Abstract
COVID-19 may predispose patients to an increased risk of thrombotic complications through various pathophysiological mechanisms. Most of the reports on a high incidence of thrombotic complications are in relation to deep vein thrombosis and pulmonary embolism, while the evidence about arterial thrombosis in patients with COVID-19 is limited. We describe 4 cases of aortic thrombosis and associated ischemic complications in patients with severe SARS-CoV-2 infection.
Introduction
Coronavirus disease 2019 (COVID-19) is a viral respiratory illness caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which was first described in Wuhan, China, and later declared by the World Health Organization as pandemic.1 Acute respiratory distress syndrome is the main complication in patients with severe disease, but other complications, including thromboembolic events, have been also described.2 , 3 COVID-19 may predispose patients to an increased risk of thrombotic complications through various pathophysiological mechanisms, such as inflammation, immobilization, endothelial dysfunction, and a hypercoagulable state.3
Among the large number of patients treated in our hospital with COVID-19 in the last months, we describe 4 cases that have presented a severe form of thrombotic event with aortic involvement.
Case Series
Characteristics of the 4 patients (3 men and 1 woman) are summarized in Table I . The mean age of patients was 65.5 years (range, 50–76 years) and their medical history was consistent for hypertension (case 1), and hypertension, dyslipidemia, and psoriasis (case 3), the other 2 patients (cases 2 and 4) were otherwise healthy. All the patients were admitted to our hospital by respiratory symptoms compatible with COVID-19 (i.e., fever, cough, and dyspnea) during March and April 2020, and the diagnosis of SARS-CoV-2 infection was confirmed by reverse transcriptase polymerase chain reaction testing in all cases. During the hospital stay, all patients received empirical antibiotic and antiviral treatment, supportive therapies, and antithrombotic prophylaxis with low-molecular-weight heparins (LMWHs).
Table I.
Demographic and clinical characteristics, and laboratory findings at the time of the thrombotic event
| Characteristics | Case 1 | Case 2 | Case 3 | Case 4 |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Age (years) | 67 | 50 | 76 | 69 |
| Sex | Male | Male | Female | Male |
| Medical history | Hypertension | None | Hypertension, dyslipidemia, psoriasis | None |
| Clinical characteristics | ||||
| Aortic thrombosis | Occlusion | Occlusion | Floating thrombus | Floating thrombus |
| Clinical event | ALI, AMI | ALI, DVT, Stroke | Stroke | PE |
| Days from disease onset to thrombotic event | 17 | 12 | 15 | 15 |
| Anticoagulation before thrombotic event | Prophylactic | Prophylactic | Prophylactic | Prophylactic |
| Surgery (Thrombectomy) | Yes | Yes | No | No |
| Outcome | Dead | Discharged | Discharged | Discharged |
| Days of hospital stay | 11 | 22 | 24 | 20 |
| Laboratory findings | ||||
| Hemoglobin (g/dL) | 15.3 | 16.1 | 14.3 | 14.7 |
| Hematocrit (%) | 47.1 | 46.3 | 41 | 43 |
| Platelet count (/μL) | 209,000 | 401,000 | 481,000 | 241,000 |
| White-cell count (/μL) | 12,500 | 17,000 | 9,000 | 10,600 |
| Differential count (/μL) | ||||
| Total neutrophils | 11,900 | 15,800 | 8,000 | 10,000 |
| Total lymphocytes | 200 | 400 | 500 | 300 |
| Total monocytes | 400 | 700 | 300 | 200 |
| Creatinine (mg/dL) | 1.36 | 0.97 | 0.84 | 0.97 |
| EGFR (ml/min/1.73 m2) | 53 | >90 | 68 | 79 |
| Creatine kinase (U/L) | 1,565 | 3,866 | 44 | 42 |
| Albumin (g/dL) | 3 | 3.5 | 3.5 | 2.9 |
| Alanine aminotransferase (U/L) | 54 | 150 | 30 | 63 |
| Aspartate aminotransferase (U/L) | 49 | 95 | 34 | 30 |
| Lactate dehydrogenase (U/L) | 893 | 823 | 391 | 510 |
| Prothrombin time (sec) | 14.3 | 13.1 | 14.8 | 14.3 |
| Activated partial-thromboplastin time (sec) | 25 | 21 | 29 | 23 |
| Fibrinogen (mg/dL) | 624 | 513 | 903 | 509 |
| D-dimer (ng/mL) | 7,756 | 19,289 | 1,077 | 31,336 |
| Serum ferritin (ng/mL) | 8,203 | 1,585 | 568 | 1,700 |
| Interleukin-6 (pg/mL) | 657 | 507 | 176 | 1,138 |
| hs-C-reactive protein (mg/dL) | 8.1 | 3.8 | 28.6 | 3.4 |
| Antiphospholipid antibodiesa | ND | Normal | Normal | ND |
Anticardiolipin IgG and IgM, anti-b2-glycoprotein I IgA, IgG, and IgM. EGFR, estimated glomerular filtration rate (CKD-EPI); ALI, acute limb ischemia; AMI, acute mesenteric ischemia; DVT, deep vein thrombosis; PE, pulmonary embolism; ND, not determined.
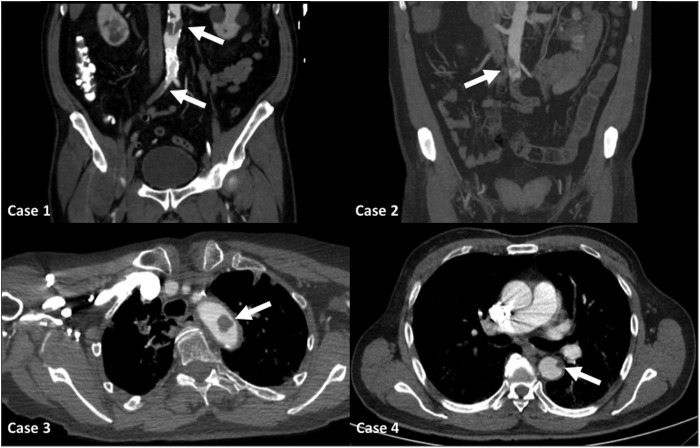
Despite the antithrombotic prophylaxis, the patients developed aortic thrombosis (Fig. 1 ) and subsequent diverse ischemic events. The mean time from disease onset to thrombotic event was 14.7 days (range, 12–17 days). Case 1 presented pain, coldness, and paleness of sudden onset in both legs suggesting acute limb ischemia (ALI), and the computed tomographic angiography (CTA) confirmed an aortoiliac thrombosis. Case 2 also presented aortoiliac thrombosis and ALI, and concomitantly, the CTA revealed femoropopliteal deep vein thrombosis (DVT) of the left lower limb. Later, this patient presented a confusional state and the brain computed tomography demonstrated an infarction in the left cerebellar hemisphere. Case 3 presented mixed dysphasia and on clinical suspicion of stroke neurovascular imaging studies were performed. The brain computed tomography revealed an acute infarct in the left frontal lobe, and the CTA of the supra-aortic trunks demonstrated a free-floating thrombus in the aortic arch and left common carotid. Case 4 developed sudden shortness of breath and hypoxia, and the pulmonary CTA confirmed acute pulmonary embolism (PE). Moreover, concomitant small filling defects were identified in the descending thoracic aorta. An echocardiography ruled out the presence of structural cardiac abnormalities, and this dismissed the possibility of paradoxical embolisms.
Fig. 1.
Computed tomographic image of aortic thrombosis in the four reported cases.
At the time of the thrombotic event, all patients had normal prothrombin time, activated partial-thromboplastin time, and platelets count. Conversely, fibrinogen (mean 637.2 mg/dL; range 509–903 mg/dL), D-dimer (mean 14,864.5 ng/mL, range 1,077–31,336 ng/mL), serum ferritin (mean 3,014 ng/mL, range 568–8,203 ng/mL), and interleukin-6 (mean 619.5, range 176–1,138 pg/mL) were significantly increased in all patients (Table I).
A bilateral retrograde transfemoral thrombectomy was performed in cases 1 and 2 with acute lower limb ischemia. Posteriorly, case 1 presented episodes of rethrombosis, requiring a major limb amputation due to persistent critical ischemia and died 5 days later from acute mesenteric ischemia and multiorgan failure. Case 2 evolved uneventful for the leg ischemia, but with neurological deficit by the stroke. Case 3 evolved with neurological deficit and the thrombus was washed with the heparin. Case 4 was also treated conservatively with full anticoagulation and evolved uneventful.
Discussion
COVID-19 is associated with an increased risk of thromboembolic events and numerous mechanisms may be involved in the pathogenesis of these complications.3, 4, 5 Most of the reports on a high incidence of thrombotic complications are in relation to DVT and PE,6 while the evidence about arterial thrombosis in patients with COVID-19 is limited.7 An acute thrombosis of an aortic prosthetic graft in a patient with COVID-19 has been also described.8 We reported 4 cases of aortic thrombosis and associated ischemic complications in patients with severe SARS-CoV-2 infection.
In our series, thrombotic events were more common in men than in women (3:1). The cases described in the present article were otherwise healthy patients, except for hypertension and dyslipidemia in 2 cases, and had no history of known prothrombotic disease or atrial fibrillation. All thrombotic events occurred despite the use of antithrombotic prophylaxis, and even episodes of rethrombosis occurred despite full anticoagulation (e.g., case 1). Therefore, it seems reasonable to attribute these thrombotic complications to a severe hypercoagulable state associated with COVID-19. To note, all of our patients had a severe presentation of the SARS-CoV-2 infection and required admission to the intensive care unit (ICU). The 2 cases of aortoiliac thrombosis and ALI underwent surgery, and given the limitations caused by infection, we opted for the simplest option to restore blood flow (thrombectomy). The other 2 cases with free-floating thrombus evolved satisfactorily with full anticoagulation.
Although, there are several factors that may favor the COVID-19-related thrombotic complications, the pathogenesis of hypercoagulability in this disease has yet to be fully elucidated. SARS-CoV-2 infection can produce endothelial dysfunction due to direct invasion of endothelial cells by the virus or mediated by the presence of cytokines (e.g., interleukin-6) and other acute phase reactants.9 Various changes in circulating prothrombotic factors have been reported in patients with severe COVID-19,3, 4, 5 such as elevated D-dimer and fibrinogen as in our series. It is noteworthy that D-dimer was elevated in all of our patients, but it does not seem to be directly related with the severity of the event (e.g., case 1). Antiphospholipid antibodies may also be related to the increased thrombotic risk,10 but, in the 2 patients in our series who underwent this determination, the result was normal. Furthermore, hypoxia and immobilization can cause an increase in blood viscosity and stasis, respectively, and have an important role in hospitalized and critically ill patients, such as those reported in this work.
Conclusions
COVID-19 is associated with an increased thrombotic risk, and those events in the venous system are the most frequent. Although aortic thrombosis is a rare event, its possible appearance underscores the need to initiate complete anticoagulation in patients at risk (i.e., ICU, high levels of D-dimer and interleukin-6) and to maintain it over time. According to our experience, we suggest maintaining full anticoagulation for at least 1-2 months after hospital discharge with LMWHs due to its anti-inflammatory effect and to facilitate the ambulatory control. After normalization of the analytical parameters, particularly D-dimer, the long-term treatment must be individualized. Nonetheless, further research is required to better understand the potential mechanisms by which the infection of SARS-CoV-2 is associated with a prothrombotic state, and also to establish the best strategy for the prevention and treatment of the thrombotic complications.
Footnotes
Authors' contributions: All authors contributed substantially to the article.
Declarations of interest: none.
Financial support: none.
References
- 1.World Health Organization Coronavirus disease (COVID-19) pandemic. https://www.who.int/emergencies/diseases/novel-coronavirus-2019 Available at:
- 2.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bikdeli B., Madhavan M.V., Jimenez D. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. J Am Coll Cardiol. 2020;75:2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spiezia L., Boscolo A., Poletto F. COVID-19-Related severe hypercoagulability in patients admitted to intensive care unit for acute respiratory failure. Thromb Haemost. 2020;120:998–1000. doi: 10.1055/s-0040-1710018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang N., Li D., Wang X. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Middeldorp S., Coppens M., van Haaps T.F. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020 doi: 10.1111/jth.14888. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Le Berre A., Marteau V., Emmerich J. Concomitant acute aortic thrombosis and pulmonary embolism complicating COVID-19 pneumonia. Diagn Interv Imaging. 2020;101:321–322. doi: 10.1016/j.diii.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giacomelli E., Dorigo W., Fargion A. Acute thrombosis of an aortic prosthetic graft in a patient with severe COVID-19 related pneumonia. Ann Vasc Surg. 2020 doi: 10.1016/j.avsg.2020.04.040. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang N., Bai H., Chen X. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18:1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y., Xiao M., Zhang S. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N Engl J Med. 2020;382:e38. doi: 10.1056/NEJMc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]