Abstract
The increasing use of gadolinium-based contrast agents (GBCAs) for magnetic resonance imaging is leading to widespread contamination of freshwater and drinking water systems. Contrary to previous assumptions that GBCAs are stable throughout the water cycle, they can degrade. The stability of GBCAs depends largely on their organic ligands, but also on the physicochemical conditions. There is specific concern regarding UV end-of-pipe water treatments, which may degrade GBCAs. Degradation products in drinking water supplies can increase the risk of adverse health effects. This is of particular relevance where the raw water for drinking water production has a higher proportion of recycled wastewater. GBCAs concentrations in aquatic systems, often referred to as anthropogenic gadolinium, are determined using a variety of calculation methods. Where anthropogenic gadolinium concentrations are low, the inconsistent use of these methods results in high discrepancies and high levels of uncertainty. The current COVID-19 crisis will, in the short-term, drastically decrease the input of GBCAs to freshwater systems. Temporal variations in anthropogenic gadolinium concentrations in river water can be used to better understand river-aquifer interactions and groundwater flow velocities. Collecting urine from all patients following MRI examinations could be a way forward to halt the generally increasing concentrations of Gd in drinking water systems and recover this technologically critical element.
Keywords: Anthropogenic gadolinium, Gadolinium-based contrast agents, Gadolinium anomaly, Drinking water, Micropollutants, COVID-19
Graphical abstract
Highlights
-
•
Rising concentration of gadolinium-based contrast agents (GBCAs) in drinking water.
-
•
Stability of GBCAs is determined by their organic ligands.
-
•
UV end-of-pipe treatment may enhance the risks posed by GBCAs in drinking water.
-
•
Inconsistent use of methods to calculate Gd anomalies and anthropogenic Gd.
-
•
Temporal Gd patterns in rivers can improve understanding of subsurface systems.
1. Magnetic resonance imaging and the increasing use of gadolinium-based contrast agents
Magnetic resonance imaging (MRI) is a versatile radiological tool for generating detailed images that can be used to assist in the diagnosis of a variety of diseases affecting the brain, spinal cord, heart, blood vessels, bones, and joints; it can also be used to check the health of organs such as e.g. breasts, kidneys, ovaries, the liver, the pancreas, and the prostate. In contrast to X-rays and computerized tomography (CT) scans, MRI does not employ cell-damaging ionizing radiation, but the relaxation time of hydrogen nuclei excited by a strong external magnetic field to image organs and physiological processes in the body. Gadolinium-based contrast agents (GBCAs) are administered in MRI to increase the contrast of these images and allow radiologists to more accurately identify neoplastic, inflammatory, and functional abnormalities (Bellin, 2006).
GBCAs are strong chelate complexes in which the trivalent gadolinium ion (Gd3+) is chelated by polyaminocarboxylic acid to avoid the toxic effects of free Gadolinium (Gd). Because the ionic radius of Gd is close to that of calcium (Ca) (107.8 pm for Gd3+ and 114 pm for Ca2+), Gd inhibits physiological processes that rely on Ca (Bellin and Van Der Molen, 2008). Two general types of GBCA have been used to date: linear (“open chain”) chelates and macrocyclic chelates, both of which can exist in either ionic or non-ionic forms (Fig. S1). The ligands of clinically approved GBCAs are all octadentate, with all donor atoms coordinated.
GBCAs shorten the longitudinal (T1) and transverse (T2 and T2∗) relaxation time constants of adjacent hydrogen nuclei in MRI and thereby enhance contrast (Bellin and Van Der Molen, 2008; Caravan et al., 1999). Gd is specifically well suited for the desired signal enhancement due to its strong magnetic moment and the presence of an additional water molecule in the inner sphere, which completes the nine-fold coordination of Gd within GBCA complexes (Burai et al., 1997; Chan and Wong, 2007; Micskei et al., 1993). Because of its versatility, availability, and importance in modern diagnostics, the use of MRI and consequently also of GBCAs is constantly increasing (Organisation for Economic Cooperation and Development (OECD), 2017).
GBCAs are administered in 33–50% of all MRI examinations, either intravenously or by intra-articular injection (Idée et al., 2008; Thomsen, 2017). Due to their small size (around 500 Da), they are rapidly cleared from the intravascular space into the interstitial space, and their biodistribution is therefore non-specific (Bellin and Van Der Molen, 2008). GBCAs are eliminated unmetabolized, mostly by passive glomerular filtration in the kidney. The residence time of a GBCA within the body depends on the type of complex administered and the renal function of the patient. The plasma elimination half-lives of GBCAs range from 1.5 h for healthy individuals up to 34 h for patients with reduced renal function (Idée et al., 2008; Joffe et al., 1998).
GBCAs are generally considered to be safe and adverse effects are rarely observed (Kanda et al., 2016). However, in 2006 first links were established between nephrogenic systemic fibrosis (NSF) and GBCAs (Grobner, 2006; Marckmann, 2006). Concerns have since increased, especially as Gd deposits have been reported in brains, bone, skin, and other tissues following GBCA administration, even in healthy patients (Kanda et al., 2016; Le Fur and Caravan, 2019; Montagne et al., 2016; Nehra et al., 2018; Xia et al., 2010). The European Medicines Agency (EMA) therefore released a warning and suspended the use within the EU of certain linear GBCAs (Table 1 ) that are considered to present the highest risk of inducing NSF (European Medicines Agency, 2017). This has been legislated in all EU member states. The US Food and Drug Administration (FDA) has issued an update of their Medication Guides in relation to the use of GBCAs, requiring a new class warning especially for patients with kidney dysfunction (Food and Drug Administration, 2018, 2017). Other regulatory bodies in Australia, Japan, and Canada have also released revised precautionary advices (Le Fur and Caravan, 2019). The use of GBCAs is not recommended for patients with renal failure.
Table 1.
Characteristics and stability properties of approved gadolinium-based contrast agents (worldwide).
| Name |
Acronym |
Gd-DTPA |
Gd-DTPA-BMA |
Gd-DTPA-BMEA |
Gd-BOPTA |
Gd-EOB-DTPA |
Gd-DOTA |
Gd-HP-DO3A |
Gd-BT-DO3A |
|---|---|---|---|---|---|---|---|---|---|
| Generic name |
Gadopentetate dimeglumine |
Gadodiamide |
Gadoversetamide |
Gadobenate dimeglumine |
Gadoxetate disodium |
Gadoterate meglumine |
Gadoteridol |
Gadobutrol |
|
| Trade name | Magnevist® | Omniscan® | OptiMARK® | MultiHance® | Primovist®/Eovist® | Dotarem®/Artirem® | ProHance® | Gadovist®/Gadavist® | |
| Manufacturer | Bayer HealthCare | GE Healthcare | Guerbet | Bracco | Bayer HealthCare | Guerbet | Bracco | Bayer HealthCare | |
| Year of FDA approval | 1988 | 1993 | 1999 | 2004 | 2008 | 2013 | 1992 | 2011 | |
| Current legal status (Europe) | restricted to intra-articular | suspended | suspended | restricted to liver scans | maintained | maintained | maintained | maintained | |
| Type | linear | linear | linear | linear | linear | macrocyclic | macrocyclic | macrocyclic | |
| Charge | di-ionic | non-ionic | non-ionic | di-ionic | di-ionic | ionic | non-ionic | non-ionic | |
| Concentration | [M] | 0.5 | 0.5 | 0.5 | 0.5 | 0.25 | 0.5 | 0.5 | 1 |
| Standard dose | [mmol/kg] | 0.1 | 0.1 | 0.1 | 0.1 | 0.025 | 0.1 | 0.1 | 0.1 |
| Excess Ligand | 0.10% | 5% | 10% | none | 0.50% | none | 0.10% | 0.10% | |
| log Ktherm | [1] | 22.1 | 16.9 | 16.6 | 22.6 | 23.46 | 25.6 | 23.8 | 21.8 |
| log Kcond | [1] | 17.7 | 14.9 | 15 | 18.4 | 18.7 | 19.3 | 17.1 | 14.7 |
| log Kcond | [2] | 18.4 | 14.9 | 15 | 18.4 | 18.7 | 17.2 | 17.1 | 16.1 |
| log Kcond calculated for pH 4.0 | [2] | 11.2 | 10.8 | 10.8 | 11.1 | 11.5 | 9.5 | 9.9 | 9 |
| log Kcond (modelled) | [3] | 6.30 | 4.17 | not available | not available | not available | 7.24 | not available | not available |
| Kinetic stability (dissociation half-life at pH 1.0) | [1] | 10 min | 35 s | not available | not available | not available | >1 month | 3 h | 24 h |
Log Ktherm and log Kcond are the thermodynamic and conditional stability constants, respectively; [1] Morcos (2008) and references therein, [2] Le Fur and Caravan (2019), [3] Prybylski et al. (2017).
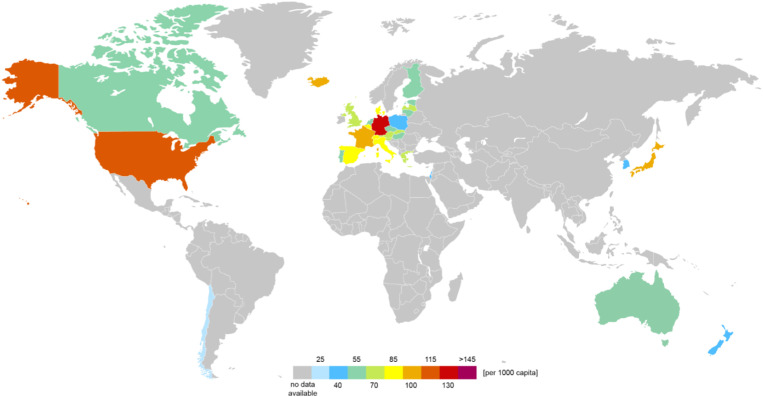
Since GBCA’s use was first approved in 1988, more than 460 million doses have been administered worldwide up to 2018 (Gibby et al., 2019). In 2016, almost 200,000 MRI scans using GBCAs were conducted per day within the EU and the US combined (Organisation for Economic Cooperation and Development (OECD), 2017). In Germany, the country with the highest MRI use per capita (Fig. 1 ), more than 32,000 MRI scans are carried out per day, resulting in an estimated annual emission of 4 tons of gadolinium (assuming 50% of all MRIs use GBCAs with 1.1 g Gd per MRI scan); the corresponding figure for the whole of the EU is 19 tons, 21 tons for the US, and 0.3 tons for Switzerland (data from 2016). Vriens et al. (2017) calculated a total river-borne discharge of 0.78 tons of Gd from the concentrations of the four major rivers leaving Switzerland, with a sewage treatment plant (STP) contribution of around 83% for the same year. Compared to the late 1990s, the Gd emission in Germany has doubled over the last 20 years (Bau and Dulski, 1996; Kümmerer and Helmers, 2000). Increasing concentrations of Gd, which can be attributed to the use of GBCAs, have been found in rivers, groundwater, and estuaries around the world (Hatje et al., 2016; Lerat-Hardy et al., 2019; Tepe et al., 2014). In the vicinity of STP outlets Gd concentrations are substantially higher than the geogenic background (in general about a few nanograms per liter) with up to 86 μg per liter (Parant et al., 2018).
Fig. 1.
Total annual MRI examinations: global distribution based on the latest data available (Organisation for Economic Cooperation and Development (OECD), 2017). Data from Austria, Great Britain, New Zealand, Switzerland, and Portugal are restricted to in-hospital data.
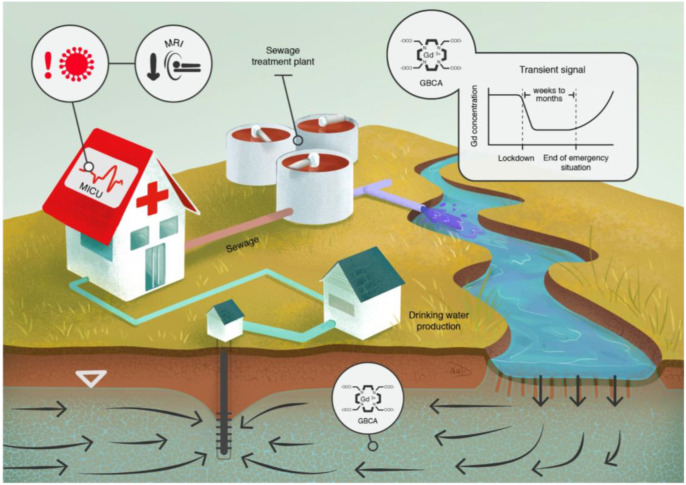
At present, the global spread of the SARS-CoV-2 coronavirus and the COVID-19 disease lead to a temporal decrease in GBCAs use. The paralysis in economic production and socialization leaves strong signals in the environment (Callaway et al., 2020). In addition to the closure of factories and businesses, a major concern for all countries has been the drastic effect on their health systems. Hospitals around the world have been preparing for this unprecedented situation for weeks and ramped up the number of medical intensive care units (MICUs). Therefore, non-essential medical consultations, examinations and surgeries, including the use of MRI, have been postponed in order to free up hospital capacities and personnel. Medical facilities are currently performing about 80% fewer MRI examinations and a significant reduction of GBCA emissions is to be expected. This will result in a temporal reduction of Gd concentrations in freshwater systems (Fig. 2 ). Measuring and analyzing this transient Gd signal could improve our understanding of environmental systems and provide a better database for the risk assessment of waterworks regarding inputs of xenobiotics or pathogens.
Fig. 2.
Due to COVID-19 and its implications, changing anthropogenic gadolinium concentrations in surface water and groundwater could be used to improve the understanding of complex environmental systems.
In future, GBCA concentrations in aquatic environments are likely to continue to rise due to the constantly increasing use of MRI. Even though actual increasing GBCA concentrations are still low, this could become of concern in settings where drinking water is produced from raw water resources with a high proportion of recycled wastewater. In this paper, we review on the stabilities of GBCAs, their behavior in freshwater systems, and the importance of the method to differentiate between naturally occurring and anthropogenic Gd. We conclude our review by suggestions how to reduce GBCAs emissions.
2. Stability of gadolinium-based contrast agents determined by their organic ligands
It is not yet fully clear whether GBCAs themselves, their transformation products, or the release of free Gd are causing adverse health effects; these remain fundamental topics for a better risk assessment (Le Fur and Caravan, 2019). Every substitution of Gd3+ from GBCA complexes by other metal ions, known as transmetallation, can also increase the toxicity of GBCAs by releasing toxic Gd3+. Iron (Fe3+), zinc (Zn2+) and copper (Cu2+) in particular, are in focus to be possible substituents because of their similar (or higher) thermodynamic stability and in vivo concentrations (Le Fur and Caravan, 2019; Semelka et al., 2019; Swaminathan, 2016). The degree of transmetallation of GBCAs in humans has been assessed from zinc enhancement in urine following GBCA administration, which yielded evidence of possible reactions with other trace metals (Rogowska et al., 2018). The inner sphere coordination of a single water molecule within the Gd complex could be a critical aspect because it is potentially substituted by other, preferably negatively charged ligands (Burai et al., 1997). Whether this reduces the stability of GBCAs remains unclear and would be an important subject for future research. Gd complexes are susceptible to dissociation and in vivo dissociation can be favored by any interaction with biological competitors for the original GBCA ligands, such as the adenosine triphosphate (ATP) present in human serum (Bazzicalupi et al., 2012; Port et al., 2008). The same principle also applies in freshwater systems, where other competitive ligands exist.
The kinetic, thermodynamic, or conditional stabilities of GBCAs are measures that are often used to compare different species of GBCAs regarding their possible transformation, but their use is inconsistent and questionable. The thermodynamic stability of Gd complexes in solution is described as an equilibrium between Gd ions [Gd], their ligands [L], and the complexed form [GdL]. For all GBCAs the number of involved ligands is one, which simplifies the equation to:
| Equation 1 |
and the thermodynamic stability constant Ktherm is defined as
| Equation 2 |
The kinetic stability depends on the activation energy (Ea) and Gd complexes are kinetically stable/inert if Ea is high enough to prevent the formation of any other complexes. The “inertness” or “stability” of metal complexes is operationally defined. Beyer and Angulo Cornejo (2012) regard metal complexes to be inert if the exchange of ions takes longer than a minute at 25 °C. Because the Ea cannot include any possibly present catalyst which potentially lower reaction barriers, Morcos (2007) used the dissociation half-life at pH 1.0 to assess and quantify the kinetic stability of GBCAs. The half-lives vary from a few seconds to more than one month (Table 1). It may be convenient to compare kinetic stabilities with biological half-lives, but neither is able to indicate whether or not GBCAs are transformed within the human body not to quantify the extent of such processes. The dissociation half-life depends on both pH and temperature. For example, the dissociation half-life of the macrocyclic Gd-DOTA at pH 1 is 26.4 h at 37 °C but 338 h at 25 °C (Idée et al., 2009), and the DOTA ligand has a dissociation half-life of 85 days at pH 2 and more than 200 days at pH 5 (Wang et al., 1992). Idée et al. (2009) used a comparative subdivision of the kinetic inertness (into low, medium, or high) to compare different GBCAs, but cannot resolve the question of GBCA transformation within the human body.
The toxicological need for a measure that applies at the physiological pH of 7.4 has been tackled using the concept of conditional stability, expressed as Kcond. It can be measured in vivo and considers the protonation constants of the ligand. These constants can vary slightly, depending on the methods used, i.e., potentiometric measurement or competition experiments (Idée et al., 2009). Frenzel et al. (2008) confirmed Kcond as an good indicator for the in vivo stability of GBCAs based on the release kinetics in human serum at physiological temperature and pH. Prybylski et al. (2017) came to a different conclusion; remodeling the data from Frenzel et al. (2008) using the dissociation rate and the balancing aspect of the association between Gd and the GBCA ligand, they found that GBCAs were less stable in humans than previously indicated by Kcond.
The stability constants in Table 1 show that macrocyclic GBCAs are more stable than linear GBCAs, and that the linear ionic GCBAs are more stable than linear non-ionic GBCAs. The weakness in all stability concepts, however, is the assumption of equilibrium conditions since environmentally relevant conditions are likely to be more complex and not always in equilibrium (Prybylski et al., 2017). The inversely proportional relationship between the release of Gd from GBCAs and their stability is generally accepted, but the extent to which GBCAs can be transformed within the human body and the exact processes involved still remains unresolved.
3. The concentration of gadolinium-based contrast agents is increasing in freshwater environments
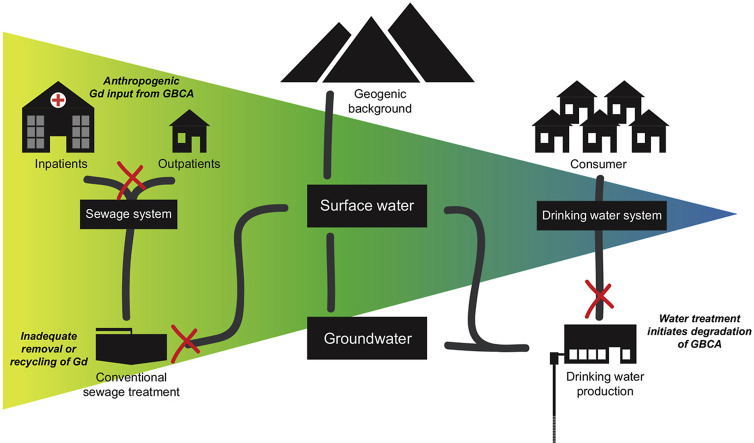
Following their excretion via urine after the MRI, GBCAs enter the sewage system and are released into surface waters as they are not removed by conventional sewage treatment plants. Anomalous occurrences of Gd in rivers were first reported in 1996 (Bau and Dulski, 1996). Since then GBCAs have been found in the effluents from hospitals, sewage water treatment plants, as well as other surface waters (Birka et al., 2013; Künnemeyer et al., 2009; Lindner et al., 2013). Increased Gd concentrations have even been reported in rural areas where there are no MRI facilities in the catchments of the local sewage treatment plants (Brünjes et al., 2016; Rabiet et al., 2009). This is due to the large number of patients who receive MRI scans as outpatients and are then sent home. Elevated Gd concentrations have been found in drinking water and are of increasing concern to waterworks, as well as to the general public (Kulaksiz and Bau, 2011a; Lindner et al., 2015; Morteani et al., 2006; Richardson and Kimura, 2016; Schmidt et al., 2019; Tepe et al., 2014). Gadolinium originating from GBCAs is one of a series of emerging contaminants released from sewage systems, which makes it a suitable indicator for other sewage water-borne xenobiotics (Reoyo-Prats et al., 2018).
In general, most studies in freshwater systems pool GBCAs under the term “anthropogenic Gd” (Gdanth), which does not imply the transformation of GBCAs. We hereafter refer to Gdanth when we do not distinguish between GBCAs and their transformation products. Anthropogenic Gd has been globally detected, mainly in densely populated areas with highly developed health systems; in Europe (Bau and Dulski, 1996; Dia et al., 2000; Elbaz-Poulichet et al., 2002; Knappe et al., 2005; Möller et al., 2003, 2000; Petelet-Giraud et al., 2009), in North America (Barber et al., 2006; Bau et al., 2006; Hatje et al., 2016; Verplanck et al., 2005), in Oceania (Lawrence et al., 2009; Lawrence and Bariel, 2010, Lawrence and Kamber, 2006), in South America (de Campos and Enzweiler, 2016; Merschel and Bau, 2015), Africa (Atinkpahoun et al., 2020), and in Asia (Nozaki et al., 2000; Ogata and Terakado, 2006; Song et al., 2017).
Due to the widespread contamination of freshwater environments with Gdanth, a variety of hydrogeological investigations have used it as an ideal tracer in different hydrochemical settings, assuming that all types of GBCAs do not undergo degradation once released from STPs (Barber et al., 2006; Bichler et al., 2016; Klaver et al., 2014; Möller et al., 2011, 2000; Petelet-Giraud et al., 2009). Depending on the operating mode of an STP, the effluent Gd concentration may show a temporal variation (daily to weekly) because most MRI scans are carried out during the daytime from Monday to Friday (Telgmann et al., 2012). This transient signal can propagate into the groundwater and can be used to calculate groundwater transit times (Brünjes et al., 2016).
Holzbecher et al. (2005) assumed an environmental half-life of 130 days for Gd-DTPA from modelling breakthrough curves of column experiments. Based on these findings, Massmann et al. (2008) used this half-life to calculate groundwater residence times from the attenuation of the Gdanth signal in the aquifer. Predictions based on this approach depend on the precise quantification of the hydrodynamic dispersion and dilution, which can be susceptible to large errors. Further, the degradation of the ligand DTPA and its inorganic complexes is in general thought to be photochemical (Hinck et al., 1997; Means et al., 1980; Metsärinne et al., 2004).
Gd-DTPA appeared relatively inert, for example in column experiments carried out over a 70 day period under a range of redox conditions (Dulski et al., 2011; Menahem et al., 2016). In the presence of other metal ions, especially Fe3+, Zn2+, Cu2+, other heavy lanthanides, or Yttrium (Y3+), transmetallation can be slow due to the low concentrations involved, but it cannot be neglected over long time scales (Möller et al., 2011; Möller and Dulski, 2010). A modeling study by Schijf and Christy (2018) has shown that elevated concentrations of calcium and magnesium can destabilize Gd-DTPA in seawater.
4. Determination of anthropogenic gadolinium and differentiation from naturally occurring gadolinium
Gadolinium is also naturally occurring in the environment as a result of the dissolution of minerals. It is one of the elements in the lanthanide series. These elements are frequently referred to as the rare earth elements (REE), a term that usually also includes the elements scandium (Sc) and yttrium (Y). Except for two elements, all lanthanides exist only in a trivalent oxidation state. Cerium (Ce) can exist as both Ce3+ and Ce4+ and europium (Eu) as both Eu2+ and Eu3+, which affects their solubility and melting temperature. Due to their different redox behavior, these two elements are decoupled from the coherent chemical behavior of the rest of the lanthanides (Goldstein and Jacobsen, 1988; Möller et al., 2002). The abundance of lanthanides follows the Oddo-Harkins rule; elements with even atomic numbers are naturally more abundant than those with odd atomic numbers. This leads to a typical zig-zag shape on logarithmic plots of concentration versus atomic number. To avoid this zig-zag shape, lanthanide concentrations are usually normalized against a geological reference which allows anthropogenically induced anomalies to be detected, as has been demonstrated for Gd, lanthanum (La) and samarium (Sm) in aqueous samples (Kulaksiz and Bau, 2013, 2011b).
For water samples, reference compositions such as those of the Post-Archean Australian Shale (PAAS) composite (McLennan, 1989), the North American Shale Composite (NASC) (Gromet et al., 1984), the Upper Continental Crust (UCC) (Taylor and McLennan, 1985), or (rarely) the Mud of Queensland (MUQ) (Kamber et al., 2005) compositions, have been used for normalization. The choice of geological reference has been a matter of debate. The use of a newly defined European Shale (EUS) reference has been recommended recently for European water samples to increase acceptance by national regulatory authorities (Bau et al., 2018). Averaging data from PAAS, NASC and EUS, the same authors have also suggested a World Shale (WSH) (Bau et al., 2018). Global-scale investigations could benefit from worldwide use of the WSH reference. However, for investigations of local element enrichment phenomena, site-specific geological reference compositions may be more appropriate as they better represent the natural background.
At present, inductively coupled plasma mass spectrometry (ICP-MS) is the technique most commonly used to measure lanthanides. To increase the analytical sensitivity and ensure precise measurements of low lanthanide concentrations in aqueous solutions with complex matrices, liquid chromatography (LC) can be directly coupled to ICP-MS systems for matrix removal and lanthanide preconcentration (Brünjes et al., 2016; Hathorne et al., 2012; Lagerström et al., 2013). Offline or online preconcentration methods often use ion exchange resins with iminodiacetate functional groups for the enrichment of lanthanides (Pyrzynska et al., 2016). A crucial step before any preconcentration by ion exchange resins is the degradation of the GBCAs, e.g., by UV and H2O2. Due to their chemical properties, GBCAs are not retained unless the chelate complexes are degraded and the Gd ions are released.
More specialized LC systems are equipped with hydrophilic interaction chromatography (HILIC), size exclusion chromatography (SEC), or reversed-phase chromatography (RPC) columns which can be coupled with MS-systems to enable the direct measurement of specific GBCAs species and transformation products (Birka et al., 2016; Clases et al., 2018b; Künnemeyer et al., 2009; Telgmann et al., 2012). The location of Gd precipitated in brains, other human tissue, or other species has commonly been determined by laser ablation coupled with ICP-MS (Clases et al., 2018a, 2018b; Fingerhut et al., 2018; Lingott et al., 2016).
In many investigations total Gd (Gdtotal) is measured. Because the anthropogenic component Gdanth cannot be measured directly, the geogenic Gd (Gd∗) background needs to be subtracted (Equation (3)). The advantage of this approach is that it has a significant lower limit of detection than methods that measure individual species of GBCAs.
| Equation 3 |
There is no standard methodology on how to quantify Gd∗ in an aqueous sample, which brought up a variety of different approaches. All calculations to determine Gdanth are based on either interpolations or extrapolations of Gd∗ from the shale-normalized lanthanides (LnSN) by means of linear, logarithmic, geometric equations, or third-order polynomial fit (Table 2 ). Slight differences, apart from the mathematical methods, consist in the assumption of the specific behavior of Gd compared to the other lanthanides. While Bau and Dulski (1996) and Kulaksiz and Bau (2011a) assume that Gd behaves as a light lanthanide in freshwater systems and preferably use the light lanthanides (praseodymium (Pr), neodymium (Nd), Sm and terbium (Tb)) within their equations, Hissler et al. (2015) and Ogata and Terakado (2006) include the heavier lanthanide dysprosium (Dy). The approach from Möller et al. (2002) is the only one with no implicit assumption about the behavior of Gd compared to that of lighter, middle or heavier lanthanides and includes all lanthanides, besides Ce and Eu due to their natural occurring anomalies (Lawrence et al., 2009).
Table 2.
Selected methods for calculating Gdanth; elements and approaches used.
| Reference | Elements used | Interpolation/extrapolation | Specifics |
|---|---|---|---|
| Bau and Dulski (1996) | Sm, Tb | linear | Gd behaves as a LREEa |
| Hissler et al. (2015) | Nd, Dy | linear | Gd behaves as a MREE |
| Ogata and Terakado (2006) | Sm, Dy | logarithmic | Gd behaves as a HREE |
| Kulaksiz and Bau (2013) | Eu, Nd | logarithmic | Not usable if MREE are enriched or water is slightly acidic, rich in organic colloids |
| Kulaksiz and Bau (2011a) | Pr, Nd, Sm | geometric | Gd behaves as a LREE |
| Möller et al. (2002) | all Ln except Ce, Eu | third grade polynomial fit | no implicit assumption about the behavior of Gd |
No specifics stated in the original manuscript but implied in (Bau et al., 2006); LREE - light rare earth element, MREE - middle rare earth element, HREE - heavy rare earth element (terminology is referring to the original literature).
Most calculation methods are avoiding these elements with naturally occurring anomalies. As an exception, the method developed by Kulaksiz and Bau (2013) uses the redox-sensitive element Eu excluding slightly acidic colloid-rich conditions, and enrichments in the middle lanthanides. The use of lanthanides with any anomalous concentrations, however, limits the applicability of the method.
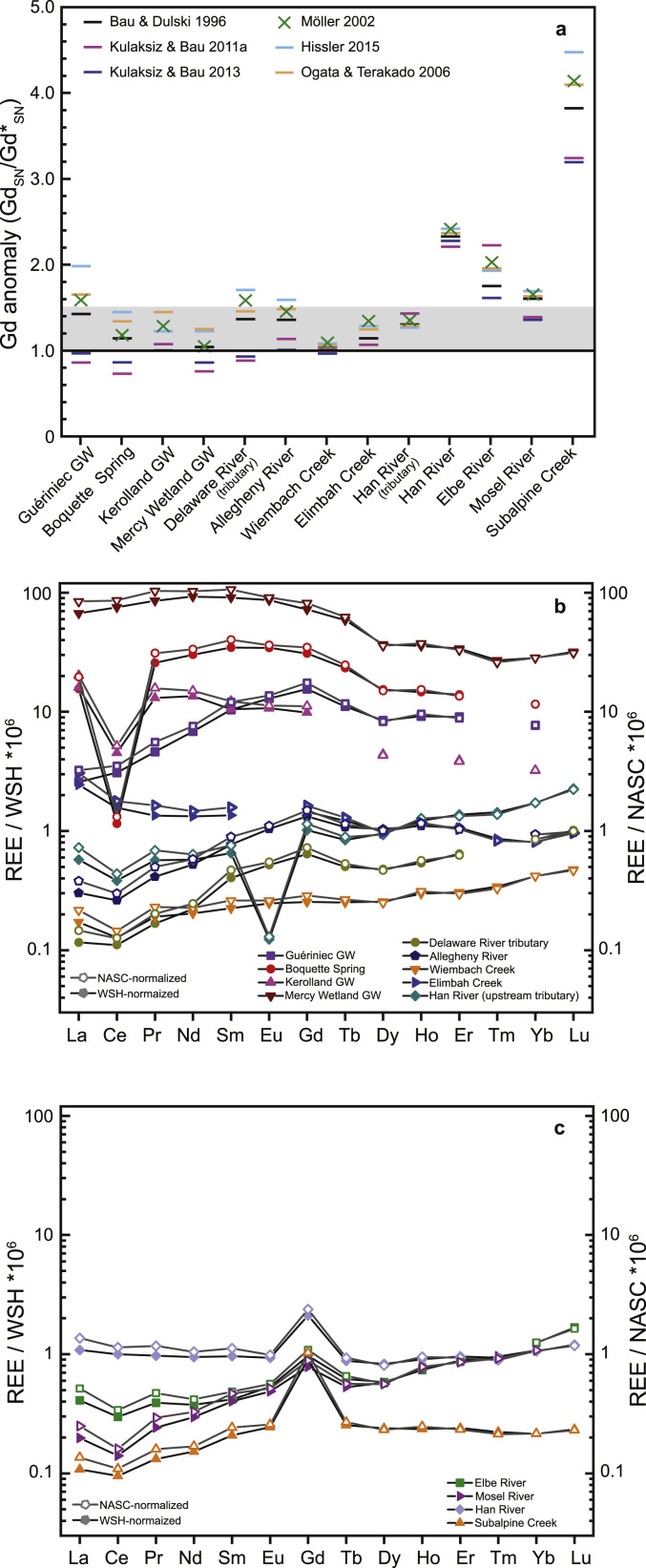
In order to compare and evaluate the different approaches, we used published data on lanthanides in freshwater systems under a variety of hydrochemical conditions. We compared the Gd anomaly, defined as the ratio between the shale-normalized measured Gd concentration and the shale-normalized geogenic background concentration (GdSN/Gd∗SN), and the concentrations obtained for Gdanth using the different normalization and interpolation/extrapolation approaches.
The results of our analysis clearly show that the geological reference used for normalization has only a minor effect up to 7% on the calculated Gdanth concentration (Fig. 3 b and c; Table S1). The comparison also elucidates that the smaller the Gd anomaly, the less important is the choice of geological reference. Nevertheless, the different approaches resulted in a broad range of amplitudes for Gd anomalies (Fig. 3a). Gadolinium anomalies calculated for Wiembach Creek varied only within a range of 4%, while those in samples from Guériniec groundwater (GW) and the Delaware River varied by up to 28% and 24%, respectively.
Fig. 3.
a Calculated gadolinium anomalies in water samples using different interpolation or extrapolation methods. Grey shading: area of reported Gd anomalies (max. 1.5) that have been assumed to be natural; b published data from water samples with Gd anomalies of less than 1.5 (averaged over selected methods); c published data from water samples with Gd anomalies greater than 1.5 (averaged over selected methods).
Any Gd anomaly greater than 1.0, in theory, implies that Gdanth is present in the sample. However, this threshold is misleading for most of the samples selected from the published literature since the authors assumed that the samples contained no Gdanth (Fig. 3a). To avoid overestimation of Gdanth, some investigators have used Gd anomaly thresholds of 1.2, 1.3, 1.4, or 1.5 (Bau et al., 2006; Petelet-Giraud et al., 2009; Rabiet et al., 2009; Rozemeijer et al., 2012). Others adjusted the geogenic Gd concentration by a factor of 1.1, 1.15, or 1.2 (Lawrence, 2010; Lawrence et al., 2009; Möller et al., 2003, respectively). Often this is justified by natural Gd anomalies found in Gdanth-uncontaminated waters, particularly in acid mine drainage environments and seawater (De Baar et al., 1985; Migaszewski and Gałuszka, 2015). However, from the above cited studies which use corrections, only Lawrence (2010) is working with seawater conditions. To avoid any bias we did not include a correction factor to our calculations.
In contrast to all other methods, the calculations based on the two methods by Kulaksiz and Bau resulted in Gd anomalies closest to 1.0, in agreement with the assumptions by the authors that no anthropogenic Gd was present in the samples (Fig. 3b; Table S1). The largest Gd anomalies were obtained using the approaches from Hissler et al. (2015), Ogata and Terakado (2006), and Möller et al. (2002), with exceptions for the Han and Elbe rivers (Fig. 3b and c). Comparing the methods, Gd anomaly differences are particularly high if concentrations of the higher lanthanides Tb and Dy are more abundant compared to the lighter lanthanides Nd and Sm (Fig. 3a, Table S1). While the influence of Tb and Dy can be decisive using approaches from Hissler et al. (2015) or Ogata and Terakado (2006), the advantage of the third-grade polynomial fit approach by Möller et al. (2002) is the larger number of elements included. This reduces the influence of minor anomalies and element-specific measurement errors on the interpolated geogenic Gd.
5. Measures to halt the increase in gadolinium concentrations in freshwater environments
Once GBCAs reach the aquatic environment, they are diluted to ng/L or to μg/L levels, which is at least an order of magnitude below the free Gd toxicity level for humans (Merbach et al., 2013). However, the potential toxicity of Gd needs to be considered together with that of the entire lanthanum series, as they form a uniform group of elements (Blinova et al., 2018). Anthropogenically elevated concentrations of other lanthanides further increase the risks to aquatic ecosystems, because aquatic organisms absorb lanthanides through their skin, gills, and digestive systems (Kulaksiz, 2012; Kulaksiz and Bau, 2013; Lingott et al., 2016).
Unaltered GBCAs have been shown not to sorb to materials such as activated carbon (contrary to the findings of Elizalde-González et al., 2017), but once acidified, they release Gd3+ with a high sorption affinity to many adsorbers (Anastopoulos et al., 2016; Elizalde-González et al., 2017; Kovalova et al., 2013; Patra et al., 2017). The sorption of Gd3+ onto activated carbon strongly depends on the pH of the solution (Pourret and Houben, 2018). The environmental mobility of Gd3+ is similar to that of the other lanthanides and mainly involves complexation with dissolved organic carbon (DOC) under slightly acidic or neutral conditions (Pédrot et al., 2010). This complexation is based on electrostatic interactions and multidentate bonding between positively charged Gd3+ and inorganic anions, or negatively charged organic ligands (Byrne and Kim, 1990; Davranche et al., 2015; Luo and Byrne, 2004).
In contrast to natural lanthanides, GBCAs have so far not been reported in sewage sludge, even though coagulation-flocculation is reported to remove at least some of the linear GBCAs (Lawrence et al., 2010; Neubert, 2008; Telgmann et al., 2012; Verplanck et al., 2010). Most of the commercially used coagulants are based on ferric or aluminum salts (e.g. FeCl3 and Al2(SO4)3), which are Lewis acids and form acidic microenvironments (Lee et al., 2014). Under these conditions, linear GBCAs are destabilized resulting in rapid transmetallation (Rabiet et al., 2014). The formation of Fe-oxide flocs further assists in the removal of Gd3+ from solution due to the high sorption affinity of lanthanides towards iron phases (Davranche et al., 2008, 2004). Once Gd3+ is released from GBCA complexes, this sorption affinity could be utilized to recover Gd from sewage water (Nassar et al., 2015; Stepka et al., 2018).
Water suppliers and STPs seek to eliminate GBCAs (Lingott et al., 2016; Rogowska et al., 2018; Thomsen, 2017). Promising results have been achieved using advanced oxidation processes based on in situ formation of •OH radicals by means of various chemical, photochemical, sonochemical, or electrochemical reactions (Oturan and Aaron, 2014). While direct reactions between GBCAs and ozone are insignificant, the •OH radicals they produce can result in degradation of GBCAs (Cyris et al., 2013). Treatment with a combination of UV-C irradiation and hydrogen peroxide (H2O2) effectively degrades GBCAs within 24 h (Bichler et al., 2016). Low energy (15 W) irradiation with UV-C over long periods of time (>12 h) has been shown to degrade all types of GBCAs, but these conditions cannot be met by most commercial water sanitation facilities (Brünjes et al., 2017). Birka et al. (2016) reported a series of degradation products from the linear Gd-BOPTA complexes after applying UV-irradiation, while other complexes such as the macrocyclic Gd-DOTA and Gd-BT-DO3A, and the linear Gd-DTPA showed no degradation even after 300 min. Because of this duration of exposure to UV, the study does not simulate conditions found in natural environments, STPs, or end-of-pipe treatments of drinking water purification (Birka et al., 2016; Thomsen, 2017). So far, there is no systematic study on the stability of GBCAs regarding end-of-pipe UV treatments. Since the human uptake of potential harmful GBCA transformation products is of special concern, further studies are recommended.
To reduce GBCAs contamination of drinking water, a few starting points can be considered. During drinking water production, improved water purification using expensive reverse osmosis would be required as this is the only efficient way to fully remove GBCAs (Lawrence et al., 2010). Reverse osmosis can be applied as drinking water treatment or as the last step of sewage water treatment to prevent Gd emissions into the aquatic environment. Because of the high percentage of outpatients undergoing MRI scans, treating only hospital effluents would not prevent the increasing input of GBCAs into freshwater water resources.
The simplest way to reduce the input of Gd into the aquatic environment and its potential health risk would be to collect urine from patients for at least 24 h following the administration of GBCAs. This would require urine collection not only in hospitals, but also at the patients’ homes. Urine collection bags can be made leak-proof by including super absorbent polymers. A trial in Germany found that although medical staff were skeptical about integrating these bags into their existing routines, there was a high level of acceptance by patients (Niederste-Hollenberg et al., 2018).
Collecting urine would also allow to recover and recycle Gd from GBCAs and prevent the technologically critical element Gd from being lost into aquatic environments (Cobelo-García et al., 2015). Extraction procedures from polymer matrices would need to involve the degradation of GBCAs but benefit from the high concentrations of the recycling target. For example, GBCAs could be degraded by a combination of H2O2 and UV radiation and recovered by using a variety of existing green technology methods such as biosorption that are already used for lanthanides from wastewater (Pereao et al., 2018). In contrast, recovering diluted Gd in sewage systems or after STPs would be economically less attractive for recycling.
6. Conclusion
Due to their ability to significantly enhance the contrast of MRI scans there is currently no viable alternative to GBCAs for medical use. MRI will develop towards higher resolutions, but the use of Gd for MRI contrast agents remains necessary. In general, GBCAs are renally excreted with plasma elimination half-lives of 1.5–34 h, however, there is increasing evidence that some of the Gd may be retained in the human body, but so far, the exact processes and amounts remain unknown.
As conventional sewage water treatment cannot eliminate GBCAs, they contaminate freshwater systems which provide our drinking water resources. GBCA concentrations in drinking water, the main pathway for unintentional human intake, are still at the nanogram per liter levels but will further increase. As soon as they enter the stomach their stability is reduced by the acidity of the gastrointestinal fluids.
Unaltered GBCAs are highly mobile and neither retained by activated carbon filters nor present in sewage sludge. Promising results to degrade and remove GBCAs have been achieved using advanced oxidation processes. Macrocyclic GBCAs are more stable against degradation than linear GBCAs, but besides reverse osmosis, no other advanced water treatment technique is capable to fully eliminate GBCAs. UV radiation can alter GBCAs and their transformation products may enhance the risk of adverse health effects if the treatment is utilized as an end-of-pipe solution.
The amount of GBCA contamination in freshwater environments, also referred to as anthropogenic gadolinium, is determined using a variety of calculation methods. In water bodies with low concentrations of Gdanth compared to the geogenic background concentration, the inconsistent use of these methods results in high discrepancies and high levels of uncertainty. Applying any corrections to account for natural Gd anomalies should be based on site-specific conditions rather than on Gd anomalies from literature. In our comparison, the approach from Möller et al. (2002) is the most holistic using a larger number of elements which reduces the influence of minor anomalies and element-specific measurement errors. The assessment of Gdanth in drinking water would benefit from a coordinated approach to determine Gd anomalies and Gdanth concentrations. A recognized standard methodology would also be beneficial for the acceptance of Gdanth as a sewage water indicator and quantitative tracer. Temporal changes of GBCA emission patterns in rivers can be used to determine groundwater residence times and river-aquifer interactions to improve our understanding of these complex subsurface aquatic systems.
Even if Gd emissions are temporarily reduced due to the COVID-19 pandemic, increasing emissions are expected in future if no measures are taken. At present, the technologically critical element Gd is being lost into the aquatic environment, for example 19 tons/year in the EU and 21 tons/year in the USA. Considering the total environmental burden of mining and extracting Gd from ore, and in the perspective of a circular economy, the collection and recycling of Gd from the urine of patients should be considered “best practice” in future.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We acknowledge the illustration of Figure 2 by Audrey Desaulniers, Orcéine, Montreal, Canada.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.watres.2020.115966.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Anastopoulos I., Bhatnagar A., Lima E.C. Adsorption of rare earth metals: a review of recent literature. J. Mol. Liq. 2016;221:954–962. doi: 10.1016/j.molliq.2016.06.076. [DOI] [Google Scholar]
- Atinkpahoun C.N.H., Pons M.N., Louis P., Leclerc J.P., Soclo H.H. Rare earth elements (REE) in the urban wastewater of Cotonou (Benin, West Africa) Chemosphere. 2020;251 doi: 10.1016/j.chemosphere.2020.126398. [DOI] [PubMed] [Google Scholar]
- Barber L.B., Murphy S.F., Verplanck P.L., Sandstrom M.W., Taylor H.E., Furlong E.T. Chemical loading into surface water along a hydrological, biogeochemical, and land use gradient: a holistic watershed approach. Environ. Sci. Technol. 2006;40:475–486. doi: 10.1021/es051270q. [DOI] [PubMed] [Google Scholar]
- Bau M., Dulski P. Anthropogenic origin of positive gadolinium anomalies in river waters. Earth Planet Sci. Lett. 1996;143:245–255. doi: 10.1016/0012-821X(96)00127-6. [DOI] [Google Scholar]
- Bau M., Knappe A., Dulski P. Anthropogenic gadolinium as a micropollutant in river waters in Pennsylvania and in Lake Erie, northeastern United States. Chem. Erde. 2006;66:143–152. doi: 10.1016/j.chemer.2006.01.002. [DOI] [Google Scholar]
- Bau M., Schmidt K., Pack A., Bendel V., Kraemer D. The European Shale: an improved data set for normalisation of rare earth element and yttrium concentrations in environmental and biological samples from Europe. Appl. Geochem. 2018;90:142–149. doi: 10.1016/j.apgeochem.2018.01.008. [DOI] [Google Scholar]
- Bazzicalupi C., Bianchi A., Giorgi C., Clares M.P., García-España E. Addressing selectivity criteria in binding equilibria. Coord. Chem. Rev. 2012;256:13–27. doi: 10.1016/j.ccr.2011.05.013. [DOI] [Google Scholar]
- Bellin M.-F. MR contrast agents, the old and the new. Eur. J. Radiol. 2006;60:314–323. doi: 10.1016/j.ejrad.2006.06.021. [DOI] [PubMed] [Google Scholar]
- Bellin M.F., Van Der Molen A.J. Extracellular gadolinium-based contrast media: an overview. Eur. J. Radiol. 2008;66:160–167. doi: 10.1016/j.ejrad.2008.01.023. [DOI] [PubMed] [Google Scholar]
- Beyer L., Angulo Cornejo J. Springer-Verlag; 2012. Koordinationschemie: Grundlagen-Synthesen-Anwendungen. [Google Scholar]
- Bichler A., Muellegger C., Brünjes R., Hofmann T. Quantification of river water infiltration in shallow aquifers using acesulfame and anthropogenic gadolinium. Hydrol. Process. 2016;30:1742–1756. doi: 10.1002/hyp.10735. [DOI] [Google Scholar]
- Birka M., Roscher J., Holtkamp M., Sperling M., Karst U. Investigating the stability of gadolinium based contrast agents towards UV radiation. Water Res. 2016;91:244–250. doi: 10.1016/j.watres.2016.01.012. [DOI] [PubMed] [Google Scholar]
- Birka M., Wehe C.A., Telgmann L., Sperling M., Karst U. Sensitive quantification of gadolinium-based magnetic resonance imaging contrast agents in surface waters using hydrophilic interaction liquid chromatography and inductively coupled plasma sector field mass spectrometry. J. Chromatogr. A. 2013;1308:125–131. doi: 10.1016/j.chroma.2013.08.017. [DOI] [PubMed] [Google Scholar]
- Blinova I., Lukjanova A., Muna M., Vija H., Kahru A. Evaluation of the potential hazard of lanthanides to freshwater microcrustaceans. Sci. Total Environ. 2018;642:1100–1107. doi: 10.1016/j.scitotenv.2018.06.155. [DOI] [PubMed] [Google Scholar]
- Brünjes R., Bichler A., Hoehn P., Lange F.T., Brauch H.J., Hofmann T. Anthropogenic gadolinium as a transient tracer for investigating river bank filtration. Sci. Total Environ. 2016;571:1432–1440. doi: 10.1016/j.scitotenv.2016.06.105. [DOI] [PubMed] [Google Scholar]
- Brünjes R., Höhn P., Hofmann T. Wasser; 2017. Stabilitätsunterschiede von Gadolinium-Komplexen; pp. 538–543. 2017. [Google Scholar]
- Burai L., Hietapelto V., Király R., Tóth É., Brücher E. Stability constants and 1H relaxation effects of ternary complexes formed between Gd-DTPA, Gd-DTPA-BMA, Gd-DOTA, and Gd-EDTA and citrate, phosphate, and carbonate ions. Magn. Reson. Med. 1997;38:146–150. doi: 10.1002/mrm.1910380120. [DOI] [PubMed] [Google Scholar]
- Byrne R.H., Kim K.H. Rare earth element scavenging in seawater. Geochem. Cosmochim. Acta. 1990;54:2645–2656. doi: 10.1016/0016-7037(90)90002-3. [DOI] [Google Scholar]
- Callaway E., Cyranoski D., Mallapaty S., Stoye E., Tollefson J. The coronavirus pandemic in five powerful charts. Nature. 2020;579:482–483. doi: 10.1038/d41586-020-00758-2. [DOI] [PubMed] [Google Scholar]
- Caravan P., Ellison J.J., McMurry T.J., Lauffer R.B. Gadolinium(III) chelates as MRI contrast agents: structure, dynamics, and applications. Chem. Rev. 1999;99:2293–2352. doi: 10.1021/cr980440x. [DOI] [PubMed] [Google Scholar]
- Chan K.W.Y., Wong W.T. Small molecular gadolinium(III) complexes as MRI contrast agents for diagnostic imaging. Coord. Chem. Rev. 2007;251:2428–2451. doi: 10.1016/j.ccr.2007.04.018. [DOI] [Google Scholar]
- Clases D., Fingerhut S., Jeibmann A., Sperling M., Doble P., Karst U. LA-ICP-MS/MS improves limits of detection in elemental bioimaging of gadolinium deposition originating from MRI contrast agents in skin and brain tissues. J. Trace Elem. Med. Biol. 2018;51:212–218. doi: 10.1016/j.jtemb.2018.10.021. [DOI] [PubMed] [Google Scholar]
- Clases D., Sperling M., Karst U. Analysis of metal-based contrast agents in medicine and the environment. TrAC Trends Anal. Chem. (Reference Ed.) 2018;104:135–147. doi: 10.1016/j.trac.2017.12.011. [DOI] [Google Scholar]
- Cobelo-García A., Filella M., Croot P., Frazzoli C., Du Laing G., Ospina-Alvarez N., Rauch S., Salaun P., Schäfer J., Zimmermann S. COST action TD1407: network on technology-critical elements (NOTICE)—from environmental processes to human health threats. Environ. Sci. Pollut. Res. 2015;22:15188–15194. doi: 10.1007/s11356-015-5221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyris M., Knolle W., Richard J., Dopp E., Von Sonntag C., Schmidt T.C. Reaction of gadolinium chelates with ozone and hydroxyl radicals. Environ. Sci. Technol. 2013;47:9942–9949. doi: 10.1021/es402219u. [DOI] [PubMed] [Google Scholar]
- Davranche M., Gruau G., Dia A., Marsac R., Pédrot M., Pourret O. Biogeochemical factors affecting rare earth element distribution in shallow wetland groundwater. Aquat. Geochem. 2015;21:197–215. doi: 10.1007/s10498-014-9247-6. [DOI] [Google Scholar]
- Davranche M., Pourret O., Gruau G., Dia A. Impact of humate complexation on the adsorption of REE onto Fe oxyhydroxide. J. Colloid Interface Sci. 2004;277:271–279. doi: 10.1016/j.jcis.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Davranche M., Pourret O., Gruau G., Dia A., Jin D., Gaertner D. Competitive binding of REE to humic acid and manganese oxide: impact of reaction kinetics on development of cerium anomaly and REE adsorption. Chem. Geol. 2008;247:154–170. doi: 10.1016/j.chemgeo.2007.10.010. [DOI] [Google Scholar]
- De Baar H.J.W., Brewer P.G., Bacon M.P. Anomalies in rare earth distributions in seawater: Gd and Tb. Geochem. Cosmochim. Acta. 1985;49:1961–1969. doi: 10.1016/0016-7037(85)90090-0. [DOI] [Google Scholar]
- de Campos F.F., Enzweiler J. Anthropogenic gadolinium anomalies and rare earth elements in the water of Atibaia River and Anhumas Creek, Southeast Brazil. Environ. Monit. Assess. 2016;188:281. doi: 10.1007/s10661-016-5282-7. [DOI] [PubMed] [Google Scholar]
- Dia A., Gruau G., Olivié-Lauquet G., Riou C., Molénat J., Curmi P. The distribution of rare earth elements in groundwaters: assessing the role of source-rock composition, redox changes and colloidal particles. Geochem. Cosmochim. Acta. 2000;64:4131–4148. doi: 10.1016/S0016-7037(00)00494-4. [DOI] [Google Scholar]
- Dulski P., Möller P., Pekdeger A. Comparison of gadopentetic acid (Gd-DTPA) and bromide in a dual-tracer field experiment. Hydrogeol. J. 2011;19:823–834. doi: 10.1007/s10040-011-0713-6. [DOI] [Google Scholar]
- Elbaz-Poulichet F., Seidel J.L., Othoniel C. Occurrence of an anthropogenic gadolinium anomaly in river and coastal waters of Southern France. Water Res. 2002;36:1102–1105. doi: 10.1016/S0043-1354(01)00370-0. [DOI] [PubMed] [Google Scholar]
- Elizalde-González M.P., García-Díaz E., González-Perea M., Mattusch J. Removal of gadolinium-based contrast agents: adsorption on activated carbon. Environ. Sci. Pollut. Res. 2017;24:8164–8175. doi: 10.1007/s11356-017-8491-x. [DOI] [PubMed] [Google Scholar]
- European Medicines Agency EMA’s final opinion confirms restrictions on use of linear gadolinium agents in body scans Recommendations conclude. EMA’s Sci. Rev. Gadolinium Deposit. 2017;44:5–8. doi: 10.1016/j.rse.2010.12.006. [DOI] [Google Scholar]
- Fingerhut S., Niehoff A.C., Sperling M., Jeibmann A., Paulus W., Niederstadt T., Allkemper T., Heindel W., Holling M., Karst U. Spatially resolved quantification of gadolinium deposited in the brain of a patient treated with gadolinium-based contrast agents. J. Trace Elem. Med. Biol. 2018;45:125–130. doi: 10.1016/j.jtemb.2017.10.004. [DOI] [PubMed] [Google Scholar]
- Food and Drug Administration Update on FDA approach to safety issue of gadolinium retention after administration of gadolinium-based contrast agents. 2018. https://www.fda.gov/media/116492/download accessed 3.5.19.
- Food and Drug Administration FDA Safety Announcement - FDA warns that gadolinium-based contrast agents (GBCAs) are retained in the body; requires new class warnings. 2017. https://www.fda.gov/downloads/Drugs/DrugSafety/UCM589442.pdf
- Frenzel T., Lengsfeld P., Schirmer H., Hütter J., Weinmann H.J. Stability of gadolinium-based magnetic resonance imaging contrast agents in human serum at 37°C. Invest. Radiol. 2008;43:817–828. doi: 10.1097/RLI.0b013e3181852171. [DOI] [PubMed] [Google Scholar]
- Gibby W., Parish W., Merrill R.M., Fernandez D., Anderson C.R., Merchel E., Parr R. The use of a binary chelate formulation: could gadolinium based linear contrast agents be rescued by the addition of zinc selective chelates? Magn. Reson. Imaging. 2019;58:76–81. doi: 10.1016/j.mri.2019.01.001. [DOI] [PubMed] [Google Scholar]
- Goldstein S.J., Jacobsen S.B. Rare earth elements in river waters. Earth Planet Sci. Lett. 1988;89:35–47. doi: 10.1016/0012-821X(88)90031-3. [DOI] [Google Scholar]
- Grobner T. Gadolinium – a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol. Dial. Transplant. 2006;21:1104–1108. doi: 10.1093/ndt/gfk062. [DOI] [PubMed] [Google Scholar]
- Gromet L.P., Dymek R.F., Haskin L.a., Korotev R.L. The “North American shale composite” - its compilation, major and trace element characteristics. G-cubed. 1984;48:2469–2482. doi: 10.1016/0016-7037(84)90298-9. [DOI] [Google Scholar]
- Hathorne E.C., Haley B., Stichel T., Grasse P., Zieringer M., Frank M. Online preconcentration ICP-MS analysis of rare earth elements in seawater. G-cubed. 2012;13:1–12. doi: 10.1029/2011GC003907. [DOI] [Google Scholar]
- Hatje V., Bruland K.W., Flegal A.R. Increases in anthropogenic gadolinium anomalies and rare earth element concentrations in san francisco bay over a 20 Year record. Environ. Sci. Technol. 2016;50:4159–4168. doi: 10.1021/acs.est.5b04322. [DOI] [PubMed] [Google Scholar]
- Hinck M.L., Ferguson J., Puhaakka J. Resistance of EDTA and DTPA to aerobic biodegradation. Water Sci. Technol. 1997;35:25–31. doi: 10.1016/S0273-1223(96)00911-0. [DOI] [Google Scholar]
- Hissler C., Hostache R., Iffly J.F., Pfister L., Stille P. Anthropogenic rare earth element fluxes into floodplains: coupling between geochemical monitoring and hydrodynamic sediment transport modelling. Compt. Rendus Geosci. 2015;347:294–303. doi: 10.1016/j.crte.2015.01.003. [DOI] [Google Scholar]
- Holzbecher E., Knappe A., Pekdeger A. Identification of degradation characteristics - exemplified by Gd-DTPA in a large experimental column. Environ. Model. Assess. 2005;10:1–8. doi: 10.1007/s10666-004-4269-x. [DOI] [Google Scholar]
- Idée J.M., Port M., Medina C., Lancelot E., Fayoux E., Ballet S., Corot C. Possible involvement of gadolinium chelates in the pathophysiology of nephrogenic systemic fibrosis: a critical review. Toxicology. 2008;248:77–88. doi: 10.1016/j.tox.2008.03.012. [DOI] [PubMed] [Google Scholar]
- Idée J.M., Port M., Robic C., Medina C., Sabatou M., Corot C. Role of thermodynamic and kinetic parameters in gadolinium chelate stability. J. Magn. Reson. Imag. 2009:1249–1258. doi: 10.1002/jmri.21967. [DOI] [PubMed] [Google Scholar]
- Joffe P., Thomsen H.S., Meusel M. Pharmacokinetics of gadodiamide injection in patients with severe renal insufficiency and patients undergoing hemodialysis or continuous ambulatory peritoneal dialysis. Acad. Radiol. 1998;5:491–502. doi: 10.1016/S1076-6332(98)80191-8. [DOI] [PubMed] [Google Scholar]
- Kamber B.S., Greig A., Collerson K.D. A new estimate for the composition of weathered young upper continental crust from alluvial sediments, Queensland, Australia. Geochem. Cosmochim. Acta. 2005;69:1041–1058. doi: 10.1016/j.gca.2004.08.020. [DOI] [Google Scholar]
- Kanda T., Oba H., Toyoda K., Kitajima K., Furui S. Brain gadolinium deposition after administration of gadolinium-based contrast agents. Jpn. J. Radiol. 2016;34:3–9. doi: 10.1007/s11604-015-0503-5. [DOI] [PubMed] [Google Scholar]
- Klaver G., Verheul M., Bakker I., Petelet-Giraud E., Négrel P. Anthropogenic rare earth element in rivers: gadoliniumand lanthanum. Partitioning between the dissolved and particulate phases in the rhine river and spatial propagation through the rhine-meuse delta (The Netherlands) Appl. Geochem. 2014;47:186–197. doi: 10.1016/j.apgeochem.2014.05.020. [DOI] [Google Scholar]
- Knappe A., Möller P., Dulski P., Pekdeger A. Positive gadolinium anomaly in surface water and ground water of the urban area Berlin, Germany. Chem. Erde - Geochem. 2005;65:167–189. doi: 10.1016/j.chemer.2004.08.004. [DOI] [Google Scholar]
- Kovalova L., Siegrist H., Von Gunten U., Eugster J., Hagenbuch M., Wittmer A., Moser R., McArdell C.S. Elimination of micropollutants during post-treatment of hospital wastewater with powdered activated carbon, ozone, and UV. Environ. Sci. Technol. 2013;47:7899–7908. doi: 10.1021/es400708w. [DOI] [PubMed] [Google Scholar]
- Kulaksiz S. Jacobs University; 2012. Rare Earth Elements as Emerging Contaminants in the Rhine River , Germany and its Tributaries. [Google Scholar]
- Kulaksiz S., Bau M. Anthropogenic dissolved and colloid/nanoparticle-bound samarium, lanthanum and gadolinium in the Rhine River and the impending destruction of the natural rare earth element distribution in rivers. Earth Planet Sci. Lett. 2013;362:43–50. doi: 10.1016/j.epsl.2012.11.033. [DOI] [Google Scholar]
- Kulaksiz S., Bau M. Anthropogenic gadolinium as a microcontaminant in tap water used as drinking water in urban areas and megacities. Appl. Geochem. 2011;26:1877–1885. doi: 10.1016/j.apgeochem.2011.06.011. [DOI] [Google Scholar]
- Kulaksiz S., Bau M. Rare earth elements in the Rhine River, Germany: first case of anthropogenic lanthanum as a dissolved microcontaminant in the hydrosphere. Environ. Int. 2011;37:973–979. doi: 10.1016/j.envint.2011.02.018. [DOI] [PubMed] [Google Scholar]
- Kümmerer K., Helmers E. Hospital effluents as a source of gadolinium in the aquatic environment. Environ. Sci. Technol. 2000;34:573–577. doi: 10.1021/es990633h. [DOI] [Google Scholar]
- Künnemeyer J., Terborg L., Meermann B., Brauckmann C., Möller I., Scheffer A., Karst U. Speciation analysis of gadolinium chelates in hospital effluents and wastewater treatment plant sewage by a novel HILIC/ICP-MS method. Environ. Sci. Technol. 2009;43:2884–2890. doi: 10.1021/es803278n. [DOI] [PubMed] [Google Scholar]
- Lagerström M.E., Field M.P., Séguret M., Fischer L., Hann S., Sherrell R.M. Automated on-line flow-injection ICP-MS determination of trace metals (Mn, Fe, Co, Ni, Cu and Zn) in open ocean seawater: application to the GEOTRACES program. Mar. Chem. 2013;155:71–80. doi: 10.1016/j.marchem.2013.06.001. [DOI] [Google Scholar]
- Lawrence M.G. Detection of anthropogenic gadolinium in the brisbane river plume in moreton bay, Queensland, Australia. Mar. Pollut. Bull. 2010;60:1113–1116. doi: 10.1016/j.marpolbul.2010.03.027. [DOI] [PubMed] [Google Scholar]
- Lawrence M.G., Bariel D.G. Tracing treated wastewater in an inland catchment using anthropogenic gadolinium. Chemosphere. 2010;80:794–799. doi: 10.1016/j.chemosphere.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Lawrence M.G., Kamber B.S. The behaviour of the rare earth elements during estuarine mixing-revisited. Mar. Chem. 2006;100:147–161. doi: 10.1016/j.marchem.2005.11.007. [DOI] [Google Scholar]
- Lawrence M.G., Keller J., Poussade Y. Removal of magnetic resonance imaging contrast agents through advanced water treatment plants. Water Sci. Technol. 2010;61:685–692. doi: 10.2166/wst.2010.885. [DOI] [PubMed] [Google Scholar]
- Lawrence M.G., Ort C., Keller J. Detection of anthropogenic gadolinium in treated wastewater in South East Queensland, Australia. Water Res. 2009;43:3534–3540. doi: 10.1016/j.watres.2009.04.033. [DOI] [PubMed] [Google Scholar]
- Le Fur M., Caravan P. The biological fate of gadolinium-based MRI contrast agents: a call to action for bioinorganic chemists. Metall. 2019;11 doi: 10.1039/c8mt00302e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C.S., Robinson J., Chong M.F. A review on application of flocculants in wastewater treatment. Process Saf. Environ. Protect. 2014;92:489–508. doi: 10.1016/j.psep.2014.04.010. [DOI] [Google Scholar]
- Lerat-Hardy A., Coynel A., Dutruch L., Pereto C., Bossy C., Gil-Diaz T., Capdeville M.J., Blanc G., Schäfer J. Rare Earth Element fluxes over 15 years into a major European Estuary (Garonne-Gironde, SW France): hospital effluents as a source of increasing gadolinium anomalies. Sci. Total Environ. 2019;656:409–420. doi: 10.1016/j.scitotenv.2018.11.343. [DOI] [PubMed] [Google Scholar]
- Lindner U., Lingott J., Richter S., Jakubowski N., Panne U. Speciation of gadolinium in surface water samples and plants by hydrophilic interaction chromatography hyphenated with inductively coupled plasma mass spectrometry. Anal. Bioanal. Chem. 2013;405:1865–1873. doi: 10.1007/s00216-012-6643-x. [DOI] [PubMed] [Google Scholar]
- Lindner U., Lingott J., Richter S., Jiang W., Jakubowski N., Panne U. Analysis of Gadolinium-based contrast agents in tap water with a new hydrophilic interaction chromatography (ZIC-cHILIC) hyphenated with inductively coupled plasma mass spectrometry. Anal. Bioanal. Chem. 2015;407:2415–2422. doi: 10.1007/s00216-014-8368-5. [DOI] [PubMed] [Google Scholar]
- Lingott J., Lindner U., Telgmann L., Esteban-Fernández D., Jakubowski N., Panne U. Gadolinium-uptake by aquatic and terrestrial organisms-distribution determined by laser ablation inductively coupled plasma mass spectrometry. Environ. Sci. Process. Impacts. 2016;18:200–207. doi: 10.1039/c5em00533g. [DOI] [PubMed] [Google Scholar]
- Luo Y.R., Byrne R.H. Carbonate complexation of yttrium and the rare earth elements in natural waters. Geochem. Cosmochim. Acta. 2004;68:691–699. doi: 10.1016/S0016-7037(03)00495-2. [DOI] [Google Scholar]
- Marckmann P. Nephrogenic systemic fibrosis: suspected causative role of gadodiamide used for contrast-enhanced magnetic resonance imaging. J. Am. Soc. Nephrol. 2006;17:2359–2362. doi: 10.1681/asn.2006060601. [DOI] [PubMed] [Google Scholar]
- Massmann G., Sültenfuß J., Dünnbier U., Knappe A., Taute T., Pekdeger A. Investigation of groundwater residence times during bank filtration in Berlin: a multi-tracer approach. Hydrol. Process. Int. J. 2008;22:788–801. doi: 10.1002/hyp.6649. [DOI] [Google Scholar]
- McLennan S.M. Rare earth elements in sedimentary rocks: influence of provenance and sedimentary processes. In: Lipin B.R., McKay G.A., editors. Geochemistry and Mineralogy of Rare Earth Elements. De Gruyter; Berlin, Boston: 1989. pp. 169–200. [DOI] [Google Scholar]
- Means J.L., Kucak T., Crerar D.A. Relative degradation rates of NTA, EDTA and DTPA and environmental implications. Environ. Pollut. Ser. B Chem. Phys. 1980;1:45–60. doi: 10.1016/0143-148X(80)90020-8. [DOI] [Google Scholar]
- Menahem A., Dror I., Berkowitz B. Transport of gadolinium- and arsenic-based pharmaceuticals in saturated soil under various redox conditions. Chemosphere. 2016 doi: 10.1016/j.chemosphere.2015.09.044. [DOI] [PubMed] [Google Scholar]
- Merbach A., Helm L., Tóth É. Wiley & Sons; 2013. The Chemistry of Contrast Agents. [Google Scholar]
- Merschel G., Bau M. Rare earth elements in the aragonitic shell of freshwater mussel Corbicula fluminea and the bioavailability of anthropogenic lanthanum, samarium and gadolinium in river water. Sci. Total Environ. 2015;533:91–101. doi: 10.1016/j.scitotenv.2015.06.042. [DOI] [PubMed] [Google Scholar]
- Metsärinne S., Rantanen P., Aksela R., Tuhkanen T. Biological and photochemical degradation rates of diethylenetriaminepentaacetic acid (DTPA) in the presence and absence of Fe(III) Chemosphere. 2004;55:379–388. doi: 10.1016/j.chemosphere.2003.10.062. [DOI] [PubMed] [Google Scholar]
- Micskei K., Powell D.H., Helm L., Brücher E., Merbach A.E. Water exchange on [Gd(H2O)8]3+ and [Gd(PDTA)(H2O)2]- in aqueous solution: a variable-pressure, -temperature and -magnetic field 17O NMR study. Magn. Reson. Chem. 1993;31:1011–1020. doi: 10.1002/mrc.1260311111. [DOI] [Google Scholar]
- Migaszewski Z.M., Gałuszka A. The characteristics, occurrence, and geochemical behavior of rare earth elements in the environment: a review. Crit. Rev. Environ. Sci. Technol. 2015;45:429–471. doi: 10.1080/10643389.2013.866622. [DOI] [Google Scholar]
- Möller P., Dulski P. Transmetallation of Gd-DTPA by Cu, Y and lanthanides and its impact on the hydrosphere. Appl. Geochem. 2010;25:48–59. doi: 10.1016/j.apgeochem.2009.09.027. [DOI] [Google Scholar]
- Möller P., Dulski P., Bau M., Knappe A., Pekdeger A., Sommer-Von Jarmersted C. Anthropogenic gadolinium as a conservative tracer in hydrology. J. Geochem. Explor. 2000;69–70:409–414. doi: 10.1016/S0375-6742(00)00083-2. [DOI] [Google Scholar]
- Möller P., Knappe A., Dulski P., Pekdeger A. Behavior of Gd-DTPA in simulated bank filtration. Appl. Geochem. 2011;26:140–149. doi: 10.1016/j.apgeochem.2010.11.011. [DOI] [Google Scholar]
- Möller P., Morteani G., Dulski P. Anomalous gadolinium, cerium, and yttrium contents in the adige and isarco river waters and in the water of their tributaries (provinces trento and bolzano/bozen, NE Italy) Acta Hydrochim. Hydrobiol. 2003;31:225–239. doi: 10.1002/aheh.200300492. [DOI] [Google Scholar]
- Möller P., Paces T., Dulski P., Morteani G. Anthropogenic Gd in surface water, drainage system, and the water supply of the City of Prague, Czech Republic. Environ. Sci. Technol. 2002;36:2387–2394. doi: 10.1021/es010235q. [DOI] [PubMed] [Google Scholar]
- Montagne A., Toga A.W., Zlokovic B.V. Blood-brain barrier permeability and gadolinium benefits and potential pitfalls in research. JAMA Neurol. 2016;73:13–14. doi: 10.1001/jamaneurol.2015.2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morcos S.K. Extracellular gadolinium contrast agents: differences in stability. Eur. J. Radiol. 2008;66:175–179. doi: 10.1016/j.ejrad.2008.01.025. [DOI] [PubMed] [Google Scholar]
- Morcos S.K. Nephrogenic systemic fibrosis following the administration of extracellular gadolinium based contrast agents: is the stability of the contrast agent molecule an important factor in the pathogenesis of this condition? Br. J. Radiol. 2007;80:586. doi: 10.1259/bjr/16877468. [DOI] [PubMed] [Google Scholar]
- Morteani G., Möller P., Fuganti A., Paces T. Input and fate of anthropogenic estrogens and gadolinium in surface water and sewage plants in the hydrological basin of Prague (Czech Republic) Environ. Geochem. Health. 2006;28:257–264. doi: 10.1007/s10653-006-9040-6. [DOI] [PubMed] [Google Scholar]
- Nassar N.T., Du X., Graedel T.E. Criticality of the rare earth elements. J. Ind. Ecol. 2015;19:1044–1054. doi: 10.1111/jiec.12237. [DOI] [Google Scholar]
- Nehra A.K., McDonald J.S., Bluhm A.M., Gunderson T.M., Murray D.L., Jannetto P.J., Kallmes D.F., Eckel L.J., McDonald R.J. Accumulation of gadolinium in human cerebrospinal fluid after gadobutrol-enhanced MR imaging: a prospective observational cohort study. Radiology. 2018;288:416–423. doi: 10.1148/radiol.2018171105. [DOI] [PubMed] [Google Scholar]
- Neubert C. Technical University; Berlin: 2008. Umweltverhalten und Ökotoxikologie von gadoliniumhaltigen Magnetresonanztomographie-Kontrastmitteln. [Google Scholar]
- Niederste-Hollenberg J., Eckartz K., Peters A., Hillenbrand T., Maier U., Beer M., Reszt A. Reducing the emission of X-Ray contrast agents to the environment. Gaia. 2018;27:147–155. doi: 10.14512/gaia.27.1.10. [DOI] [Google Scholar]
- Nozaki Y., Lerche D., Alibo D.S., Tsutsumi M. Dissolved indium and rare earth elements in three Japanese rivers and Tokyo Bay: evidence for anthropogenic Gd and in. Geochem. Cosmochim. Acta. 2000;64:3975–3982. doi: 10.1016/S0016-7037(00)00472-5. [DOI] [Google Scholar]
- Ogata T., Terakado Y. Rare earth element abundances in some seawaters and related river waters from the Osaka Bay area, Japan: significance of anthropogenic Gd. Geochem. J. 2006;40:463–474. doi: 10.2343/geochemj.40.463. [DOI] [Google Scholar]
- Organisation for Economic Cooperation and Development (OECD) Magnetic resonance imaging (MRI) exams [WWW Document] OECD Heal. Stat. Heal. Care Util. 2017 https://data.oecd.org/healthcare/magnetic-resonance-imaging-mri-exams.htm accessed 8.26.19. [Google Scholar]
- Oturan M.A., Aaron J.-J. Advanced oxidation processes in water/wastewater treatment: principles and applications. A review. Crit. Rev. Environ. Sci. Technol. 2014;44:2577–2641. doi: 10.1080/10643389.2013.829765. [DOI] [Google Scholar]
- Parant M., Perrat E., Wagner P., Rosin C., Py J.S., Cossu-Leguille C. Variations of anthropogenic gadolinium in rivers close to waste water treatment plant discharges. Environ. Sci. Pollut. Res. 2018 doi: 10.1007/s11356-018-3489-6. [DOI] [PubMed] [Google Scholar]
- Patra S., Roy E., Madhuri R., Sharma P.K. Removal and recycling of precious rare earth element from wastewater samples using imprinted magnetic ordered mesoporous carbon. ACS Sustain. Chem. Eng. 2017;5:6910–6923. doi: 10.1021/acssuschemeng.7b01124. [DOI] [Google Scholar]
- Pédrot M., Dia A., Davranche M. Dynamic structure of humic substances: rare earth elements as a fingerprint. J. Colloid Interface Sci. 2010;345:206–213. doi: 10.1016/j.jcis.2010.01.069. [DOI] [PubMed] [Google Scholar]
- Pereao O., Bode-Aluko C., Fatoba O., Laatikainen K., Petrik L. Rare earth elements removal techniques from water/wastewater: a review. Desalin. Water Treat. 2018;130:71–86. doi: 10.5004/dwt.2018.22844. [DOI] [Google Scholar]
- Petelet-Giraud E., Klaver G., Negrel P. Natural versus anthropogenic sources in the surface- and groundwater dissolved load of the Dommel river (Meuse basin): constraints by boron and strontium isotopes and gadolinium anomaly. J. Hydrol. 2009;369:336–349. doi: 10.1016/j.jhydrol.2009.02.029. [DOI] [Google Scholar]
- Port M., Idée J.M., Medina C., Robic C., Sabatou M., Corot C. Efficiency, thermodynamic and kinetic stability of marketed gadolinium chelates and their possible clinical consequences: a critical review. Biometals. 2008;21:469–490. doi: 10.1007/s10534-008-9135-x. [DOI] [PubMed] [Google Scholar]
- Pourret O., Houben D. Characterization of metal binding sites onto biochar using rare earth elements as a fingerprint. Heliyon e00543. 2018. [DOI] [PMC free article] [PubMed]
- Prybylski J.P., Semelka R.C., Jay M. The stability of gadolinium-based contrast agents in human serum: a reanalysis of literature data and association with clinical outcomes. Magn. Reson. Imaging. 2017;38:145–151. doi: 10.1016/j.mri.2017.01.006. [DOI] [PubMed] [Google Scholar]
- Pyrzynska K., Kubiak A., Wysocka I. Application of solid phase extraction procedures for rare earth elements determination in environmental samples. Talanta. 2016;154:15–22. doi: 10.1016/j.talanta.2016.03.022. [DOI] [PubMed] [Google Scholar]
- Rabiet M., Brissaud F., Seidel J.L., Pistre S., Elbaz-Poulichet F. Positive gadolinium anomalies in wastewater treatment plant effluents and aquatic environment in the Hérault watershed (South France) Chemosphere. 2009;75:1057–1064. doi: 10.1016/j.chemosphere.2009.01.036. [DOI] [PubMed] [Google Scholar]
- Rabiet M., Letouzet M., Hassanzadeh S., Simon S. Transmetallation of Gd-DTPA by Fe3+, Cu2+and Zn2+in water: batch experiments and coagulation-flocculation simulations. Chemosphere. 2014;95:639–642. doi: 10.1016/j.chemosphere.2013.09.102. [DOI] [PubMed] [Google Scholar]
- Reoyo-Prats B., Aubert D., Sellier A., Roig B., Palacios C. Dynamics and sources of pharmaceutically active compounds in a coastal Mediterranean river during heavy rains. Environ. Sci. Pollut. Res. 2018;25:6107–6121. doi: 10.1007/s11356-017-0880-7. [DOI] [PubMed] [Google Scholar]
- Richardson S.D., Kimura S.Y. Water analysis: emerging contaminants and current issues. Anal. Chem. 2016;88:546–582. doi: 10.1021/acs.analchem.5b04493. [DOI] [PubMed] [Google Scholar]
- Rogowska J., Olkowska E., Ratajczyk W., Wolska L. Gadolinium as a new emerging contaminant of aquatic environment. Environ. Toxicol. Chem. 2018 doi: 10.1002/etc.4116. [DOI] [PubMed] [Google Scholar]
- Rozemeijer J., Siderius C., Verheul M., Pomarius H. Tracing the spatial propagation of river inlet water into an agricultural polder area using anthropogenic gadolinium. Hydrol. Earth Syst. Sci. 2012;16:2405–2415. doi: 10.5194/hess-16-2405-2012. [DOI] [Google Scholar]
- Schijf J., Christy I.J. Effect of Mg and Ca on the stability of the MRI contrast agent Gd–DTPA in seawater. Front. Mar. Sci. 2018;5:1–17. doi: 10.3389/fmars.2018.00111. [DOI] [Google Scholar]
- Schmidt K., Bau M., Merschel G., Tepe N. Anthropogenic gadolinium in tap water and in tap water-based beverages from fast-food franchises in six major cities in Germany. Sci. Total Environ. 2019;687:1401–1408. doi: 10.1016/j.scitotenv.2019.07.075. [DOI] [PubMed] [Google Scholar]
- Semelka R.C., Prybylski J.P., Ramalho M. Influence of excess ligand on Nephrogenic Systemic Fibrosis associated with nonionic, linear gadolinium-based contrast agents. Magn. Reson. Imaging. 2019;58:174–178. doi: 10.1016/j.mri.2018.11.015. [DOI] [PubMed] [Google Scholar]
- Song H., Shin W., Ryu J., Seon H. Anthropogenic rare earth elements and their spatial distributions in the Han River, South Korea. Chemosphere. 2017;172:155–165. doi: 10.1016/j.chemosphere.2016.12.135. [DOI] [PubMed] [Google Scholar]
- Stepka Z., Dror I., Berkowitz B. The effect of nanoparticles and humic acid on technology critical element concentrations in aqueous solutions with soil and sand. Sci. Total Environ. 2018;610–611:1083–1091. doi: 10.1016/j.scitotenv.2017.08.170. [DOI] [PubMed] [Google Scholar]
- Swaminathan S. Gadolinium toxicity: iron and ferroportin as central targets. Magn. Reson. Imaging. 2016;34:1373–1376. doi: 10.1016/j.mri.2016.08.016. [DOI] [PubMed] [Google Scholar]
- Taylor S.R., McLennan S.M. Oxford & Palo Alto (Blackwell Scientific Publications); 1985. The Continental Crust: its Composition and Evolution. An Examination of the Geochemical Record Preserved in Sedimentary Rocks. [Google Scholar]
- Telgmann L., Wehe C.A., Birka M., Künnemeyer J., Nowak S., Sperling M., Karst U. Speciation and isotope dilution analysis of gadolinium-based contrast agents in wastewater. Environ. Sci. Technol. 2012;46:11929–11936. doi: 10.1021/es301981z. [DOI] [PubMed] [Google Scholar]
- Tepe N., Romero M., Bau M. High-technology metals as emerging contaminants: strong increase of anthropogenic gadolinium levels in tap water of Berlin, Germany, from 2009 to 2012. Appl. Geochem. 2014;45:191–197. doi: 10.1016/j.apgeochem.2014.04.006. [DOI] [Google Scholar]
- Thomsen H.S. Are the increasing amounts of gadolinium in surface and tap water dangerous? Acta Radiol. 2017;58:259–263. doi: 10.1177/0284185116666419. [DOI] [PubMed] [Google Scholar]
- Verplanck P.L., Furlong E.T., Gray J.L., Phillips P.J., Wolf R.E., Esposito K. Evaluating the behavior of gadolinium and other rare earth elements through large metropolitan sewage treatment plants. Environ. Sci. Technol. 2010;44:3876–3882. doi: 10.1021/es903888t. [DOI] [PubMed] [Google Scholar]
- Verplanck P.L., Taylor H.E., Nordstrom D.K., Barber L.B. Aqueous stability of gadolinium in surface waters receiving sewage treatment plant effluent Boulder Creek, Colorado. Environ. Sci. Technol. 2005;39:6923–6929. doi: 10.1021/es048456u. [DOI] [PubMed] [Google Scholar]
- Vriens B., Voegelin A., Hug S.J., Kaegi R., Winkel L.H.E., Buser A.M., Berg M. Quantification of element fluxes in wastewaters: a nationwide survey in Switzerland. Environ. Sci. Technol. 2017;7b01731 doi: 10.1021/acs.est.7b01731. acs.est. [DOI] [PubMed] [Google Scholar]
- Wang X., Jin T., Comblin V., Lopez-Mut A., Merciny E., Desreux J.F. A kinetic investigation of the lanthanide DOTA chelates. Stability and rates of formation and of dissociation of a macrocyclic gadolinium(III) polyaza polycarboxylic MRI contrast agent. Inorg. Chem. 1992;31:1095–1099. doi: 10.1021/ic00032a034. [DOI] [Google Scholar]
- Xia D., Davis R.L., Crawford J.A., Abraham J.L. Gadolinium released from MR contrast agents is deposited in brain tumors: in situ demonstration using scanning electron microscopy with energy dispersive X-ray spectroscopy. Acta Radiol. 2010;51:1126–1136. doi: 10.3109/02841851.2010.515614. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.