Abstract
The presence of cardiovascular co-morbidities and the known effects of coronaviruses on the cardiovascular system have called attention to the potential implications for patients with cardiovascular risk factors. This evidence-based viewpoint will address two questions: (a) are individuals with underlying cardiovascular risk factors (e.g. high blood pressure or diabetes) or overt disease (e.g. coronary heart disease, heart failure, kidney disease) more likely to develop severe Covid-19 and to die than those without underlying conditions? (b) does the regular use of angiotensin-converting enzyme inhibitors (ACE-i) or angiotensin-receptor blockers (ARB) make patients more likely to get infected and to die of Covid-19?
With a necessary cautionary note that the evidence around the links between Covid-19 and cardiovascular disease is accruing at a fast pace, to date we can conclude that: (a) the greater susceptibility of individuals with underlying cardiovascular conditions to develop more severe Covid-19 with higher mortality rate is likely to be confounded, in part, by age and the type of co-morbidities. Patients with heart failure or chronic kidney disease might show an excess risk; (b) neither ACE-i nor ARB are associated with greater risk of SARS-Cov2 infection, or severity or risk of death in patients with Covid-19. Patients on these drugs should not stop them, unless under strict medical supervision and with the addition of a suitable replacement medicine.
Keywords: Covid-19, SARS-CoV-2, Hypertension, Diabetes, Cardiovascular disease, ACE-Inhibitors, Angiotensin-receptor-blockers, Renin-angiotensin system
Introduction
As of 14 May 2020, there have been 4,369,410 confirmed cases of Novel Coronavirus Disease 2019 (Covid-19) due to Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection in the world, with 297,569 fatalities globally [1]. The epidemic originated in Wuhan, Hubei province of China, and spread rapidly throughout mainland China and worldwide, reaching pandemic proportions [2]. The clinical manifestations initially were of a viral pneumonia with fever, cough, fatigue and dyspnoea [2,3], severe acute respiratory syndrome (ARDS) some requiring hospitalisation, with escalation to intensive care support with oxygen, mechanical ventilation and, eventually, death. The rapid dissemination of the pandemic trough Asia, Europe and North America has revealed additional patterns of presentation features, comorbidities and factors associated with mortality [3]. The presence of cardiovascular (CV) co-morbidities and the known effects of coronaviruses on the CV system have called attention to the potential implications for patients with CV risk factors [4].
This evidence-based viewpoint will attempt to answer two questions: (a) are individuals with underlying cardiovascular risk factors (e.g. high blood pressure or diabetes) or overt disease (e.g. coronary heart disease, heart failure, kidney disease) more likely to develop severe Covid-19 and to die than those without underlying conditions? (b) does the regular use of angiotensin-converting enzyme inhibitors (ACE-i) or angiotensin-receptor blockers (ARB) make patients more likely to get infected and to die of Covid-19?
Covid-19 and underlying CV risk
The most common comorbidities among patients admitted to hospitals worldwide have been hypertension and diabetes, followed by cardiovascular and respiratory diseases [[2], [3], [4], [5]]. Table 1 summarises the prevalence of hypertension and diabetes recorded in 18 reports (10 from China [2,[6], [7], [8], [9],14,[16], [17], [18], [19], [20], [21]], 3 from USA [5,13,15], 2 from Italy [10,11] and 1 from a multicentre study from 3 continents [12]) and, where available, the severity and mortality in hospitalised patients with confirmed Covid-19 by underlying risk factors. The reported prevalence of pre-existing hypertension varied from 12.2% [14] to 78.6% [13], and the prevalence of pre-existing diabetes varied from 7.4% [2] to 38.2% [13]. Furthermore, some studies reported an increased severity of symptoms in hospitalised patients with confirmed Covid-19 and underlying hypertension or diabetes, with a 2.7 and 3.8 folds higher severity [17] and with a fatality ratio up to 2.6 and 4.4 folds [2], respectively (Table 1). A meta-analysis of 2552 confirmed Covid-19 patients from 11 studies suggested that the severity of Covid-19 was higher in the presence of hypertension (pooled unadjusted OR 2.49, 95% CI 1.98 to 3.12) [22]. Similarly, a meta-analysis of 6452 patients with Covid-19 from 30 studies showed that pre-existing diabetes was associated with poor outcome including severity of Covid-19 (unadjusted risk ratio 2.45, 95% CI 1.79 to 3.35) and mortality (unadjusted risk ratio 2.12, 1.44 to 3.11) [23]. Meta-regression indicated age and hypertension as the statistically significant explanatory variables of such associations.
Table 1.
Prevalence of hypertension and diabetes, and severity and outcomes by comorbidity, in hospitalised patients with confirmed Covid-19.
| Author (Country) | Sample size (n) | Age (yrs) | Hypertension (%) | Diabetes (%) | Hypertension |
Diabetes |
||
|---|---|---|---|---|---|---|---|---|
| Survivor vs Death (%) | Not severe vs Severe (%) | Survivor vs Death (%) | Not severe vs Severe (%) | |||||
| Chen N [6] (China) | 99 | 55.6 | 40.0a | 13.0 | ||||
| Chen T [7] (China) | 274 | 68.0 | 34.0 | 17.0 | 24.0 vs 48.0 | 14.0 vs 21.0 | ||
| Conversano [11] (Italy) | 191 | 63.4 | 50.2 | 14.6 | 42.3 vs 81.0 | 11.4 vs 26.2 | ||
| Guan [2] (China) | 1099 | 47.0 | 15.0 | 7.4 | 13.7 vs 35.8 | 13.4 vs 23.7 | 6.1 vs 26.9 | 5.7 vs 16.2 |
| Guo [8] (China) | 187 | 58.5 | 32.6 | 15.0 | ||||
| Li [9] (China) | 1178 | 55.5 | 30.7 | n/a | ||||
| Mancia [10] (Italy) | 6272 | 68.0 | 57.9b | 19.1b | ||||
| Mehra [12] (Global) | 8910 | 49.0 | 26.3 | 14.3 | 26.4 vs 25.2 | 14.0 vs 18.8 | ||
| Mehta [13] (USA) | 1735 | 49.0 | 78.6 | 38.2 | ||||
| Meng [14] (China) | 417 | 64.5 | 12.2 | n/a | ||||
| Reynolds [15] (USA) | 12,594 | 49.0 | 34.6 | 18.0 | ||||
| Richardson [5] (USA) | 5700 | 63.0 | 56.6 | 33.8 | ||||
| Shi [16] (China) | 416 | 64.0 | 30.5 | 14.4 | ||||
| Wang [17] (China) | 138 | 56.0 | 31.2 | 10.1 | 21.6 vs 58.3 | 5.9 vs 22.2 | ||
| Yang [18] (China) | 52 | 51.9 | n/a | 17.0 | 10.0 vs 22.0 | |||
| Zhang [19] (China) | 140 | 57.0 | 30.0 | 12.1 | 24.4 vs 37.9 | 13.8 vs 11.0 | ||
| Zhou [20] (China) | 191 | 56.0 | 30.0 | 19.0 | 23.0 vs 48.0 | 14.0 vs 31.0 | ||
| Zhu [21] (China) | 7337 | 62 | 24.0 | 12.9 | 2.7 vs 7.8 | |||
CVD.
on medication for either BP or diabetes.
Are these early figures a reflection of a true causal link between the presence of these risk factors and both the propensity to acquire the infection and the prospect of a worse outcome?
In the case series of 1099 patients with confirmed Covid-19 admitted to 552 hospitals in mainland China, 15% had hypertension and 7% had diabetes [2]. Covid-19 was twice as severe in them and the mortality was almost three times as high. In a larger multi-centre case series of 5700 patients with Covid-19 admitted to 12 hospitals New York City, Long Island and Westchester County in the USA, the prevalence was much higher, with one in two patients with hypertension and a third of them with diabetes [4]. Finally, a large study of 7337 confirmed cases of Covid-19 patients admitted to 19 hospitals in the Hubei Province of China between 30 December 2019 and 20 March 2020, of whom 952 had type 2 diabetes, reported a 28-days greater mortality from Covid-19 in people with diabetes compared to non-diabetics (7.8% vs 2.7%) [21]. The association was independent of age, sex and severity of Covid-19 (HR 1.49; 95% CI 1.13 to 1.96). However, no further adjustments were made for concomitant comorbidities.
The groups with more comorbidities and worse outcomes were, as one would expect in an unselected population, older that the groups with fewer comorbidities and better outcome. The majority of the case series reported unadjusted results for either age or presence of comorbidities (Table 1). Covid-19 case fatality rates and CV risk both increase with age and with the presence of concomitant comorbidities, so that the association between Covid-19 and CV risk factors might reflect the confounding effect of these variables. More recently, a large study of 8910 hospitalised patients with Covid-19 from 169 hospitals in North America, Europe and Asia confirmed in a multivariate analysis that significant ‘independent’ predictors of in-hospital death, in addition to age ≥65 years, are presence of coronary heart disease, heart failure, cardiac arrhythmias, COPD, current smoking, but not hypertension or diabetes [13]. Similarly, a smaller study from Italy confirmed age, heart failure and chronic kidney disease (but not hypertension and diabetes) as independent predictors of death in hospitalised Covid-19 patients [11]. Finally, a meta-regression analysis of the association between diabetes and severity and outcome of Covid-19 indicated that the association was explained primarily by age and concomitant presence of hypertension [23]. Further analyses of incoming data will undoubtedly refine our understanding of the interplay between underlying cardiovascular risk and Covid-19.
Is there any plausible explanation for the potential increased susceptibility to SARS-CoV-2 infection and the excess risk of death from Covid-19 of patients with underlying CV disease? Severe infections are recognised triggers for CV disease expression [24], increasing the incidence, severity and mortality from infectious diseases [25]. Numerous mechanisms have been suggested that would explain an increased risk of CV disease [4,26]. They include vascular endothelial cell dysfunction, myocardial depression, stress cardiomyopathy, myocarditis and arrhythmias, coagulopathies and thrombosis. Early predictive markers of severity and worse prognosis in Covid-19 patients have been detected in raised levels of high-sensitivity cardiac troponin I (hs-TnI) levels [18] and high D-dimer levels on admission [20].
Covid-19 and the renin-angiotensin-aldosterone system
The interactions between Coronaviruses (SARS-CoV-1, MERS-CoV and SARS-CoV-2) and the renin-angiotensin-aldosterone system (RAAS), via the angiotensin-converting enzyme 2 (ACE2), have been postulated to modulate the infectivity of these viruses and to influence the prognosis of the ensuing respiratory diseases [27,28].
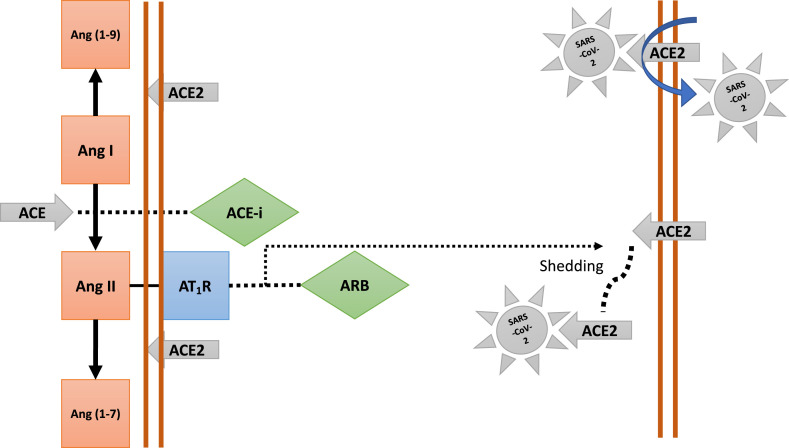
Unlike ACE, ACE2 does not convert Ang I to Ang II, nor do ACE inhibitors (ACE-i) block its activity [29]. The primary substrate of ACE2 is Angiotensin II (Ang II) that is converted to Ang (1–7), thereby attenuating its effects on vasoconstriction, sodium retention and fibrosis (Fig. 1 ). ACE2 also converts Angiotensin I to Ang (1–9). ACE2 is primarily a membrane-bound enzyme, and its soluble circulating levels in the blood are very low. Membrane-bound ACE2 has been identified as a functional receptor for coronaviruses [29,30]. The spike protein of the virus binds to the membrane ACE2, highly expressed in lungs and heart, acting as a carrier for the entry (endocytosis) of the virus into the cell and its replication [27,28] (Fig. 1). Ang II activation of AT1 receptors (type 1) increases soluble ACE2 levels by cleaving the anchoring of ACE2 to the cell membrane (shedding). SARS-CoV-2 bound to soluble ACE2 is, then, kept in solution and prevented from entering the cells [29,30]. Theoretically, ACE-i, might reduce the shedding of membrane ACE2, increasing the chance of SARS-CoV-2 binding and endocytosis. Conversely, ARB would increase shedding of ACE2, increasing the amount of soluble ACE2 to which SARS-CoV-2 would bind and kept soluble (not entering the cell) [29,30] (Fig. 1).
Figure 1.
Schematic interaction between the renin-angiotensin-aldosterone system and SARS-CoV-2. On the left side the role of circulating ACE and ACE2 in the cleavage of angiotensin polypeptides and the site of actions of ACE-inhibitors and ARBs. On the right the binding of SARS-CoV-2 spike proteins to membrane-bound ACE2 that allows the virus to enter the cell (endocytosis) and replicate. Blockade of the AT1R by ARB will increase Ang II circulating levels that will shed the membrane-bound ACE2 resulting in an increase in the levels of soluble ACE2 that will not mediate the entry of the virus in to the cell.
Is there evidence that the use of ACE-i and ARB increase susceptibility to infection by SARS-CoV-2 and worsen the prognosis of patients with Covid-19? Patients with hypertension and diabetes have been seen to experience a high case-fatality rate. This observation has led to concerns about the safety and the potential effects of therapies that block the RAAS [31], commonly used in patients with hypertension, diabetes, heart failure and other CV conditions in which they are indicated. This theory, without direct evidence in support, has led to dangerous calls by some to advise patients to stop ACE-i and ARB therapies [32]. Table 2 summarises the studies to date that have addressed these questions with different study designs and in different case-series. All, but one [10], are retrospective case-series or observational studies of hospitalised patients with confirmed Covid-19 or positivity to SARS-CoV-2 infection. Two of them were in hypertensive patients only [14,34]. In all of them the results indicate, with different degree of confidence, that neither the use of ACE-i or ARB are associated with a greater risk of infection from SARS-CoV-2, or of severity or death in Covid-19 patients. A separate mention is necessary for a population-based case–control study carried out in the Lombardy region of Italy, one of the hardest hit by the pandemic in Europe [10]. In this study 6272 cases of SARS-CoV-2 infection confirmed between 21 February and 11 March 2020 identified through the Regional Health Authority register were matched by sex, age and municipality (1:5) with 30,759 controls registered with the same Health Authority. All were aged 40 years or older. Information on selected drug use and patients’ clinical characteristics were obtained from the Regional Healthcare database on healthcare utilisation. More than half of the selected participants had high blood pressure. In this highly controlled comparison, the use of ACE-i and ARB did not show any association with Covid-19 prevalence, severity or fatality, and no sex difference. Finally, in two studies there was a suggestion that the treatment with ACE-i/ARB would be associated with a lower rate of severity [14] or mortality [34]. This proposition, whilst highly speculative, finds some theoretical corroboration in a possible down-regulation of ACE2 activity in the lungs and trials with ARB as a treatment in patients with Covid-19 are underway (NCT04312009 and NCT04311177).
Table 2.
Published evidence (as to May 12, 2020) addressing the suggestions that the use of ACE-inhibitors and ARBs might be harmful in the context of SARS-CoV-2 susceptibility to infection and in the prognosis of patients with Covid-19.
| Author (Country) | Period | Population | Study design | Main results | Additional results | Authors' conclusions | Strengths and Limitations |
|---|---|---|---|---|---|---|---|
|
Bean [33] (United Kingdom) |
01-Mar to 22-Mar, 2020 | Inpatients with Covid-19 at King's College Hospital and Princess Royal University Hospital in London. Mean age 63 yrs; 52% men; 51.2% with hypertension. | Case-series (n = 205) follow-up 7-days from symptoms onset to primary end point (death or transfer to ICU). 53 patients reached end-point. | Lower death rate or transfer to ICU in patients on an ACE-i (OR 0.29, 95% CI 0.10 to 0.75, p < 0.01), adjusting for age, gender, comorbidities (hypertension, diabetes mellitus, ischaemic heart disease and heart failure). | – | No evidence for ACE-i increasing the short-term severity of Covid-19 disease. | Small sample size. Short follow-up. |
|
Conversano [11] (Italy) |
27-Feb to 17-Mar, 2020 | Patients admitted to San Raffaele Hospital in Milan with confirmed SARS-CoV-2 pneumonia. Mean age: 63.4 yrs; 68.6% men. | Retrospective case-series (n = 191), 96 (50.2%) with hypertension | Predictors of death in Cox models (HR, 95% CI). All (n = 191): Age (1.1, 1.0–1.2); HF (3.1, 1.3–7.5); CKD (2.1, 1.1–4.0). Hypertensive (n = 96): Age 1.1 (1.0–1.1); HF 2.8 (1.1–6.9). |
No association between use of ACE-i/ARB with mortality in both groups in multivariate models. | In multivariate model, age, heart failure and CKD (but not hypertension, diabetes and current use of ACE-i/ARB) were independent predictors of mortality. | Multivariate models deal with important confounders. Small sample size. Short follow-up. |
|
Guo [8] (China) |
23-Jan to 23-Feb, 2020 | Hospitalised patients with Covid-19 at the Seventh Hospital of Wuhan City. Mean age 58.5 yrs; 48.7% men. | Retrospective case series (n = 187), 32.6% with hypertension. |
– | Death rates with and without use of ACE-i/ARB 36.8% (7/19) and 25.6% (43/168), respectively. | Not primary objective of the study. | Incidental finding. Small sample. Risk of bias and confounding. |
|
Li [9] (China) |
15-Jan to 15-Mar, 2020 | Hospitalised patients with Covid-19 at the Central Hospital of Wuhan. Median age 55.5 yrs; 46.3% men. | Retrospective case series (n = 1178), 362 (30.7%) with hypertension. |
In-hospital mortality 21.3%. Percentage taking ACE-i/ARB did not differ between those with severe and non-severe infections (32.9% vs 30.7%; P = 0.645) nor between non-survivors and survivors (27.3% vs 33.0%; P = 0.34). | Similar findings when data analysed for patients taking ACE-i and ARB. | ACE-i/ARB not associated with severity or mortality of Covid-19 in patients with hypertension. | Small number with hypertension taking ACE-i/ARB who were hospitalized with Covid-19. Not generalisable to all patients with hypertension. Risk of bias and confounding. |
|
Mancia [10] (Italy) |
21-Feb to 11 Mar, 2020 | Residents in Lombardy, 40+ years, beneficiaries of the Regional Health Service as target population (>6 M, ~17% of the Italian population of that age). Mean age 68 yrs; 37% women. | Population-based case–control study (6272 cases matched to 30,759 controls). Case: confirmed infection with SARS-CoV-2. Control: non-infected matched by age, sex and municipality. | [1] No association between use of RAAS blockers and Covid-19 (adjusted OR 0.95, 95% CI: 0.86 to 1.05) for ARB and 0.96, 0.87 to 1.07) for ACE-i or among patients who had severe or fatal disease (adjusted OR 0.83, 0.63 to 1.10) for ARB and 0.91, 0.69 to 1.21 for ACE-i. [2] No sex difference. | [1] Use of ACE-i and ARBs more frequent among patients with Covid-19 than controls. [2] Results apply to both sexes, younger and older. [3] Loop diuretics associated with an increased risk of Covid-19. | No evidence that ACE-i or ARB affect the risk of Covid-19 or its severity. | Large sample size, well-matched controls. Drugs prescribed to outpatients accurately recorded. Information on drug use limited to prescriptions, not actual consumption. Drugs purchased by the patients and those dispensed after Dec 31, 2019, not captured. Unmeasured confounders. |
|
Mehra [12] (Asia, Europe, N America) |
20-Dec 2019 to 15-Mar 2020, deaths at 28-Mar 2020 | Patients with Covid-19 hospitalised in 169 hospitals in 3 continents. | Observational database (n = 8910), 1536 from N America, 5755 from Europe and 1619 from Asia). | 515 died and 8395 survived to discharge. No increased risk of in-hospital death associated with the use of ACE-i (2.1% vs. 6.1%; OR 0.33; 95% CI, 0.20 to 0.54) or ARBs (6.8% vs. 5.7%; OR 1.23; 0.87 to 1.74). | Age>65 yrs, CHD, cardiac arrhythmia, COPD, smoking independently associated with increased risk of in-hospital death. | Results did not confirm concerns of harmful association of ACE-I or ARB with in-hospital death. | Large sample size. International representation of patients. Cannot exclude confounding. No pre-specified hypothesis, increased probability of chance finding. Cause–effect relationship between therapy and survival should not be inferred. |
|
Mehta [13] (USA) |
8-Mar to 12-Apr, 2020 | All patients tested for SARS-CoV-2 at the Cleveland Clinic Health System in Ohio and Florida. Mean age 49 yrs; 40% men; 69% white. | Retrospective cohort analysis (n = 18,472), 2285 taking either ACE-i or ARB, with overlap propensity score (OPS) weighting. | OPS weighting showed no significant association of ACE-i and/or ARB use with SARS-CoV-2 test positivity (OPS–weighted OR 0.97; 95% CI, 0.81–1.15). For use of ACE-i only, OPS–weighted OR 0.89; 0.72–1.10. | Higher likelihood of hospital admission in patients with positive results who were taking either ACE-i/ARB (OR 1.84; 1.22–2.79), or ACEIs only (OR 1.77; 1.07–2.92). | No association between ACE-i or ARB use and SARS-CoV-2 test positivity. | Risk of confounding. No information on duration of drug use before and after testing. Data reflect medication list in the electronic medical record, not actual use, risk of non-ascertainment bias. Most patients white, limited generalisability to other ethnic groups. Small sample size and wide confidence intervals for data on severity. |
|
Meng [14] (China) |
11-Jan to 23-Feb, 2020 | Covid-19 patients with treated hypertension. Median age 64.5 yrs; 57% men. | Retrospective review of medical records (n = 42 out of 417) admitted to Hospital in the period. | During hospitalisation, 12 patients in the non ACE-i/ARB group (48%) were severe and one died. In the ACE-i/ARB group only 4 patients (23.5%) were severe and none died. | ACE-i and ARB associated with increased CD3 and CD8 T cell counts and lower peak viral load. | Patients receiving ACE-i or ARB had lower rate of severe diseases. | Observational case-series. Small sample, open to bias and confounding. |
|
Reynolds [15] (USA) |
01-Mar to 15-Apr, 2020 | All patients in the New York University (NYU) Langone Health electronic health record who had Covid-19 test results recorded. | Observational study using Electronic Health Records (n = 12,594), 5894 with positive test and 1002 with severe illness (defined as admission to ICU, mechanical ventilation or death). | [1] Among 12,594 patients, 4357 (34.6%) had hypertension; of these, 2573 (59.1%) were Covid-19–positive and 1784 (40.9%) were Covid-19–negative. The results in matched patients ruled out an absolute difference of >10% in the likelihood of a positive test with at least 97.5% certainty for ACE inhibitors, ARBs, beta-blockers, calcium-channel blockers, and thiazide diuretics. [2] Among the 2573 patients with hypertension and positive results on Covid-19 testing, 634 (24.6%) had severe Covid-19. The likelihood of severe Covid-19 among patients who tested positive, matched on propensity score for previous treatment, ruled out a ≥10% in the likelihood of severe illness with at least 97.5% certainty for all the medication classes. | – | No increase in likelihood of a positive test for Covid-19 or risk of severe Covid-19 in patients who tested positive in association with five common classes of anti-hypertensive medications, including ACE-i and ARB. | Propensity-score matching creates balance between comparison groups. Variations in diagnostic characteristics for Covid-19 testing methods may have led to misclassification of Covid-19 status. Some patients underwent multiple tests, increased likelihood of identifying disease. Possible Covid-19 are not tested, particularly those who are not hospitalised. Results may overestimate proportion of severe Covid-19. Ascertainment of medication use may not reflect actual drug exposure. |
|
Richardson [5] (USA) |
01-Mar to 04-Apr, 2020 | Patients admitted to 12 hospitals in NYC, Long Island and Westchester County, within the Northwell Health system with Covid-19 confirmed positive to SARA-CoV-2. Median age 63 yrs; 39.7% women; 56.6% with hypertension. | Case-series (n = 5700). Outcomes assessed in 2634 who were either discharged or died (latter n = 553). | – | [1] Of 2,411, 189 were on ACE-i and 267 on ARBs. During admission, 91 and 136, respectively continued their RAAS blocker as in-patients. [2] Suppl. Table 2. Hypertensives on ACE-i: died 55, discharged 113; on ARB: died 75, discharged 170. Not on ACE-i/ARB: died 254; discharged 699. | This case series design cannot address the complexity of the question. | Results are unadjusted for common confounders. Study population only included patients within the NYC metropolitan area. Electronic health record database. Brief post-discharge follow-up. Clinical outcomes only available in 46.2% of patients. |
|
Zhang [34] (China) |
31-De, 2019 to 20-Feb, 2020. Final date of follow up 07-Mar, 2020 | Patients with hypertension diagnosed with Covid-19 and admitted to 9 hospitals in Hubei Province. | Retrospective multi-center study (n = 1128), 18 taking ACE-I/ARB and 940 not on them. | Risk for all-cause mortality lower in the ACE-i/ARB group vs non-ACE-i/ARB group (adjusted HR 0.42; 95% CI 0.19–0.92; P = 0.03). In a propensity score-matched analysis followed by adjusting imbalanced variables, lower risk of Covid-19 mortality in patients who received ACE-i/ARB vs those who did not receive ACE-i/ARB (adjusted HR 0.37; 0.15–0.89; P = 0.03). | Compared to use of other antihypertensive drugs, ACEI/ARB was associated with decreased mortality (adjusted HR, 0.30; 95% CI, 0.12–0.70; P = 0.01). | Inpatient use of ACE-i/ARB was associated with lower risk of all-cause mortality compared with ACE-i/ARB non-users | May not reflect outpatient management. Modest sample size in those on ACE-i/ARB. Some parameters not available in all patients. Antihypertensive drugs not matched or adjusted when comparing ACE-i/ARB and non-ACE-i/ARB groups. [5] Not retrieving pre-hospital self-medications. |
Conclusions
Can we formulate evidence-based answers to the questions set in the introduction? With a necessary cautionary note that the evidence around the links between Covid-19 and CV disease is accruing at a fast pace, to date we can conclude that:
-
(a)
the greater susceptibility of individuals with underlying CV risk factors or overt disease to develop more severe Covid-19 with higher mortality rate is likely to be confounded, in part, by age and the type of co-morbidities. However, patients with heart failure or chronic kidney disease might show an excess risk and vigilance and further research is necessary to establish a causality link.
-
(b)
Neither ACE-i nor ARB are associated with greater risk of SARS-Cov2 infection, or severity or risk of death in patients with Covid-19. The evidence to date does not support the discontinuation of ACE-i or ARB. Patients on these drugs, whether for high blood pressure, heart failure, coronary heart disease or renal protection in diabetes, should not stop them for fears related to vulnerability to Covid-19 unless advised by a doctor and under strict medical supervision and with the addition of a suitable replacement medicine. These conclusions are concordant with International position statements on the issue [[35], [36], [37], [38], [39]].
Declaration of Competing Interest
No conflict of interest to declare.
Handling Editor: A. Siani
References
- 1.John Hopkins Coronavirus Research Centre 2020. https://coronavirus.jhu.edu/map.html
- 2.Guan W., Hu N.Y., Liang W., Ou C., He J., Liu L. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang J., Zheng Y., Gou X., Pu K., Chen Z., Guo Q. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Madjid M., Safavi-Naeini P., Solomon S.D. Potential effects of Coronaviruses on the cardiovascular system. A review. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1286. online March 27. [DOI] [PubMed] [Google Scholar]
- 5.Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W. Presenting characteristics, comorbidities and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. J Am Med Assoc. 2020 doi: 10.1001/jama.2020.6775. online April 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen N., Zhou M., Dong X., Qu J., Gang F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen T., Wu D., Chen H., Yan W., Yang D., Chen G. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo T., Fan Y., Chen M., Wu X., Zhang L., He T. Cardiovascular implications of fatal outcomes of patients with Coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1017. online March 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li J., Wang X., Chen J., Zhang H., Deng A. Association of Renin-Angiotensin system inhibitors with severity or risk of death in patients with hypertension hospitalized for Coronavirus Disease 2019 (COVID-19) infection in Wuhan, China. JAMA Cardiology. 2020 doi: 10.1001/jamacardio.2020.1624. online April 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mancia G., Rea F., Ludergnani M., Apolone G., Corrao G. Renin-angiotensin-aldosterone system blockers and the risk of covid-19. N Engl J Med. 2020 doi: 10.1056/NEJMoa2006923. online May 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conversano A., Melillo F., Napolano A., Fominskiy E., Spessot M., Ciceri F. RAAs inhibitors and outcome in patients with SARS-CoV-2 pneumonia. A case series study. Hypertension. 2020 doi: 10.1161/HYPERTENSIONAHA.120.15312. online May 8. [DOI] [PubMed] [Google Scholar]
- 12.Mehra M.R., Desai S.S., Kuy S.R., Henry T.D., Patel A.N. Cardiovascular disease, drug therapy, and mortality in Covid-19. N Engl J Med. 2020 doi: 10.1056/NEJMoa2007621. online May 1. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Mehta N., Kalra A., Nowacki A.S., Anjewierden S., Han Z., Bhat P. Association of use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers with testing positive for Coronavirus Disease 2019 (COVID-19) JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1855. online May 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meng J., Xiao G., Zhang J., He X., Ou M., Bi J. Renin-angiotensin-system inhibitors improve the clinical outcome of COVID-19 patients with hypertension. Emerg Microb Infect. 2020;9(1) doi: 10.1080/22221751.2020.1746200. online March 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reynolds H.R., Adhikari S., Pulgarin C., Troxel A.B., Iturrate E., Johnson S.B. Renin-Angiotensin-Aldosterone system inhibitors and risk of Covid-19. N Engl J Med. 2020 doi: 10.1056/NEJMoa2008975. online May 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi S., Qin M., Shen B., Cai Y., Liu T., Yang F. Association of cardian injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.0950. online March 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. J Am Med Assoc. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang J., Dong X., Cao Y., Yuan Y., Yang Y., Yan Y. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020 doi: 10.1111/all.14238. online February 19. [DOI] [PubMed] [Google Scholar]
- 20.Zhou F., Yu T., Du R., Fan G., Liu Y., Xiang J. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu L., She Z.G., Cheng X., Qin J.-J., Zhang X.-J., Cai J. Association of blood glucose control and outcomes in patients with covid-19 and pre-existing type 2 diabetes. Cell Metabol. 2020 doi: 10.1016/j.cmet.2020.04.021. online May 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lippi G., Wong J., Henry B.M. Hypertension and its severity or mortality in Coronavirus Disease 2019 (COVID-19): a pooled analysis. Pol Arch Intern Med. 2020 doi: 10.20452/pamw.15272. online April 2. [DOI] [PubMed] [Google Scholar]
- 23.Huang I., Lim M.A., Pranata R. Diabetes mellitus is associated with increased mortality and severity of disease in COVID-19 pneumonia. A systematic review, meta-analysis, and meta-regression. Diabetes Metab Syndr. 2020;14:395–403. doi: 10.1016/j.dsx.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cowan L.T., Lutsey P.L., Pankow J.S., Matsushita K., Ishigami J., Lakshminarayan K. Inpatient and outpatient infection as a trigger of cardiovascular disease: the ARIC study. J Am Heart Assoc. 2018;7(22) doi: 10.1161/JAHA.118.009683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dhainaut J.-F., Claessens Y.-E., Janes J., Nelson D.R. Underlying disorders and their impact on the host response to infection. Clin Infect Dis. 2005;41(suppl 7):S481–S489. doi: 10.1086/432001. [DOI] [PubMed] [Google Scholar]
- 26.Li G., Hu R., Gu X. A close-up on COVID-19 and cardiovascular diseases. Nutr Metabol Cardiovasc Dis. 2020 doi: 10.1016/j.numecd.2020.04.001. online Apr 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuba K., Imai Y., Rao S., Gao H., Guo F., Guan B. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11(8):875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zheng Y.-Y., Ma Y.-T., Zhang J.-Y., Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17:259–260. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vaduganathan M., Vardeny O., Michel T., McMurray J.J.V., Pfeffer M.A., Solomon S.D. Renin-Angiotensin-Aldosterone system inhibitors in patients with Covid-19. N Engl J Med. 2020;382:1653–1659. doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Danser A.H.J., Epstein M., Batlle D. Renin-Angiotensin System blockers and the COVID-19 pandemic. At present there is no evidence to abandon renin-angiotensin system blockers. Hypertension. 2020;75 doi: 10.1161/HYPERTENSIONAHA.120.15082. online May 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fang L., Karakiulakis G., Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;4:e1. doi: 10.1016/PII. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aronson J.K., Ferner R.E. Drugs and the renin-angiotensin system in covid-19. Br Med J. 2020;369:m1313. doi: 10.1136/bmj.m1313. [DOI] [PubMed] [Google Scholar]
- 33.Bean D.M., Kraljevic Z., Searle T., Bendayan R., Pickles A., Folarin A. Treatment with ACE-inhibitors is associated with less severe disease with SARS-Covid-19 infection in a multi-site UK acute hospital trust. MedRxiv. 2020 doi: 10.1002/ejhf.1924. www.medrxiv.org/content/10.1101/2020.04.07.20056788v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang P., Zhu L., Cai J., Lei F., Qin J.-J., Xie J. Association of inpatient use of angiotensin converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID-19. Circ Res. 2020 doi: 10.1161/CIRCRESAHA.120.317134. online April 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.https://www.eshonline.org/spotlights/esh-statement-covid-19/ 2020. (Accessed 11 May 2020).
- 36.2020. https://www.escardio.org/Councils/Council-on-Hypertension-(CHT)/News/position-statement-of-the-esc-council-on-hypertension-on-ace-inhibitors-and-ang
- 37.2020. http://www.ccs.ca/images/Images_2020/CCS_CHFS_statement_regarding_COVID_EN.pdf
- 38.2020. https://ish-world.com/news/a/A-statement-from-the-International-Society-of-Hypertension-on-COVID-19/
- 39.2020. https://www.acc.org/latest-in-cardiology/articles/2020/03/17/08/59/hfsa-acc-aha-statement-addresses-concerns-re-using-raas-antagonists-in-covid-19