Abstract
The data presented here are related to the research article entitled “Differential expression of the angiotensin-(1-12) [Ang-(1-12)]/chymase axis in human atrial tissue [1]. We have showed that chymase gene transcripts, chymase activity, and immunoreactive- Ang-(1-12) expression levels were higher in left compared to right atrial tissue, irrespective of cardiac disease. This article presents the echocardiographic characteristics of 111 patients undergoing heart surgery for the correction of valvular heart disease, resistant atrial fibrillation or ischemic heart disease. Left atrial chymase mRNA expression and activity, and left atrial Ang-(1-12) levels were compared between patients with stroke vs. non-stroke, congestive heart failure vs. non-heart failure, and in cardiac surgery patients who had a history of postoperative atrial fibrillation vs. non-atrial fibrillation.
Keywords: chymase, angiotensin-(1-12), angiotensin II, human, heart disease, atria
Specifications table
| Subject | Cardiology and Cardiovascular Medicine |
| Specific subject area | Physiology, Molecular biology |
| Type of data | Table and Figure |
| How data were acquired | Preoperative transthoracic echocardiograms were performed in patients using a 1-5 MHz phased array transducer (Philips S5-1) and Philips iE33 sector scanner (Philips Medical Systems, Andover, MA). Real-time PCR by QuantStudio 3 Real-Time PCR Systems (Thermo Fisher Scientific Inc.). Chymase activity was measured by Shimadzu Prominence HPLCs (Model# LC-20AD) using angiotensin-(1-12) as the substrate. Atrial angiotensin-(1-12) was determined using immunohistochemistry assay. |
| Data format | Raw data (Excel file) and Analyzed (Table and Figure) |
| Parameters for data collection | Atrial tissues were collected from patients undergoing heart surgery for the correction of valvular heart disease, resistant atrial fibrillation or ischemic heart disease. |
| Description of data collection | Left atrial appendages were collected during heart surgery, and then snap-frozen in liquid nitrogen and kept in -80°C freezer for future use. The tissues were cut into three pieces on dry ice for chymase mRNA measurement by real-time PCR, chymase activity assay by HPLC, and immunohistochemistry assay for angiotensin-(1-12). |
| Data source location | Wake Forest School of Medicine, Winston-Salem, NC, USA |
| Data accessibility | With the article |
| Related research article | Wang H, Varagic J, Nagata S, Kon ND, Ahmad S, VonCannon JL, Wright KN, Sun X, Deal D, Groban L, Ferrario CM. Differential Expression of the Angiotensin-(1-12)/chymase Axis in Human Atrial Tissue Journal of Surgical Research |
Value of the data
-
•
These data obtained directly from human samples suggests Ang-(1-12)/chymase might be involved in the development of cardiovascular disease.
-
•
The data provide a basis for further investigation of cardiac angiotensin II production through a renin-independent pathway.
-
•
These data has highly significant clinical implications because intracellular chymase-mediated Ang II formation is unaffected by ACE inhibitors or angiotensin II type 1 (AT1) receptor antagonists.
1. Data Description
Table 1 documents the state of cardiac function prior to surgical intervention. Mean left ventricular ejection fraction (LVEF) was preserved across all groups of patients except in those patients who underwent coronary artery bypass graft (CABG) surgery, where it was slightly reduced. When compared to the CABG only group, peak velocities of early mitral blood flow were higher in mitral valve repair groups with and without concomitant CABG. There were no other differences in echocardiographic parameters of left ventricle function or morphology between patients undergoing the different cardiac surgery procedures.
Table 1.
Main Echocardiographic Variables
| Surgical Procedures |
|||||
|---|---|---|---|---|---|
| Variable | Aortic Valve Repair (only) (n = 23) | Mitral Valve Repair (only) (n = 19) | Coronary Artery Bypass Graft + Mitral Valve Repair (n = 6) | Coronary Artery Bypass Graft + Aortic Valve Repair (n = 7) | Coronary Artery Bypass Graft (n = 56) |
| LVEF, % | 56.70 ± 1.25* | 55.89 ± 1.69 | 53.33 ± 1.68 | 55.57 ± 2.65 | 49.19 ± 1.58 |
| LA diameter, cm | 4.15 ± 0.20 | 4.57 ± 0.36 | 5.70 ± 0.71 | 4.04 ± 0.19 | 4.00 ± 0.13 |
| E max, cm/s | 95.69 ± 7.20 | 128.61 ± 12.13* | 120.60 ± 15.00* | 82.92 ± 12.34 | 77.30 ± 3.75 |
| E/A ratio | 1.42 ± 0.25 | 1.89 ± 0.40 | 1.36 ± 0.21 | 1.00 ± 0.12 | 1.28 ± 0.11 |
| IVS diameter, cm | 1.28 ± 0.05 | 1.15 ± 0.07 | 1.06 ± 0.11 | 1.41 ± 0.20 | 1.24 ± 0.04 |
| LVID diastolic, cm | 4.75 ± 0.13 | 4.69 ± 0.25 | 4.83 ± 0.57 | 4.65 ± 0.25 | 4.59 ± 0.12 |
| LVID systolic, cm | 3.34 ± 0.15 | 3.11 ± 0.18 | 3.53 ± 0.67 | 3.15 ± 0.30 | 3.31 ± 0.15 |
| LVPW diameter, cm | 1.45 ± 0.17 | 1.10 ± 0.07 | 1.12 ± 0.08 | 1.32 ± 0.10 | 1.22 ± 0.04 |
Abbreviations are; LVEF, left ventricular ejection fraction; LA, left atrium; RA, right atrium; IVS, Interventricular septum; LVID, left ventricular internal diameter; LVPW, left ventricular posterior wall. * p < 0.05 compared with coronary artery bypass graft.
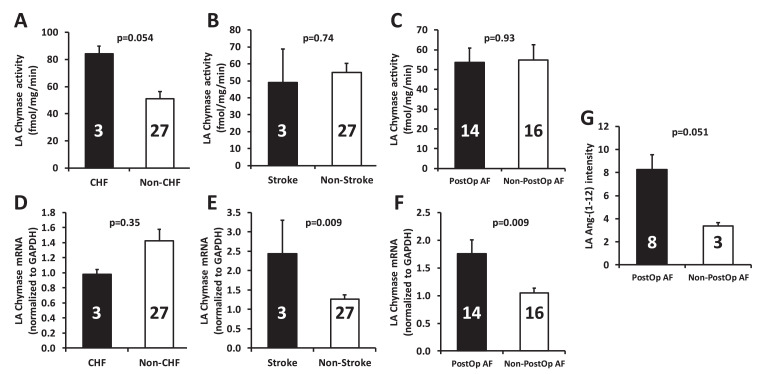
We combined all the male and female patients for the data analysis. As shown in Fig. 1, the left atrial (LA) chymase activity tended to be higher in congestive heart failure (CHF) versus non-CHF patients (p=0.054), while there was no statistical difference at chymase mRNA level between these two groups. In comparison of patients with stroke vs. non-stroke, chymase activity did not differ between these two groups, but LA chymase mRNA level was markedly higher in patients with stroke compared with non-stroke patients (p=0.009). Similarly, although there was no difference of LA chymase activity between patients with history of postoperative atrial fibrillation (PostOp AF) and patients without PostOp AF, LA chymase mRNA was significantly higher in PostOp AF patients vs. non-PostOp AF patients (p=0.009). LA Ang-(1-12) level tended to be higher in the patients with PostOp AF in comparison to non-PostOP AF patients (8.27 ± 1.29 vs. 3.36 ± 0.30, p=0.051).
Fig. 1.
Left atrial (LA) chymase mRNA, activity, and Ang-(1-12) in diseased hearts. (A-C) Comparisons of LA chymase activity in patients with CHF vs. non-CHF, stroke vs. non-stroke, and PostOp AF vs. non PostOp AF. (D-F) Comparisons of LA chymase mRNA levels in patients with CHF vs. non-CHF, stroke vs. non-stroke, and PostOp AF vs. non PostOp AF. (G) LA Ang-(1-12) levels in patients with and without PostOp AF. Numbers in the bars represent the sample size for the corresponding data. Values are means ± SE. Ang-(1-12): angiotensin-(1-12); CHF: congestive heart failure; PostOp AF: postoperative atrial fibrillation.
2. Experimental Design, Materials, and Methods
2.1. Ethic statement
The study was approved by the Wake Forest University Health Sciences (IRB 22619). Atrial appendages were obtained from 111 patients undergoing cardiac surgery at the Wake Forest Baptist Medical Center (Winston-Salem, NC, USA). Left atrial appendages were resected during cardiopulmonary bypass for correction of left cardiac valve replacement [aortic valve replacement (AVR), mitral valve regurgitation (MVR)], or coronary artery bypass grafting (CABG). Preoperative transthoracic echocardiograms and consent forms were obtained prior to cardiac surgery.
2.2. Echocardiography
Preoperative transthoracic echocardiograms were performed by highly trained sonographers using a 1-5 MHz phased array transducer (Philips S5-1) and Philips iE33 sector scanner (Philips Medical Systems, Andover, MA). Digitally-stored images were reviewed and final reports were completed off-line (Xcelera 3.1; Koninklijke Philips Electronics, Amsterdam, The Netherlands) by cardiologists board certified in adult echocardiography. The preoperative transthoracic echocardiograms reported here were the studies most proximate to the patient's surgery. An experienced investigator trained in perioperative echocardiography (LG), who was masked to biochemical and histological findings, manually reviewed all stored images in conjunction with the archived echocardiographic report. Transthoracic echocardiograms were performed and analyzed according to American Society of Echocardiography recommendations [2]. Left ventricular end-diastolic and end-systolic internal diameters (LVID and LVIS, respectively), and end diastolic LV posterior wall (LVPW) and interventricular septal diameters (IVS) and left ventricular end-systolic left atrial diameter measurements were acquired and measured from the parasternal long-axis view using two-dimensional guided M-mode echocardiography by the leading edge-to-leading edge technique. Left ventricular end diastolic and end systolic volumes (EDV and ESV, respectively) and left atrial volume at end ventricular systole were measured by the biplane method of disks (modified Simpson's rule) using apical 4-chamber and apical 2-chamber. Left ventricular ejection fraction was calculated as LVEF (%) = [(EDV-ESV/EDV)] × 100%. Mitral inflow measurements of early and late filling velocities were obtained using pulsed Doppler, with the sample volume placed at the tips of mitral leaflets from an apical four-chamber orientation. The early-to-late filing velocity ratio (E/A) was calculated in those patients who were in sinus rhythm at the time of the examination.
2.3. Ang-(1-12) immunohistochemistry
Human angiotensin-(1-12) was synthetized for us by AnaSpec Inc. (San Jose, CA). Immunohistochemistry was performed using an affinity purified polyclonal antibody directed to the COOH-terminus of the full length of the sequence of human Ang-(1-12) [Asp1-Arg2-Val3-Tyr4-Ile5-His6-Pro7-Phe8-His9-Leu10-Val11-Ile12]. Excised segments of the left and right atrial appendages were immediately immersed in a solution of 4% paraformaldehyde for 24 h and then transferred into 70% ethanol. After dehydration, the tissues were embedded in paraffin and cut into 5 µm thick sections. Slides were warmed for 1 h (55°C), deparaffinized in xylene, and, after being subsequently dipped in serial solutions of ethanol (100%, 95%, 85% and 70%), were rinsed in phosphate buffered saline (PBS). The slides were incubated in an antigen retrieval buffer (Antigen Unmasking Solution H-3300; Vector Laboratories Inc., Burlington, CA) and washed with double distilled water. Slides were then incubated for 5 min in 3% hydrogen peroxide to block the endogenous peroxidase. The sections were blocked with 1% bovine serum in PBS with 5% normal goat serum for 1 h at room temperature and then incubated with the affinity-purified human Ang-(1-12) primary antibody (1:1000 dilution in 1% BS in PBS with 5% normal goat serum) overnight at 4°C. Sections independently treated with 5% normal goat serum in the absence of the primary antibody served as negative controls. Additional controls included sections treated with the primary antibody preincubated with a 20-fold excess of human Ang-(1-12) peptide. In prior studies, we documented that this human Ang-(1-12) antibody does not cross-react with either Ang I or Ang II [3,4]; however, we observed cross reactivity with human angiotensinogen (AGT). Although literature describes low, if any, expression of angiotensinogen in the human heart [5], additional sections were incubated with human angiotensinogen antibody (IBL, MN, 1:50). After incubating with the primary antibody, each section was washed three times in PBS. The sections were blocked with 1% BS in PBS with 5% normal goat serum for 1 h at room temperature and then incubated with biotinylated goat anti-rabbit secondary antibody (1:400 dilutions in 1% bovine serum in PBS with 5% normal goat serum; Vector Laboratories Inc., Burlington, CA) for 3 h. After washing the secondary antibody with PBS, sections were stained with 3,3’-diaminobenzidine (DAB, Sigma-Aldrich Chemical Co. St. Louis, MO) in Tris-buffered saline (0.05 mol/L, pH 7.65), and counterstained with hematoxylin before being dehydrated and mounted. The Ang-(1-12) staining-positive rates were calculated using Image J software (http://imagej.nih.gov/ij/), a public domain Java image processing program developed by the National Institutes of Health.
2.4. Chymase activity assay
Native plasma membranes (PMs) were prepared as described previously [3,4]. Frozen atrial tissue (30-60 mg) was homogenized at 4°C in 1 mL reaction buffer (25 mM HEPES, 125 mM NaCl, and 10 mM ZnCl2, pH 7.4) using a Tissue Lyzer (Qiagen, Inc., Valencia, CA) for 90 seconds at 20 Hz. The homogenate was centrifuged at low spin (200 g) for 1 minute at 4°C to remove the connective tissue and cell debris. The supernatant was transferred into a new tube and centrifuged at 28,000 g for 20 minutes at 4°C. The pellet (native membranes) was resuspended in the reaction buffer and stored at -80°C until assayed for chymase activity.
125I-Ang-(1-12) metabolism by human atrial tissue PMs were studied in the absence or presence of the chymase inhibitor chymostatin. Highly purified human 125I-Ang-(1-12) substrate [1 nmol/L of 125I-Ang-(1-12)] was added to native PMs in the presence of a mixture of enzyme and peptidase inhibitors (chymostatin, lisinopril, MLN-4760, SCH39370, amastatin, bestatin, benzyl succinate and p-chloromercuribenzoate; each 50 µM) or in the absence of chymostatin only for 30 minutes at 37°C. At the end of the incubation time the reaction was stopped by adding equal volume of 1% phosphoric acid, mixed well, centrifuged (28,000 g for 20 minutes to remove the native PMs) and stored at 4°C. On the day of analysis, the samples were filtered before separation by reverse-phase high-performance liquid chromatography (RP-HPLC). We used a linear gradient from 10% to 50% mobile phase B at a flow rate of 0.35 mL/minute at 32°C. The solvent system consisted of 0.1% phosphoric acid (mobile phase A) and 80% acetonitrile/0.1% phosphoric acid (mobile phase B). Eluted 125I products, monitored by an in-line flow-through gamma detector (BioScan Inc., Washington, DC), were identified by comparing them with the retention time of synthetic standard 125I peptides. Data were analyzed using Shimadzu LC Solution acquisition software (Kyoto, Japan). The metabolic products are documented as the percent of Ang peptide fractions generated from the parent substrate by chymase. Chymase activity was calculated as the amount of parent 125I-Ang-(1-12) hydrolyzed into specific 125I-Ang II in the absence or presence of chymostatin and reported as fmoles of Ang II generated from the parent 125I-Ang-(1-12) in fmol/mg/min.
2.5. Analysis of gene expression by quantitative real-time PCR
Quantitative real-time PCR was used to detect gene mRNA levels in human atrial tissue. Total RNA was extracted from frozen, pulverized atria using TRIzol Reagent and processed according to the manufacturer's recommendations. The quality and quantity of RNA samples were determined by spectrometry and agarose gel electrophoresis. Complementary first strand DNA was synthesized from oligo (dT)-primed total RNA, using the Omniscript RT kit (Qiagen Inc, CA). Relative quantification of mRNA levels by real-time PCR was performed using a SYBR Green PCR kit (Qiagen Inc. CA). Amplification and detection were performed with QuantStudio 3 Real-Time PCR Systems (Thermo Fisher Scientific Inc.). Only one peak from the dissociation curve was found from each pair of oligonucleotide primers tested. Real-time PCR was carried out in duplicate; a no-template control was included in each run to check for contamination. It was also confirmed that no amplification occurred when samples were not subjected to reverse transcription. Sequence-specific oligonucleotide primers were designed according to published GenBank sequences (www.ncbi.nlm.nih.gov/Genbank) and confirmed with Oligo-Analyzer 3.0. The relative target mRNA levels in each sample were normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH).
2.6. Statistics
Differences between groups were evaluated by the Student's t-test for unpaired analysis. In evaluating differences among echocardiographic variables, Kruskal-Wallis test followed by Dunn's Multiple Comparison Test were employed. The results are presented as mean ± SE. Values of p < 0.05 were considered significant. Statistical analysis was carried out using GraphPad Prism 8 (GraphPad Software, Inc., La Jolla, CA).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relation-ships that could have appeared to influence the work reported in this paper.
Acknowledgment
This research was supported by grant HL-051952 from the National Heart Lung and Blood Institute (NHLBI) of the National Institutes of Health (CMF) and grants AG042758 and AG033727 (LG) from the National Institute on Aging (NIA), National Institutes of Health.
Footnotes
Authors’ contributions: H.W., J.V., N.D.K., and C.M.F. designed the study. H.W., J.V., S.N., N.D.K., L.G., S.A., J.L.V., K.N.W., X.S., and D.D. contributed to acquisition of data. H.W., J.V., S.N., N.D.K., L.G., and C.M.F. contributed to analysis and interpretation of data. H.W., J.V., S.N., L.G., and C.M.F. drafted the manuscript and contributed to critical revision. Each author has made final approval of the manuscript.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.dib.2020.105744.
Appendix. Supplementary materials
References
- 1.H. Wang, J. Varagic, S. Nagata, N.D. Kon, L. Groban, S. Ahmad, J.L. VonCannon, K.N. Wright, X. Sun, D. Deal, C.M. Ferrario, Differential expression of the angiotensin-(1-12)/chymase axis in human atrial tissue, Journal of Surgical Research (Submitted for publication). [DOI] [PMC free article] [PubMed]
- 2.Lang R.M., Bierig M., Devereux R.B. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology, J. Am. Soc. Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 3.Ahmad S., Simmons T., Varagic J., Moniwa N., Chappell M.C., Ferrario C.M. Chymase-dependent generation of angiotensin II from angiotensin-(1-12) in human atrial tissue. PLoS One. 2011;6:e28501. doi: 10.1371/journal.pone.0028501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahmad S., Wei C.C., Tallaj J. Chymase mediates angiotensin-(1-12) metabolism in normal human hearts. J. Am. Soc. Hypertens. 2013;7:128–136. doi: 10.1016/j.jash.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Urata H., Hoffmann S., Ganten D. Tissue angiotensin II system in the human heart. Eur. Heart. J. 1994;15(Suppl D):68–78. doi: 10.1093/eurheartj/15.suppl_d.68. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.