by SiO2 in the silica and the silica+agomir-NC groups. Treatment with agomir miR-411-3p could reduce silicotic lesions compared to the silica+agomir-NC group (scale bars, 50 μm). (B) The miR-411-3p expression was shown by in situ hybridization (scale bars, 100 μm). (C) The level of miR-411-3p was reduced in the silica and the silica+agomir-NC groups and was increased in the agomir miR-411-3p group. The mRNA levels of Col I, α-SMA, SRF, and MRTF-A were increased in the silica and the silica+agomir-NC groups and were decreased in the agomir miR-411-3p group. (D) The protein levels of Col I, α-SMA, SRF, and MRTF-A were increased in the silica and the silica+agomir-NC groups and were decreased in the agomir miR-411-3p group. (Data indicate mean ± SD; n = 6 independent experiments.)
Figure 13.
Agomir of miR-411-3p Regulated Lung Function in Mice Exposed to Silica
Agomir of miR-411-3p upregulated the level of Rpef and downregulated the level of EEP, PAU, and Penh. (Data indicate mean ± SD; n = 6 independent experiments.)
Discussion
In this study, we report on the miRNA expression profile associated with pulmonary silicosis in the rat lung. Although several studies have described changes in miRNAs in mouse models,8,9 our study performed a detailed assessment of miRNA expression in a chronic dust inhalation model that mimics the exposure and histological findings in humans.13 From this work, we identified 70 miRNAs that are differentially expressed in the silicotic rat lung when compared with controls. Furthermore, we verified these findings through a variety of approaches and also demonstrated similar changes for some of these miRNAs in lung fibroblasts exposed to TGF-β1. Mechanistic studies confirmed that many of these miRNAs are involved in regulating extracellular matrix production and/or myofibroblast differentiation, and we showed that miR-411-3p has potent anti-fibrotic effects in vivo and in vitro.
Among the miRNAs dysregulated in our study are several that have been shown to be involved in tissue fibrosis in other organs. For example, miR-21-5p has been implicated as a serum biomarker in patients with idiopathic pulmonary fibrosis.20 Moreover, miR-146b-5p and miR-146b-5p have been shown to be upregulated in various models of kidney injury,21 while miR-183-5p has been shown to be a significant predictor of experimentally induced liver fibrosis.22
Although the focus of our study was pulmonary silicosis, we believe that our findings may have broader implications for the pulmonary fibrosis field. This is because several of the miRNAs that were altered in the silicotic lung were also altered in cultured fibroblasts exposed to TGF-β1. Moreover, we have shown that mimics and inhibitors of these miRNAs have profound effects on either extracellular matrix production or myofibroblast formation.
Recently, a miR-292-5p inhibitor was shown to protect against myocardial ischemia reperfusion injury through activation of the peroxisome-proliferator-activated receptor signaling pathway.23 Also, miR-292-3p was noted to be one of the most abundant miRNAs in syncytial-exosome-enriched extracellular vesicles.24 Embryonic stem cells have been reported to package miR-291-3p into exosomes that demonstrate anti-senescence activity upon transfer to human dermal fibroblasts, thereby accelerating the excisional skin wound-healing process in aged mice.25 In monocytes and macrophages induced by lipopolysaccharide (LPS), a miR-155-3p mimic promoted increased levels of interleukin (IL)-6.26 miR-411-3p has been reported to suppress the hypoxia-inducible factor 1α/vascular endothelial growth factor/p38 pathway and to exert an anti-cancer role by promoting apoptosis and inhibiting cell growth, invasion, and migration.27 A potential association between miR-370-3p and pneumonia has been identified from studies in which the normal human fibroblast WI-38 cell line was induced with LPS.28 In the present study, we provide preliminary experimental evidence for roles for 10 miRNAs in silicosis and fibroblast activation, following their identification as the top differentially expressed genes in a rat model of pulmonary silicosis. We have also identified target genes regulated by these miRNAs that are associated with fibrosis-related signaling pathways, highlighting the potential utility of miRNA-based therapies in the treatment of silicosis.
Myofibroblast differentiation, which is characterized by increased cellular expression of α-SMA, is one of the causes of SRF-dependent collagen deposition in silicosis.14 MRTF-A, functioning as a co-activator for the SRF-mediated transcription of CArG-box-containing genes, plays a central role in fibroblast activation, myofibroblast differentiation, and collagen deposition.29 MRTF-A also physically interacts with the promoter of the COL1A1 gene to facilitate histone acetylation and RNA polymerase II recruitment.30 In the present study, we identified the 3′ UTR of Mrtfa mRNA as a potential target binding site for miR-411-3p, using the TargetScan online tool. Our subsequent in vitro assays demonstrated that miR-411-3p is a negative regulator of TGF-β1-dependent lung fibroblast induction. Given these findings, we examined the therapeutic effects of mir-411-3p in vivo and found that it had potent anti-fibrotic effects, not only reducing the histological and biochemical indicators of lung fibrosis but also restoring physiological lung function in animals. We found that miR-411-3p attenuated silicosis by regulating Mrtfa expression, resulting in the downregulation of α-SMA and Col I protein levels. In a previous study, we have demonstrated that miR-411-3p inhibits lung fibroblast viability and migration induced by Smurf2/TGF-β1 signaling.31 Taken together, these findings provide strong support for the notion of miRNA targeting as a therapeutic strategy in the treatment of pulmonary silicosis.
Our study demonstrates that miRNA expression is significantly altered in pulmonary silicosis and suggests that therapeutic approaches aimed at restoring miR-411-3p levels may be effective in the treatment of this occupational lung disease. Whether targeting other miRNAs identified in our study would have similar therapeutic effects on pulmonary silicosis is a focus of future investigation.
Materials and Methods
Animal Experiments
Three-week-old male Wistar rats, weighing approximately 80 g, were purchased from Vital River Laboratory Animal Technology (Beijing, China). Pulmonary silicosis was induced using previously described methods.13 In brief, rats were placed in a HOPE MED 8050 exposure apparatus (HOPE Industry and Trade, Tianjin, China) for 3 h/day for 24 consecutive weeks. Cabinet temperature, humidity and pressure were set at 20°C–25°C, 70%–75%, and −50 to +50 Pa, respectively. The oxygen concentration was maintained at 20%–23%, and the air flow rate was set at 3.0–3.5 mL/min. SiO2 dust particle concentration in the cabinet was 50 ± 10 μg/m3, with 80% of particles less than 5 μm in size (S5631, Sigma-Aldrich). Rat lung tissues were harvested at week 24 from the start of the protocol. All animal experiments were approved by the Ethics Committee for Animal Experimentation of North China University of Science and Technology (2013-038) and were in accordance with the guidelines set by the National Institutes of Health.
In vivo therapeutic studies using miR-411-3p were performed in mice. Mice were divided into four groups: (1) control, (2) silica,32,33 (3) silica plus agomir-Negative control (NC), and (4) silica plus agomir miR411-3P (n = 6 animals per group).31,34 In these studies, pulmonary silicosis was induced by administering a one-time dose of SiO2 dust into the posterior oropharynx.35 Silica dust (5-μm silica particles, S5631, Sigma-Aldrich) was administered at a concentration of 100 mg/kg. One week after silica dust administration, 0.05 mL control (agomir-NC) or agomir miR411-3P (5 nmol/mL, RiboBio, Guangzhou,China) was administered by tail vein injection.31 Animals were sacrificed at day 14 after silica administration, and harvested lungs were then stored at −80°C for subsequent analysis.
Cell Culture Studies
Rat lung fibroblasts were cultured in 25 cm2 dishes in Dulbecco’s modified Eagle’s medium (BI-SH0019, Biological Industries, Kibbutz Beit-Haemek, Israel) containing 10% fetal bovine serum (FBS; 10099141, GIBCO, Thermo Fisher Scientific) and 1% penicillin-streptomycin.14 Cell cultures were maintained at 37°C in humidified chambers containing a mixture of 5% CO2 and 95% atmospheric air. Experiments were performed when cell cultures reached 80% confluence. Transfection was performed according to standard methods. Sequences for each miRNA or siRNA are shown in Table S2. TGF-β1 was administered at a concentration of 5 ng/mL (PHG9204, Invitrogen, Waltham, MA, USA).
miRNA Sequence Analysis
High-throughput screening for miRNAs was performed on control and silicotic tissues. Total RNA was extracted with TRIzol reagent (Invitrogen, Waltham, MA, USA) in accordance with the manufacturer’s recommendations. Proprietary adaptors (Illumina, San Diego, CA, USA) were then bound to the 5′ and 3′ ends of small RNAs, and reverse transcription was performed. Small cDNA libraries were generated by applying primers complementary to the adaptor sequences. Denaturing polyacrylamide-gel electrophoresis was performed to separate 18- to 40-nt-long RNAs from total RNA samples by size fractionation. Deep sequencing was performed on cDNA libraries using the HiSeq 2500 system (Illumina, San Diego, CA, USA) at RiboBio (Guangzhou, China) as per the manufacturer’s instructions. As an initial filtering step, unqualified reads, 30 adaptor reads, reads with 50 adaptor contaminants, and reads shorter than 15 nt were excluded. Post-filtering, sequences were matched to the Rattus norvegicus (Norway rat) genome applying the Burrow-Wheeler Aligner (BWA) procedure with a common difference of two mismatches. The mapped sequences were compared with the miRBase database (v.21; http://www.mirbase.org/) to identify rRNAs, tRNAs, small nuclear RNAs (snRNAs), and small nucleolar RNAs (snoRNAs). Sequence reads were then categorized according to sequence similarity and aligned to the miRBase database to identify conservative miRNAs. miRNA expression levels were estimated by base mean value, and the normalization process was realized by software. The fold change in miRNA expression levels between groups was calculated by the formula: fold change = |log2 (test RPM/control RPM)|, where RPM refers to reads per million mapped reads. Only those miRNAs demonstrating a |log2 (fold change)| ≥1 (p < 0.05) difference in expression between samples were considered as differentially expressed.
Quantitative Real-Time PCR Analysis
Reverse transcription (TakaRa, Clontech) was performed according to the manufacturer’s instructions. Target amplification by quantitative real-time PCR was carried out using the TB Green Premix Ex Taq II (TakaRa, Clontech) system. Each 20-μL reaction contained cDNA (2 μL), TB Green Premix Ex TaqII Mix (10 μL), and specific forward and reverse miRNA primers (5 μM, 0.8 μL). Primer sequence details are shown in Tables S3 and S4. Thermocycling was conducted as follows: initial denaturation at 95°C for 30 s followed by 40 cycles of denaturation at 95°C for 5 s and annealing at 60°C for 30 s. U6 snRNA was used as the internal reference for miRNA, and GAPDH was used as the internal reference for Col I, α-SMA, SRF, and MATF-A. The results were calculated using the 2−ΔΔCt method.
Analysis of Differentially Expressed miRNAs
Differentially expressed miRNAs were determined using the TargetScan program (http://www.targetscan.org/). Functional categories were ascertained using GO analysis (http://www.geneontology.org) and the KEGG pathway analysis (https://www.genome.jp/kegg/pathway.html).
In Situ Hybridization of miR-411-3p
Paraffin-embedded lung sections were dewaxed and re-hydrated. Pepsin was used to expose nucleic acid fragments. Hybridization reactions were performed overnight at 38°C–42°C using the miR-411-3p probe sequence 5′-GGTTA GTGGACCGTGTTACATA-3′. Sections were washed several times and then incubated with blocking solution. Digoxin-labeled biotinylated antibody was applied for 2 h at 37°C, followed by several washing steps. Cy3-labeled streptavidin-biotin complex was then applied for 1 h at 37°C. Visualization of miR-411-3p-stained cells was performed using standard fluorescence microscopy.
Luciferase Reporter Assay
MRTF-A (MKL1), WT, and mutant (Mut) 3′ UTRs were synthesized and cloned into the Xhol/SalI restriction sites of the pmirGLO vector, downstream of the Firefly luciferase reporter gene. The vector also contains a control Renilla luciferase gene for the normalization of the Firefly luciferase signal. pmirGLO-MKL1-3′ UTR-WT or pmirGLO-MKL1-3′ UTR-Mut constructs were transfected into 293T cells using Lipofectamine 2000 Reagent (Thermo Fisher Scientific, Waltham, MA, USA). Cells were lysed at 48 h post-transfection, and luciferase activity was then measured using the Dual-Luciferase Reporter Assay System (Promega, E1910, Madison, WI, USA). Firefly luciferase activity was normalized to that of Renilla luciferase. All assays were performed in triplicate. Ratios of Firefly luciferase and Renilla luciferase relative light unit (RLU) values were used for comparisons between treatment groups.
Histology and Immunostaining
Lung tissues were stained with hematoxylin and eosin (H&E), Van Gieson’s solution (VG), or Sirius red for the semiquantitative analysis of collagen levels. Immunostaining was performed according to published methods.14,18 In brief, dewaxed tissue sections were treated with 0.3% H2O2 to quench endogenous peroxidases and then subjected to antigen retrieval using a high-pressure method. Samples were incubated overnight at 4°C with a primary antibody against α-SMA (ab32575, Abcam), followed by incubation with a secondary antibody (PV-6000, Beijing Zhongshan Jinqiao Biotechnology, Beijing, China) at 37°C for 30 min before the application of 3,3′-diaminobenzidine tetrahydrochloride substrate (ZLI-9018, ZSGB-BIO, Beijing, China). Brown staining, signifying α-SMA-positive cells, was visualized by light microscopy. Co-staining of α-SMA and MATF-A was performed according to our previously published method18 using primary antibodies against α-SMA (ab7817, Abcam) and MATF-A (A12598, ABclonal).
Western Blotting
Western blotting was performed according to standard protocols using a Bicinchonininc acid (BCA) protein concentration determination kit (70-PQ0012, MultiSciences) and primary antibodies against Col I (ab34710, Abcam), α-SMA (ab32575, Abcam), SRF (AF6160, Affinity), MATF-A (A12598, ABclonal), and α-tubulin (sc-53029, Santa Cruz Biotechnology).13,18 Membranes were probed with a peroxidase-conjugated, affinity-purified secondary antibody to rabbit/mouse IgG (H+L, 074-1506/074-1806, Kirkegaard and Perry Laboratories, Gaithersburg, MD). Target bands were visualized by the addition of ECL Prime Western Blotting Detection Reagent (RPN2232, GE Healthcare, Hong Kong, China). The expression of proteins of interest was normalized to that of the α-tubulin control.
Lung Function
Respiratory parameters were assessed in whole-body plethysmograph chambers (Buxco FinePointe Whole Body Plethysmography, Buxco Research Systems, St. Paul, MN, USA) according to published methods.36 In brief, rats were placed in the chambers and allowed to acclimatize for 10 min before measurements were performed (respiratory parameters are shown in Table S5). Lung function in mice was normalized to body weight.37
Statistical Analyses
The SPSS v.22.0 software package (SPSS, Chicago, IL, USA) was used to perform statistical analyses. All results are presented as mean ± standard deviation (SD). Analysis of variance (ANOVA) was performed when comparing more than two groups, using the least significant difference test where variance was constant and Tamhane’s test where variance was unequal. The independent samples t test was used to compare the means of two groups. Statistical significance was set at p < 0.05, with a 95% confidence interval.
Author Contributions
H.X.: data collection, analysis, drafting the manuscript, and final approval of the manuscript; X.G., D.X., Z.W., Shumin Li, W.C., Shifeng Li, F.J., Y.L., and X.Y.: data collection, analysis, and interpretation; D.X., Z.W., Shifeng Li, and H.L.: collection of experimental samples; X.G. and H.X.: writing the manuscript; H.X. and F.Y.: study concept and design. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Conflicts of Interest
The authors declare no competing interests.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81972988), the Scientific Research Project of Hebei Province Department of Education (ZD2019077), and the Open Fund of Key Laboratory of Functional and Clinical Translational Medicine of Xiamen Medical College (XMMC-FCTM201902). We thank James Monypenny, PhD, from Liwen Bianji, Edanz Group China (https://www.liwenbianji.cn/ac), for editing the English text of the manuscript.
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.omtn.2020.05.005.
Contributor Information
Hong Xu, Email: xuhong@ncst.edu.cn.
Fang Yang, Email: fangyang@ncst.edu.cn.
Supplemental Information
References
- 1.Kawasaki H. A mechanistic review of silica-induced inhalation toxicity. Inhal. Toxicol. 2015;27:363–377. doi: 10.3109/08958378.2015.1066905. [DOI] [PubMed] [Google Scholar]
- 2.Zhao J.Q., Li J.G., Zhao C.X. Prevalence of pneumoconiosis among young adults aged 24-44 years in a heavily industrialized province of China. J. Occup. Health. 2019;61:73–81. doi: 10.1002/1348-9585.12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leung C.C., Yu I.T., Chen W. Silicosis. Lancet. 2012;379:2008–2018. doi: 10.1016/S0140-6736(12)60235-9. [DOI] [PubMed] [Google Scholar]
- 4.Rajasekaran S., Rajaguru P., Sudhakar Gandhi P.S. MicroRNAs as potential targets for progressive pulmonary fibrosis. Front. Pharmacol. 2015;6:254. doi: 10.3389/fphar.2015.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bowen T., Jenkins R.H., Fraser D.J. MicroRNAs, transforming growth factor beta-1, and tissue fibrosis. J. Pathol. 2013;229:274–285. doi: 10.1002/path.4119. [DOI] [PubMed] [Google Scholar]
- 6.Creemers E.E., van Rooij E. Function and Therapeutic Potential of Noncoding RNAs in Cardiac Fibrosis. Circ. Res. 2016;118:108–118. doi: 10.1161/CIRCRESAHA.115.305242. [DOI] [PubMed] [Google Scholar]
- 7.Fernandez I.E., Eickelberg O. New cellular and molecular mechanisms of lung injury and fibrosis in idiopathic pulmonary fibrosis. Lancet. 2012;380:680–688. doi: 10.1016/S0140-6736(12)61144-1. [DOI] [PubMed] [Google Scholar]
- 8.Ji X., Wu B., Fan J., Han R., Luo C., Wang T., Yang J., Han L., Zhu B., Wei D. The Anti-fibrotic Effects and Mechanisms of MicroRNA-486-5p in Pulmonary Fibrosis. Sci. Rep. 2015;5:14131. doi: 10.1038/srep14131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Faxuan W., Qin Z., Dinglun Z., Tao Z., Xiaohui R., Liqiang Z., Yajia L. Altered microRNAs expression profiling in experimental silicosis rats. J. Toxicol. Sci. 2012;37:1207–1215. doi: 10.2131/jts.37.1207. [DOI] [PubMed] [Google Scholar]
- 10.Yuan J., Li P., Pan H., Li Y., Xu Q., Xu T., Ji X., Liu Y., Yao W., Han L., Ni C. miR-542-5p Attenuates Fibroblast Activation by Targeting Integrin α6 in Silica-Induced Pulmonary Fibrosis. Int. J. Mol. Sci. 2018;19:E3717. doi: 10.3390/ijms19123717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yan W., Wu Q., Yao W., Li Y., Liu Y., Yuan J., Han R., Yang J., Ji X., Ni C. MiR-503 modulates epithelial-mesenchymal transition in silica-induced pulmonary fibrosis by targeting PI3K p85 and is sponged by lncRNA MALAT1. Sci. Rep. 2017;7:11313. doi: 10.1038/s41598-017-11904-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Souma K., Shichino S., Hashimoto S., Ueha S., Tsukui T., Nakajima T., Suzuki H.I., Shand F.H.W., Inagaki Y., Nagase T., Matsushima K. Lung fibroblasts express a miR-19a-19b-20a sub-cluster to suppress TGF-β-associated fibroblast activation in murine pulmonary fibrosis. Sci. Rep. 2018;8:16642. doi: 10.1038/s41598-018-34839-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shifeng L., Hong X., Xue Y., Siyu N., Qiaodan Z., Dingjie X., Lijuan Z., Zhongqiu W., Xuemin G., Wenchen C. Ac-SDKP increases α-TAT 1 and promotes the apoptosis in lung fibroblasts and epithelial cells double-stimulated with TGF-β1 and silica. Toxicol. Appl. Pharmacol. 2019;369:17–29. doi: 10.1016/j.taap.2019.02.015. [DOI] [PubMed] [Google Scholar]
- 14.Xu H., Yang F., Sun Y., Yuan Y., Cheng H., Wei Z., Li S., Cheng T., Brann D., Wang R. A new antifibrotic target of Ac-SDKP: inhibition of myofibroblast differentiation in rat lung with silicosis. PLoS ONE. 2012;7:e40301. doi: 10.1371/journal.pone.0040301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Na M., Hong X., Fuyu J., Dingjie X., Sales D., Hui Z., Zhongqiu W., Shifeng L., Xuemin G., Wenchen C. Proteomic profile of TGF-β1 treated lung fibroblasts identifies novel markers of activated fibroblasts in the silica exposed rat lung. Exp. Cell Res. 2019;375:1–9. doi: 10.1016/j.yexcr.2019.01.010. [DOI] [PubMed] [Google Scholar]
- 16.Small E.M. The actin-MRTF-SRF gene regulatory axis and myofibroblast differentiation. J. Cardiovasc. Transl. Res. 2012;5:794–804. doi: 10.1007/s12265-012-9397-0. [DOI] [PubMed] [Google Scholar]
- 17.Luchsinger L.L., Patenaude C.A., Smith B.D., Layne M.D. Myocardin-related transcription factor-A complexes activate type I collagen expression in lung fibroblasts. J. Biol. Chem. 2011;286:44116–44125. doi: 10.1074/jbc.M111.276931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li S., Wei Z., Li G., Zhang Q., Niu S., Xu D., Mao N., Chen S., Gao X., Cai W. Silica Perturbs Primary Cilia and Causes Myofibroblast Differentiation during Silicosis by Reduction of the KIF3A-Repressor GLI3 Complex. Theranostics. 2020;10:1719–1732. doi: 10.7150/thno.37049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Penke L.R., Huang S.K., White E.S., Peters-Golden M. Prostaglandin E2 inhibits α-smooth muscle actin transcription during myofibroblast differentiation via distinct mechanisms of modulation of serum response factor and myocardin-related transcription factor-A. J. Biol. Chem. 2014;289:17151–17162. doi: 10.1074/jbc.M114.558130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Makiguchi T., Yamada M., Yoshioka Y., Sugiura H., Koarai A., Chiba S., Fujino N., Tojo Y., Ota C., Kubo H. Serum extracellular vesicular miR-21-5p is a predictor of the prognosis in idiopathic pulmonary fibrosis. Respir. Res. 2016;17:110. doi: 10.1186/s12931-016-0427-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pellegrini K.L., Gerlach C.V., Craciun F.L., Ramachandran K., Bijol V., Kissick H.T., Vaidya V.S. Application of small RNA sequencing to identify microRNAs in acute kidney injury and fibrosis. Toxicol. Appl. Pharmacol. 2016;312:42–52. doi: 10.1016/j.taap.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chandel R., Saxena R., Das A., Kaur J. Association of rno-miR-183-96-182 cluster with diethyinitrosamine induced liver fibrosis in Wistar rats. J. Cell. Biochem. 2018;119:4072–4084. doi: 10.1002/jcb.26583. [DOI] [PubMed] [Google Scholar]
- 23.Zhu Z.D., Ye J.Y., Niu H., Ma Y.M., Fu X.M., Xia Z.H., Zhang X. Effects of microRNA-292-5p on myocardial ischemia-reperfusion injury through the peroxisome proliferator-activated receptor-α/-γ signaling pathway. Gene Ther. 2018;25:234–248. doi: 10.1038/s41434-018-0014-y. [DOI] [PubMed] [Google Scholar]
- 24.Stefanski A.L., Martinez N., Peterson L.K., Callahan T.J., Treacy E., Luck M., Friend S.F., Hermesch A., Maltepe E., Phang T. Murine trophoblast-derived and pregnancy-associated exosome-enriched extracellular vesicle microRNAs: Implications for placenta driven effects on maternal physiology. PLoS ONE. 2019;14:e0210675. doi: 10.1371/journal.pone.0210675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bae Y.U., Son Y., Kim C.H., Kim K.S., Hyun S.H., Woo H.G., Jee B.A., Choi J.H., Sung H.K., Choi H.C. Embryonic stem cell-derived mmu-miR-291a-3p inhibits cellular senescence in human dermal fibroblasts through the TGF–receptor 2 pathway. J. Gerontol. A Biol. Sci. Med. Sci. 2019;74:1359–1367. doi: 10.1093/gerona/gly208. [DOI] [PubMed] [Google Scholar]
- 26.Marques-Rocha J.L., Garcia-Lacarte M., Samblas M., Bressan J., Martínez J.A., Milagro F.I. Regulatory roles of miR-155 and let-7b on the expression of inflammation-related genes in THP-1 cells: effects of fatty acids. J. Physiol. Biochem. 2018;74:579–589. doi: 10.1007/s13105-018-0629-x. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y., Huang Y., Liu H., Su D., Luo F., Zhou F. Long noncoding RNA CDKN2B-AS1 interacts with miR-411-3p to regulate ovarian cancer in vitro and in vivo through HIF-1a/VEGF/P38 pathway. Biochem. Biophys. Res. Commun. 2019;514:44–50. doi: 10.1016/j.bbrc.2019.03.141. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y., Zhu Y., Gao G., Zhou Z. Knockdown XIST alleviates LPS-induced WI-38 cell apoptosis and inflammation injury via targeting miR-370-3p/TLR4 in acute pneumonia. Cell Biochem. Funct. 2019;37:348–358. doi: 10.1002/cbf.3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Y., Jia L., Hu Z., Entman M.L., Mitch W.E., Wang Y. AMP-activated protein kinase/myocardin-related transcription factor-A signaling regulates fibroblast activation and renal fibrosis. Kidney Int. 2018;93:81–94. doi: 10.1016/j.kint.2017.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meng C., He Y., Wei Z., Lu Y., Du F., Ou G., Wang N., Luo X.G., Ma W., Zhang T.C., He H. MRTF-A mediates the activation of COL1A1 expression stimulated by multiple signaling pathways in human breast cancer cells. Biomed. Pharmacother. 2018;104:718–728. doi: 10.1016/j.biopha.2018.05.092. [DOI] [PubMed] [Google Scholar]
- 31.Gao X., Xu H., Xu D., Li S., Wei Z., Li S., Cai W., Mao N., Jin F., Li Y. MiR-411-3p alleviates Silica-induced pulmonary fibrosis by regulating Smurf2/TGF-β signaling. Exp. Cell Res. 2020;388:111878. doi: 10.1016/j.yexcr.2020.111878. [DOI] [PubMed] [Google Scholar]
- 32.Li S., Li C., Zhang Y., He X., Chen X., Zeng X., Liu F., Chen Y., Chen J. Targeting Mechanics-Induced Fibroblast Activation through CD44-RhoA-YAP Pathway Ameliorates Crystalline Silica-Induced Silicosis. Theranostics. 2019;9:4993–5008. doi: 10.7150/thno.35665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou Q., Guan Y., Hou R., Wang J., Gao H., Li H., Zhao Y., Liu N., Wang Y., Li N., Yao S. PolyG mitigates silica-induced pulmonary fibrosis by inhibiting nucleolin and regulating DNA damage repair pathway. Biomed. Pharmacother. 2020;125:109953. doi: 10.1016/j.biopha.2020.109953. [DOI] [PubMed] [Google Scholar]
- 34.Chen Y., Xu D., Yao J., Wei Z., Li S., Gao X., Cai W., Mao N., Jin F., Li Y. Inhibition of miR-155-5p Exerts Anti-Fibrotic Effects in Silicotic Mice by Regulating Meprin α. Mol. Ther. Nucleic Acids. 2020;19:350–360. doi: 10.1016/j.omtn.2019.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun J., Li Q., Lian X., Zhu Z., Chen X., Pei W., Li S., Abbas A., Wang Y., Tian L. MicroRNA-29b Mediates Lung Mesenchymal-Epithelial Transition and Prevents Lung Fibrosis in the Silicosis Model. Mol. Ther. Nucleic Acids. 2019;14:20–31. doi: 10.1016/j.omtn.2018.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schafer M.J., White T.A., Iijima K., Haak A.J., Ligresti G., Atkinson E.J., Oberg A.L., Birch J., Salmonowicz H., Zhu Y. Cellular senescence mediates fibrotic pulmonary disease. Nat. Commun. 2017;8:14532. doi: 10.1038/ncomms14532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Du S., Li C., Lu Y., Lei X., Zhang Y., Li S., Liu F., Chen Y., Weng D., Chen J. Dioscin Alleviates Crystalline Silica-Induced Pulmonary Inflammation and Fibrosis through Promoting Alveolar Macrophage Autophagy. Theranostics. 2019;9:1878–1892. doi: 10.7150/thno.29682. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.