Abstract
Objectives
To confirm whether a relationship exists between male sex and coronavirus disease 2019 (COVID-19) mortality and whether this relationship is age dependent.
Patients and Methods
We queried the COVID-19 Research Network, a multinational database using the TriNetX network, to identify patients with confirmed COVID-19 infection. The main end point of the study was all-cause mortality.
Results
A total of 14,712 patients were included, of whom 6387 (43%) were men. Men were older (mean age, 55.0±17.7 years vs 51.1±17.9 years; P<.001) and had a higher prevalence of hypertension, diabetes, coronary disease, obstructive pulmonary disease, nicotine dependence, and heart failure but a lower prevalence of obesity. Before propensity score matching (PSM), all-cause mortality rate was 8.8% in men and 4.3% in women (odds ratio, 2.15; 95% CI, 1.87 to 2.46; P<.001) at a median follow-up duration of 34 and 32 days, respectively. In the Kaplan-Meier survival analysis, the cumulative probability of survival was significantly lower in men than in women (73% vs 86%; log-rank, P<.001). After PSM, all-cause mortality remained significantly higher in men than in women (8.13% vs 4.60%; odds ratio, 1.81; 95% CI, 1.55 to 2.11; P<.001). In the Kaplan-Meier survival analysis, the cumulative probability of survival remained significantly lower in men than in women (74% vs 86%; log-rank, P<.001). The cumulative probability of survival remained significantly lower in propensity score–matched men than in women after excluding patients younger than 50 years and those who were taking angiotensin-converting enzyme inhibitor or angiotensin receptor blocker medications on admission.
Conclusion
Among patients with COVID-19 infection, men had a significantly higher mortality than did women, and this difference was not completely explained by the higher prevalence of comorbidities in men.
Abbreviations and Acronyms: ACE2, angiotensin-converting enzyme 2; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; COVID-19, coronavirus disease 2019; PSM, propensity score matching; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2
The coronavirus disease 2019 (COVID-19) epidemic has introduced unprecedented challenges to health care systems worldwide owing to the rapidly progressive nature of its biological manifestation: severe acute respiratory syndrome coronavirus (SARS-CoV-2).1 As the outbreak spread rapidly to many countries, concerns were raised about susceptible cohorts (older patients, immunocompromised patients, women, racial minorities) who may be disproportionally affected by the highly aggressive virus.2, 3, 4 In this realm, reports on sex-based disparities in the incidence, severity, and outcomes of COVID-19 have recently surfaced.3 , 5, 6, 7 Although women are often found to have worse outcomes in various acute illnesses than do men, those studies paradoxically suggested better outcomes in COVID-19–infected women than in men.8, 9, 10 In the largest survey of 72,314 COVID-19 suspected or confirmed cases in China (men, 63.8% [6387]; women, 36.2% [8325]), the case fatality rate was significantly higher in men than in women (2.8% vs 1.7%).6 In another study from Italy, among the first 827 COVID-19–related deaths in the country, 80% were men.5 Similar results were found in a study of 3912 patients from England and Wales who died of SARS-CoV-2 in March 2020. In this report, the mortality rates were higher in men than in women (97.5 cases per 100,000 vs 46.5 cases per 100,000).11 Whether those sex differences in mortality are also observed in other countries and whether an interaction between age and sex exist remain unknown. We used a multinational COVID-19 registry to assess difference in baseline characteristics and outcomes between men and women in a large sample of patients with laboratory-confirmed COVID-19 infection.
Patients and Methods
Data Source
TriNetX is a global federated health research network providing access to statistics on electronic medical records (diagnoses, procedures, medications, laboratory data, and genomic information) from patients in predominantly large health care organizations. The TriNetX database (COVID-19 Research Network) is a network of 41 global health care organizations (36% based in the United States and 64% outside the United States). Patients were divided into 2 cohorts of male and female patients on the basis of their reported sex in electronic medical records. This study was supported by the Charleston Area Medical Center Health Education and Research Institute. The Institutional Review Board at the Charleston Area Medical Center Institute granted approval for the study before study initiation.
Study Population and End Points
We queried the TriNetX research network to select adult patients (age, ≥18 years) with COVID-19 infection in the database between January 20, 2020, and April 20, 2020. Patients were identified as COVID-19 positive if they had a billable code for COVID-19 and had a positive laboratory confirmation of the infection (Supplemental Table, available online at http://www.mayoclinicproceedings.org). The main objective of this study was to compare crude and risk-adjusted mortality between men and women.
Statistical Analyses
Descriptive statistics were presented as frequencies with percentages for categorical variables and as mean ± SD for continuous measures. Baseline characteristics were compared using a Pearson chi-square test for categorical variables and an independent-samples t test for continuous variables. To account for differences in baseline characteristics between the 2 groups, a propensity score–matching (PSM) model was developed using logistic regression to derive 2 well-matched groups for comparative outcome analysis. Variables included in the PSM model included age, race, and key comorbidities (hypertension, diabetes, chronic obstructive lung disease, heart failure, obesity, nicotine dependence, and history of stroke).
The TriNetX program uses logistic regression to obtain the listed propensity scores within each covariate selected with the use of the Python libraries NumPy and sklearn (Python Software Foundation, Wilmington, DE). The platform also runs PSM in R code to compare and verify the outputs. The final step in verification uses a nearest-neighbor matching algorithm with the tolerance level of 0.01, and the difference between their propensity scores must not be greater than 0.1. All-cause mortality was displayed in the propensity score–matched cohorts using the Kaplan-Meier method, and the statistical significance of the differences between the 2 groups was assessed using the log-rank test. To protect patient health information from TriNetX, patient counts are rounded up to the nearest 10. We made every effort to mitigate these results by using a large sample size.
Sensitivity analyses
It has been hypothesized that age or the use of angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin receptor blockers (ARBs) might explain the differences in the reported outcomes between men and women.12 , 13 Therefore, we performed 2 sensitivity analyses of the primary end point (all-cause mortality) to test these hypotheses. First, we compared propensity score–matched cohorts of men and women 50 years and older. This would have excluded most women of childbearing age in whom the proposed protective effect of estrogen is likely to be present. Second, we excluded patients who were taking ACEI or ARB medications on admission to mitigate the potential confounding effect of those medications on SARS-CoV-2. Finally, given the possibility of residual confounding affecting the results, a falsification end point of bleeding was tested in both matched groups. This end point was selected to serve as a negative control as gastrointestinal bleeding rates were unlikely to be affected by the administration of these drugs.
Results
A total of 14,712 patients with COVID-19 were identified in the TriNetX COVID-19 Research Network. Of those, 6387 (43.4%) were men and 8325 (56.6%) were women. The patient population was proportionally split between the United States (47%) and outside the United States (53%). In the US cohort, 14.6% were in northeast, 27% were in the Midwest, 33.3% were in the south, and 22.9% were in the west. In the overall cohort, 37% of patients were hospitalized and 63% were managed in the outpatient setting. Men were more likely to be hospitalized than women (44% vs 31%; P<.001). Men were older (mean age, 55.0±17.7 years vs 51.1±17.9 years; P<.001) and had a significantly higher prevalence of key comorbidities including hypertension, diabetes, coronary disease, chronic obstructive pulmonary disease, nicotine dependence, and heart failure. However, the prevalence of obesity was higher in women (Table 1 ). Differences in key baseline laboratory test results between the 2 groups are listed in Table 2 .
Table 1.
| Baseline characteristic | Unmatched cohorts |
Propensity score–matched cohorts |
||||
|---|---|---|---|---|---|---|
| Male cohort (n=6387) | Female cohort (n=8325) | P value | Male cohort (n=5990) | Female Cohort (n=5990) | P value | |
| Age (y) | 55.0±17.7 | 51.1 ±17.9 | <.011 | 53.9±17.6 | 53.5±17.4 | .22 |
| Whitec | 23.0 | 26.0 | <.01 | 22.8 | 23.0 | .87 |
| Black or African American | 16.1 | 19.5 | <.01 | 16.4 | 17.5 | .37 |
| Asian | 1.3 | 1.1 | .24 | 1.3 | 1.3 | .99 |
| American Indian or Alaska Native | 0.2 | 0.1 | .20 | 0.2 | 0.2 | .99 |
| Native Hawaiian | 0.2 | 0.1 | .55 | 0.2 | 0.2 | .99 |
| COPD | 5.8 | 3.4 | <.01 | 4.1 | 4.3 | .71 |
| CAD | 7.3 | 3.5 | <.01 | 4.5 | 4.8 | .54 |
| Hypertension | 28.1 | 25.1 | <.01 | 25.5 | 25.0 | .58 |
| Diabetes mellitus | 15.1 | 12.3 | <.01 | 13.1 | 13.1 | .89 |
| Nicotine dependence | 10.7 | 7.1 | <.01 | 7.8 | 7.5 | .49 |
| Heart failure | 7.4 | 5.8 | <.01 | 6.1 | 6.3 | .79 |
| History of stroke | 3.2 | 2.9 | .24 | 2.9 | 2.9 | .99 |
| Obesityd | 13.4 | 16.6 | <.01 | 13.1 | 12.4 | .2 |
CAD = coronary artery disease; COPD = chronic obstructive pulmonary disease.
Data are presented as mean ± SD or as percentage.
Race was not recorded in ∼56% of patients.
Obesity was defined as body mass index ≥ 30 kg/m2.
Table 2.
Differences in Baseline Laboratory Data and Medication Use Between Male and Female Cohorts
| Laboratory data | Male cohort (n=6387) | Female cohort (n=8325) | % of the cohort with the available test |
|---|---|---|---|
| Sodium level | 140±3.75 | 140±3.43 | 90 (62/69) |
| Potassium level | 4.32±0.52 | 4.19±0.531 | 90 (62/69) |
| Chloride level | 102±4.68 | 102±4.5 | 90 (61/68) |
| Bicarbonate level | 25.4±4.06 | 25.3±3.69 | 93 (43/46) |
| Urea nitrogen level | 18.4±11.4 | 14±8.07 | 75 (18/24) |
| Creatinine level | 1.32±1.43 | 0.908±0.83 | 92 (68/74) |
| Glucose level | 124±60 | 111±50.6 | 92 (68/74) |
| Calcium level | 9.2±0.68 | 9.3±0.62 | 88 (56/64) |
| Magnesium level | 1.96±0.36 | 2.02±0.66 | 100 (13/13) |
| Phosphate level | 3.31±1.03 | 3.39±0.86 | 94 (31/33) |
| Leukocyte level | 7.59±6.13 | 7.39±4.42 | 91 (68/75) |
| Hemoglobin level | 14.1±2.2 | 12.9±1.8 | 91 (67/74) |
| Platelet count | 226±84.3 | 256±81.5 | 91 (68/75) |
| Alanine aminotransferase level | 35±53.6 | 23.9±30.2 | 93 (66/71) |
| Aspartate aminotransferase level | 36.8±80.5 | 27.9±47.9 | 93 (65/70) |
| Alkaline phosphatase level | 82.6±58.3 | 80.1±46.3 | 92 (60/65) |
| Lactate dehydrogenase level | 316±225 | 279±220 | 108 (40/37) |
| Total bilirubin level | 0.654±0.51 | 0.488±0.44 | 93 (65/70) |
| Albumin level | 3.96±0.80 | 4.06±0.71 | 92 (60/65) |
| Troponin level | 0.685±8.45 | 0.139±0.81 | 120 (12/10) |
| B-type natriuretic peptide level | 227±613 | 191±563 | 113 (9/8) |
| N-terminal pro–B-type natriuretic peptide level | 3027±697 | 2562±735 | 120 (6/5) |
Data are presented as mean ± SD unless specified otherwise.
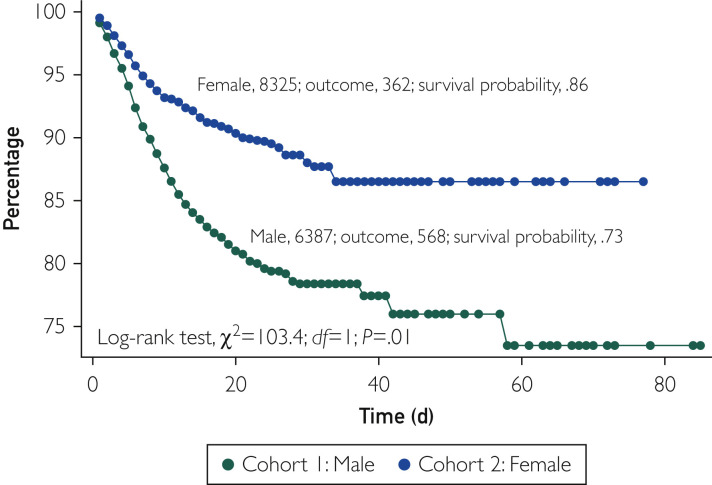
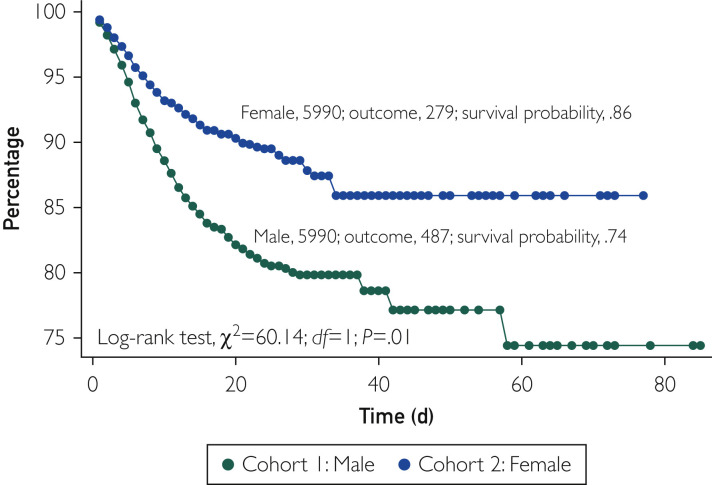
Before PSM, the all-cause mortality rate was 8.8% (568 of 6387) in men and 4.3% (363 of 8325) in women (odds ratio, 2.15; 95% CI, 1.87 to 2.46; P<.001) at a follow-up duration of 34 and 32 days in the 2 cohorts, respectively. In the Kaplan-Meier survival analysis, the cumulative probability of survival was significantly lower in men than in women (73% vs 86%; log-rank, P<.001) (Figure 1 ). After PSM, 5990 well-matched pairs of men and women were compared. The baseline characteristics of the 2 cohorts were well matched as illustrated in Table 1. The median follow-up duration in the matched cohorts was 33.5 days in men and 32 days in women. In these propensity score–matched cohorts, all-cause mortality remained significantly higher in men than in women (8.13% vs 4.60%; odds ratio, 1.81; 95% CI, 1.55 to 2.11; P<.001). In the Kaplan-Meier survival analysis, the cumulative probability of survival remained significantly lower in men than in women (74% vs 86%; log-rank, P<.001) (Figure 2 ).
Figure 1.
Kaplan-Meier survival analysis of the study groups before propensity score matching.
Figure 2.
Kaplan-Meier survival analysis of the study groups after propensity score matching. Variables used for propensity score matching included hypertension, diabetes, chronic obstructive lung disease, heart failure, obesity, nicotine dependence, and history of stroke.
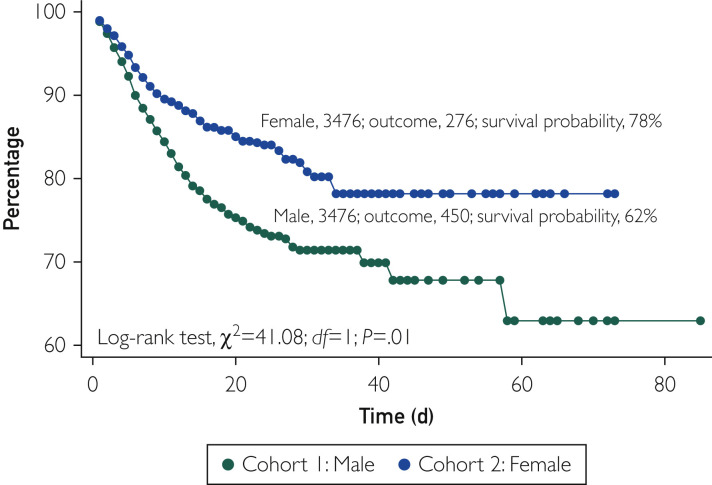
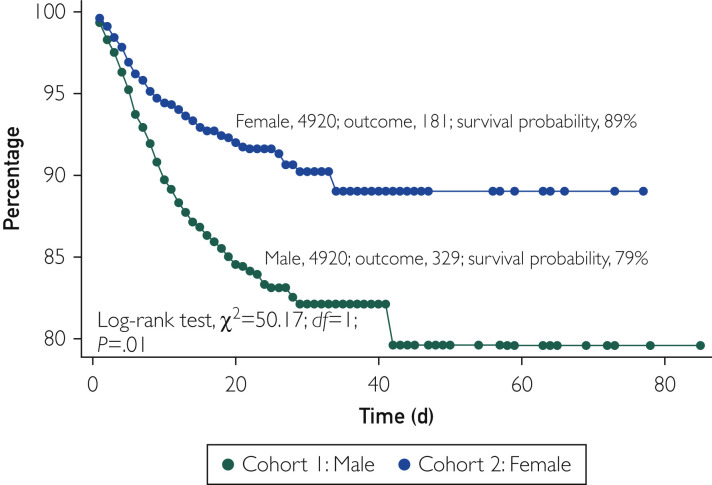
Sensitivity analyses confirmed the association between male sex and higher COVID-19–related mortality. In a subset of propensity score–matched pairs that excluded patients younger than 50 years and included 3476 patients, the cumulative probability of survival remained significantly lower in men than in women (62% vs 78%; P<.001) (Figure 3 ). In another subset of propensity score–matched pairs that excluded patients taking ACEI or ARB medications and included 4920 patients, the cumulative probability of survival remained lower in men (79% vs 89%; P<.001) (Figure 4 ).4 Finally, there was no difference in the falsification end point of bleeding between men and women (3.8% vs 3.8%; P=.99), suggesting the absence of a significant unmeasured confounder that would explain the primary outcome (Supplemental Figure, available online at http://www.mayoclinicproceedings.org).
Figure 3.
Kaplan-Meier survival in propensity score–matched patients 50 years and older. Variables used for propensity score matching included hypertension, diabetes, chronic obstructive lung disease, heart failure, obesity, nicotine dependence, and history of stroke, with exclusion of patients older than 50 years.
Figure 4.
Kaplan-Meier survival excluding patients taking angiotensin-converting enzyme inhibitor or angiotensin receptor blocker medications. Variables used for propensity score matching included hypertension, diabetes, chronic obstructive lung disease, heart failure, obesity, nicotine dependence, and history of stroke, with exclusion of patients taking angiotensin-converting enzyme inhibitor or angiotensin receptor blocker medications.
Discussion
The global SARS-CoV-2 pandemic prompted crisis teams around the globe to identify the most susceptible subgroups of patients to better understand the disease pathophysiology and inform treatment strategies to enhance outcomes.14 , 15 There was an early interest in understanding the effect of sex on COVID-19 outcomes because women are known to suffer worse outcomes in various acute illnesses than do men, and if this held true in this pandemic, there would also be increased occupational risk as a large proportion of the frontline health care workers are women.16 However, early observations from China and from limited samples in Italy and the United Kingdom indicated that women actually have lower COVID-19 mortality than do men. We sought to further confirm those observations using a large multinational data set of laboratory-confirmed COVID-19 infections and test whether those differences were age related or affected by the use of ACEI or ARB medications.
Our study documented significantly higher mortality rates in men than in women with COVID-19 infection. In addition to confirming previous observations from China, Italy, and the United Kingdom, our analysis provides additional important insights.5, 6, 7 First, to our knowledge, this is the first study to assess sex-related outcomes with COVID-19 that included a large cohort of patients from the United States. Second, this study found that the higher mortality rates from COVID-19 in men were not completely explained by the older age and the higher prevalence of comorbidities in men at presentation, as the higher mortality in men persisted after PSM. Third, our sensitivity analyses revealed that the differences in mortality between the 2 groups was independent of age, suggesting that the protective effects of sex-specific hormones in women are not adequate to explain the lower mortality in them. It also revealed that the lower mortality in women were also not explained by the use of ACEI or ARB medications, which have been thought to have a potential differential effect on the outcomes of SARS-CoV-2.17 , 18
These findings call for additional studies to understand the mechanism of the observed association between female sex and lower mortality in patients with COVID-19. However, several previous studies have proposed plausible mechanisms for the protective effect of female sex in sepsis.13 Male sex steroids appear to be immunodepressive, whereas female sex steroids increase the activity of humoral immune responses.19 Sex-specific expression of pro- and anti-inflammatory cytokines has been found in patients with trauma and septic shock at the molecular level.20 , 21 The X-chromosome mosaicism that exists naturally in women diversifies leukocyte responses during endotoxemia and may contribute to the dimorphic character of the inflammatory response.22 Finally, in childbearing age women, estrogen promotes a higher density of angiotensin-converting enzyme 2 (ACE2) receptors. The COVID-19 virus uses ACE2 receptors to penetrate endothelial lung cells, and hence a higher density of ACE2 receptors in childbearing age women might have led to the tendency to develop severe lung injury in them.12 , 17
Limitations
This study has a number of limitations. (1) Because of the retrospective nature of the study, the findings are subject to the inherent limitations of retrospective observational studies such as selection bias, differences in care-seeking behaviors, and other residual confounders. Furthermore, the accuracy of the results relies on the accuracy of coding in this database, which has not been previously independently verified. (2) Granular data on medication use, viral loads, and need for intensive care were not available because of the nature of the database. (3) The overall mortality rate in this database is higher than the case fatality rate of COVID-19 infection in some countries. However, this is likely due to the inherent selection bias associated with the inclusion of a large percentage of hospitalized patients with COVID-19 and multinational contribution to the database. (4) Granular data to perform a systematic assessment of the differences in the severity of illness between men and women were not available. (5) Although we accounted for race in our PSM analysis, the interaction between age, sex, and COVID-19–related mortality could not be addressed in this study because race was not recorded in a large proportion of patients. Our study, albeit retrospective and observational, is the largest multinational study to date to report on the outcomes of patients with SARS-CoV-2 stratified by sex.
Conclusion
In this multinational registry of patients with laboratory-confirmed COVID-19 infection, men had significantly higher mortality than did women. The effect of sex was not completely explained by the higher prevalence of comorbidities in men.
Footnotes
Grant Support: The work was supported by the Charleston Area Medical Center.
Potential Competing Interests: Dr Bates discloses holding patents and being a consultant for CeloNova BioSciences and W.L. Gore and Associates. Dr Bhatt discloses the following relationships: Advisory Board: Cardax, Cereno Scientific, Elsevier Practice Update Cardiology, Level Ex, Medscape Cardiology, PhaseBio, PLx Pharma, Regado Biosciences; Board of Directors: Boston VA Research Institute, Society of Cardiovascular Patient Care, TobeSoft; Chair: American Heart Association Quality Oversight Committee; Data Monitoring Committees: Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Cleveland Clinic (including for the ExCEED trial, funded by Edwards), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi Sankyo), Population Health Research Institute; Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org; Vice-Chair, ACC Accreditation Committee), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE-DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim; AEGIS-II executive committee funded by CSL Behring), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Duke Clinical Research Institute (clinical trial steering committees, including for the PRONOUNCE trial, funded by Ferring Pharmaceuticals), HMP Global (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), Medtelligence/ReachMD (CME steering committees), Level Ex, MJH Life Sciences, Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and USA national coleader, funded by Bayer), Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees); Other: Clinical Cardiology (Deputy Editor), NCDR-ACTION Registry Steering Committee (Chair), VA CART Research and Publications Committee (Chair); Research Funding: Abbott, Afimmune, Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Cardax, Chiesi, CSL Behring, Eisai, Ethicon, Ferring Pharmaceuticals, Forest Laboratories, Fractyl, Idorsia, Ironwood, Ischemix, Lexicon, Lilly, Medtronic, Pfizer, PhaseBio, PLx Pharma, Regeneron, Roche, sanofi-aventis, Synaptic, The Medicines Company; Royalties: Elsevier (Editor, Cardiovascular Intervention: A Companion to Braunwald’s Heart Disease); Site Coinvestigator: Biotronik, Boston Scientific, CSI, St. Jude Medical (now Abbott), Svelte; Trustee:American College of Cardiology; Unfunded Research: FlowCo, Merck, Novo Nordisk, Takeda. The other authors report no competing interests.
Supplemental Online Material
Supplemental material can be found online at: http://www.mayoclinicproceedings.org. Supplemental material attached to journal articles has not been edited, and the authors take responsibility for the accuracy of all data.
References
- 1.Carenzo L., Costantini E., Greco M., et al. Hospital surge capacity in a tertiary emergency referral centre during the COVID-19 outbreak in Italy [published online ahead of print April 4, 2020] https://doi.org/10.1111/anae.15072 Anaesthesia. [DOI] [PubMed]
- 2.Yancy C.W. COVID-19 and African Americans [published online ahead of print April 15, 2020] https://doi.org/10.1001/jama.2020.6548 JAMA. [DOI] [PubMed]
- 3.Gausman J., Langer A. Sex and gender disparities in the COVID-19 pandemic. J Womens Health (Larchmt) 2020;29(4):465–466. doi: 10.1089/jwh.2020.8472. [DOI] [PubMed] [Google Scholar]
- 4.Chung R.Y.N., Dong D., Li M.M. Socioeconomic gradient in health and the COVID-19 outbreak. BMJ. 2020;369:m1329. doi: 10.1136/bmj.m1329. [DOI] [PubMed] [Google Scholar]
- 5.Remuzzi A., Remuzzi G. COVID-19 and Italy: what next? Lancet. 2020;395(10231):1225–1228. doi: 10.1016/S0140-6736(20)30627-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Epidemiology Working Group for NCIP Epidemic Response, Chinese Center for Disease Control and Prevention The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China [in Chinese] Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41(2):145–151. doi: 10.3760/cma.j.issn.0254-6450.2020.02.003. [DOI] [PubMed] [Google Scholar]
- 7.Karlberg J., Chong D.S.Y., Lai W.Y.Y. Do men have a higher case fatality rate of severe acute respiratory syndrome than women do? Am J Epidemiol. 2004;159(3):229–231. doi: 10.1093/aje/kwh056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alkhouli M., Alqahtani F., Elsisy M.F., Kawsara A., Alasnag M. Incidence and outcomes of acute ischemic stroke following percutaneous coronary interventions in men versus women. Am J Cardiol. 2020;125(3):336–340. doi: 10.1016/j.amjcard.2019.10.045. [DOI] [PubMed] [Google Scholar]
- 9.Cenko E., Yoon J., Kedev S., et al. Sex differences in outcomes after STEMI: effect modification by treatment strategy and age. JAMA Intern Med. 2018;178(5):632–639. doi: 10.1001/jamainternmed.2018.0514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chaker Z., Badhwar V., Alqahtani F., et al. Sex differences in the utilization and outcomes of surgical aortic valve replacement for severe aortic stenosis. J Am Heart Assoc. 2017;6(9):e006370. doi: 10.1161/JAHA.117.006370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campbell A., Caul S. Deaths involving COVID-19, England and Wales: deaths occurring in March 2020. Office for National Statistics website. https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/bulletins/deathsinvolvingcovid19englandandwales/deathsoccurringinmarch2020 Accessed April 25, 2020.
- 12.Kuba K., Imai Y., Rao S., et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11(8):875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Angele M.K., Pratschke S., Hubbard W.J., Chaudry I.H. Gender differences in sepsis: cardiovascular and immunological aspects. Virulence. 2014;5(1):12–19. doi: 10.4161/viru.26982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giannakeas V., Bhatia D., Warkentin M.T., Bogoch, Stall N.M. Estimating the maximum capacity of COVID-19 cases manageable per day given a health care system’s constrained resources [published online ahead of print April 16, 2020] https://doi.org/10.7326/M20-1169 Ann Intern Med. [DOI] [PMC free article] [PubMed]
- 15.Laurencin C.T., McClinton A. The COVID-19 pandemic: a call to action to identify and address racial and ethnic disparities. J Racial Ethn Health Disparities. 2020;7(3):398–402. doi: 10.1007/s40615-020-00756-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wenham C., Smith J., Morgan R. Gender and COVID-19 Working Group. COVID-19: the gendered impacts of the outbreak. Lancet. 2020;395(10277):846–848. doi: 10.1016/S0140-6736(20)30526-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoffmann M., Kleine-Weber H., Schroeder S., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280.e278. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuster G.M., Pfister O., Burkard T., et al. SARS-CoV2: should inhibitors of the renin-angiotensin system be withdrawn in patients with COVID-19? Eur Heart J. 2020;41(19):1801–1803. doi: 10.1093/eurheartj/ehaa235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sakiani S., Olsen N.J., Kovacs W.J. Gonadal steroids and humoral immunity. Nat Rev Endocrinol. 2013;9(1):56–62. doi: 10.1038/nrendo.2012.206. [DOI] [PubMed] [Google Scholar]
- 20.Shimizu T., Yu H.P., Hsieh Y.C., et al. Flutamide attenuates pro-inflammatory cytokine production and hepatic injury following trauma-hemorrhage via estrogen receptor-related pathway. Ann Surg. 2007;245(2):297–304. doi: 10.1097/01.sla.0000232523.88621.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frink M., Pape H.C., van Griensven M., Krettek C., Chaudry I.H., Hildebrand F. Influence of sex and age on mods and cytokines after multiple injuries. Shock. 2007;27(2):151–156. doi: 10.1097/01.shk.0000239767.64786.de. [DOI] [PubMed] [Google Scholar]
- 22.Chandra R., Federici S., Haskó G., Deitch E.A., Spolarics Z. Female X-chromosome mosaicism for gp91phox expression diversifies leukocyte responses during endotoxemia. Crit Care Med. 2010;38(10):2003–2010. doi: 10.1097/CCM.0b013e3181eb9ed6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.