Abstract
When facing stressful conditions, some people tend to be impulsively aggressive whereas others are not. However, the causes and underlying mechanisms remain elusive. It has been reported that acute stress induces outbursts of aggression in post-weaning social isolation (SI) mice but not in group housing (GH) mice. Here we report epigenetic regulation of impulsive aggression in SI mice. At post-natal day 21, mice were randomly assigned to GH or SI groups. We found that SI mice exhibited a higher level of microRNA 206 (miR-206) compared with GH mice. Intra-hippocampal injection of AM206, an antagomir of miR-206, decreased stress-induced attack behavior in SI mice and increased BDNF expression. Moreover, BDNF expression was required for AM206 effects on the reduction of aggression. On the other hand, miR-206 overexpression in GH mice induced attack behavior. Intranasal administration of AM206 rather than a scramble control significantly reduced attack behavior and depression-like behavior in SI mice. Our results suggest that miR-206 mediates development of maladaptive impulsive aggression in early life adversity and that its antagomir could potentially be a therapeutic target against stress-exacerbated aggressive behavior.
Keywords: epigenetics, microRNA, miR-206, aggression, social isolation, BDNF, ventral hippocampus, antagomir, AM206, intranasal
Graphical Abstract
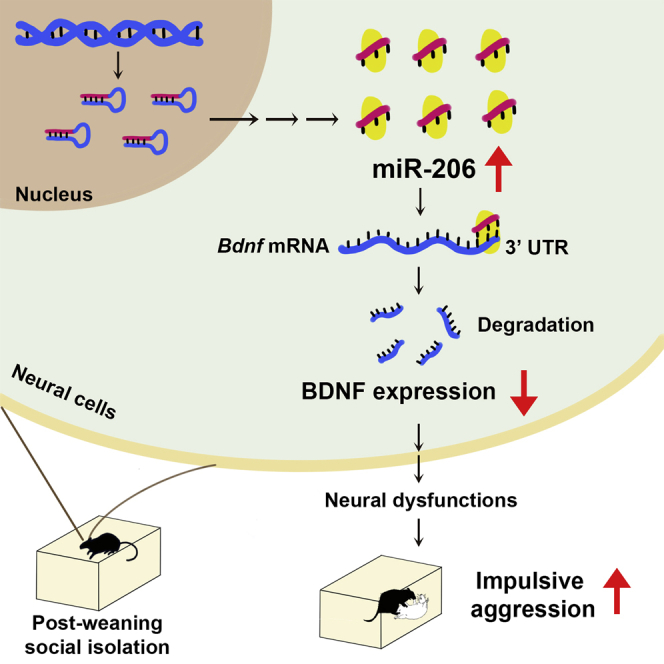
miR-206 is known to regulate skeletal muscle development. Chang et al. showed that socially isolated mice exhibited higher levels of miR-206 and aggressive behavior. Intranasal administration of miR-206 antagomir significantly reduced attack behavior in social isolation mice. miR-206 is a potential therapeutic target for impulsive aggression.
Introduction
Maladaptive impulsive aggression is a major school and social problem and contributes to a cost burden on society. Impulsive aggression is characterized by unplanned and reactive aggression that exceeds appropriate levels in an event-induced emotional status. Under acutely stressful conditions, some people are susceptible to stress and have a tendency to attack another being with out-of-context and exaggerated levels, but other people do not. Post-weaning social isolation (SI) mice have been reported to exhibit violent attack behavior after acute stress, but group housing (GH) mice do not attack intruder mice.1 However, little is known about the mechanism whereby early life influences maladaptive impulsive aggression.
The external environment and genes interplay to shape brain development and neural plasticity and affect adult behaviors. It is through epigenetic regulation that the environment modulates gene expression and even mediates development of neuropsychiatric disorders.2,3 One of these epigenetic mechanisms is RNA translation repression through microRNA (miRNA). miRNAs are small, evolutionary conserved, non-coding RNA molecules (∼22 nt) that negatively regulate gene expression by binding to the 3′ untranslated region (3′ UTR) of their target mRNAs.4,5 miR-206, a member of the muscle-specific miR-1 family, was originally known to regulate embryonic development of skeletal muscle.6 Recently, studies have shown that miR-206 is also involved in neurological diseases through its target, brain-derived neurotrophic factor (BDNF). miR-206 is increased in olfactory mucosal tissues7 and in the temporal cortex of human Alzheimer disease (AD) patients and in brains of Tg2576 mice.8 miR-206 has been shown to regulate BDNF expression and is involved in the pathogenesis of AD.8 In dorsal root ganglia, miR-206 specifically targets long 3′ UTRs to regulate BDNF expression without affecting short 3′ UTR counterparts.9 These studies suggest that miR-206 could post-transcriptionally regulate BDNF expression.
BDNF plays a crucial role in brain development and is also associated with neuroplasticity10,11 and neuroprotection.12 In human studies, there is a single-nucleotide polymorphism in the BDNF gene, leading to a valine-to-methionine substitution (Val66Met). The Met allele of BDNF Val66Met exhibits significantly decreased hippocampal gray matter volumes compared with Val homozygotes.13 In addition, an increased number of BDNF Met alleles is associated with increased aggressive behavior in a population of schizophrenic patients.14 It has also been shown that people who carry the BDNF Met-Met variant are vulnerable to environmental risk and have increased risk for aggressive behavior in adolescence compared with Val-Val carriers.15,16 These clinical studies suggest that BDNF can potentially intervene in aggression. In rodents, reduction of hippocampal hyperactivity blunted attack behavior via upregulation of BDNF in SI mice.17 Additionally, mice with hippocampus-restricted BDNF knockout show increased aggression.18 The purpose of this study was to investigate the involvement of epigenetic regulation in impulsive aggression in SI mice. We demonstrated that miR-206 levels of the ventral hippocampus (vHip) was significantly higher in SI mice compared with GH mice. AM206, a specific miR-206 antagomir, decreased stress-provoked attack behavior, and the effect required increased BDNF expression. Moreover, intranasal delivery of AM206 significantly reduced attack behavior and depression-like behavior in SI mice. Our results demonstrate that miR-206-dependent epigenetic regulation promotes impulsive aggression in post-weaning SI model and suggest a potential therapeutic approach for impulsive aggression, such as noninvasive intranasal administration of AM206.
Results
The Level of miR-206 Is Higher in SI Mice
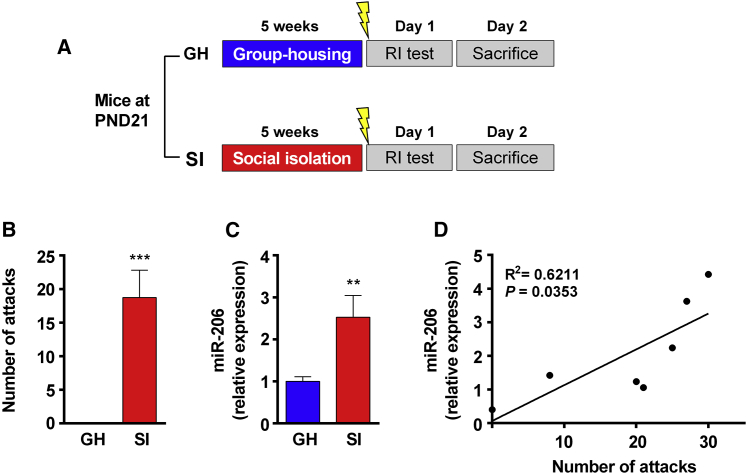
miR-206 has been shown to specifically target long 3′ UTRs to repress BDNF expression,9 and we have reported previously that BDNF expression is involved in regulation of impulsive aggression in SI mice.17 We investigated a role of miR-206 in stress-provoked aggression. Mice on post-natal day 21 (P21) were randomly assigned to GH or SI experimental groups. After 5 weeks, they were subjected to five footshocks 30 min before the resident-intruder (RI) test (Figure 1A). The next day, vHip tissues were dissected out for quantitative real-time PCR analysis. Figure 1B shows that SI mice had higher attack numbers (Figure 1B) and higher miR-206 levels (Figure 1C) compared with GH mice. Furthermore, the number of attacks was significantly correlated with the levels of miR-206 in SI mice (Figure 1D; the linear regression of correlation, R2 = 0.6211). The results indicated that there was a positive correlation between attack levels and miR-206 levels after post-weaning SI.
Figure 1.
SI Mice with Higher miR-206 Levels Exhibit a Higher Level of Aggression
(A) The experimental design and procedures. Mice at P21 were randomly assigned to group housing (GH) or social isolation (SI) groups. 5 weeks later, the mice were subjected to five footshocks 30 min before the RI test. (B) SI mice showed a higher number of biting attacks toward intruders compared with GH mice. Unpaired Student’s t test, t(13) = 4.90, ∗∗∗p < 0.001 versus GH; n = 8 and 7 in GH and SI mice, respectively. (C) vHip tissues were dissected out for quantitative real-time PCR analysis. SI mice showed higher levels of miR-206 compared with GH mice. Unpaired Student’s t test, t(13) = 3.074, ∗∗p < 0.01 versus GH. (D) There was a significant positive correlation when the number of attacks was plotted against the levels of miR-206 in SI mice. R2 = 0.62, ∗p < 0.05, n = 7 in SI mice. Data represent mean ± SEM.
AM206 Decreases the SI-Induced Increase in miR-206 and Stress-Provoked Attack Numbers
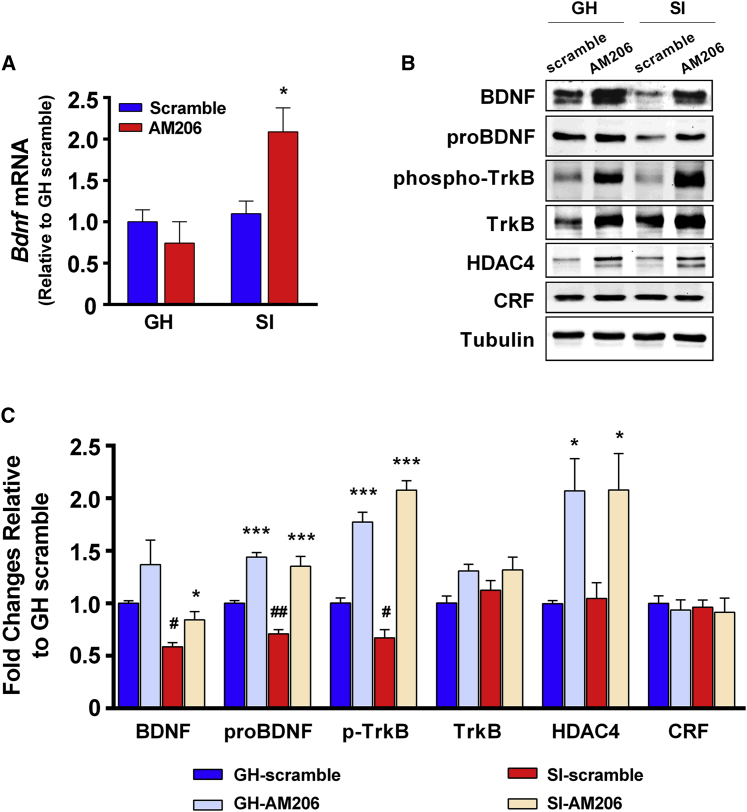
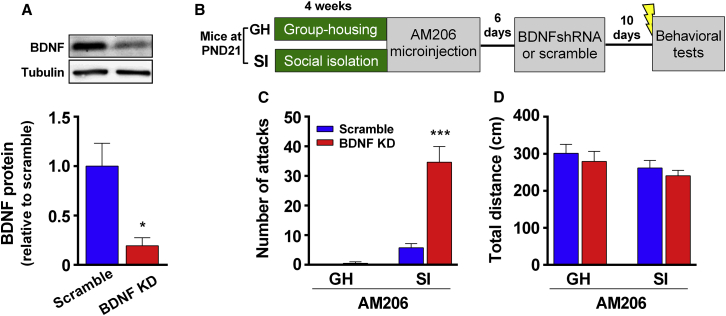
To investigate whether miR-206 was involved in the aggression trait of SI mice, we infused the antagomir of miR-206, AM206 (2′-O-methylated antisense oligonucleotides), into the vHip, a brain area that mediates stress-induced aggression in SI mice.19 We used fluorescent Cy3 to attach the 5′ end of the AM206 oligonucleotide to visualize the AM206 injection sites. First we examined whether AM206 improved BDNF expression in vivo and then tested whether inhibiting miR-206 with AM206 reduced attack behavior. On P21, mice were assigned to GH or SI groups. On P49, scramble or AM206 (500 μM, 0.8 μL/side) was infused into the bilateral vHip of mice. Two weeks later, the vHip tissues of these mice were analyzed after the RI test. Two-way ANOVA revealed a significant group (SI versus GH) × drug (AM206 versus scramble) interaction effect in Bdnf mRNA levels (Figure 2A). Intra-vHip infusion of AM206 significantly increased Bdnf mRNA in SI mice but not in GH mice (Figure 2A). In a western blot analysis (Figures 2B and 2C), intra-vHip infusion of AM206 increased hippocampal BDNF protein expression in vivo (Figure 2C). AM206 significantly increased proBDNF and BDNF expression in both SI and GH mice, indicating that AM206 did not involve the process of proBDNF into mature BDNF. Interestingly, in scramble-transduced mice, proBDNF and BDNF protein levels were lower in SI mice compared with GH mice (Figures 2B and 2C), whereas Bdnf mRNA levels were comparable between SI and GH mice (Figure 2A). These results suggested that SI involved modification of Bdnf mRNA rather than its pre-RNA production. In addition, the SI-decreased BDNF protein level in scramble mice (Figure 2C) is consistent with the result showing that SI increased the miR-206 level (Figure 1C).
Figure 2.
The miR-206 Antagomir Increases BDNF Protein Expression
(A) AM206 had no effect on Bdnf mRNA in GH mice but increased Bdnf mRNA in SI mice. Two-way ANOVA, F(1,22) = 9.18, ∗∗p < 0.01 in the interaction effect, Bonferroni’s post hoc test, scramble versus AM206, ∗∗p< 0.01 in SI mice, p = 0.72 in GH mice. n = 10, 5, 6, and 5 in scramble-GH, AM206-GH, scramble-SI, and AM206-SI mice, respectively. (B) The representative images of Western blotting in (C). (C) AM206 significantly increased the protein expression of miR-206 downstream targets. Two-way ANOVA, significant main effects of AM206: F(1,22) = 9.72, ∗∗p < 0.01 in BDNF protein levels; F(1,22) = 118.6, ∗∗p < 0.001 in proBDNF protein levels; F(1,22) = 180.2, ∗∗p < 0.001 in p-TrkB levels; F(1,22) = 18.6, ∗∗p < 0.001 in HDAC4 protein levels. In scramble-treated mice, BDNF, proBDNF, and p-TrkB protein levels were significantly lower in SI mice compared with GH mice. GH versus SI, t(14) = 9.55, #p< 0.05, t(14) = 6.34, ##p< 0.01, and t(11) = 3.46, ##p< 0.01 in BDNF, proBDNF, and p-TrkB of scramble control, respectively. However, the HDAC4 level of scramble-treated GH mice was not different from the one of scramble-treated SI mice (t(11) = 0.30, p = 0.77). In total TrkB and CRF protein expression, there was no difference among groups (one-way ANOVA, F(3,22) = 2.9, p > 0.05 and F(3,22) = 0.14, p > 0.05 in TrkB and CRF, respectively). For BDNF and proBDNF protein, n = 10, 5, 6, and 5 in scramble-GH, AM206-GH, scramble-SI, and AM206-SI mice, respectively. For p-TrkB, TrkB, HDAC4, and CRF protein, n = 6, 7, 7, and 6 in scramble-GH, AM206-GH, scramble-SI, and AM206-SI mice, respectively. Data represent mean ± SEM.
The tropomyosin receptor kinase B (TrkB) receptor is a receptor for BDNF. Consistent with the BNDF results, the levels of phospho-TrkB (p-TrkB; the active form of the TrkB receptor) in scramble-treated SI mice was lower than those of scramble-treated GH mice (Figures 2B and 2C). It suggested that BDNF signaling was less activated in SI mice compared with GH mice. Moreover, AM206 treatment increased p-TrkB levels in GH and SI mice (Figures 2B and 2C), indicating that AM206 treatment activated BNDF signaling. There was no difference in total TrkB protein expression among groups.
Histone deacetylase 4 (HDAC4) is one of the downstream targets of miR-206 in skeletal muscle.20,21 We found that AM206 treatment significantly increased HDAC4 levels in the vHip of SI and GH mice. However, in scramble-treated groups, the HDAC4 level was not different between SI and GH mice (Figures 2B and 2C). In addition, protein expression of corticotropin-releasing factor (CRF), which regulates functions of the hypothalamic-pituitary-adrenal (HPA) axis, was not different among groups, indicating that AM206 treatment has no effects on CRF expression (Figures 2B and 2C). Thus, intra-vHip infusion of AM206 efficiently increased BDNF levels, the downstream target of miR-206, in vivo.
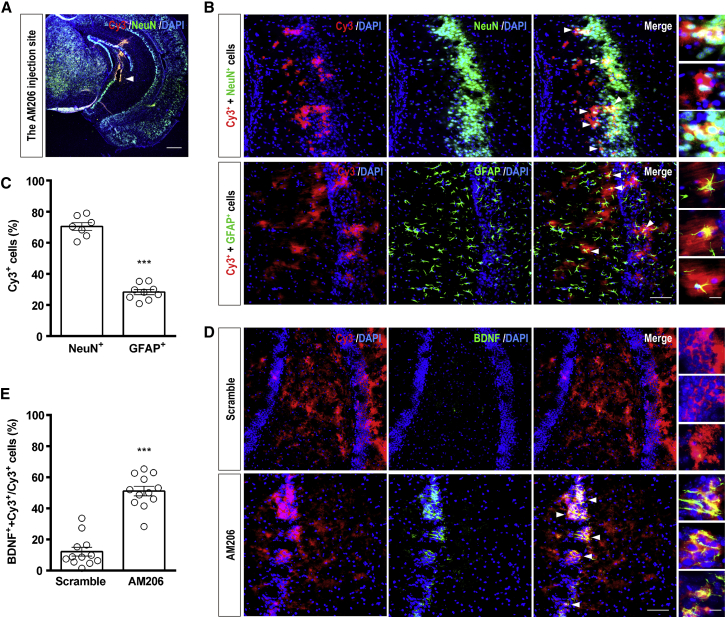
We visualized the distribution of AM206 by checking the injection sites of Cy3-labeled AM206 (500 μM, 0.8 μL/side) in the vHip of SI mice (Figure 3A). The results confirmed the presence of Cy3-AM206 in the vHip. Cy3-AM206 was present in NeuN-positive (a neuronal marker, Figure 3B, top; 70.46% ± 2.5%) and GFAP-positive cells (a glial cell marker, Figure 3B, bottom; 28.29% ± 1.67%), indicating that Cy3-AM206 was mainly distributed in neurons (Figure 3C). Importantly, BDNF signals (green) were highly co-localized with Cy3-positive (red) cells in the vHip of AM206-treated mice (Figure 3D, bottom). Cy3-positive cells with BDNF were 51.08% ± 3.06% in Cy3-positive cells with AM206 treatment (Figures 3D and 3E, bottom) and 12.14% ± 2.78% in Cy3-positive cells with scramble treatment (Figures 3D and 3E, top), indicating that AM206 treatment induced BDNF expression in Cy3-positive cells compared with scramble treatment.
Figure 3.
Distribution of Cy3-AM206 after Intra-hippocampal Injection
(A) The infusion site of Cy3-labeled AM206. The scale bar represents 550 μm. (B) The fluorescence of Cy3-AM206 was distributed throughout the vHip and present in NeuN-positive (top) and glial fibrillary acidic protein (GFAP)-positive (bottom) cells. The scale bar represents 50 μm. In the insets on the right, the scale bar represents 20 μm. (C) The percentage of Cy3+-NeuN+ cells among NeuN+ cells is higher than that of Cy3+-GFAP+ cells among GFAP+ cells. Unpaired Student’s t test, t(14) = 14.47, ∗∗∗p < 0.001. n = 7 and 9 brain slices in 3 mice for NeuN and GFAP, respectively. (D) BDNF expression (green) was highly colocalized with Cy3-positive cells (red) in the vHip of AM206-treated mice (bottom). The scale bar represents 50 μm. In the insets on the right, the scale bar represents 20 μm. (E) AM206 treatment increased BDNF protein expression in Cy3-positive cells compared with the scramble control. Unpaired Student’s t test, t(22) = 9.42, ∗∗∗p < 0.001. n = 12 brain slices in 3 mice per group. Data represent mean ± SEM.
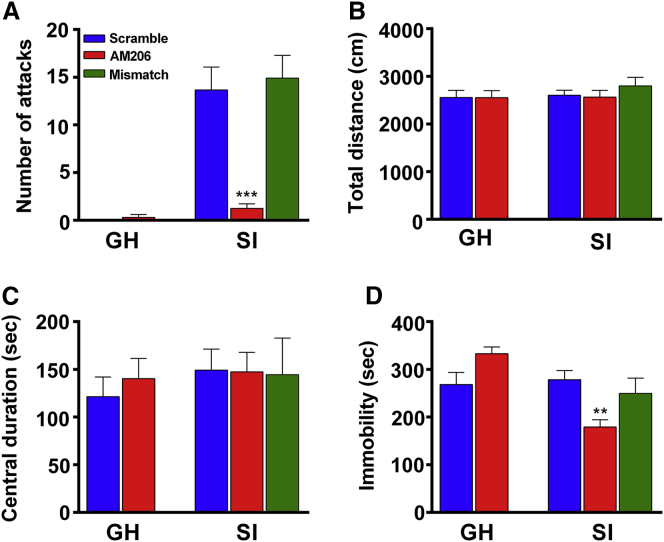
Regarding aggressive behavior, two-way ANOVA showed a significant group × drug interaction in attack numbers (Figure 4A). Bonferroni’s test showed that AM206 significantly reduced attack numbers in SI mice but not in GH mice (Figure 4A). In an open field test, AM206 had no effects on total distance traveled (Figure 4B) and central duration (Figure 4C) in GH and SI mice, indicating that AM206 did not affect locomotor function in mice. In the forced swimming test (FST), two-way ANOVA revealed a significant group × drug interaction effect (Figure 4D). AM206 significantly reduced immobility duration in SI mice but not in GH mice (Figure 4D), suggesting that AM206 produces antidepressant effects in SI mice but not in GH mice. In contrast, mismatch of the AM206 sequence has no effect on SI mice. The effects of the mismatch antagomir were similar to those of the of scramble control in the RI test, FST test, and open field test. Thus, AM206 infusion into the vHip reduced attack behavior and depression-like behavior in SI mice.
Figure 4.
Intra-hippocampal Infusion of AM206 Produces Anti-aggression Effects in SI Mice
(A) In the RI test, there was a significant interaction effect in AM206 and SI mice (two-way ANOVA, F(1,70) = 21.84, ∗∗∗p < 0.001). AM206-treated SI mice (500 μM, 0.8 μL/side) had significantly lower attack numbers than scramble-treated SI mice (500 μM, 0.8 μL/side) (Bonferroni’s post hoc test, p < 0.001). In contrast, intra-hippocampal AM206 infusion had no effect in GH mice (Bonferroni’s post hoc test, p > 0.1). For mismatch control of the AM206 sequence, mismatch-treated SI mice had significantly higher attack behavior than AM206-treated SI mice (one-way ANOVA, F(2,46) = 16.47, ∗∗∗p < 0.001; Bonferroni’s post hoc test, p < 0.05), but mismatch-treated SI mice showed no difference from scramble-treated SI mice (Bonferroni’s post hoc test, p > 0.9). (B and C) AM206 had no effect on the locomotion activity (B) and central duration (C) in the open field test in GH and SI mice. Two-way ANOVA, F(1,70) = 0.02, p = 0.89 in the interaction effect of total distance traveled (B). F(1,70) = 0.22, p = 0.63 in the interaction effect of central duration (C). For mismatch control of the AM206 sequence, mismatch-treated SI mice showed no difference from AM206- and scramble-treated SI mice (one-way ANOVA, F(2,46) = 0.59, p > 0.05 in total distance; F(2,46) = 0.01, p > 0.05 in duration traveling in the central area). (D) In the FST, AM206 significantly reduced immobility time in SI mice but not in GH mice. Two-way ANOVA, F(1,70) = 19.60, ∗∗∗p < 0.001 in the interaction effect of immobility duration. Bonferroni’s post hoc test, AM206 versus scramble, ∗∗p < 0.01 in SI mice. For mismatch control of the AM206 sequence, mismatch-treated SI mice showed no difference from scramble-treated SI mice (p > 0.9).n = 14 and 20 in scramble- and AM206-GH mice, respectively. n = 20, 20, and 9 in scramble-treated, AM206-treated, and mismatch SI mice, respectively. Data represent mean ± SEM.
Knockdown of BDNF in the vHip Prevents AM206-Mediated Reduction in Attack Behavior
To examine whether the AM206 effect depended on hippocampal BDNF protein, we combined Bdnf small hairpin RNA (shRNA) transduction with microinjection of AM206 into the vHip. We confirmed that mice with transduction of Bdnf shRNA had reduced expression levels of BDNF protein in the hippocampus (Figure 5A), indicating the efficiency of BDNF knockdown. Another group of GH and SI mice was microinjected with AM206 and, 6 days later, transduced with a lentivirus carrying scramble or Bdnf shRNA. Ten days later, these mice were subjected to five footshocks 30 min before the RI test (Figure 5B). Two-way ANOVA revealed a significant transduction (shBdnf versus scramble) × group (GH versus SI) interaction in attack numbers (Figure 5C). Bonferroni’s test showed that attack numbers were not different between scramble- and Bdnf shRNA-transduced GH mice, indicating that BDNF knockdown per se did not induce attack behavior. However, attack numbers in Bdnf shRNA-transduced SI mice were significantly higher than in scramble-transduced SI mice, indicating that the anti-aggression effect of AM206 was reversed by BDNF knockdown. In the open field test, there were no effects on total distance traveled (Figure 5D), indicating that BDNF knockdown did not involve general locomotion ability. These results suggested that the anti-aggression effect of AM206 depended on BDNF protein expression.
Figure 5.
Knockdown of BDNF Prevents the Effects of AM206 in SI Mice
(A) Transduction with Bdnf shRNA into the vHip significantly decreased BDNF protein expression. Unpaired Student’s t test, t = 3.29, ∗p < 0.05, n = 3 per group. (B) The experimental procedure of combination of AM206 with BDNF knockdown. Six days after mice received a microinjection of AM206, the mice were transduced with a lentivirus carrying scramble or Bdnf shRNA through the cannula. Ten days later, the mice were given footshocks 30 min before the RI test. (C) BDNF knockdown increased the attack behavior of AM206-treated SI mice but did not have effects on AM206-treated GH mice. Two-way ANOVA, F(1,28) = 26.63, ∗∗∗p < 0.001 in the interaction effect of the attack number. Bonferroni’s test, BDNF knockdown versus scramble, ∗∗∗p < 0.001 in AM206-treated SI mice, p > 0.05 in AM206-treated GH mice. n = 8 per group. (D) BDNF knockdown had no effects on total distance traveled in the open field test. Two-way ANOVA, F(1,28) = 0.001, p > 0.05 in the interaction effect. n = 8 per group. All data represent mean ± SEM.
Overexpression of miR-206 in GH Mice Provokes Attack Behavior
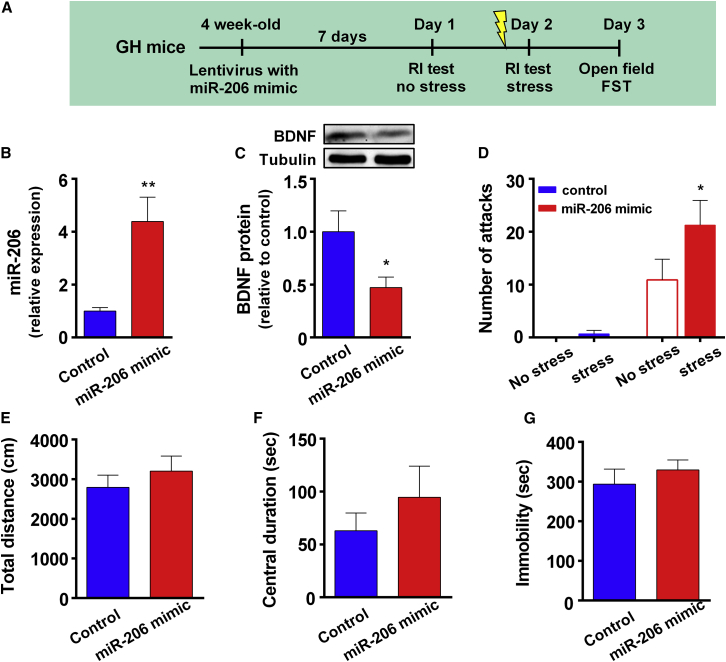
Because SI mice exhibited increased miR-206 levels, we next determined whether miR-206 overexpression in GH mice produced effects similar to SI. Four-week-old GH mice were infused with a lentivirus carrying a miR-206 mimic into the vHip. Seven days later, these mice were given RI tests without and with footshocks on sequential days (Figure 6A). To ensure overexpression of miR-206, we harvested vHip tissues for quantitative real-time PCR. As shown in Figure 6B, the levels of miR-206 were significantly higher in miR-206 mimic-transduced mice compared with control-transduced mice. Correspondingly, the expression of BDNF protein was lower in miR-206 mimic-transduced mice (Figure 6C). Mixed two-way ANOVA revealed a significant transduction × stress interaction effect in attack behavior in GH mice (Figure 6D). miR-206 mimic-transduced mice exhibited higher levels of attack behavior than control mice. Moreover, acute stress induced higher levels of attack behavior in miR-206 mimic-transduced GH mice but not in control GH mice. It indicated that miR-206 overexpression produced attack behavior in GH mice. In the open field test, miR-206 overexpression had no effect on total distance traveled (Figure 6E) or central duration (Figure 6F). In the FST, transduction with the miR-206 mimic did not significantly affect immobility duration (Figure 6G). These results suggest that an increase in miR-206 in the vHip induced attack behavior but not depression-like behavior in mice.
Figure 6.
Overexpression of miR-206 in the vHip of GH Mice Provokes Attack Behavior
(A) The experimental procedure of miR-206 overexpression in GH mice. 4-week-old GH mice were infused with a lentivirus carrying a miR-206 mimic to the vHip. Seven days later, the mice were given RI tests without receiving footshocks. The next day, the mice were given footshocks 30 min before receiving RI tests. (B) vHip tissues were harvested for quantitative real-time PCR, and the levels of miR-206 were significantly higher in lenti-miR-206-transduced mice compared with lenti-blank-transduced mice. Unpaired t test, t(14) = 3.65, ∗∗p < 0.01, n = 8 per group. (C) The expression of BDNF protein was lower in lenti-miR-206 transduced mice than in lenti-blank-transduced mice. Unpaired t test, t(8) = 2.40, ∗∗p < 0.05, n = 5 per group. (D) Transduction with the miR-206 mimic significantly increased the attack behavior of GH mice. Mixed two-way ANOVA, F(1,15) = 20.66, ∗∗∗p < 0.001 in the main effect of miR-206 overexpression. Acute stress also exacerbated the level of attack behavior in miR-206 mimic-transduced GH mice but not in blank-transduced GH mice. Mixed two-way ANOVA, F(1,15) = 4.98, ∗p < 0.05 in the miR-206 overexpression × acute stress interaction effect in attack behavior. Bonferroni’s test, stress versus no stress, ∗p < 0.05 in miR-206 mimic-transduced GH mice, p > 0.05 in blank-transduced GH mice. (E and F) In the open field test, transduction with the miR-206 mimic had no effect on total distance traveled (unpaired Student’s t test, t(15) = 0.85, p = 0.41; E) and central duration (unpaired Student’s t test, t(15) = 0.96, p = 0.35; F). (G) In the FST, transduction with the miR-206 mimic did not significantly affect immobility duration. Unpaired Student’s t test, t(15) = 0.77, p = 0.45. n = 9 and 8 in blank- and miR-206 mimic-transduced GH mice, respectively. Data represent mean ± SEM.
Intranasal Administration of AM206 Produces Anti-aggression and Antidepressant Effects
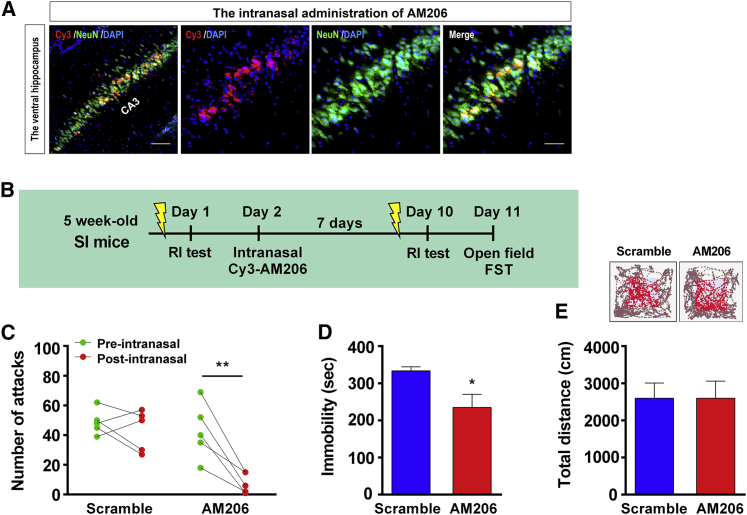
We used intranasal administration to deliver AM206 from the nasal cavity directly to the brain. First we determined whether AM206 could reach the brain via this route of administration. Twenty-four hours after administration of Cy3-AM206, the mice were sacrificed, and vHips were sectioned for immunofluorescence staining. Figure 7A shows the presence of Cy3-AM206 in the vHip, and Cy3-AM206 was in NeuN-positive cells. After confirming that intranasal Cy3-AM206 delivery could reach the vHip, another group of SI mice received intranasal administration of AM206 after a pre-intranasal RI test. 7 days later, these mice received a post-intranasal RI test and other behavioral tests (Figure 7B). Mixed two-way ANOVA revealed a significant interaction effect on attack behavior (Figure 7C). Intranasal administration of AM206 rather than the scramble control significantly reduced the attack numbers (Figure 7C). In the FST, AM206-administered mice had lower immobility duration than scramble-administered mice (Figure 7D). In the open field test, there was no difference in total distance traveled between AM206 and scramble mice (Figure 7E). These results suggested that intranasal administration of AM206 decreased attack behavior and depression-like behavior in SI mice.
Figure 7.
Intranasal Administration of AM206 Reaches the vHip and Ameliorates Attack Behavior
(A) Twenty-four hours after intranasal administration of Cy3-AM206, the mice were sacrificed, and vHips were sectioned for immunofluorescence staining. Cy3-AM206 was present in NeuN-positive cells of the vHip. The scale bar in the left image represents 50 μm. The scale bar in the other image represents 25 μm. n = 3 intranasal AM206-SI mice. (B) Experimental timeline of intranasal AM206 administration. After the RI test (the pre-intranasal test), SI mice received intranasal delivery of AM206. 7 days later, the mice received the second RI test (the post-intranasal test). n = 5 per group. (C) Intranasal delivery of AM206 rather than a scramble control significantly reduced the attack behavior of SI mice. Mixed two-way ANOVA, F(1,8) = 9.01,∗p < 0.05 in the interaction effect. Bonferroni’s test, pre-intranasal versus post-intranasal test, ∗∗p < 0.01 in AM206 delivery, p > 0.05 in scramble delivery. (D) Intranasal delivery of AM206 decreased immobility duration in the FST. Unpaired Student’s t test, t(8) = 2.69, p < 0.05. (E) In the open field test, intranasal AM206 delivery had no effect on total distance traveled. Unpaired Student’s t test, t(8) = 0.0007, p > 0.05. Data represent mean ± SEM.
Discussion
The major findings of this study are as follows: (1) miR-206 levels were higher in SI mice than in GH mice; (2) miR-206 levels positively correlated with the levels of attack behavior in SI mice; (3) miR-206 overexpression in GH mice induced attack behavior; (4) intra-vHip infusion of AM206 decreased attack behavior, which depended on the downstream target of miR-206, BDNF; and (5) intranasal administration of AM206 rather than a scramble control reduced attack behavior and ameliorated depression-like behavior. These results suggest that miR-206 mediates stress-provoked attack behavior in SI mice.
miR-206 is known to inhibit proliferation and promote differentiation of skeletal muscle.22 This study found that, in the central nervous system, post-weaning SI promotes impulsive aggression through epigenetic regulation of miR-206. miR-206 degrades its target mRNA and subsequently regulates protein expression. One of miR-206’s downstream targets is BDNF, which promotes survival of neurons and neuroplasticity. Thus, upregulation of miR-206 may impair neuronal function and induce maladaptive behavior. Consistent with this scenario, SI mice had increased miR-206 levels and decreased BDNF protein levels in the vHip. Moreover, SI mice with higher levels of miR-206 exhibited higher levels of attack behavior. To determine the relationship between miR-206 and attack behavior, we overexpressed miR-206 in the vHip of GH mice. Under our experimental conditions, GH mice rarely attacked the intruders, even when they encountered stress (5 footshocks).1,17 However, after miR-206 overexpression, GH mice attacked the intruders, and attack behavior was further aggravated when the mice encountered stress. In addition, miR-206 overexpression directly repressed BDNF translation in GH mice. These results imply that social isolation results in increased expression of miR-206 and decreased expression of BDNF in the vHip, leading to promotion of attack behavior.
An antagomir is a chemically engineered oligonucleotide used to silence endogenous miRNA. In agreement with previous reports that proved that AM206 silences miR-206 effects,8 infusion of AM206 into the vHip of SI mice increased BDNF mRNA and protein levels and subsequently ameliorated aggressive behavior. Moreover, AM206 treatment induced phosphorylation of the TrkB receptor, an active form of BDNF receptor. In vivo, AM206 treatment also increased BDNF expression in Cy3-labeled cells of the vHip. Importantly, knockdown of BDNF protein abolished the AM206 effect, indicating that inhibition of miR-206 by AM206 decreased attack behavior, which depended on BDNF signaling. Conversely, miR-206 overexpression in GH mice promoted attack behavior. These results demonstrate a novel role of miR-206 in the development of maladaptive impulsive aggression underlying post-weaning SI and suggest a possible therapeutic approach using AM206.
HDAC4 is a class IIa HDAC and modulates the expression of multiple genes with neuroprotective effects.23 HDAC4 is one of the downstream targets of miR-206 and regulates the expression of fibroblast growth factor (FGF)21 and glial cell-derived neurotrophic factor (GDNF).24 In the present study, although the AM206 antagomir increased HDAC4 expression, there were no different HDAC4 protein levels between scramble-treated GH and scramble-treated SI mice. Thus, HDAC4 is likely not a contributor to the impulsive aggression of SI mice. In addition, CRF is a peptide hormone that regulates the HPA axis and modulates stress responses. CRF is not on the list of putative targets of miR-206 in the TargetScanMouse database. Indeed, this study found that AM206 did not increase CRF expression as a negative control for AM206 effects. These results suggest that AM206 decreases stress-induced attack behavior through the BDNF signaling pathway. Although our data show that HDAC4 is likely not a contributor to impulsive aggression, the role of increased HDAC4 and its downstream targets in AM206 effects requires future investigation.
It was noted that miR-206 overexpression, which decreased BDNF levels in GH mice, did not significantly affect immobility duration in the FST (Figure 6G). Although preclinical and clinical studies suggest that BDNF is critically involved in antidepressants, some controversial studies show that mice with adulthood BDNF knockdown do not exhibit baseline depression-like behavior.25,26 It is currently accepted that BDNF is critical for antidepressant effects but not necessary for depression pathophysiology.27, 28, 29 Moreover, administration of BDNF into the nucleus accumbens (NAc) has a prodepression-like effect in the FST, and blockade of BDNF in NAc produces an antidepressant-like effect.30,31 The discrepancies could be attributed to the location of BDNF in different brain regions.
Bypassing the blood-brain barrier (BBB), noninvasive intranasal administration of drugs can rapidly reach the brain.32, 33, 34, 35 In the present study, we detected intranasally delivered Cy3-AM206 in the pyramidal cell layer of the vHip, a brain area that mediates maladaptive impulsive aggression.19 Moreover, 1 week after intranasal delivery, AM206, but not a scramble control, decreased the attack behavior of SI mice. These results suggest that intranasal delivery of the AM206 antagomir may be convenient for maladaptive impulsive aggression.
In conclusion, we found that SI mice exhibited a higher level of miR-206 compared with GH mice. Intra-hippocampal injection of AM206 decreased the SI-induced increase in miR-206 and attack behavior without affecting locomotion activity. In parallel, hippocampal BDNF protein levels were increased by AM206 and required for AM206 effects on aggression and depression. Intranasal administration of AM206, but not a scramble control, significantly reduced attack behavior and immobility duration in the FST. Our results suggest that AM206 potentially has therapeutic effects against stress exacerbation of aggressive behavior.
Materials and Methods
Animals
Male C57BL/6JNarl mice on P21 were purchased from the National Laboratory Animal Center, Taiwan, and randomly assigned to GH (5 mice per cage) or SI (one mouse per cage). 3-week-old male BALB/c mice were purchased from the National Laboratory Animal Center, Taiwan, and group-housed under the same conditions with C57BL/6JNarl mice. The animals were housed on a 12 h/12 h light/dark cycle (room temperature of 24°C ± 2°C) with food and water available ad libitum. The experimental procedures were based on National Institutes of Health guidelines and approved by the National Cheng Kung University Medical Center Animal Care and Use Committee.
RI Test
To assess aggressive behavior in mice, we used the RI test. The day before and during the RI test, the bedding of cage-resident mice was not renewed. The resident mice were given five 0.1-mA footshocks (as acute stress) 30 min before the RI test. The intruder, a male BALB/c mouse, was placed into the home cage of the resident mice for 15 min. A Sony digital camera (HDR-XR150) recorded videos. The number of attacks was defined by the bites of the resident mice to the intruder.
Open Field Test and Locomotor Activity
The open field test was used to measure the locomotor activity and anxiety-like behavior of the mice. After 1-h habituation, mice were placed into the corner of a 40 cm × 40 cm × 40 cm square box with a white floor and black walls. The trials were recorded by the Noldus video tracking system for 10 min. The total distance traveled in the box showed the locomotor activity of the mice, whereas the duration in the central zone showed the anxiety-like behavior of the mice. The box was cleaned with 75% ethanol to prevent olfactory cues after every trial. All data were analyzed using EthoVision XT5.1 software.
FST
The FST is used to assess depression-like behavior in rodents. Mice were placed in a transparent Pyrex cylinder (height, 25 cm; diameter, 20 cm) filled with water to a height of 20 cm at 20°C ± 1°C. 10 min later, the mice were removed from the bath and placed in a clean cage. The water was changed between trials. The videos were recorded by a Sony digital camera (HDR-XR150). All data were analyzed with EthoVision XT5.1 software.
Drugs
A miR206-neutralizing antagomir (AM206, 5′-Cy3-[2′-O-methylated-5′-dC]CACACACUUCCUUACAUUCCA-3′), a scrambled sequence control (5′-Cy3-[2′-O-methylated-5′-A]AGGCAAGCUGACCCUGAAGUU-3′),8 or a mismatch of AM206 sequence control (5′-Cy3[2′-O-methylated-5′-dC]CAAACAAUUCAUUACCUUCAA-3′) was dissolved in diethyl pyrocarbonate (DEPC)-treated phosphate-buffered saline.
Surgery
Mice were anesthetized with chloral hydrate (50 mg/kg, intraperitoneally [i.p.]) and placed on a stereotaxic apparatus (Kopf). The mice were infused with 500 μM of AM206, mismatch, or scramble into the vHip (anteroposterior, −3.0 mm; mediolateral, ± 3.2 mm; dorsoventral, −3.8 mm) for 0.8 μL/side at a rate of 0.1 μL/min through a stainless cannula.
Quantitative Real-Time PCR Analysis
We used the commercial Quik-RNA Mini Prep kit (Zymo Research, catalog number R2024) for total and microRNA extraction. cDNA synthesis was done by TaqMan Small RNA Assay kit (Applied Biosystems, code 15591044). The thermal cycler conditions followed the manufacturer’s protocol. The levels of miR-206 and Bdnf mRNA were measured using a StepOnePlus real-time PCR system (Applied Biosystems) and analyzed using StepOne software v.2.3 (Applied Biosystems) via comparative cycle threshold (Ct) method. The probes used in the experiments were purchased from Applied Biosystems: BDNF (Mm04230607), miR-206 (000510), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH, Mm99999915). The levels of miR-206 and bdnf mRNA were normalized by Gapdh mRNA for each sample.
Western Blot Analysis
vHip tissues were lysed in 0.32 M sucrose buffer (0.32 M sucrose, 10 mM Tris-HCl [pH 7.4], 1 mM EDTA, 2 mM sodium fluoride (NaF), 5 mM vanadate, 1 mM PMSF, 1.5 g/mL leupeptin, and protease and phosphatase inhibitor cocktail [Roche]). Supernatants were collected after 800 × g centrifugation for 10 min. To obtain the synaptosomal fraction, the supernatants were centrifuged at 9200 × g for 20 min. The samples (40 μg total proteins) were mixed with sample buffer (1.54% Dithiothreitol (DDT), 25% glycerol, 4% SDS, 12.5 mM Tris, and 0.02% bromophenol blue) and denatured at 98°C for 10 min. Equal amounts of protein samples were loaded to SDS-PAGE with the appropriate gel concentration, depending on the molecular weight of the target protein. Then they were transferred to a polyvinylidene fluoride (PVDF) membrane (Millipore) using a semi-dry transfer system (Bio-Ray). Rabbit anti-BDNF (N20, 1:1,000, Santa Cruz Biotechnology), rabbit anti-p-TrkB (1:1,000, Millipore), rabbit anti-TrkB (1:1,000, GeneTex), rabbit anti-HDAC4 (1:1,000, Cell Signaling Technology), rabbit anti-CRF (1:10,000, Millipore), and mouse anti-tubulin (1:2,000, Millipore) were used. After horseradish peroxidase (HRP)-conjugated secondary antibodies (1:5000, Jackson ImmunoResearch Laboratories) and chemiluminescence detection reagent (enhanced chemiluminescence [ECL], Perkin Elmer), images were acquired by UVP BiospectrumTM. The images were analyzed and quantified using ImageJ software. The protein levels were first normalized to the internal control (tubulin) for each sample. All experiments were performed at least in triplicate.
Immunofluorescence
Mice were anesthetized with chloral hydrate (50 mg/kg, i.p.) and perfused with 4% paraformaldehyde. Brains were fixed in 4% paraformaldehyde for an additional 24 h and incubated with 30% sucrose. Coronal sections (25-μm thickness) were obtained by cryostat (CM3050S, Leica). After washing with PBST (PBS with 0.1% Tween 20), floating sections were incubated in 1 × saline sodium citrate (SSC) with 85°C for 15 min and then incubated in 1% Triton X-100 for 1 h. 30 min after blocking with 1% normal goat serum and 5% BSA dissolved in CAS-blockTM reagent (Invitrogen), the sections were incubated in 0.1% Fab goat anti-mouse immunoglobulin G (IgG) dissolved in CAS-block reagent for 2 h. The sections were then incubated overnight in primary antibodies dissolved in CAS-block reagent at 4°C. Primary antibodies used in immunofluorescence were rabbit anti-GFAP (1:1,000, Millipore), rabbit anti-NeuN (1:1,000, GeneTex), rabbit anti-BDNF (1:500, GeneTex), and mouse anti-Cy3 (1:2,500, Abcam). The sections were incubated in Alexa 594 goat anti-mouse and Alexa 488 goat anti-rabbit antibody (1:500) in CAS-block reagent for 90 min and then incubated in 0.1% DAPI (Sigma, USA) dissolved in PBS for 15 min. Images were acquired by a Leica DM2500 microscope coupled to a Zyla sCMOS sensor (ANDORTM, Oxford Instruments) with MetaMorph software (Molecular Devices).
Lentivirus Injection
For BDNF knockdown, mice were implanted with a cannula and infused with AM206 as described above. 6 days later, lentiviruses with shRNA of BDNF (5′-TGAGCGTGTGTGACAGTATTA-3′) or scramble (5′-TCCTAGAGAAAGTCCCGGTAT-3′) were administered bilaterally into the vHip with a volume of 0.8 μL at a rate of 0.2 μL/min. The Bdnf shRNA and scramble plasmids were purchased and packed into lentiviruses from the National RNAi Core Facility (Institute of Molecular Biology, Academia Sinica, Taiwan). After resting for 10 days, the mice performed behavior tests. For miR-206 overexpression, the pLenti-miRa-GFP-mmu-miR-206-3p vector (catalog number mm10215) and pLenti-III-mir-GFP-Blank control vector (catalog number m001) were purchased from Applied Biological Materials (Vancouver, Canada) and packed into lentiviruses by the National RNAi Core Facility (Institute of Molecular Biology, Academia Sinica, Taiwan). The target sequence of the miR-206-3p mimic was as follows: 5′-UGGAAUGUAAGGAAGUGUGUGG-3′. Lentiviruses with the miR-206 mimic or with a blank control were infused bilaterally into the vHip of GH mice as described above.
Intranasal Delivery of AM206
One day after the pre-intranasal RI test, mice were handled in a supine position with the head upright. AM206 or a scramble control (5 nmol in 24 μL DEPC-treated PBS buffer) was administrated through a pipette in 4 drops (total of 6 fractions with a 2-min interval).
Statistical Analysis
GraphPad Prism 6 (GraphPad, San Diego, CA, USA) was used for data analysis. Data were analyzed using a Student’s t test or ANOVA and expressed as mean ± SEM. Bonferroni’s multiple comparisons test was used for post hoc comparisons of ANOVA. The level of significance was p <0.05.
Author Contributions
C.-H.C. and E.J.W.K. conducted experiments and analyzed data. C.-H.C. designed behavioral experiments, analyzed immunofluorescence, and wrote the manuscript. C.-L.S. designed some experiments and wrote the manuscript. P.-W.G. designed experiments and wrote the manuscript.
Conflicts of Interests
The authors declare no competing interests.
Acknowledgments
We thank the National RNAi Core Facility (Institute of Molecular Biology, Academia Sinica, Taiwan) for technical support with the lentivirus package. This work was supported by the National Health Research Institutes (NHRI-EX108-10730NI and NHRI-EX109-10730NI) and the Ministry of Science and Technology of Taiwan (MOST 108-2320-B-006-010, MOST 108-2320-B-006-008, MOST 107-2811-B-006-011, and MOST 108-2811-B-006-523).
References
- 1.Chang C.H., Hsiao Y.H., Chen Y.W., Yu Y.J., Gean P.W. Social isolation-induced increase in NMDA receptors in the hippocampus exacerbates emotional dysregulation in mice. Hippocampus. 2015;25:474–485. doi: 10.1002/hipo.22384. [DOI] [PubMed] [Google Scholar]
- 2.Palumbo S., Mariotti V., Iofrida C., Pellegrini S. Genes and Aggressive Behavior: Epigenetic Mechanisms Underlying Individual Susceptibility to Aversive Environments. Front. Behav. Neurosci. 2018;12:117. doi: 10.3389/fnbeh.2018.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuehner J.N., Bruggeman E.C., Wen Z., Yao B. Epigenetic Regulations in Neuropsychiatric Disorders. Front. Genet. 2019;10:268. doi: 10.3389/fgene.2019.00268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 5.Bartel D.P. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 6.Horak M., Novak J., Bienertova-Vasku J. Muscle-specific microRNAs in skeletal muscle development. Dev. Biol. 2016;410:1–13. doi: 10.1016/j.ydbio.2015.12.013. [DOI] [PubMed] [Google Scholar]
- 7.Moon J., Lee S.T., Kong I.G., Byun J.I., Sunwoo J.S., Shin J.W., Shim J.Y., Park J.H., Jeon D., Jung K.H. Early diagnosis of Alzheimer’s disease from elevated olfactory mucosal miR-206 level. Sci. Rep. 2016;6:20364. doi: 10.1038/srep20364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee S.T., Chu K., Jung K.H., Kim J.H., Huh J.Y., Yoon H., Park D.K., Lim J.Y., Kim J.M., Jeon D. miR-206 regulates brain-derived neurotrophic factor in Alzheimer disease model. Ann. Neurol. 2012;72:269–277. doi: 10.1002/ana.23588. [DOI] [PubMed] [Google Scholar]
- 9.Shrestha S., Phay M., Kim H.H., Pouladvand P., Lee S.J., Yoo S. Differential regulation of brain-derived neurotrophic factor (BDNF) expression in sensory neuron axons by miRNA-206. FEBS Open Bio. 2019;9:374–383. doi: 10.1002/2211-5463.12581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Korte M., Carroll P., Wolf E., Brem G., Thoenen H., Bonhoeffer T. Hippocampal long-term potentiation is impaired in mice lacking brain-derived neurotrophic factor. Proc. Natl. Acad. Sci. USA. 1995;92:8856–8860. doi: 10.1073/pnas.92.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pang P.T., Teng H.K., Zaitsev E., Woo N.T., Sakata K., Zhen S., Teng K.K., Yung W.H., Hempstead B.L., Lu B. Cleavage of proBDNF by tPA/plasmin is essential for long-term hippocampal plasticity. Science. 2004;306:487–491. doi: 10.1126/science.1100135. [DOI] [PubMed] [Google Scholar]
- 12.Hetman M., Kanning K., Cavanaugh J.E., Xia Z. Neuroprotection by brain-derived neurotrophic factor is mediated by extracellular signal-regulated kinase and phosphatidylinositol 3-kinase. J. Biol. Chem. 1999;274:22569–22580. doi: 10.1074/jbc.274.32.22569. [DOI] [PubMed] [Google Scholar]
- 13.Pezawas L., Verchinski B.A., Mattay V.S., Callicott J.H., Kolachana B.S., Straub R.E., Egan M.F., Meyer-Lindenberg A., Weinberger D.R. The brain-derived neurotrophic factor val66met polymorphism and variation in human cortical morphology. J. Neurosci. 2004;24:10099–10102. doi: 10.1523/JNEUROSCI.2680-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spalletta G., Morris D.W., Angelucci F., Rubino I.A., Spoletini I., Bria P., Martinotti G., Siracusano A., Bonaviri G., Bernardini S. BDNF Val66Met polymorphism is associated with aggressive behavior in schizophrenia. Eur. Psychiatry. 2010;25:311–313. doi: 10.1016/j.eurpsy.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 15.Kretschmer T., Vitaro F., Barker E.D. The Association Between Peer and own Aggression is Moderated by the BDNF Val-met Polymorphism. J. Res. Adolesc. 2014;24:177–185. doi: 10.1111/jora.12050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Musci R.J., Bradshaw C.P., Maher B., Uhl G.R., Kellam S.G., Ialongo N.S. Reducing aggression and impulsivity through school-based prevention programs: a gene by intervention interaction. Prev. Sci. 2014;15:831–840. doi: 10.1007/s11121-013-0441-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang C.H., Su C.L., Gean P.W. Mechanism underlying NMDA blockade-induced inhibition of aggression in post-weaning socially isolated mice. Neuropharmacology. 2018;143:95–105. doi: 10.1016/j.neuropharm.2018.09.019. [DOI] [PubMed] [Google Scholar]
- 18.Ito W., Chehab M., Thakur S., Li J., Morozov A. BDNF-restricted knockout mice as an animal model for aggression. Genes Brain Behav. 2011;10:365–374. doi: 10.1111/j.1601-183X.2010.00676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang C.H., Gean P.W. The Ventral Hippocampus Controls Stress-Provoked Impulsive Aggression through the Ventromedial Hypothalamus in Post-Weaning Social Isolation Mice. Cell Rep. 2019;28:1195–1205.e3. doi: 10.1016/j.celrep.2019.07.005. [DOI] [PubMed] [Google Scholar]
- 20.Choi W., Lee J., Lee J., Ko K.R., Kim S. Hepatocyte Growth Factor Regulates the miR-206-HDAC4 Cascade to Control Neurogenic Muscle Atrophy following Surgical Denervation in Mice. Mol. Ther. Nucleic Acids. 2018;12:568–577. doi: 10.1016/j.omtn.2018.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Williams A.H., Valdez G., Moresi V., Qi X., McAnally J., Elliott J.L., Bassel-Duby R., Sanes J.R., Olson E.N. MicroRNA-206 delays ALS progression and promotes regeneration of neuromuscular synapses in mice. Science. 2009;326:1549–1554. doi: 10.1126/science.1181046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma G., Wang Y., Li Y., Cui L., Zhao Y., Zhao B., Li K. MiR-206, a key modulator of skeletal muscle development and disease. Int. J. Biol. Sci. 2015;11:345–352. doi: 10.7150/ijbs.10921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mielcarek M., Zielonka D., Carnemolla A., Marcinkowski J.T., Guidez F. HDAC4 as a potential therapeutic target in neurodegenerative diseases: a summary of recent achievements. Front. Cell. Neurosci. 2015;9:42. doi: 10.3389/fncel.2015.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu Y., Hou F., Wang X., Kong Q., Han X., Bai B. Aberrant Expression of Histone Deacetylases 4 in Cognitive Disorders: Molecular Mechanisms and a Potential Target. Front. Mol. Neurosci. 2016;9:114. doi: 10.3389/fnmol.2016.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Autry A.E., Adachi M., Nosyreva E., Na E.S., Los M.F., Cheng P.F., Kavalali E.T., Monteggia L.M. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475:91–95. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Monteggia L.M., Barrot M., Powell C.M., Berton O., Galanis V., Gemelli T., Meuth S., Nagy A., Greene R.W., Nestler E.J. Essential role of brain-derived neurotrophic factor in adult hippocampal function. Proc. Natl. Acad. Sci. USA. 2004;101:10827–10832. doi: 10.1073/pnas.0402141101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Groves J.O. Is it time to reassess the BDNF hypothesis of depression? Mol. Psychiatry. 2007;12:1079–1088. doi: 10.1038/sj.mp.4002075. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y., Shi J., Li J., Liu R., Yu Y., Xu Y. Role of brain-derived neurotrophic factor in the molecular neurobiology of major depressive disorder. Transl. Perioper. Pain Med. 2017;4:20–30. [PMC free article] [PubMed] [Google Scholar]
- 29.Choi S.H., Bylykbashi E., Chatila Z.K., Lee S.W., Pulli B., Clemenson G.D. Combined adult neurogenesis and BDNF mimic exercise effects on cognition in an Alzheimer’s mouse model. Science. 2018;361:eaan8821. doi: 10.1126/science.aan8821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berton O., McClung C.A., Dileone R.J., Krishnan V., Renthal W., Russo S.J., Graham D., Tsankova N.M., Bolanos C.A., Rios M. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–868. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- 31.Yu H., Chen Z.Y. The role of BDNF in depression on the basis of its location in the neural circuitry. Acta Pharmacol. Sin. 2011;32:3–11. doi: 10.1038/aps.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mai H., Fan W., Wang Y., Cai Y., Li X., Chen F., Chen X., Yang J., Tang P., Chen H. Intranasal Administration of miR-146a Agomir Rescued the Pathological Process and Cognitive Impairment in an AD Mouse Model. Mol. Ther. Nucleic Acids. 2019;18:681–695. doi: 10.1016/j.omtn.2019.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grassin-Delyle S., Buenestado A., Naline E., Faisy C., Blouquit-Laye S., Couderc L.J., Le Guen M., Fischler M., Devillier P. Intranasal drug delivery: an efficient and non-invasive route for systemic administration: focus on opioids. Pharmacol. Ther. 2012;134:366–379. doi: 10.1016/j.pharmthera.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 34.Fan L.W., Carter K., Bhatt A., Pang Y. Rapid transport of insulin to the brain following intranasal administration in rats. Neural Regen. Res. 2019;14:1046–1051. doi: 10.4103/1673-5374.250624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Falcone J.A., Salameh T.S., Yi X., Cordy B.J., Mortell W.G., Kabanov A.V., Banks W.A. Intranasal administration as a route for drug delivery to the brain: evidence for a unique pathway for albumin. J. Pharmacol. Exp. Ther. 2014;351:54–60. doi: 10.1124/jpet.114.216705. [DOI] [PMC free article] [PubMed] [Google Scholar]