Abstract
Severe acute respiratory syndrome coronavirus 2 infection and development of coronavirus disease 2019 presents a major health care challenge of global dimensions. Laboratory diagnostics of infected patients, and the assessment of immunity against severe acute respiratory syndrome coronavirus 2, presents a major cornerstone in handling the pandemic. Currently, there is an increase in demand for antibody testing and a large number of tests are already marketed or are in the late stage of development. However, the interpretation of test results depends on many variables and factors, including sensitivity, specificity, potential cross-reactivity and cross-protectivity, the diagnostic value of antibodies of different isotypes, and the use of antibody testing in identification of acutely ill patients or in epidemiological settings. In this article, the recently established COVID-19 Task Force of the German Society for Clinical Chemistry and Laboratory Medicine (DGKL) addresses these issues on the basis of currently available data sets in this rapidly moving field.
Key words: Antibody response, COVID-19, diagnostic pathway, external quality assurance, immunity, immunoassay, neutralization assay, respiratory tract infections, serologic tests, severe acute respiratory syndrome coronavirus 2
Abbreviations used: COVID-19, Coronavirus disease 2019; EQA, External quality assessment; NPV, Negative predictive value; PPV, Positive predictive value; SARS, Severe acute respiratory syndrome; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2
The infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and the development of coronavirus disease 2019 (COVID-19) represents a major health care challenge of global dimensions. The current SARS-CoV-2 pandemic feels partly like a reminiscence of the earlier severe acute respiratory syndrome (SARS) epidemic in 2002/2003. Only in part because similar requirements and developments in diagnostics were necessary and similar challenges existed with regard to the evaluation of test results.1 A major difference to that time is the strong political and economic pressure to insist on the most reliable high-throughput diagnostics. There is an urgent need for the development of appropriate laboratory tests to identify infected patients, to follow the course of viral shedding and clearance, and to assess immunity against SARS-CoV-2. Laboratory testing is built on 2 different pillars: on the one side, the detection and measurement of viral RNA, and on the other side measuring antibodies of various isotypes against SARS-CoV-2 components, reflecting the host immune response. Although antibodies are developing quite early during the course of the disease, the serological response is not suitable for early detection of infected patients. Furthermore, the clinical and immunologic meaning of these antibody responses is unclear, because the many available tests do not necessarily prove protective immunity against SARS-CoV-2. It is also still unclear to what extent serological tests can be used as surrogate markers for viral encounter. In this regard, it remains unclear whether oligo- or monosymptomatic cases—which are still the majority of all SARS-CoV-2–infected patients—also develop this type of immune response. In addition, the longevity of the persistence of these antibodies is still not clear. There is increasing interest to use antibody testing to assess the immune status of larger populations and also of the risk population such as health care workers and others, to help to draw conclusions from drastic measures such as economic and social lockdown, social distancing, and other restrictive actions. These key questions require immediate attention, to appreciate the strength and weakness of antibody testing against SARS-CoV-2. This article summarizes the currently available knowledge and literature in this extremely rapidly moving area.
What are the approved indications to perform a COVID-19 serology?
In most patients, antibodies against SARS-CoV-2 become detectable within the first 10 days after the onset of symptoms of COVID-19. Also, the kinetics of the class switch of different isotypes of SARS-CoV-2–specific immunoglobulins is comparable to other coronavirus infections.2, 3, 4, 5, 6, 7, 8, 9, 10 IgM, IgA, and IgG antibodies were detectable in some patients as early as day 1 after onset of symptoms. The interquartile ranges of the first antibody detection for IgM and IgA are between day 3 and 6, and for IgG between day 10 and 18. IgA reached a plateau up to day 7, whereas IgM and IgG continuously increased until day 14 and day 21, respectively.5 Therefore, serological testing could be useful in several different aspects of COVID-19.11
First, and perhaps most important, serological testing could supplement standard RT-PCR assays for the diagnosis of COVID-19 in symptomatic patients. There is accumulating evidence that viral shedding in the upper respiratory system profoundly decreases 7 to 10 days after infection, leading to negative swab results in at least 30% to 50% of COVID-19 cases.6 , 12, 13, 14 Measurement of SARS-CoV-2–specific antibodies, which begin to be detectable in a significant proportion of patients 5 to 7 days after infection and later in almost all cases, could help to detect cases with negative RT-PCR test results.5 , 15 However, antibody tests will not replace direct pathogen detection because the immunologic response triggered by an acute infection such as COVID-19 has a certain latency.
Second, serological testing is considered to be used to retrospectively determine SARS-CoV-2 infections in people who previously have not tested positive by RT-PCR for whatever reasons. However, the kinetics and the magnitude of the antibody response seems to correlate with the clinical severity of the disease.4 , 5 Preliminary data suggest that an yet unknown number of asymptomatic infected and even oligosymptomatic patients with COVID-19 do not develop seroconversion.16 , 17
There is a lack of validation data from in vitro diagnostics manufacturers who have systematically examined asymptomatically infected patients. Therefore, it is currently challenging to establish cutoff values that are sensitive enough to determine the prevalence of infection at the population level without running the risk of too high rates of false-positive results. Performance data about the Roche antibody assay have been currently released.18 The assay exhibited no cross-reactivity with 40 endemic human coronavirus convalescence sera; that is, it yielded a specificity of 100% (95% CI, 91.2%-100%). More striking, among 5272 pre–COVID-19 sera collected from routine laboratories (n = 3420) and blood donors (n = 1772), only 10 reactive sera were identified; that is, a specificity of 99.81% (95% CI, 99.65%-99.91%) was achieved. With increasing knowledge about SARS-CoV-2, the problem of specificity could fade into the background in the future and the use of serology as an epidemiological instrument becomes the next challenge.
Third, and of utmost importance for the health care system and political decisions on lockdown measures, is the ability of serological testing to establish indicators of protection against (re-)infection with SARS-CoV-2. Indeed, sera from patients with COVID-19 show neutralizing activity in vitro and recently published case series on plasma transfer from convalescent patients with COVID-19 also demonstrate in vivo effects.4 , 19, 20, 21 However, the efficacy of this therapy has not yet been confirmed in sufficiently large, controlled studies. Furthermore, no direct conclusion can be drawn about a reliable protective effect of the antibodies individually acquired during an infection. It is therefore conceivable that anti–SARS-CoV-2 antibodies can protect against the virus. However, demonstrating a neutralizing activity of an antibody against a virus requires assays using live or pseudotyped virus, which cannot be performed in a high-throughput fashion. It is necessary to determine the targets of protective antibodies to develop simple immunoassays that best reflect virus neutralization. This is especially important because certain target epitopes of antibodies might also enhance virus entry.22 Therefore, total antibody measurements do not necessarily reflect protection after infection, nor do they indicate the efficacy of a vaccination to ascertain immunity.
How valuable is SARS-CoV-2 antibody testing in diagnostic pathways?
In a cross-validation of 22 assays (lateral-flow tests and ELISAs) to detect IgM and IgG antibodies in patients with COVID-19, a significant number of positive results were also found in historic sera from the pre–COVID-19 era and from non–SARS-CoV-2 infections,23 , 24 resulting in test specificities ranging from 84% to 100% for both isotypes (95% CI, 76%-91% and 97%-100%, respectively). The reported specificity of 100% for both IgG and IgM was yielded by one of the lateral-flow assays; however, especially evident for IgM, sensitivity within the first 10 days after patient-reported symptom onset was lower as compared with the other assays.
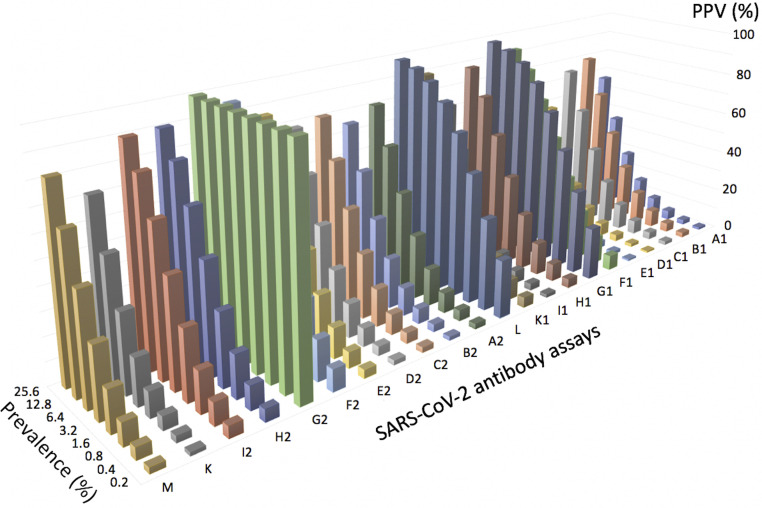
In case of a positive test result, the prevalence of the disease at the population level is the main determinant of the positive predictive value (PPV). The recently reported prevalence of COVID-19 in the population25 , 26 of 1% to 4% will result in a PPV between 25% and 58% assuming a specificity of 97% and between 4% and 15% for 76% specificity, respectively, at an artificial sensitivity of 100% in all scenarios. It is therefore not possible to infer protection against SARS-CoV-2 from a positive result of an immunoassay (see Fig 1 ).
Fig 1.
Positive predictive values for 21 commercial SARS-CoV-2 immunoassays and 1 laboratory-developed assay detecting IgM and IgG antibodies (total of 14 test systems) in patient sera and controls. Data were extracted from Whitman et al24 and plotted against various prevalence settings (0.08%-25.6%). Letters on the horizontal axis refer to the following assays: M: Inhouse; K: Epitope Diagnostics IgG; I2: VivaChek IgG; H2: UCP IgG; G2: Sure IgG; F2: Premier IgG; E2: Innovita IgG; D2: DeepBlue IgG; C2: Decombio IgG; B2: Bioperfectus IgG; A2: Biomedomics IgG; L: Wondito IgG/IgM; K1: Epitope Diagnostics IgM; I1: VivaChek IgM; H1: UCP IgM; G1: Sure IgM; F1: Premier IgM; E1: Innovita IgM; D1: DeepBlue IgM; C1: Decombio IgM; B1: Bioperfectus IgM; A1: BioMedomics IgM.
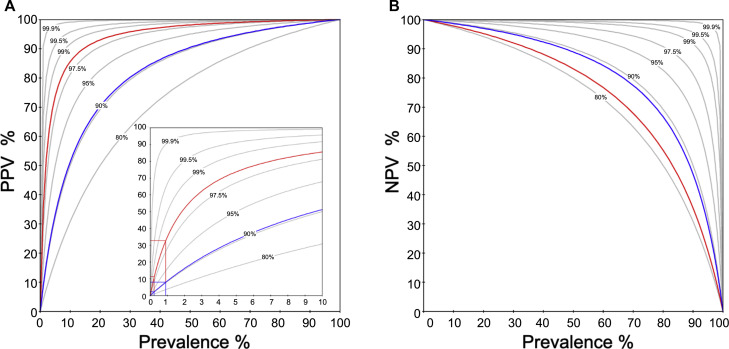
Fig 2 shows an example of PPV/negative predictive values (NPV) (y-axis) as a function of prevalence (x-axis) for theoretically assumed test sensitivities and specificities from 80% to 99.9%, respectively, and for 2 commercially available SARS-CoV-2 IgG tests with sensitivities of 88.7% and 80.0% and specificities of 90.6% and 98.5%, respectively.
Fig 2.
Examples of PPV (A) and NPV (B) (y-axis) as a function of prevalence (x-axis). Gray lines illustrate a theoretically assumed range of test sensitivities/specificities from 80/80% to 99.9/99.9%, as indicated, respectively. Two commercially available SARS-CoV-2 IgG tests are shown with (Fig 2, A) specificities of 90.6% (blue) and 98.5% (red), and (Fig 2, B) sensitivities of 88.7% (blue) and 80.0% (red), respectively. PPV for a population-based prevalence of 0.24% for COVID-19 (Regensburg, Bavaria) and 0.9%26 are illustrated in the insert of plot (Fig 2, A). As obvious in Fig 2, B, even though assay sensitivity is only 80%, due to its higher specificity the red line is located above the gray line that indicates prevalence-dependent NPV for sensitivities/specificities of 80%, respectively.
Applying these assay performance figures to testing strategies in the general population, predictive values of 2.2% to 7.9% (PPV) and 99.97% to 99.89% (NPV) or 11.4% to 32.6% (PPV) and 99.95 to 99.82% (NPV) can be calculated for a prevalence of 0.24% (Regensburg, Bavaria, Germany) or 0.9%,26 respectively. Clinical triage for COVID-19 symptoms increased the pretest probabilities toward 48% in hospitalized settings and will raise the PPV for the same tests to 89.73% and 98.01% while the NPV slightly decreases to 89.65% and 84.33%, respectively.
In the latter case, patients were questioned about COVID-19 symptoms when admitted to the emergency center, and tested only in cases of abnormalities (Rockmann and Ambrosch, personal communication, 2020). From the exemplary calculations of PPV/NPV with known sensitivity/specificity and different prevalence, it thus becomes clear under which basic conditions and prerequisites (pretest probability) a serological test can basically be carried out and the result interpreted sensibly.
The dynamics of the respective antibody classes (IgA/IgM vs IgG) in the course of the infection and their dependence on the severity of the infection represent additional factors that contribute significantly to the indication and interpretation of results for serological antibody testing. While in infections with clear respiratory symptoms caused by SARS-CoV-2, only 50% of the seroconversion seems to occur on day 7 after the onset of symptoms (IgA/IgG or IgM/IgG, SARS-CoV-2 spike protein as antigen26, 27, 28) and is completed on day 14, in severe cases of adult respiratory distress syndrome, seroconversion seems to occur earlier4; in mild or asymptomatic cases, seroconversion may even be absent.26
Do SARS-CoV-2–specific antibodies indicate the end of infectivity?
The detection of persistent infectivity cannot be conclusively verified by commercially available RT-PCR because it is not possible to distinguish between replicable virus components and inert genome fragments. It is therefore assumed that RT-PCR results lag behind the actual elimination of SARS-CoV-2 in infected individuals.
The virological criterion standard to prove infectivity is virus isolation in cell culture.13 In addition, novel molecular methods for detection of subgenomic RNA can be used to prove the end of active replication of SARS-CoV-129 and also SARS-CoV-2 in infected cells.14 In general, innate and adaptive defense mechanisms are involved in virus elimination and prevent further infections. As yet, only limited data are available on antibody responses during SARS-CoV-2 infection. Looking at the course of the virus load in COVID-19 IgA and IgM antibodies, seroconversion is not accompanied by an abrupt elimination of SARS-CoV-2. Rather, a slow but steady decrease in the viral load in the sputum coincides with the course of seroconversion at the beginning of week 2.14 , 30 At this time, there is not sufficient evidence to conclude that the detection of SARS-CoV-2–specific antibodies can be linked to the end of the virus’s infectivity. Further studies are needed to better understand the role of the various types of antibodies for different disease courses of COVID-19.
What does the detection of neutralizing antibodies imply about the immunity against SARS-CoV-2?
SARS-CoV-2 targets the mucous membranes and induces the release of secretory IgA within the first week of symptoms, followed by IgM and IgG in the second week. As with SARS and Middle East respiratory syndrome, IgM cannot be detected significantly earlier than IgG.8 Those antibodies that bind specifically to surface structures of SARS-CoV-2, like the spike protein, prevent the virus from interacting with its target cell and are called neutralizing antibodies. These antibodies play an important role in virus clearance because they have the ability to block viral infection and are assumed to protect patients. Serological tests for SARS-CoV-2 that are intended to confirm such neutralizing antibodies must therefore be robust to the detection of other, non-neutralizing antibodies. Besides interfering factors that also occur in many other assays, such as heterophilic antibodies or human anti-animal antibodies, immunogenic proteins of closely related human coronaviruses can trigger cross-reactive antibodies in the host. This has been known for many decades and led to the earlier categorization of coronaviruses into serogroups.31 Cross-reactivity with serum samples from patients with human coronavirus has been shown for serological SARS-CoV-2 IgA and IgG antibody assays.4 Therefore, to make a valid serological diagnosis of SARS-CoV-2–neutralizing antibodies, it is essential to exclude cross-reactivity by a second confirmatory test. This is even more important when, as in some commercial immunologic test systems, the SARS-CoV-2 nucleocapsid protein or parts thereof are used as an antigen. Unlike antibodies against the spike protein, antibodies against the nucleocapsid protein do not have a neutralizing effect on SARS-CoV-2 because the target protein is located inside the virus and is therefore not directly accessible for antibodies.
Widely accepted confirmatory tests, such as the virus neutralization test recommended by the World Health Organization during the SARS outbreak,32 are labor intensive, resulting in slow sample throughput in diagnostic laboratories. The establishment of highly specific primary screening assays that avoid false-positive results and thus the need for further confirmation is therefore an important objective. Surrogate neutralization assays using pseudotyped virus particles that bear the spike protein of SARS-CoV-2 do not require work inside high-containment laboratories and therefore might offer an alternative testing option in the near future.20 , 33
Another challenge for the serological detection of SARS-CoV-2 immunity is the possibility of a low antibody response in mildly infected or even asymptomatic COVID-19 cases. Most severe SARS-CoV-2 infections lead to a robust immune response,10 but on the other hand, PCR-diagnosed mild or asymptomatic infections can cause variable humoral immune responses that might not be detected by serological tests20 , 34 or even fall below the detection limit in several patients within a few weeks (Wölfel, unpublished data, 2020).
Cross-reactivity and cross-protectivity may be 2 sides of the same coin in COVID-19, too. SARS-CoV-2 is closely related to human coronavirus OC43 (another betacoronavirus), the most prevalent seasonal coronavirus detected among patients younger than 5 years.16 It has been hypothesized before that such a preexisting cross-immunity may confer protection and/or attenuate the severity of COVID-19.35 Preexisting cross-protective immunity in individuals previously exposed to antigenically related pathogens has already been demonstrated for pandemic influenza A H1N1 in 2009.36 Polyclonal antibodies against SARS-CoV spike protein significantly inhibit the entry of SARS-CoV-2 into the cell in mice,37 suggesting the possibility of a mechanism analogous to influenza. Finally, it should be mentioned that relatively nonspecific antibodies, such as those that might be produced by certain vaccination strategies, are suspected of being able to enhance a pathological immune response.22 , 38 However, first studies on vaccine antigens based on the receptor-binding domain subunit of the spike protein did not show any evidence of such an antibody-dependent enhancement.39
How do the available assay technologies differ in their conclusiveness?
A growing number of in vitro diagnostic companies are developing SARS-CoV-2–specific antibody tests (see https://www.finddx.org/covid-19/pipeline/). In addition to the differences and problems with test performance described above, the different assay techniques differ in the conclusions that can be drawn from the results. Table I gives an overview of assay techniques used in COVID-19 serology. Different antigens (receptor-binding domain, N, S1) have already been evaluated in various proprietary and commercial ELISA methods.4 Antigen selection is one of the crucial aspects of assay development that determines specificity, availability, and scalability for mass production. Recombinant proteins are produced either by prokaryotic or by eukaryotic expression systems.40 Prokaryotic systems achieve higher production rates, but the spectrum of suitable antigens is limited because of the lack of posttranslational modification and may also influence their diagnostic performance.41 Antigen extraction from complete virus lysate is technically less complex, but requires the availability of ultracentrifugation and a biosafety level 3 containment. Raw lysates are of particular interest in the early stages of outbreaks when purified proteins are not yet available. After separation of the protein fractions, virus lysates for Western blotting are used as a viable option for the validation of immunoassays and are also suitable as confirmatory tests. Because of the high safety requirements, these approaches for antigen collection and diagnostic application are reserved for specialized laboratories.42
Table I.
Synopsis of available SARS-CoV-2 serological techniques
| Technique | Rationale for usage | Advantages | Disadvantages |
|---|---|---|---|
| EIA | Monitoring of seroconversion; contact tracing; seroprevalence studies | High throughput; availability, easy to perform | Lack of knowledge on utilization and quality; inability to confirm antibodies (neutralization) functionality |
| IFT | Monitoring of seroconversion; seroprevalence studies | No analyzer (but IF microscope) needed | Low throughput; experience required; discrimination of other coronavirus antibodies; time-consuming |
| DB/WB | Confirmatory; proof of specificity/cross-reactivity; research use | Discrimination of other coronavirus antibodies | Not commonly available; experience required (WB) |
| VNT | Confirmatory; proof of specificity/cross-reactivity; virological reference method | Functional information | Biosafety level 3 laboratory necessary |
| LFA | Lack of other resources | Independent from laboratory equipment | Questionable sensitivity and specificity |
DB/WB, Dot blot/Western blot; EIA, enzyme immunoassay; IFT, immunofluorescence test; LFA, immunochromatographic lateral-flow assays; VNT, virus neutralization test.
The general issue of low PPV demands either robust sensitivities above 99.99% or a 2-tier diagnostic process; that is positive screening test results have to be confirmed, for example, by Western blot, which is a serological standard for many decades.
Neutralization assays are the virological reference method for confirmation of neutralizing antibodies. Plaque reduction neutralization tests and also more rapid microneutralization tests have been described for SARS-Cov-2 antibody testing.14 , 42 Because all these techniques rely on usage of whole-virus preparations, they are limited to biosafety level 3 laboratories. Recently, an alternative assay that can be performed under biosafety level 2 conditions was reported, using a pseudovirus-based assay to detect neutralizing antibodies against SARS-CoV-2.20 The selection of immunoglobulin isotypes is another feature that influences the informative value of an assay. The direct comparison is still limited because at present only a few studies have examined all 3 isotypes (that are IgG, IgA, and IgM) in parallel.15 , 43 IgA is supposed to have a higher sensitivity compared with IgG antibody, whereas IgG is superior in specificity.4 This observation mirrors the physiological importance of IgA as a polyreactive antibody. Although polyreactivity is primarily considered a risk for autoimmune diseases, it also offers superior defensive capabilities in the detection, neutralization, and elimination of pathogens.44
How to ensure the quality of available assays?
As of early April 2020, 101 SARS-CoV-2–specific antibody tests, most of them rapid point-of-care systems, have been Conformitè Europëenne marked under European Union Directive 98/79/EC, highlighting the currently still increasing diversity on the market.45 , 46 The globally acting nongovernmental organization Foundation of Innovative New Diagnostics provides an overview of current market readiness of different tests (see https://www.finddx.org/covid-19/pipeline/).
To ensure a high quality of diagnostic performance, laboratories have to adhere to certain requirements comprising, for example, conduction of verification studies of commercially available tests, use of internal quality controls, and participation in external quality assessment schemes (EQA). The rapid spread of COVID-19 and the associated pandemic health crisis have put an intense time pressure on test development by manufacturers and approval by governments and national regulators. These circumstances justified the rapid declaration of kits by emergency use authorization systems.47 , 48 As a consequence, laboratories might now be forced to perform clinical validation studies to ensure the quality of emergency use authorization kits. Thus, anti–SARS-CoV-2 cross-reactivity with other types of coronaviruses3 as a cause of false-positive test results as well as the influence of other interfering factors such as rheumatoid factors38 must be clarified by the respective service provider. Furthermore, the dynamic of the immune responses needs to be studied in detail to determine the optimal time of diagnostics because false-negative test results might be attributed to interindividual differences in the immune response. To date, it has not been sufficiently proven what influence the severity of the disease (asymptomatic, mild, severe) has on the extent and course of detectable antibody responses.49 The determination and ideally standardization of cutoffs is one of the essential quality criteria that will affect the intended use of COVID-19 serology. This is emphasized by the World Health Organization recommending, as of April 24, 2020, scientific report, to restrict the use of SARS-CoV-2 antibody testing to research settings until its diagnostic reliability is proven by peer-reviewed large-scale studies and EQA schemes.40 With the former currently being conducted by the World Health Organization and the Foundation of Innovative New Diagnostics,50 reference material and EQA schemes are currently available only for molecular-based SARS-CoV-2 testing.51, 52, 53, 54, 55 The need is further highlighted by former SARS-CoV EQA schemes that revealed a poor sensitivity of 53% of enzyme immunoassay–based tests.56 To meet this urgent demand, United Kingdom National External Quality Assessment Service and the German Reference Institute for Bioanalytics (Referenzinstitut für Bioanalytik) announced an upcoming EQA scheme.57 , 58 The Referenzinstitut für Bioanalytik recently conducted the first pilot-scheme (unpublished data, 2020). Here, 8 serum samples were provided for enzyme immunoassay–based testing of IgG, IgA, and IgM. Preliminary results of testing for IgG and IgA revealed a moderate concordance of assays, with 66% and 75% agreement for IgG and IgA results between the laboratories, respectively. Results submitted for IgM diverged substantially, with only 25% of laboratories reporting correct results for all samples provided (Haselmann et al, unpublished data, 2020). Notably, none of the participants correctly analyzed all samples. Hence, further schemes providing serial dilutions of samples to stress analytical test performance are mandatory.
Accuracy and reproducibility of the test formats is particularly important in the so-called gray zone in which immunity may not have developed completely. Facing a high number of rapid lateral-flow tests with questionable quality flooding the diagnostic market, certification by EQA schemes is one approach to select assays with poor quality. In contrast, analytical and clinical validation of new test formats require comprehensive testing in cohorts mirroring the natural prevalence of diverse antibodies after a season of respiratory diseases and the indication of PPVs under defined situations of varying prevalence.
With regard to the quality assurance of SARS-CoV-2 antibody tests, there is an urgent need for suitable reference material, for large-scale validation studies involving various available test systems, and for international proficiency testing initiatives.
Why should baseline samples be collected from still asymptomatic or healthy individuals?
There are different definitions of “baseline” samples and baseline studies. A blood draw to obtain a baseline serum sample is recommended for contacts of infected persons as early as possible within the incubation period of contact.59 , 60 For patients, paired samples are necessary for confirmation with the initial (baseline) sample collected in the first week of illness and the second ideally collected 2 to 4 weeks later (the optimal timing for convalescent sample needs to be established).59
In a representative baseline study, a demographically representative cohort is repeatedly tested to determine the rate of spread of the virus. This can be done by serological analysis on blood donors, by studies in particularly affected places (“hotspots”), or nationwide in a carefully controlled population-representative study. Baseline samples from noninfected healthy individuals are particularly important for future validation purposes. Such stored serum samples can be used for future usage because it can support diagnostics once validated serology tests are available.59 , 60
The proactive storage of baseline samples, that is, serum from individuals who were COVID-19–naive at the time of blood collection, could speed up diagnostics because seroconversion can be detected by parallel analysis of postexposure samples together with those initially collected. The absence of preformed cross-reacting factors in baseline samples reduces the probability of unspecific positive results in the follow-up sample in case of a suspected infection. Especially for the large number of studies initiated at high speed for the prevention of COVID-19, there is an urgent need to collect baseline samples. Although accurate serological tests are still under development, the need for study participants is urgent so as to collect blood from study participants awaiting such tests in the near future. These tests could become crucial to obtain fully interpretable and unbiased results from these studies. For example, it has recently been proposed to collect samples and data in advance to test the hypothesis that resilience of the elderly during a pandemic can be improved by countering chronic inflammation (inflammaging) and cellular senescence.61
Although this procedure is straightforward within studies, some countries may need special regulations for implementation in the field of health care. At present, it is conceivable that biobanks are established with noble intentions but may then be opened for purposes for which prior consent of the patient would have been required. Similarly, this problem could also affect stored sera from employees. At this point, the officials should verify the legitimacy of a proactive blood collection.
Can laboratories estimate the medium-term demand for SARS-CoV-2 antibody tests?
Following the introduction of PCR methods, it soon became apparent that the demand for test kits far exceeded their availability. A major difference between molecular and serological diagnostics is that the latter can be performed in almost all diagnostic laboratories; usually, equipment is readily available. Personal communication with the in vitro diagnostics industry currently estimates a demand only for a single country such as Germany between 2,000,000 and 5,000,000 tests per month. The needed capacities may double because it can be assumed that most of the tested persons have to be re-tested within 1 month. The assumed increase is also triggered by the examination of contacts of persons tested positive in a low-prevalence setting. Production capacities of “high double-digit millions” per month have already been announced by one manufacturer. It therefore remains to be seen whether the forecasts for both demand and availability will be met.
Mathematical models can help to estimate the period of increased demand on the basis of duration of the pandemic. The German Robert Koch Institute modulated a susceptible-exposed-infected-resistant model on the rate of successfully isolated patients and the seasonality of disease progression.62 Seasonality leads to fluctuations of the basic reproduction number R63 and thereby markedly determines the length or even the end of an epidemic. Risks such as uncertainty about the duration of the pandemic or failing postmarket surveillance may lead some manufacturers to withdraw from the market. However, this will not prevent others from capitalizing on the current supply shortages by fake products.64
Perspective and conclusions
Given an appropriate assay design, the serological testing of confirmed COVID-19 convalescent individuals can be expected to be accurate in detecting an anti–SARS-CoV-2 response (importantly, a false-negative result due to imperfect sensitivity will not endanger the convalescent patient). All other positive results are due to asymptomatic, previously undetected COVID-19 cases or are caused by non–SARS-CoV-2-related cross-reactivities or unspecific test interferences. In general, a specificity below 99.99%, that is, 1 false-positive result within 10,000 true-positive test results, in a low-prevalence setting (<1%) will generate a number of false positives inversely related to the prevalence of the biomarkers tested. This may lead to a systematic overestimation of the prevalence of immunity in the population as well as lower estimates of virus mortality rate and pose a challenge for any subsequent clinical, societal, and economic decision making.
Future studies therefore need to concentrate on 3 aspects: (1) using test systems with 100% SARS-CoV-2 patient antibody specificities, preferably capable to detect antibodies blocking virus-cell interaction as candidates for protective immunity. Although there is some promise with the immune testing systems available, the current tests have not shown the specificities needed to warrant the interpretation of positive results in screening situations. (2) Controlling the prevalence in the population groups tested in a dynamic fashion. This may be accomplished by contact tracing in the case of a positive virus finding, thus allowing to improve the prevalence in the social surroundings of the individual tested positive (confirmed niche testing). (3) Furthermore, overestimation of prevalence can be quickly corrected by avoiding selection bias in the study cohort.
Key messages.
-
•
Seroconversion when SARS-CoV-2 is detected by RT-PCR indicates a SARS-CoV-2–specific humoral immune response.
-
•
In screening situations, the number of false-positive results is inversely correlated to the prevalence of the disease for any test with specificity below 100%.
-
•
The response characteristics in sub- and oligo-symptomatic clinical infections, a significant proportion of SARS-CoV-2 infections, remain a key gap in the literature.
-
•
It is currently unknown whether the available serological assays can be used to confirm immunity against SARS-CoV-2.
-
•
Even though more than 100 different antibody tests are currently available, global and territorial seroprevalence of COVID-19 remains unknown.
Acknowledgments
We gratefully acknowledge Tobias Kiehntopf for developing and providing the software that was used to create Fig 2 and to calculate the prevalence-dependent predictive values shown therein. We thank Claudia Trier for excellent editorial assistance.
Footnotes
H.R. is funded by the Universities Giessen Marburg Lung Center (UGMLC) and the German Center for Lung Disease (DZL German Lung Center, no. 82DZL00502) for UGMLC.
Disclosure of potential conflict of interest: The authors declare that they have no relevant conflicts of interest.
References
- 1.Meyer B., Drosten C., Müller M.A. Serological assays for emerging coronaviruses: challenges and pitfalls. Virus Res. 2014;194:175–183. doi: 10.1016/j.virusres.2014.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jin Y., Wang M., Zuo Z., Fan C., Ye F., Cai Z. Diagnostic value and dynamic variance of serum antibody in coronavirus disease 2019. Int J Infect Dis. 2020;94:49–52. doi: 10.1016/j.ijid.2020.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu W., Liu L., Kou G., Zheng Y., Ding Y., Ni W. Evaluation of nucleocapsid and spike protein-based ELISAs for detecting antibodies against SARS-CoV-2. J Clin Microbiol. 2020;58 doi: 10.1128/JCM.00461-20. e00461-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Okba N.M.A., Müller M.A., Li W., Wang C., GeurtsvanKessel C.H., Corman V.M. Severe acute respiratory syndrome coronavirus 2-specific antibody responses in coronavirus disease 2019 patients. Emerg Infect Dis. 2020;26 doi: 10.3201/eid2607.200841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tan W, Lu Y, Zhang J, Wang J, Dan Y, Tan Z, et al. Viral kinetics and antibody responses in patients with COVID-19 [published online ahead of print March 26, 2020]. Preprint at medRxiv. https://doi.org/10.1101/2020.03.24.20042382.
- 6.To K.K.-W., Tsang O.T.-Y., Leung W.-S., Tam A.R., Wu T.-C., Lung D.C. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. 2020;20:565–574. doi: 10.1016/S1473-3099(20)30196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xiang F, Wang X, He X, Peng Z, Yang B, Zhang J, et al. Antibody detection and dynamic characteristics in patients with COVID-19 [published online ahead of print April 19, 2020]. Clin Infect Dis. https://doi.org/10.1093/cid/ciaa461. [DOI] [PMC free article] [PubMed]
- 8.Xiao AT, Gao C, Zhang S. Profile of specific antibodies to SARS-CoV-2: the first report [published online ahead of print March 21, 2020]. J Infect. https://doi.org/10.1016/j.jinf.2020.03.012. [DOI] [PMC free article] [PubMed]
- 9.Zeng Z, Chen L, Pan Y, Deng Q, Ye G, Li Y, et al. Re: Profile of specific antibodies to SARS-CoV-2: the first report [published online ahead of print April 10, 2020]. J Infect. https://doi.org/10.1016/j.jinf.2020.03.052. [DOI] [PMC free article] [PubMed]
- 10.Zhao J, Yuan Q, Wang H, Liu W, Liao X, Su Y, et al. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019 [published online ahead of print March 28, 2020]. Clin Infect Dis. https://doi.org/10.1093/cid/ciaa344. [DOI] [PMC free article] [PubMed]
- 11.Petherick A. Developing antibody tests for SARS-CoV-2. Lancet. 2020;395:1101–1102. doi: 10.1016/S0140-6736(20)30788-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kucirka L, Lauer S, Laeyendecker O, Boon D, Lessler J. Variation in false negative rate of RT-PCR based SARS-CoV-2 tests by time since exposure [published online ahead of print April 10, 2020]. Preprint at medRxiv. https://doi.org/10.1101/2020.04.07.20051474. [DOI] [PMC free article] [PubMed]
- 13.Wikramaratna P, Paton RS, Ghafari M, Lourenco J. Estimating false-negative detection rate of SARS-CoV-2 by RT-PCR [published online ahead of print April 7, 2020]. Preprint at medRxiv. https://doi.org/10.1101/2020.04.05.20053355.
- 14.Wölfel R, Corman VM, Guggemos W, Seilmaier M, Zange S, Müller MA, et al. Virological assessment of hospitalized patients with COVID-2019 [published online ahead of print April 1, 2020]. Nature. https://doi.org/10.1038/s41586-020-2196-x. [DOI] [PubMed]
- 15.Guo L, Ren L, Yang S, Xiao M, Chang D, Yang F, et al. Profiling early humoral response to diagnose novel coronavirus disease (COVID-19) [published online ahead of print March 21, 2020]. Clin Infect Dis. https://doi.org/10.1093/cid/ciaa310. [DOI] [PMC free article] [PubMed]
- 16.Zhou W., Wang W., Wang H., Lu R., Tan W. First infection by all four non-severe acute respiratory syndrome human coronaviruses takes place during childhood. BMC Infect Dis. 2013;13:433. doi: 10.1186/1471-2334-13-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yongchen Z., Shen H., Wang X., Shi X., Li Y., Yan J. Different longitudinal patterns of nucleic acid and serology testing results based on disease severity of COVID-19 patients. Emerg Microbes Infect. 2020;9:833–836. doi: 10.1080/22221751.2020.1756699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roche Diagnostics Elecsys Anti-SARS-CoV-2—instructions for use. https://www.fda.gov/media/137605/download Accessed May 17, 2020.
- 19.Shen C., Wang Z., Zhao F., Yang Y., Li J., Yuan J. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020;323:1582–1589. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu F, Wang A, Liu M, Wang Q, Chen J, Xia S, et al. Neutralizing antibody responses to SARS-CoV-2 in a COVID-19 recovered patient cohort and their implications [published online ahead of print April 20, 2020]. Preprint at medRxiv. https://doi.org/10.1101/2020.03.30.20047365.
- 21.Ye M, Fu D, Ren Y, Wang F, Wang D, Zhang F, et al. Treatment with convalescent plasma for COVID-19 patients in Wuhan, China [published online ahead of print April 15, 2020]. J Med Virol. https://doi.org/10.1002/jmv.25882. [DOI] [PMC free article] [PubMed]
- 22.Iwasaki A, Yang Y. The potential danger of suboptimal antibody responses in COVID-19 [published online ahead of print April 21, 2020]. Nat Rev Immunol. https://doi.org/10.1038/s41577-020-0321-6. [DOI] [PMC free article] [PubMed]
- 23.Mandavilli A. Coronavirus antibody tests: can you trust the results? New York Times April 27, 2020. https://www.nytimes.com/2020/04/24/health/coronavirus-antibody-tests.html Available at: Accessed May 17, 2020.
- 24.Whitman JD, Hiatt J, Mowery CT, Shy BR, Yu R, Yamamoto TN, et al. Test performance evaluation of SARS-CoV-2 serological assays [published online ahead of print May 17, 2020]. Preprint at medRxiv. https://doi.org/10.1101/2020.04.25.20074856.
- 25.Bendavid E, Mulaney B, Sood N, Shah S, Ling E, Bromley-Dulfano R, et al. COVID-19 antibody seroprevalence in Santa Clara County, California [published online ahead of print April 30, 2020]. Preprint at medRxiv. https://doi.org/10.1101/2020.04.14.20062463.
- 26.Gudbjartsson DF, Helgason A, Jonsson H, Magnusson OT, Melsted P, Norddahl GL, et al. Spread of SARS-CoV-2 in the Icelandic population [published online ahead of print April 14, 2020]. N Engl J Med. https://doi.org/10.1056/nejmoa2006100. [DOI] [PMC free article] [PubMed]
- 27.Ai T, Yang Z, Hou H, Zhan C, Chen C, Lv W, et al. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases [published online ahead of print February 26, 2020]. Radiology. https://doi.org/10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed]
- 28.Kabesch M, Roth S, Brandstetter S, Häusler S, Juraschko E, Weigl M, et al. Successful containment of COVID-19 outbreak in a large maternity and perinatal center while continuing clinical service [published online ahead of print April 22, 2020]. Pediatr Allergy Immunol. https://doi.org/10.1111/pai.13265. [DOI] [PMC free article] [PubMed]
- 29.Hussain S., Pan J., Chen Y., Yang Y., Xu J., Peng Y. Identification of novel subgenomic RNAs and noncanonical transcription initiation signals of severe acute respiratory syndrome coronavirus. J Virol. 2005;79:5288–5295. doi: 10.1128/JVI.79.9.5288-5295.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang W., Du R.-H., Li B., Zheng X.-S., Yang X.-L., Hu B. Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerg Microbes Infect. 2020;9:386–389. doi: 10.1080/22221751.2020.1729071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McIntosh K., Kapikian A.Z., Hardison K.A., Hartley J.W., Chanock R.M. Antigenic relationships among the coronaviruses of man and between human and animal coronaviruses. J Immunol. 1969;102:1109–1118. [PubMed] [Google Scholar]
- 32.World Health Organization. WHO guidelines for the global surveillance of severe acute respiratory syndrome (SARS): updated recommendations, October 2004. Publication Server of WHO. 2004. Vol 2013.
- 33.Grzelak L, Temmam S, Planchais C, Demeret C, Huon C, Guivel F, et al. SARS-CoV-2 serological analysis of COVID-19 hospitalized patients, pauci-symptomatic individuals and blood donors [published online ahead of print April 24, 2020]. Preprint at medRxiv. https://doi.org/10.1101/2020.04.21.20068858.
- 34.van der Heide V. Neutralizing antibody response in mild COVID-19 [published online ahead of print April 28, 2020]. Nat Rev Immunol. https://doi.org/10.1038/s41577-020-0325-2. [DOI] [PMC free article] [PubMed]
- 35.Nickbakhsh S, Ho A, Marques DFP, McMenamin J, Gunson RN, Murcia PR. Epidemiology of seasonal coronaviruses: establishing the context for the emergence of coronavirus disease 2019 [published online ahead of print April 14, 2020]. J Infect Dis. https://doi.org/10.1093/infdis/jiaa185. [DOI] [PMC free article] [PubMed]
- 36.Kerkhove MD Van, Hirve S., Koukounari A., Mounts A.W. Estimating age-specific cumulative incidence for the 2009 influenza pandemic: a meta-analysis of A(H1N1)pdm09 serological studies from 19 countries. Influenza Other Respi Viruses. 2013;7:872–886. doi: 10.1111/irv.12074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walls A.C., Park Y.-J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181:281–292.e6. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Quinlan BD, Mou H, Zhang L, Guo Y, He W, Ojha A, et al. The SARS-CoV-2 receptor-binding domain elicits a potent neutralizing response without antibody-dependent enhancement [published online ahead of print April 12, 2020]. SSRN Electron J. https://doi.org/10.2139/ssrn.3575134.
- 40.Stadlbauer D, Amanat F, Chromikova V, Jiang K, Strohmeier S, Arunkumar GA, et al. SARS-CoV-2 seroconversion in humans: a detailed protocol for a serological assay, antigen production, and test setup [published online ahead of print April 17, 2020]. Curr Protoc Microbiol. https://doi.org/10.1002/cpmc.100. [DOI] [PMC free article] [PubMed]
- 41.Zhang P, Gao Q, Wang T, Ke Y, Mo F, Jia R, et al. Evaluation of recombinant nucleocapsid and spike proteins for serological diagnosis of novel coronavirus disease 2019 (COVID-19) [published online ahead of print March 20, 2020]. Preprint at medRxiv. https://doi.org/10.1101/2020.03.17.20036954.
- 42.Haveri A., Smura T., Kuivanen S., Österlund P., Hepojoki J., Ikonen N. Serological and molecular findings during SARS-CoV-2 infection: the first case study in Finland, January to February 2020. Euro Surveill. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.11.2000266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang Z, Xiao T, Wang Y, Yuan J, Ye H, Wei L, et al. Early viral clearance and antibody kinetics of COVID-19 among asymptomatic carriers [published online ahead of print May 2, 2020]. Preprint at medRxriv. https://doi.org/10.1101/2020.04.28.20083139. [DOI] [PMC free article] [PubMed]
- 44.Dimitrov J.D., Planchais C., Roumenina L.T., Vassilev T.L., Kaveri S.V., Lacroix-Desmazes S. Antibody polyreactivity in health and disease: Statu Variabilis. J Immunol. 2013;191:993–999. doi: 10.4049/jimmunol.1300880. [DOI] [PubMed] [Google Scholar]
- 45.Commission of the European Union. Communication from the Commission—guidelines on COVID-19 in vitro diagnostic tests and their performance. Off J Eur Union 2020/C 122 I/01.
- 46.Vashist S.K. In vitro diagnostic assays for COVID-19: recent advances and emerging trends. Diagnostics. 2020;10:202. doi: 10.3390/diagnostics10040202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Food and Drug Administration Policy for coronavirus disease-2019 tests during the public health emergency (Revised) 2020.. https://www.fda.gov/media/135659/download Available at: Accessed May 11, 2020.
- 48.Hong K.H., Lee S.W., Kim T.S., Huh H.J., Lee J., Kim S.Y. Guidelines for laboratory diagnosis of coronavirus disease 2019 (COVID-19) in Korea. Ann Lab Med. 2020;40:351–360. doi: 10.3343/alm.2020.40.5.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang AT, Garcia-Carreras B, Hitchings MDT, Yang B, Katzelnick L, Rattigan SM, et al. A systematic review of antibody mediated immunity to coronaviruses: antibody kinetics, correlates of protection, and association of antibody responses with severity of disease [published online ahead of print April 17, 2020]. Ann Lab Med. https://doi.org/10.1101/2020.04.14.20065771.
- 50.Foundation for Innovative New Diagnostics FIND evaluation update: SARS-CoV-2 immunoassays. 2020.. https://www.finddx.org/covid-19/sarscov2-eval-immuno/ Available at: Accessed April 27, 2020.
- 51.AccuPlex SARS-CoV-2 reference material kit. 2020.. https://www.seracare.com/AccuPlex-SARSCoV2-Reference-Material-Kit-0505-0126/ Available at: Accessed April 28, 2020.
- 52.Reichhardt H., Kammel M. Gesellschaft zur Förderung der Qualitätssicherung in medizinischen Laboratorien e.V. Novel coronavirus SARS-CoV-2 (2019-nCoV) includeding the next INSTAND EQA scheme. 2020.. https://www.instand-ev.de/fileadmin/uploads/user_upload/Dokumente/Virologie/20200214_INSTAND_announcement_EQAS_novel_coronavirus_SARS-CoV-2.pdf Available at: Accessed April 28, 2020.
- 53.Laboratory of the Government Chemist Group COV - SARS-CoV-2 molecular proficiency test. 2020.. https://us.lgcstandards.com/US/en/COV-SARS-CoV-2-molecular/p/PT-CL-MI-COV Available at: Accessed April 28, 2020.
- 54.Randox Quality control for molecular diagnostics. Coronavirus outbreak preparedness EQA pilot study. 2020.. https://www.randox.com/sars-cov-2-eqa-scheme/ Available at: Accessed April 28, 2020.
- 55.College of American Pathologists SARS-CoV-2 molecular proficiency testing program. 2020.. https://www.cap.org/laboratory-improvement/proficiency-testing/sars-cov-2-molecular Available at: Accessed April 28, 2020.
- 56.Niedrig M., Leitmeyer K., Lim W., Peiris M., Mackenzie J.S., Zambon M. First external quality assurance of antibody diagnostic for SARS-new coronavirus. J Clin Virol. 2005;34:22–25. doi: 10.1016/j.jcv.2005.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.United Kingdom National External Quality Assessment Service New COVID-19 UK NEQAS pilot scheme developments. 2020.. https://ukneqas.org.uk/news/new-covid-19-uk-neqas-pilot-scheme-developments/ Available at: Accessed March 19, 2020.
- 58.Referenzinstitut für Bioanalytik Application survey SARS-CoV-2 immunology. 2020.. https://rfb.bio/pdf/2020/Ankuendigung_Anmeldung_CoV_2020_de_en.pdf Available at: Accessed March 19, 2020.
- 59.World Health Organization. Laboratory testing for 2019 novel coronavirus (2019-nCoV) in suspected human cases, Interim guidance, 19 March 2020. Publication Server of WHO.
- 60.European Centre for Disease Prevention and Control Laboratory support for COVID-19 in the EU/EEA. 2020.. https://www.ecdc.europa.eu/en/novel-coronavirus/laboratory-support Available at: Accessed April 27, 2020.
- 61.Fuellen G, Liesenfeld O, Kowald A, Barrantes I, Bastian M, Simm A, et al. The preventive strategy for pandemics in the elderly is to collect in advance samples & data to counteract chronic inflammation (inflammaging) [published online ahead of print May 24, 2020]. Ageing Res Rev. https://doi.org/10.1016/j.arr.2020.101091. [DOI] [PMC free article] [PubMed]
- 62.an der Heiden M, Buchholz U. Modellierung von Beispielszenarien der SARS-CoV-2-Epidemie 2020 in Deutschland. Publication Server of Robert Koch Institute. 2020. https://doi.org/10.25646/6571.2.
- 63.Kissler S.M., Tedijanto C., Goldstein E., Grad Y.H., Lipsitch M. Projecting the transmission dynamics of SARS-CoV-2 through the postpandemic period. Science. 2020;368:860–868. doi: 10.1126/science.abb5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.World Health Organization Medical Product Alert N°3/2020. Falsified medical products, including in vitro diagnostics, that claim to prevent, detect, treat or cure COVID-19. 2020. https://www.who.int/news-room/detail/31-03-2020-medical-product-alert-n-3-2020 Available at: Accessed March 31, 2020.