Abstract
Cardiomyocytes differentiated from human induced pluripotent stem cells (hiPSCs) have great potential for regenerative medicine and drug discovery. In this study, we developed a novel protocol to more reproducibly and efficiently induce cardiomyocytes. A large quantity of uniformly sized spheroids were generated from hiPSCs using microfabricated vessels and induced into cardiac differentiation. In the middle of the cardiac differentiation process, spheroids were then dissociated into single cells and reaggregated into smaller spheroids using the microfabricated vessels. This reaggregation process raised WNT5A and WNT11 expression levels and improved the quality of cardiomyocyte population compared to that in a control group in which dissociation and reaggregation were not performed.
Keywords: Microfabricated vessels, Human induced pluripotent stem cells, Cardiac differentiation, WNT signal, Spheroid, Reaggregation
Abbreviations: human pluripotent stem cells (hPSCs), human embryonic stem cells (hESCs); human induced pluripotent stem cells (hiPSCs), cardiomyocytes (CMs)
Highlights
-
•
Microfabricated culture vessels were used to form large number of hiPSC spheroids.
-
•
High purity cardiomyocytes were obtained by reaggregation of hiPSC-derived spheroids.
-
•
Maturation of cardiomyocyte was promoted by the reaggregation process.
-
•
WNTs were also increased the reaggregation process during cardiac differentiation.
1. Introduction
Because of their ability to proliferate indefinitely and differentiate into any cell type, human pluripotent stem cells (hPSCs), such as human embryonic stem cells (hESCs) and induced pluripotent stem cells (hiPSCs), have great potential in the fields of regenerative medicine and drug discovery. In particular, hiPSC-derived cardiomyocytes (hiPSC-CMs) are expected to be used in regenerative medicine to replenish cardiomyocytes that are lost during heart failure [1] and as a source of cells for novel drug cardio-toxicity and pharmacology screening; a wide range of related studies are being carried out [2]. Therefore, it is of paramount importance that an efficient and robust protocol be developed for cardiomyocyte differentiation from hiPSCs.
High-purity cardiomyocytes can be obtained quickly by activating and suppressing activin/nodal, BMP, and WNT signaling at the proper time [3]. Cardiac differentiation proceeds in 3 main stages: first, hPSCs are treated with BMP4/FGF2/activin A or the WNT activator CHIR and induced into primitive streak-like populations; second, the cells are treated with DKK1 or the WNT inhibitor IWP and induced into cardiac progenitor-like populations; finally, they are differentiated into cardiomyocytes [[4], [5], [6]].
Nevertheless, cell culture technology is also important for cardiac differentiation. Compared with traditional 2D adherent cultures, 3D cultures of multicellular aggregates (spheroids) have many advantages, including ease of scale-up, no requirement of an expensive extracellular matrix (ECM), and promotion of cardiac maturation [7,8]. The efficiency of cardiac differentiation of hESC spheroids is affected by spheroid size and initial seeding density [9]. WNT5A and WNT11 expression levels change with the size of mouse ES cell spheroids [10,11]. WNT5A, WNT5B, and WNT11, which are associated with the non-canonical WNT signaling pathway, are essential to cardiac differentiation after treatment with a WNT inhibitor [12].
We assumed that controlling spheroid size and cell seeding density in each stage of cardiac differentiation from hiPSCs would promote production of cardiomyocytes. In general differentiation methods using hiPSC spheroids, initial spheroid size can be controlled by initial seeding density. During the differentiation process, spheroid size can be easily altered by increasing cell number and fusion of the spheroids themselves. In this study, we developed a unique cardiac differentiation method using microfabricated EZSPHERE vessels designed for fast formation and culture of high-density, uniformly sized spheroids. We previously reported that the microfabricated EZSPHERE vessels are very useful for high-efficiency hiPSC spheroid formation and cell growth in hiPSC maintenance medium while maintaining their uniformity and pluripotency, thereby allowing promotion of rapid neural stem cell differentiation [13].
Thus, we attempted to develop a novel method for inducing cardiac differentiation from hiPSCs using EZSPHERE vessels.
2. Materials and methods
2.1. Cardiac differentiation of hiPSCs
The hiPSC line 253G2 and 201B7 provided by iPS Academia Japan, Inc. was used in all experiments. The hiPSCs were cultured and maintained in mTeSR 1 maintaining medium (Stemcell Technologies, Vancouver, Canada) on Matrigel (Corning, Inc., Corning, NY, USA) or Laminin-521 (BioLamina AB, Sundbyberg, Sweden)-coated dishes, according to the manufacturer's instructions. For cardiac differentiation, we used modified StemPro-34 medium (Thermo Fisher Scientific, Waltham, MA, USA), containing penicillin/streptomycin (1%, Thermo Fisher Scientific), l-glutamine (2 mM, Thermo Fisher Scientific), transferrin (150 μg/mL, Sigma-Aldrich, St. Louis, MO, USA), ascorbic acid (50 μg/mL, Sigma-Aldrich), monothioglycerol (0.000039%, Sigma-Aldrich). To start the cardiac differentiation, 2D-cultured hiPSCs on the dishes were harvested by treatment with TrypLE Select (Thermo Fisher Scientific) for 4–5 min and dissociated into single cells by gentle pipetting 2–5 times. The harvested cells were re-suspended in an EB medium containing BMP4 (1 ng/mL, R&D Systems, Minneapolis, MN, USA) and Y-27632 (10 μM, Wako Pure Chemical Industries, Ltd., Tokyo, Japan) in the modified StemPro-34. Five or 10 mL of the cell suspension (containing 3 × 106 cells) was then seeded into a 100 mm EZSPHERE dish (#4020-900, approximately 14,000 micro-wells per dish; AGC Techno Glass Co., Ltd., Yoshida, Japan) to form spheroids (initial seeding density approximately 3 × 106 cells/dish). After 24 h, 5 or 10 mL of the stage-1 differentiation medium (equal volume with EB medium) containing BMP4 (20 ng/mL), bFGF (10 ng/mL; R&D Systems) and activin A (12 ng/mL) in modified StemPro-34 was added to the culture. On day 4, the spheroids were harvested and washed with IMDM, and then transferred into low-adhesion 100 mm EZ-bindshut II dishes (AGC Techno Glass) after being suspended in stage-2 differentiation medium containing IWP-4 (2.5 μM; Stemgent, Cambridge, MA, USA) and VEGF (10 ng/mL; R&D Systems) in modified StemPro-34. On day 6 or 7, the obtained spheroids were washed with IMDM and transferred to stage-3 differentiation medium containing bFGF (5 ng/mL) and VEGF (10 ng/mL) in modified StemPro-34 and cultured until day 14. Media was changed every 2–3 days. Cell number and viability was counted using an automated cell counter (TC10 Automated Cell Counter; BioRad, Hercules, CA).
2.2. Reaggregation of cardiac mesoderm/progenitors spheroids
Spheroids obtained on day 6 or 7 of the differentiation process were washed with DPBS and treated with Accutase (Innovative Cell Technologies, San Diego, CA, USA) at 37 °C for 8 min for dissociation to occur. During Accutase treatment, the spheroids were vortexed for approximately for 10 s during a 4 min duration to dissociate them into single cells. To stop Accutase treatment, stage-3 differentiation medium was added to the dissociated cells. The cell suspension was centrifuged (200×g, 4 min), re-suspended with stage-3 differentiation medium, and seeded onto 35 mm EZSPHERE dishes (3 mL/dish; #4000-903 type, approximately 1000 micro-wells per dish; AGC Techno Glass) at cell densities of 0.5 × 106, 1.0 × 106, 2.0 × 106, and 3.0 × 106 cells/dish. Whole or half volumes of the culture media were changed every 2–3 days.
2.3. Flow cytometry analysis
The spheroids obtained on days 6–7 during the cardiac differentiation process were dissociated into single cells as described above, and then suspended with the HBSS flow cytometry buffer containing 3% FBS and 0.03 mM EDTA. The following antibodies were labeled with florescent dyes and used for immunostaining in flow cytometry: PE anti-human PDGFRα (5:100; BioLegend, San Diego, CA), APC anti-human VEGFR2 (KDR) (2.5:100; BioLegend). The cell immunostaining process was performed for 30–45 min at room temperature using the flow cytometry buffer.
The cardiac spheroids obtained on days 8–14 were treated with the dissociation solution (1:3 Acuumax [Innovative Cell Technologies]:0.05% trypsin [Thermo Fisher] at 37 °C for 4–16 min, and vortexed every 4 min for dissociation into single cells. An equal volume of quenching buffer [DPBS:FBS 1:1] containing 200 units/mL of DNase I (Merck & Co., Kenilworth, NJ, USA) was then added to the dissociated cells. For the flow cytometry analysis, the obtained cells were fixed with 4% paraformaldehyde (Wako Pure Chemical Industries) for 20 min, blocked and permeabilized with Flow Cytometry Permeabilization/Wash Buffer I (R&D Systems) for 30 min, and finally stained with PE anti-cardiac troponin T antibody (BD Biosciences, Franklin Lakes, NJ, USA) for 30 min at room temperature using Flow Cytometry Permeabilization/Wash Buffer I.
The BD FACSVerse flow cytometer (BD Biosciences) was used for all flow cytometry analysis and acquired raw data were analyzed using the FlowJo software (FlowJo LLC, Ashland, OR, USA).
2.4. Quantitative RT-PCR (qRT-PCR)
Total RNA was isolated from each cell or spheroid sample using the PureLink RNA Mini Kit (Thermo Fisher Scientific). Approximately 200 ng to 1 μg of isolated total RNA was reverse transcribed using the High Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific). qRT-PCR was performed (n = 3 for each cDNA sample as technical triplicate) on the Stratagene M × 3000P (Agilent Technologies, Santa Clara, CA, USA) instrument using the THUNDERBIRD SYBR qPCR Mix (Toyobo, Osaka, Japan). The mRNA level of each target gene was normalized to that of GAPDH. Nucleotide sequences of the primers used in PCR are listed in Table 1.
Table 1.
Primers used for quantitative RT-PCR.
| GAPDH | F R |
TGCACCACCAACTGCTTAGC GGCATGGACTGTGGTCATGAG |
|---|---|---|
| TNNT2 | F R |
TTCGACCTGCAGGAGAAGTT GCGGGTCTTGGAGACTTTCT |
| NKX2-5 | F R |
GCCTTCTATCCACGTGCCTA CCTCTGTCTTCTCCAGCTCC |
| MYL2 | F R |
GCAGGCGGAGAGGTTTTC AGTTGCCAGTCACGTCAGG |
| MYL7 | F R |
CTTGTAGTCGATGTTCCCCG TCAAGCAGCTTCTCCTGACC |
| WNT5A | F R |
GCCCAGGTTGTAATTGAAGC TGGCACAGTTTCTTCTGTCC |
| WNT5B | F R |
CGGGAGCGAGAGAAGAACT CGTCTGCCATCTTATACACAGC |
| WNT11 | F R |
GGCCAAGTTTTCCGATGCTC CACCCCATGGCACTTACACT |
2.5. Immunofluorescent staining
Cardiac spheroids obtained on days 8–14 were dissociated into single cells as described above and re-suspended with DMEM (Nacalai Tesque, Inc., Kyoto, Japan) containing FBS (10%, MP Biomedicals, Santa Ana, CA). The re-suspended cells were plated onto fibronectin-coated EZVIEW 96-well glass bottom plates LB (AGC Techno Glass) and cultured for one day. The cells were then fixed with 4% paraformaldehyde for 15 min at room temperature. To stain for the cardiomyocyte markers CTNT and NKX 2–5, the cells were treated with the Human Cardiomyocyte Immunocytochemistry Kit (A25973; Thermo Fisher Scientific). To stain for the other markers, the cells were permeabilized with 0.1% Triton X-100 and 3% BSA in DPBS. The cells were then blocked with DPBS blocking buffer containing 3% BSA and were stained with primary antibodies in the blocking buffer overnight at 4 °C. The following primary antibodies were used for the immunostaining process: anti-α-actinin (sarcomeric, 1:800; Sigma-Aldrich) and anti-vimentin (1:200; Thermo Fisher Scientific). The cells were washed 3 times with DPBS for 2 min, and then further stained with the fluorescence-labeled secondary antibodies anti-rabbit IgG Alexa Fluor 488 (Abcam, Cambridge, UK) and anti-mouse IgG Alexa Fluor 555 (Abcam) in blocking buffer. The immunofluorescent-stained cells were washed again as described above. Cell nuclei were counterstained using DAPI (Thermo Fisher Scientific). Stained cells were analyzed using the EVOS FL auto fluorescence microscope (Thermo Fisher Scientific).
2.6. Statistical analysis
Quantitative data are presented as means ± SD (unless indicated otherwise).
3. Results
3.1. Differentiation of cardiac mesoderm cells from hiPSC spheroids using microfabricated EZSPHERE vessels
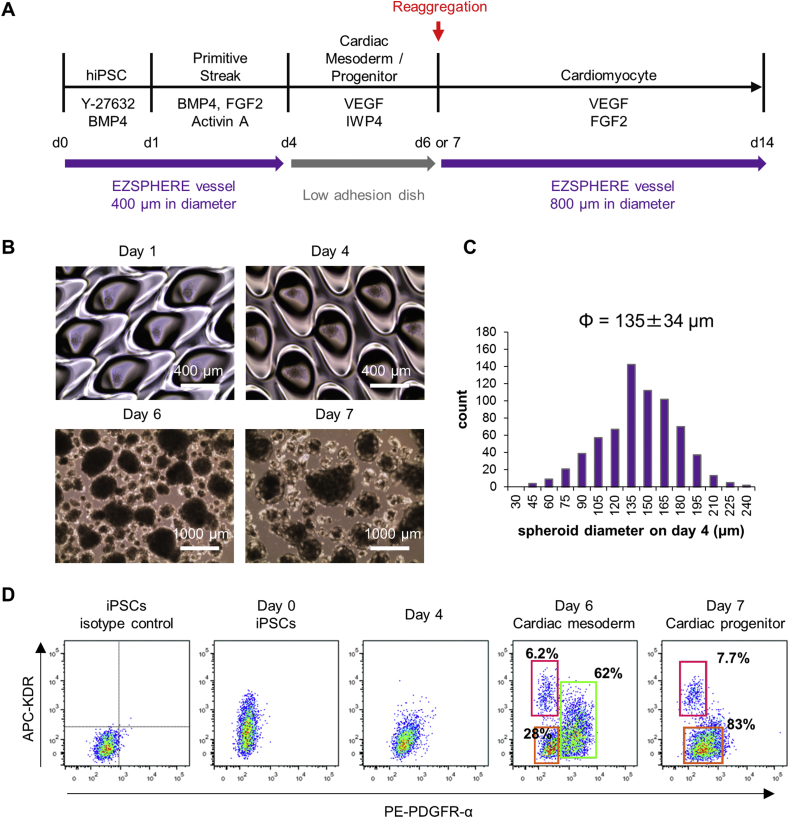
To combine the high-density spheroid formation and cultivation method of hiPSCs using EZSPHERE vessels with Yang's well-known protocol for embryonic body (EB)/spheroid-based cardiac differentiation [4], 3 × 106 single cell, dissociated hiPSCs (of the 253G1 cell line [14]) were seeded into a standard 100 mm EZSPHERE dish (#900, with approximately 14,000 individual micro-wells 400–500 μm in diameter and approximately 100 μm in depth) in EB formation medium (Fig. 1A). Initial cell number in each spheroid was calculated to be 200–220 cells. Twenty-four hours after seeding, uniform spheroids were observed in each micro-well. Activin A, BMP4, and FGF2 were then added into the EZSPHERE vessels to induce differentiation into a primitive streak-like population (Fig. 1B). On day 4, to completely exclude activin A from the culture medium, the spheroids were harvested from the vessels, washed, and then suspended in differentiation medium containing the WNT inhibitors IWP-4 and VEGF. The spheroid suspension was then transferred to a flat, low-adhesion dish, where spheroid size (average diameter) was measured as 135 ± 34 μm with a Gaussian distribution (Fig. 1C). However, the sizes varied during the subsequent culture process due to fusion of the spheroids to one another (Fig. 1B). After 4–7 days of culture, the obtained spheroids were dissociated into single cells and analyzed by flow cytometry to detect the markers PDGFR-α and KDR; double-positive cells are known to be cardiac mesoderm cells [15,16]. The results showed that 62% of the cells were PDGFR-α+/KDRlow, 28% PDGFR-α-/KDR−, and 6.2% PDGFR-α-/KDRhigh on day 6, while only PDGFR-α- and KDR− cells were detected on day 4 (Fig. 1D). On day 7, 83% of cells were PDGFR-αlow/KDR− and 7.7% PDGFR-α-/KDRhigh. The cell viability was 88% and 76% on day 6 and day 7 respectively. Therefore, the main populations present on days 6 and 7 were considered cardiac mesoderm-like and cardiac progenitor-like cells, respectively.
Fig. 1.
Cardiac mesoderm/progenitor differentiation from hiPSCs using microfabricated EZSPHERE vessels. (A) Schematic outline of the procedure used to differentiate hiPSCs to cardiomyocytes. (B) Phase-contrast microscopy images of spheroids obtained on days 1, 4, 6, and 7. (C) Size distribution of spheroids obtained on the microfabricated EZSPHERE vessels on day 4. (D) Flow cytometric analysis of the markers KDR and PDGFR-α for detection of the cardiac mesoderm-like cell population on days 0–7.
3.2. Enrichment of cardiomyocytes by reaggregation of cardiac mesoderm/progenitor spheroids
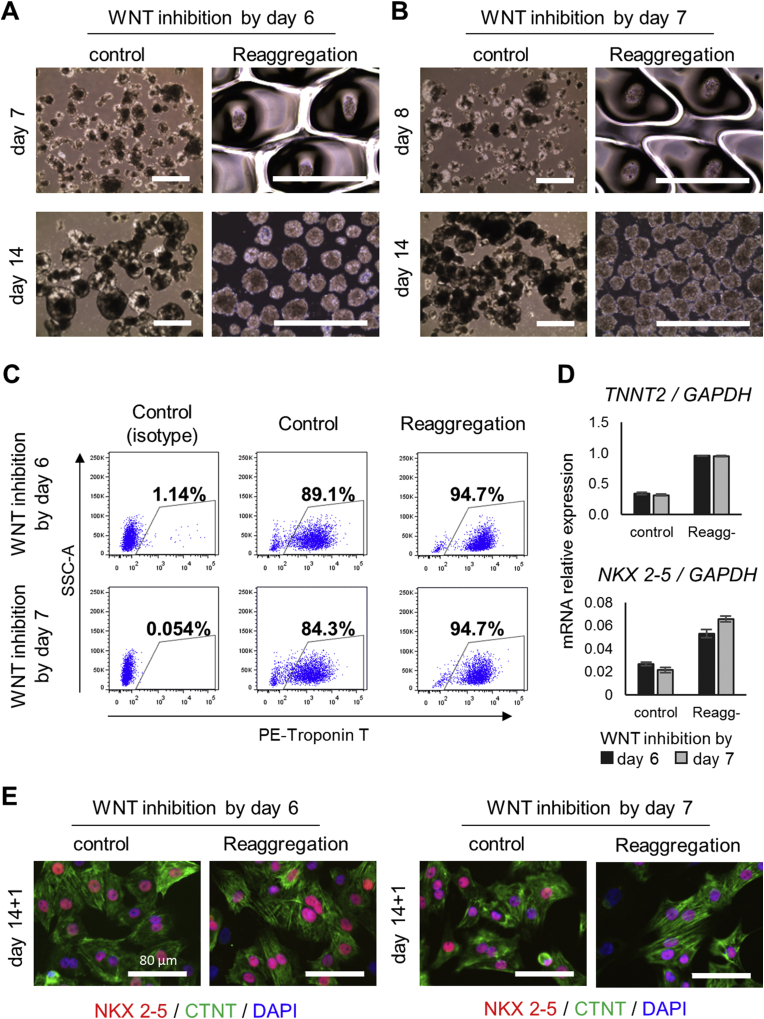
To induce cardiac differentiation by the general method, cardiac progenitor spheroids were cultured in differentiation medium containing VEGF and FGF2 from day 7 (Fig. 2B). On day 14, the spheroids were harvested and analyzed for expression of the cardiac isoform of troponin T (CTNT) by flow cytometry or re-seeded on a fibronectin-coated plate for immunofluorescence staining. 84.3% of the cells, were CTNT+ cardiomyocytes (Fig. 2C). Immunofluorescence staining revealed that most cells expressed both CTNT and NKX 2–5, and the well-characterized sarcomere structure of cardiomyocytes was observed (Fig. 2E).
Fig. 2.
Cardiomyocyte enrichment by reaggregation of cardiac progenitor spheroids on day 7. (A, B) Phase-contrast microscopy images of spheroids treated with or without reaggregation on day 6 (A) and day 7 (B). Scale bar indicates 1000 μm. (C) Flow cytometric analysis of the CTNT + cardiomyocyte population in each type of spheroids obtained on day 14. (D) qRT-PCR analysis of the cardiac markers TNNT2 (gene coding for CTNT) and NKX 2–5 in each type of spheroid obtained on day 14. (E) Fluorescence microscopy of immunostaining for cardiomyocyte markers CTNT and NKX 2–5 in spheroids harvested and reseeded on day 14 (CTNT: green, NKX 2–5: red, nuclei/DAPI: blue).
To determine whether reaggregation of cardiac progenitor cells promotes cardiac differentiation, the spheroids obtained on day 7, note that reaggregation is on day 7, were dissociated into single cells and seeded into a 35 mm EZSPHERE dish with larger pores (#903, containing approximately 1000 individual micro-wells 800 μm in diameter and 400 μm in depth) at a density of 1000 cells/micro-well in differentiation medium containing VEGF and FGF2. The next day, uniform spheroids approximately 150 μm in diameter had formed in each micro-well (Fig. 2B). On day 14, the spheroids were collected from the EZSPHERE and found to be uniformly sized and almost all were beating (Figs. 2B and S1 Movie). The harvested spheroids were then analyzed by flow cytometry and immunofluorescence as described above. 94.7% of the cells from the reaggregated spheroids were CTNT+ cardiomyocytes, 11% more than in non-reaggregated control spheroids (Fig. 2C). In addition, qRT-PCR analysis of reaggregated and control spheroids obtained on day 14 revealed that the mRNA expression levels of both TNNT2 (the gene coding for CTNT) and NKX2-5 in the reaggregated spheroids were more than double those in control spheroids (Fig. 2D). These findings suggest that reaggregation of the cardiac progenitor cells improved hiPSC-CM purity and probably maturation supported by the changes in the expression levels of cardiac-related genes. Although immunofluorescence staining revealed that most cells expressed both CTNT and NKX 2–5 and exhibited slightly clearer cardiac-specific sarcomere structure than did the control cells (Fig. 2E).
Supplementary video related to this article can be found at https://doi.org/10.1016/j.reth.2020.04.008
The following are the supplementary data related to this article:
Movie. Beating of reaggregated spheroid shown in Fig 2B (1000 cells/micro-well).
Similarly, on day 6 spheroids were dissociated into single cells and were re-seeded onto EZSPHERE dishes to reaggregate (Fig. 2A). On day 14, the reaggregated spheroids were 95% CTNT+ cardiomyocytes (Fig. 2C). In contrast, in the non-reaggregated control group, in which only the cardiac differentiation medium was replaced on day 6, only 89% of the cells were CTNT+. Moreover, TNNT2 and NKX2-5 expression levels in the reaggregated spheroids were approximately double those in control spheroids (Fig. 2D). Even in the case of reaggregation on day 6, cardiomyocyte purity and cardiomyocyte related mRNA expression was improved as in the case where reaggregation performed on day 7, and no difference in effect was observed.
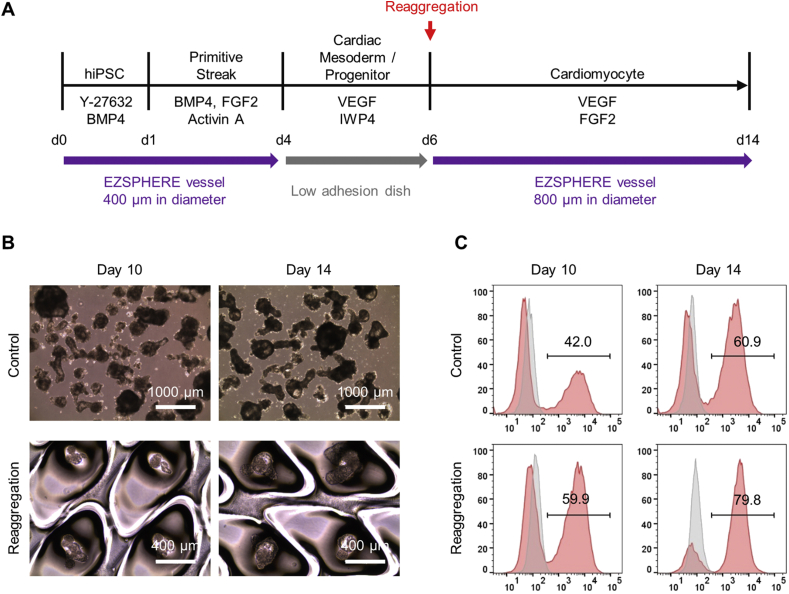
We then tested the reaggregation protocol using the 201B7 hiPSC line [17] (S1A and S1B Fig.). Flow cytometry specific to CTNT revealed 60.9% and 79.8% cardiomyocyte populations in the non-reaggregated and reaggregated cells, respectively, indicated a purity improvement of approximately 31% by the result of reaggregation on day 6 S1C Fig.(S1C Fig.). These results suggest that the reaggregation of cardiac mesoderm/progenitor-like cells robustly improves cardiomyocyte purity and probably maturation.
3.3. Reaggregation after WNT inhibition promotes expression of cardiac-related genes
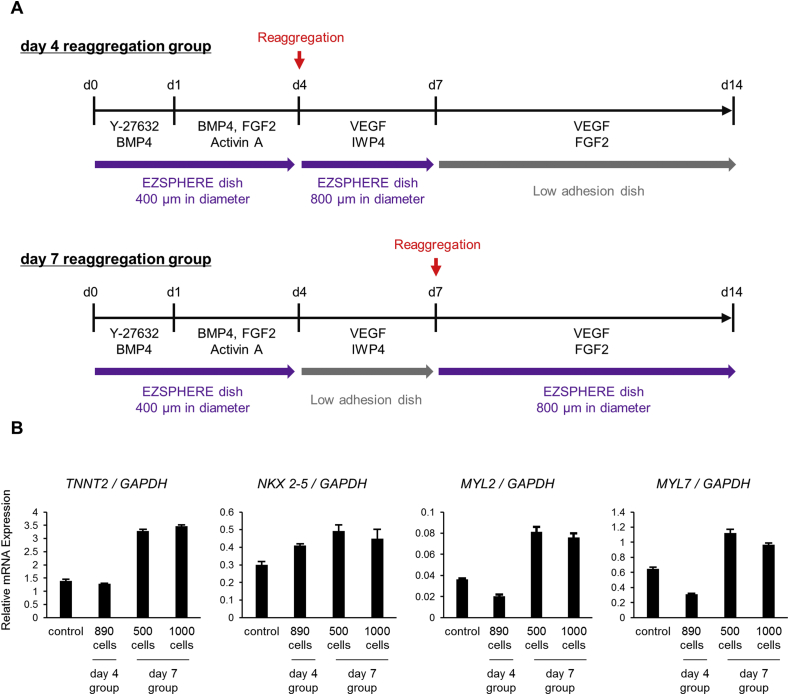
Next, we examined in detail how cardiac differentiation is affected by the order and timing of WNT inhibitor treatment, single cell dissociation, and reaggregation. We compared reaggregation on day 4 (before IWP-4 treatment) and day 7 (after treatment) (S2A Fig.). In the day 4 reaggregation group, on day 4, before the cells were treated with IWP-4, the spheroids were dissociated into single cells and allowed to reaggregate. After 3 days of WNT inhibition, IWP-4 was removed from the culture medium without spheroid reaggregation. On the other hand, in the day 7 reaggregation group, IWP-4 was added to spheroids without reaggregation on day 4. After 3 days of WNT inhibition, dissociation and reaggregation were performed. A control group subjected to neither dissociation nor reaggregation was also maintained. Expression levels of cardiac-related genes were analyzed in all groups. The expression levels of cardiomyocyte marker TNNT2, cardiomyocyte maturation/ventricle-specific marker MYL2, and cardiomyocyte maturation/atrium-specific marker MYL7 were approximately 2–4 times higher in the day 7 reaggregation group than in the day 4 reaggregation group (S2B Fig.). In addition, the day 4 reaggregation group had almost the same or lower TNNT2, MYL2, and MYL7 expression levels as did the control group. This suggests that after WNT inhibition, disassociation of the spheroids and reaggregation on day 7 is an effective way to promote expression of cardiac-related genes.
3.4. Importance of number of cells seeded and spheroid size during reaggregation to expression of cardiac-related genes
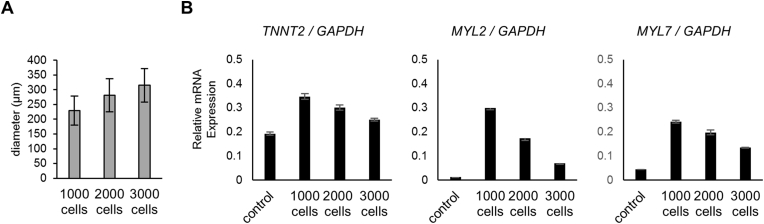
We next investigated whether cardiac-related gene expression is influenced by the number and density of cells seeded and the size of the reformed spheroids. After being treated with IWP-4 from days 4–7, the obtained spheroids were dissociated into single cells, and then seeded onto EZSPHERE dishes at 1000, 2000, or 3000 cells/micro-well for reaggregation at seeding densities of 0.33 × 106, 0.67 × 106, and 1 × 106 cells/mL, respectively. A group that received no dissociation or reaggregation on day 7 was used as a control. The cells were cultured under cardiac differentiation conditions from day 7. To further maturate, the cardiac spheroids differentiation were extended until day 18. As expected, when more cells were seeded for reaggregation, spheroid size was larger (Fig. 3A). Quantitative RT-PCR analysis revealed that a seeding density of 1000 cells/micro-well resulted in the highest MYL2 and MYL7 expression levels, approximately 20 and 5 times higher, respectively, than levels in the non-reaggregated controls. A seeding density of 3000 cells/micro-well resulted in the lowest MYL2 and MYL7 expression levels (Fig. 3B). Although, comparing a seeding density of 500 and 1000 cells/micro-well, there was no difference in MYL2 and MYL7 expression levels on day 14 (S2B Fig.). These results suggest that maturation during cardiac differentiation is promoted in smaller spheroids than a certain size. The TNNT2 expression level was also highest when seeding density was 1000 cells/micro-well; however, the differences were not as drastic as they were in MYL2 and MYL7 levels.
Fig. 3.
Effects of spheroid size and cell seeding density on cardiomyocyte enrichment. (A) Histogram of re-seeding cell density-dependent average size of reaggregated cardio spheroids on day 18. Each type of cardiac spheroid was re-seeded onto EZSPHERE vessels on day 7 at cell seeding densities of 1,000, 2,000, and 3000 cells/micro-well. (B) qRT-PCR analysis of spheroid size effects on cardiomyocyte-related gene expression of TNNT2, MYL2, and MYL7 after the reaggregation process.
3.5. Promotion of non-canonical WNT signaling by cardiac mesoderm cell reaggregation
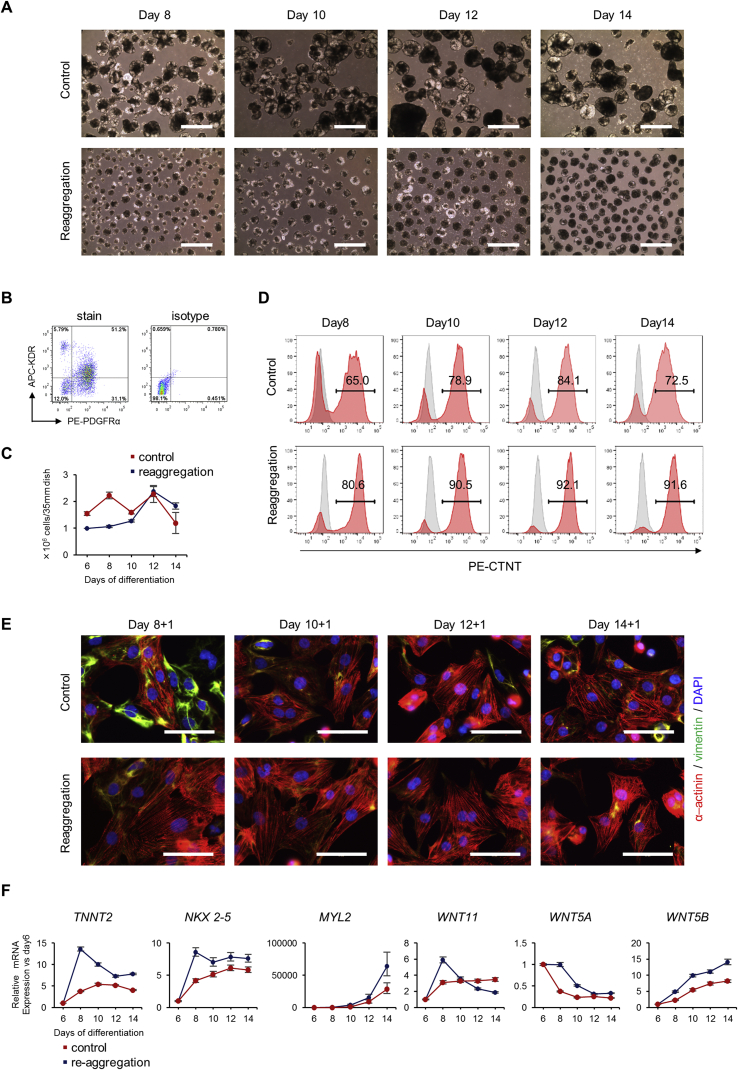
In cardiac differentiation, the expression of non-canonical WNT signal-related genes, such as WNT5A, WNT5B, and WNT11, increases after WNT inhibition [12]. Therefore, we investigated whether reaggregation of cardiac mesoderm-like spheroids changes non-canonical WNT signaling behavior over time. On day 6, cardiac mesoderm-like spheroids were dissociated into single cells and allowed to reaggregate at a seeding cell density of 1000 cells per micro-well (Fig. 4A and B). Every two days, the change in the population of CTNT+ cardiomyocytes, the internal structure of the cardiomyocytes, and the expression levels of several genes related to cardiomyocytes and non-canonical WNT signaling were analyzed by flow cytometry, immunofluorescence staining, and qRT-PCR, respectively. A control group not subjected to reaggregation on day 6 was also analyzed. The dissociated cell counts in the reaggregated spheroids gradually increased from days 6–12, suggesting that the cells were proliferating continuously (Fig. 4C).
Fig. 4.
Morphology of reaggregated cardiac mesoderm spheroids and changes in expression levels of cardiomyocyte-related genes and WNT signaling-related factors over time. (A, D, E, and F) Changes in spheroid morphology and marker expression levels observed every two days in cardiac mesoderm spheroids reaggregated on day 6 at cell seeding density of 1,000 cells/micro-well. The control spheroids did not undergo reaggregation on day 6; only the culture medium was replaced. (A) Phase-contrast microscopy images of cardiac mesoderm spheroids on days 8–14. Scale bar = 1,000 μm. (B) Flow cytometric analysis of the markers KDR and PDGFR-α for detection of the cardiac mesoderm-like population of the dissociated single cells on day 6 just before reaggregation. (C) Time course of cell counts from day 8 to 14 for reaggregated (on day 6) and non-reaggregated (control) spheroids. (D) Flow cytometric analysis of CTNT+ cardiomyocyte populations from day 8 to 14 to compare differences between reaggregation (on day 6) and non-reaggregation (control). (E) Immunofluorescent images of intracellular sarcomere structures of α-actinin (red) and vimentin (green) in cardiomyocytes after re-seeding onto fibronectin-coated plates between day 8 and 14. (F) qRT-PCR analysis of time course (day 6–14) of mRNA levels of several gene factors related to cardiac differentiation and WNT signaling.
In the control group, the population of CTNT+ cardiomyocytes on days 8–14 was 65.0–84.1% (Fig. 4D). In contrast, 80.6–92.1% of cells in the reaggregated group were CTNT+ cardiomyocytes on days 8–14. Immunofluorescence staining for α-actinin and vimentin (a protein that forms mesenchymal cell-specific type-III intermediate filaments) showed that reaggregated cells re-seeded on fibronectin-coated plates on day 8 definitively exhibited α-actinin sarcomere structures (Fig. 4E). Control cells re-seeded on day 8 showed unclear α-actinin sarcomere structures, and half of the cell population was positive for vimentin. Cells re-seeded on days 10–14 in both the control and reaggregation groups showed fewer vimentin-positive cells and clearer sarcomere structures.
Cardiac-related gene expression was analyzed by qRT-PCR in reaggregated spheroids; TNNT2 and NKX 2–5 expression levels rapidly increased from days 6–8, and then decreased or remained constant from days 8–14 (Fig. 4F). In contrast, TNNT2 and NKX 2–5 expression levels were lower in non-reaggregated spheroids and tended to gradually increase from days 6–14. Expression levels of MYL2, a mature ventricle cardiomyocyte marker, were steady from days 6–10, and then increased from days 10–14. By day 14, MYL2 expression levels in reaggregated spheroids were twice those in non-reaggregated spheroids.
qRT-PCR analysis of several WNT genes related to non-canonical WNT signaling showed that WNT11 expression levels spheroids rapidly increased in reaggregated spheroids from days 6–8, and then decreased or remained constant from days 8–14, as observed for TNNT2 and NKX 2–5 (Fig. 4F). In contrast, WNT11 expression levels increased in non-reaggregated spheroids from days 6–8 until they were equal to approximately half the levels in reaggregated spheroids, and then remained constant from days 8–14. WNT5A expression levels in reaggregated spheroids remained constant from day 6 to day 8, then decreased from day 8 to day 12. However, WNT5A expression levels in non-reaggregated spheroids decreased from day 6 to day 10. WNT5B expression levels continuously increased at approximately the same rate from day 6 to day 14. WNT5B expression levels in reaggregated spheroids were approximately 1.5 times greater than those in non-reaggregated spheroids.
These results suggest that single-cell dissociation and spheroid reaggregation after treatment with a WNT inhibitor promote cardiac differentiation and maturation. In addition, expression levels were elevated not only in cardiomyocyte-related genes (TNNT2, NKX 2–5, MYL2), but also in genes associated with non-canonical WNT signaling and heart development (WNT5A, WNT5B, WNT11).
4. Discussion
Aiming for a more efficient and robust protocol, we developed a technique that promotes cardiac differentiation by forming a large number of uniformly sized spheroids in microfabricated EZSPHERE vessels, dissociating the cardiac mesoderm/progenitor-like spheroids into single cells, and then allowing them to reaggregate.
Based on general embryonic body/spheroid-based cardiac differentiation protocols, we first generated uniform spheroids from hiPSCs using microfabricated EZSPHERE vessels. The spheroids were then treated with activin A, BMP-4, and FGF2. WNT inhibition then induced differentiation into cardiac mesoderm/progenitor-like cells. After temporarily dissociating the obtained spheroids into single cells, we seeded them into EZSPHERE vessels again to allow reaggregation into uniformly sized spheroids. This reaggregation in the middle of the cardiac differentiation process improved the cardiomyocyte population compared to that in a control group in which dissociation and reaggregation were not performed. Flow cytometric analysis using the cardiac mesoderm makers KDR and PDGFR-α shows different patterning at days 6 and 7 (Fig. 1D). However, by this reaggregation process either on day 6 and 7 we succeeded in generating uniformly sized cardiac spheroids with an up to 95% CTNT+ cardiomyocyte population (Fig. 2C). This high cardiomyocyte purity was only observed when dissociation and reaggregation were performed after treatment with the WNT inhibitor IWP-4 for 2–3 days (S1 Fig.), suggesting the importance of performing the reaggregation after the WNT inhibition process.
Another study reported single-cell dissociation and reaggregation during cardiac differentiation in pluripotent stem cells, such as ES/iPS cells, but performed reaggregation before WNT inhibition [19]. However, the present study verified that dissociation and reaggregate after WNT inhibition is important for strongly elevating the expression of the cardiomyocyte maturation markers MYL2 and MYL7 and increasing differentiation efficiency (S2 Fig.). Furthermore, we found that the number of cells seeded affected MYL2 and MYL7 expression levels, with the highest expression levels achieved using 1000 cells/micro-well and the lowest levels using 3000 cells/micro-well (Fig. 3A and B). This suggests that cardiomyocyte maturation is promoted when cardiac mesoderm/progenitor-like spheroids are dissociated into single cells and then allowed to reaggregate and continue differentiation as relatively small spheroids at an adequate density. To identify the molecular mechanism of the efficient maturation in small cardiac spheroids, detail analysis of the difference between inside and outside of spheroids is needed. Because, the differences of oxygen gradients, glucose distribution, lactate accumulation, and proliferation rate between outside and inside of spheroids should be less in small spheroids. These conditions might contribute the maturation of small cardiac spheroids.
Activation of non-canonical WNT signaling is important to maturation of cardiomyocytes from cardiac mesoderm/progenitor cells [12]. Therefore, after dissociating cardiac mesoderm/progenitor -like spheroids into single cells and allowing them to reaggregate, we analyzed the cardiomyocyte population and expression levels of several genes associated with cardiac function and the non-canonical WNT signaling pathway over time and compared the results with those obtained using non-reaggregated control spheroids. The percentage of CTNT+ cardiomyocytes increased more quickly in reaggregated than in non-reaggregated spheroids (Fig. 4D). Furthermore, two days after reaggregation, there was a sharp rise in the expression levels of the cardiac-related genes TNNT2 and NKX 2–5, which are expressed at the beginning of heart development, compared to levels in non-reaggregated spheroids. After reaggregation, expression levels of the cardiomyocyte maturation marker MYL2 increased exponentially and were approximately those in non-reaggregated controls. As seen with TNNT2 and NKX 2–5, WNT11 expression also increased sharply two days after reaggregation. WNT11 is essential for Xenopus heart development and is also known for its role in generating myocardial electrical gradient patterns through the activation of non-canonical Wnt/Ca2+ signaling during zebrafish heart development and regulation of L-type Ca2+ signaling [20,21]. Furthermore, WNT5A expression levels in non-reaggregated control spheroids immediately decreased, whereas WNT5A expression levels in reaggregated spheroids remained constant for two days. In mice, WNT5A and WNT11 are both essential to the generation of second heart field progenitor cells [22]. WNT5B expression levels continuously increased at approximately the same rate after reaggregation. The role of WNT5B is not well understood, but it is expressed in both embryonic and adult mice [23,24]. From these results, it might be presumed that the promotion of cardiomyocyte purification and maturation by single cell dissociation and reaggregation of cardiac mesoderm spheroids is connected to the promotion of non-canonical WNT pathway gene expression. However, there are only qPCR date to address this point, further study like protein levels analysis or experiments using specific inhibitors are needed to elucidate the mechanism of our speculation.
It has been reported that hPSC-CMs exclusively bind and reaggregate with one another via the cell adherent factor NCAM-1, dissociating terminally differentiated cardiomyocytes into single cells and re-seeding them onto microfabricated vessels, thereby promoting their purification [8]. From this, it might be possible that dissociation and reaggregation spheroids led to preferential aggregation of cardiac mesoderm/progenitor cells under specific conditions via a specific adhesion molecule, thereby promoting selection and purification of the cardiomyocytes. In addition, the expression levels of WNT5A and WNT11 have been shown to change depending on spheroid (or EB) size in mouse ES cell aggregates [10,11]. Hence, it is also possible that due to reaggregation of spheroids following single-cell dissociation, spheroid size and cell density were temporarily reset and homogenized, thereby maintaining and increasing uniform expression of WNT5A and WNT11 in each spheroid and promoting cardiac differentiation. In our study, however, we were unable to conclusively determine whether cardiac mesoderm/progenitor cell reaggregation or the regulation of spheroid size or cell density is the critical factor responsible for efficient cardiac differentiation, and we suspect that a synergistic effect may exist. Another possibility is that dissociation and reaggregation of the spheroids mixed and uniformly redistributed cells in different differentiation states into each micro-well to reform homogenized spheroids, which may have been related to total cardiac differentiation efficiency. Flow cytometry for KDR and PDGFR-α antigens of the cardiac mesoderm on day 6 demonstrated that the total cell population was already divided into three groups [16], which may have been heterogeneously distributed in each spheroid before reaggregation (Fig. 1D). The three different cell populations would have been equally redistributed into each reformed spheroid after reaggregation, regenerating homogeneous spheroids. As embryos proceed through development in vivo, various cells affect one another in a spatiotemporal manner. For example, cells inside reaggregated spheroids secreting WNT11 in the early stage of differentiation presumably act as organizers and stimulate the surrounding cells to differentiate into cardiomyocytes. However, to verify this assumption, further research analyzing the distribution and transcription/secretion profiles of cells within the inner structure of reaggregated spheroids will be necessary. Furthermore, we cannot completely eliminate the possibility that the effects of reaggregation could be simply attributed to the washing away of IWP-4 from the cells.
As mentioned above, WNT11 expression levels change depending on spheroid size and cell seeding density. However, cell proliferation (about 2–3 fold growth) during the cardiac differentiation process was observed in the present study, meaning that some constant changes in culture/differentiation conditions occurred. Cardiac differentiation from hPSCs via spheroid/EB formation using bioreactors has great potential in large-scale production; however, wide variations in cell density and cardiomyocyte purity between experiments still need to be resolved [5]. Our method overcomes these limitations; when the non-canonical WNT signal is required (after WNT inhibition), the cells are dissociated and reaggregated using the EZSPHERE vessels, whereby spheroid size and cell density resetting is expected to contribute to the robustness of the cardiac differentiation protocol. Additionally, it has been reported that mesenchymal stem cells (MSCs) can be differentiated into cardiomyocytes by WNT inhibition in the early stages of differentiation [25]. Therefore, in addition to hPSCs, this reaggregation-based cardiac differentiation method could be also applied to MSCs, and further investigation in this direction is warranted.
5. Conclusions
In summary, the microfabric vessel EZSPHERE-based 3D culture system is found to be very useful for high efficient differentiation of the cardiomyocyte. It was found in the cardiomyocyte differentiation process performed on the EZSPHERE that reaggregation of the induced cardiac mesoderm/progenitor is effective for producing cardiomyocytes with high purity and maturation levels.
Declaration of Competing Interest
The authors declare no conflicts of interest associated with this manuscript.
Acknowledgements
We thank Dr. Mayumi Okamoto for her technical assistance. A part of this work was supported by the “Network Program for Realization of Regenerative Medicine” from the Japan Agency for Medical Research and Development (AMED).
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.reth.2020.04.008.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
S1 Fig.
Cardiac mesoderm/progenitor cell differentiation from hiPSC line 201B7 using microfabricated EZSPHERE vessels. (A) Schematic outline of the procedure used to differentiate hiPSC 201B7 cells to cardiomyocytes. (B) Phase-contrast microscopy images of spheroids obtained on days 10 and 14. There was no reaggregation (1000 cells/microwell re-seeding onto EZSPHERE vessels) performed for the control group on day 6; only the culture medium was replaced. (C) Flow cytometric analysis of CTNT+ cardiomyocyte population detected in reaggregated (on day 6) and non-reaggregated spheroids on days 10 and 14.
S2 Fig.
Changes in cardiomyocyte-related marker expression due to reaggregation after treatment with a WNT inhibitor. (A) Spheroids obtained before (on day 4) or after (on day 7) treatment with a WNT inhibitor in the cardiac differentiation process were dissociated into single cells and allowed to reaggregate at a density of 500, 890, or 1000 cells/micro-well. (B) The expression levels of cardiomyocyte markers (TNNT2, NKX 2-5), cardiomyocyte maturation/atrium marker (MYL7), and cardiomyocyte maturation/ventricle marker (MYL2) were analyzed by qRT-PCR in the resultant spheroids on day 14. The control was not subjected to reaggregation.
References
- 1.Shiba Y., Gomibuchi T., Seto T., Wada Y., Ichimura H., Tanaka Y. Allogeneic transplantation of iPS cell-derived cardiomyocytes regenerates primate hearts. Nature. 2016;538:388–391. doi: 10.1038/nature19815. [DOI] [PubMed] [Google Scholar]
- 2.Yamamoto W., Asakura K., Ando H., Taniguchi T., Ojima A., Uda T. Electrophysiological characteristics of human iPSC-derived cardiomyocytes for the assessment of drug-induced proarrhythmic potential. PloS One. 2016;11 doi: 10.1371/journal.pone.0167348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loh K.M., Chen A., Koh P.W., Deng T.Z., Sinha R., Tsai J.M. Mapping the pairwise choices leading from pluripotency to human bone, heart, and other mesoderm cell types. Cell. 2016;166:451–467. doi: 10.1016/j.cell.2016.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang L., Soonpaa M.H., Adler E.D., Roepke T.K., Kattman S.J., Kennedy M. Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population. Nature. 2008;453:524–528. doi: 10.1038/nature06894. [DOI] [PubMed] [Google Scholar]
- 5.Kempf H., Olmer R., Kropp C., Rückert M., Jara-Avaca M., Robles-Diaz D. Controlling expansion and cardiomyogenic differentiation of human pluripotent stem cells in scalable suspension culture. Stem Cell Rep. 2014;3:1132–1146. doi: 10.1016/j.stemcr.2014.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kempf H., Kropp C., Olmer R., Martin U., Zweigerdt R. Cardiac differentiation of human pluripotent stem cells in scalable suspension culture. Nat Protoc. 2015;10:1345–1361. doi: 10.1038/nprot.2015.089. [DOI] [PubMed] [Google Scholar]
- 7.Pal R., Mamidi M.K., Das A.K., Bhonde R. Comparative analysis of cardiomyocyte differentiation from human embryonic stem cells under 3-D and 2-D culture conditions. J Biosci Bioeng. 2013;115:200–206. doi: 10.1016/j.jbiosc.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen D.C., Hookway T.A., Wu Q., Jha R., Preininger M.K., Chen X. Microscale generation of cardiospheres promotes robust enrichment of cardiomyocytes derived from human pluripotent stem cells. Stem Cell Rep. 2014;3:260–268. doi: 10.1016/j.stemcr.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ng E.S., Davis R., Stanley E.G., Elefanty A.G. A protocol describing the use of a recombinant protein-based, animal product-free medium (APEL) for human embryonic stem cell differentiation as spin embryoid bodies. Nat Protoc. 2008;3:768–776. doi: 10.1038/nprot.2008.42. [DOI] [PubMed] [Google Scholar]
- 10.Hwang Y.S., Chung B.G., Ortmann D., Hattori N., Moeller H.C., Khademhosseini A. Microwell-mediated control of embryoid body size regulates embryonic stem cell fate via differential expression of WNT5a and WNT11. Proc Natl Acad Sci U S A. 2009;106:16978–16983. doi: 10.1073/pnas.0905550106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen M., Qian C., Bi L.L., Zhao F., Zhang G.Y., Wang Z.Q. Enrichment of cardiac differentiation by a large starting number of embryonic stem cells in embryoid bodies is mediated by the Wnt11-JNK pathway. Biotechnol Lett. 2015;37:475–481. doi: 10.1007/s10529-014-1700-5. [DOI] [PubMed] [Google Scholar]
- 12.Mazzotta S., Neves C., Bonner R.J., Bernardo A.S., Docherty K., Hoppler S. Distinctive roles of canonical and noncanonical Wnt signaling in human embryonic cardiomyocyte development. Stem Cell Rep. 2016;7:764–776. doi: 10.1016/j.stemcr.2016.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sato H., Idiris A., Miwa T., Kumagai H. Microfabric vessels for embryoid body formation and rapid differentiation of pluripotent stem cells. Sci Rep. 2016;6:31063. doi: 10.1038/srep31063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakagawa M., Koyanagi M., Tanabe K., Takahashi K., Ichisaka T., Aoi T. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol. 2008;26:101–106. doi: 10.1038/nbt1374. [DOI] [PubMed] [Google Scholar]
- 15.Kattman S.J., Witty A.D., Gagliardi M., Dubois N.C., Niapour M., Hotta A. Stage-specific optimization of activin/nodal and BMP signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines. Cell stem cell. 2011;8:228–240. doi: 10.1016/j.stem.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 16.Birket M.J., Ribeiro M.C., Verkerk A.O., Ward D., Leitoguinho A.R., den Hartogh S.C. Expansion and patterning of cardiovascular progenitors derived from human pluripotent stem cells. Nat Biotechnol. 2015;33:970–979. doi: 10.1038/nbt.3271. [DOI] [PubMed] [Google Scholar]
- 17.Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 19.Funakoshi S., Miki K., Takaki T., Okubo C., Hatani T., Chonabayashi K. Enhanced engraftment, proliferation, and therapeutic potential in heart using optimized human iPSC-derived cardiomyocytes. Sci Rep. 2016;6:19111. doi: 10.1038/srep19111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pandur P., Lasche M., Eisenberg L.M., Kuhl M. Wnt-11 activation of a non-canonical Wnt signalling pathway is required for cardiogenesis. Nature. 2002;418:636–641. doi: 10.1038/nature00921. [DOI] [PubMed] [Google Scholar]
- 21.Panakova D., Werdich A.A., Macrae C.A. Wnt11 patterns a myocardial electrical gradient through regulation of the L-type Ca(2+) channel. Nature. 2010;466:874–878. doi: 10.1038/nature09249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cohen E.D., Miller M.F., Wang Z., Moon R.T., Morrisey E.E. Wnt5a and Wnt11 are essential for second heart field progenitor development. Development. 2012;139:1931–1940. doi: 10.1242/dev.069377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gavin B.J., McMahon J.A., McMahon A.P. Expression of multiple novel Wnt-1/int-1-related genes during fetal and adult mouse development. Genes Dev. 1990;4:2319–2332. doi: 10.1101/gad.4.12b.2319. [DOI] [PubMed] [Google Scholar]
- 24.Yamaguchi T.P. Genetics of Wnt signaling during early mammalian development. Methods Mol Biol. 2008;468:287–305. doi: 10.1007/978-1-59745-249-6_23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mansson-Broberg A., Rodin S., Bulatovic I., Ibarra C., Löfling M., Genead R. Wnt/beta-Catenin stimulation and laminins support cardiovascular cell progenitor expansion from human fetal cardiac mesenchymal stromal cells. Stem Cell Rep. 2016;6:607–617. doi: 10.1016/j.stemcr.2016.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Movie. Beating of reaggregated spheroid shown in Fig 2B (1000 cells/micro-well).