Abstract
The lymphopenia exhibited in patients with COVID-19 has been associated with a worse prognosis in the development of the disease.
To understand the factors associated with a worse evolution of COVID-19, we analyzed comorbidities, indicators of inflammation such as CRP and the ratio of neutrophils/lymphocytes, as well as the count of blood cells with T-lymphocyte subtypes in 172 hospitalized patients with COVID-19 pneumonia. Patients were grouped according to their needs for mechanical ventilation (ICU care) or not.
Within the comorbidities studied, obesity was the only associated with greater severity and ICU admission. Both the percentage and the absolute number of neutrophils were higher in patients needing ICU care than non-ICU patients, whereas absolute lymphocyte count, and especially the percentage of lymphocytes, presented a deep decline in critical patients. There was no difference between the two groups of patients for CD4 T-lymphocytes, neither in percentage of lymphocyte nor in absolute number, however for CD8 T-cells the differences were significant for both parameters which were in decline in ICU patients. There was a firm correlation between the highest values of inflammation indicators with the decrease in percentage of CD8 T-lymphocytes. This effect was not seen with CD4 cells.
Obesity together with lymphopenia, especially whether preferentially affects to CD8 T- lymphocytes, are factors that can predict a poor prognosis in patients with COVID-19.
Highlights
-
•
Among the comorbidities studied, only obesity is associated with a worse prognosis in patients with COVID-19.
-
•
The number of lymphocytes in blood, especially expressed as a percentage, is the main analytic factor that is associated with severity in COVID-19.
-
•
COVID-19 patients with ICU care have a selective decrease in blood CD8 cells that does not happen with CD4 cells.
-
•
There was a correlation in COVID-19 patients between inflammation indicators with the decrease in percentage of CD8 T-lymphocytes.
1. Introduction
Severe acute respiratory syndrome coronavirus (SARS-CoV-2) which causes the COVID-19 has rapidly evolved from an epidemic outbreak to a pandemic that affects virtually everyone. SARS-CoV-2 has a great similarity with to SARS-CoV and invades host human cells by binding to the angiotensin-converting enzyme 2 (ACE2) receptor [1]. In addition the cellular serine protease TMPRSS2 is also required to properly process the SARS-CoV-2 spike protein and facilitate host cell entry [2]. Although it is established that COVID-19 manifests itself mainly as an infection of the respiratory tract, COVID-19 behaves as a systemic disease affecting multiple organs including the gastrointestinal, cardiovascular, neurological, hematopoietic and immune system. SARS-CoV-2 viremia affects the organs where ACE2 is expressed. From several days after the onset of symptoms, the infection becomes more systemic, affecting various organs and with a clear evidence of inflammation development. Associated with the systemic involvement of the disease, the presence of lymphopenia is evident in many patients [3]. Lymphopenia could be explained due to the direct lethal effect of SARS-CoV-2 on lymphocytes, since expression of ACE2 in leukocytes has been described, although at low level [4]. Another possibility to explain lymphopenia is that the inflammation caused by the infection and the release of pro-inflammatory cytokines, such as TNF alpha and IL-6, could also induce apoptosis in lymphocytes [5]. This phenomenon has been clearly demonstrated in the sepsis.
The lymphopenia exhibited in patients with COVID-19 along with the rise in neutrophil leukocytes have been associated with a worse prognosis in the development of the disease. Indeed, in patients who needed intensive care units (ICU) and who presented acute respiratory distress syndrome, the lymphocyte count levels were lower than those without these requirements [6]. Likewise lymphopenia has been associated with increased mortality and mechanical ventilation requirements [7].
The objective of the present study was to investigate whether the subpopulations of T-lymphocytes (CD4+ and CD8+) are affected in a greater way in lymphopenia induced by SARS-CoV-2, as well as to determinate the associations with clinical features. To this end, we studied the lymphocyte populations, inflammation markers, as well as comorbidities in patients with COVID-19 pneumonia admitted in ICU and patients with a less severe condition (without request invasive mechanical ventilation and without severe multi-organ involvement).
2. Patient selection and methods
A retrospective case-control study was conducted in patients suffering from COVID-19 pneumonia admitted to University Hospital of Ciudad Real (Spain) from March 1 to April 15, 2020. A total number of one hundred and seventy two patients (N = 172) were investigated. Hospitalized patients without acute respiratory failure (N = 145) were included as the control group, and patients supporting mechanic ventilation at intensive care unit (ICU-patients, N = 27) represented the group of cases. The infection by SARS-Cov-2 was confirmed by the real-time (rt) reverse transcriptase (RT) polymerase chain reaction (PCR) from Abbott Laboratories (Abbott RealTime SARS-COV-2 assay, Abbott Park, Illinois, U.S.A) from upper respiratory tract samples after admission. Demographic and clinical features (comorbidities) obtained from patient's medical records as well as laboratory determinations were analyzed between cases and controls. The absolute number and percentage of lymphocytes and neutrophils were measured in an automated analyzer of blood cell count (UniCel DxH800 Beckman Coulter Miami FL) from EDTA-anticoagulated samples. For the flow cytometry assay of the lymphocyte subpopulations (CD3+, CD3 + CD4+ and CD3 + CD8+ lymphocytes) in peripheral blood samples the following monoclonal antibodies were used: PerCP conjugated anti-human CD3, FITC anti-CD4 and PE anti-CD8 purchased from Becton Dickinson (BD San José, USA). Lymphocytes were acquired in a FACScan cytometer (BD) and analyzed with the CellQuest Pro software (BD). The prevalence of categorical variables (gender and comorbidities) was calculated by Chi2 with the Fisher's exact test, with 2 × 2 contingency tables. For the continuous variables (age at admission, laboratory findings, and lymphocyte subpopulations) the non-parametric Mann-Whitney U test was used. The influence of the parameters under study on the defined groups of patients were determined by the odds ratios (ORs) with the confidence intervals (CIs) at 95% in a univariate regression analysis. The relationships between the percentage of lymphocyte subpopulations (CD3+, CD3 + CD4+ and CD3 + CD8+) and laboratory determinations were analyzed by the Spearman's correlation coefficient linear regresion. All the statistical determinations were analyzed using SPSS version 23.0 (SPSS Inc., Chicago, Ill., USA). The differences were considered statistically significant at p-values of less than 0.05.
3. Results
3.1. Clinical analytical and demographic characteristics of patients
The demographic and clinical characteristics of the patients introduced in the study are summarized in Table 1 . We found differences between the groups of patients studied in terms of biochemical and blood cell parameters, in agreement with the results previously described in other series [7,8]. As is reflected in Table 1, only the presence of obesity showed significant differences between both groups of patients in terms of comorbidities, with an increased risk respect to the control group in the univariate regression analysis performed (OR = 4.72, 95% CI 1.614–13.830, p = .005). The remaining comorbidities did not show substantial differences between both groups of patients.
Table 1.
Demographic, clinical and analytical data of 172 patients with COVID-19 grouped by severity of illness according to ICU care requirements. In quantitative variables the p value was calculated by the non-parametric U Mann-Withney test and in qualitative variables by the test Chi2 with the Fisher's exact test, with 2 × 2 contingency tables.
| ICU patients (N = 27) | Non ICU (N = 145) | p value | |
|---|---|---|---|
| Demographic features and comorbidities | |||
| Age (years) | 65.64 ± 14.1 | 57.89 ± 13.1 | 0.018 |
| Male | 20 (74.1%) | 84 (57.9%) | ns |
| Female | 7 (25.9%) | 61(42.1%) | ns |
| Hypertension | 13 (48.1%) | 74 (51.03%) | ns |
| Diabetes | 8 (29.6%) | 31 (21.3%) | ns |
| Dyslipidemia | 5 (18.5%) | 54 (37.2%) | 0.06 |
| Obesity | 7 (25.9%) | 10 (6.8%) | 0.002 |
| COPD | 2 (7.4%) | 15 (10.3%) | ns |
| Cardiovascular disease | 4 (14.8%) | 24 (16.5%) | ns |
| Thrombotic disease | 1 (3.7%) | 11 (7.5%) | ns |
| Cancer | 3 (11.1%) | 16 (11.0%) | ns |
| Autoimmune disease | 5 (18.5%) | 14 (9.6%) | ns |
| Mortality | 6 (22.2%) | 21 (14.4%) | ns |
| Cellular and biochemical features | |||
| CRP (mg/dL) | 15.2 ± 9.0 | 10.4 ± 8.0 | 0.011 |
| D Dimer (ng/mL) | 7582 ± 10,922 | 22,317 ± 5613 | <0.0001 |
| Neutrophils % | 88.1 ± 5.7 | 73.5 ± 15.4 | <0.0001 |
| Neutrophils x 103/μL | 12.5 ± 4.6 | 5.1 ± 3.2 | <0.0001 |
| Platelets x 103/μL | 258.9 ± 138.7 | 235.0 ± 124.8 | ns |
| Lymphocytes % | 5.7 ± 3.6 | 16.9 ± 10.5 | <0.0001 |
| Lymphocytes/μL | 733.3 ± 473.9 | 971.5 ± 529.0 | ns |
| % Lymphopenia (< 1000/μL) | 81.5 | 54.9 | 0.01 |
| Neutrophil/Limphocytes ratio | 22.1 ± 13.2 | 7.3 ± 6.7 | <0.0001 |
| Platelets/Limphocytes ratio | 297.6 ± 211.6 | 428.1 ± 297.3 | ns |
| T Lymphocytes subpopulations | |||
| CD3 (% lymphocytes) | 69.3 ± 13.2 | 72.0 ± 9.7 | ns |
| CD3/μL | 528.3 ± 350.9 | 701.0 ± 408.5 | 0.018 |
| CD4 (% lymphocytes) | 44.1 ± 10.9 | 41.5 ± 12.4 | ns |
| CD4/μL | 340.30 ± 251.9 | 395.9 ± 241.0 | ns |
| CD8 (% lymphocytes) | 23.1 ± 9.4 | 28.4 ± 11.8 | 0.039 |
| CD8/μL | 172.4 ± 123.9 | 287.6 ± 223.8 | 0.001 |
| CD4/CD8 | 2.4 ± 1.4 | 1.9 ± 1.6 | 0.037 |
Abbreviations: COPD, Chronic Obstructive Pulmonary Disease; CRP, C-reactive protein; ns, not significant.
3.2. Parameters associated with inflammation
Inflammation is the clue determinant and primary basic mechanism resulting in disability and increased mortality in COVID-19 [9]. Systemic inflammation is associated with changes in circulating blood cells quantity and composition. In the blood cell analysis, both the percentage and the absolute number of neutrophils were higher in ICU patients, whereas on the other hand, absolute lymphocyte count, and especially the percentage of lymphocytes, presented a deep decline in critical patients (OR = 0.769, 95% CI 0.687–0.861, p < .0001). The Neutrophil -Lymphocyte ratio (NLR) and platelet-lymphocyte ratio (PLR) have emerged as excellent indicators of systemic inflammation [10]. As is indicated in Table 1, NLR was higher in patients requiring ICU care, as well as the biochemical indicator of inflammation, C-reactive protein (CRP), demonstrating a higher rate of inflammation in the most critical patients.
3.3. T-lymphocyte subpopulations
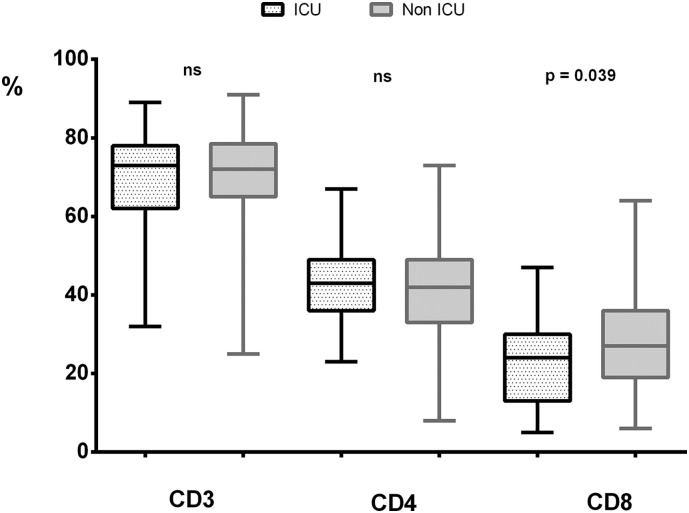
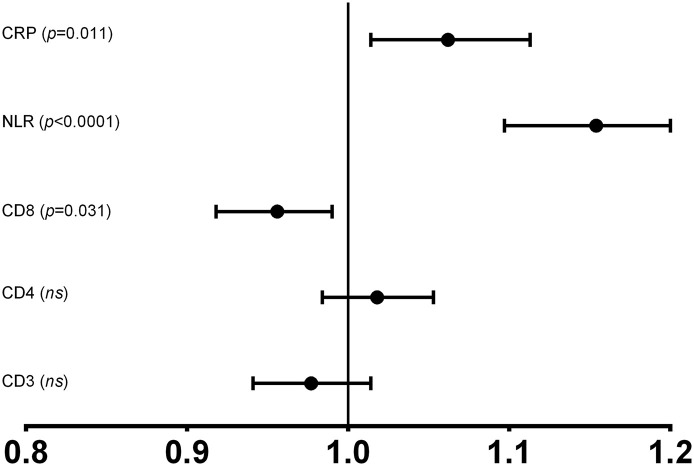
When lymphopenia is analyzed in terms of studies of T lymphocyte subpopulations, as shown in Table 1, there was no difference between the two groups of patients studied for CD4 T-lymphocytes, neither in percentage of lymphocyte nor in absolute number. However for CD8 T-cell the differences were significant for both parameters which are in decline in the most severe patients (Fig. 1 ). Alteration in CD8 T lymphocyte population is partially transferred to the results observed in T lymphocytes and the CD4/CD8 ratio. However, the decrease observed in CD8 T-cells (OR = 0.956, 95% CI 0.918–0.996, p < .031) is less evident than the global data of lymphopenia. Similarly, as is shown in the univariate regression analysis (OR) in Fig. 2 , the T-lymphocyte populations showed that only the decrease in the CD8 lymphocyte subpopulation is associated with more severity in disease. Whether we set a random cut-off point for the absolute number of CD8 cells at a value ≤100 cells/μL, the univariate regression study will give us a significant association between having fewer CD8 T-cells and ICU care requirements (OR = 2.630, 95% CI 1.053–6.574, p < .039). Finally, there was a group of 31 patients with an inverted CD4/CD8 ratio (CD4/CD8 < 0.9). Of these only one was in the group of patients with ICU care (3.7% vs 20.7%; p = .023).
Fig. 1.
Mean values ± standard deviation, with maximum and minimum in the percentages of CD3, CD4 and CD8 lymphocytes in patients with or without ICU care.
Fig. 2.
Odds ratios (ORs) with the confidence intervals (CIs) at 95% in a univariate regression analysis for T-lymphocyte subpopulations and indicators of inflammation C-reactive protein (CRP) and Neutrophil/ Lymphocyte ratio (NLR) depending on the ICU requirements in patients with COVID-19.
3.4. Correlation between T-lymphocytes and inflammation
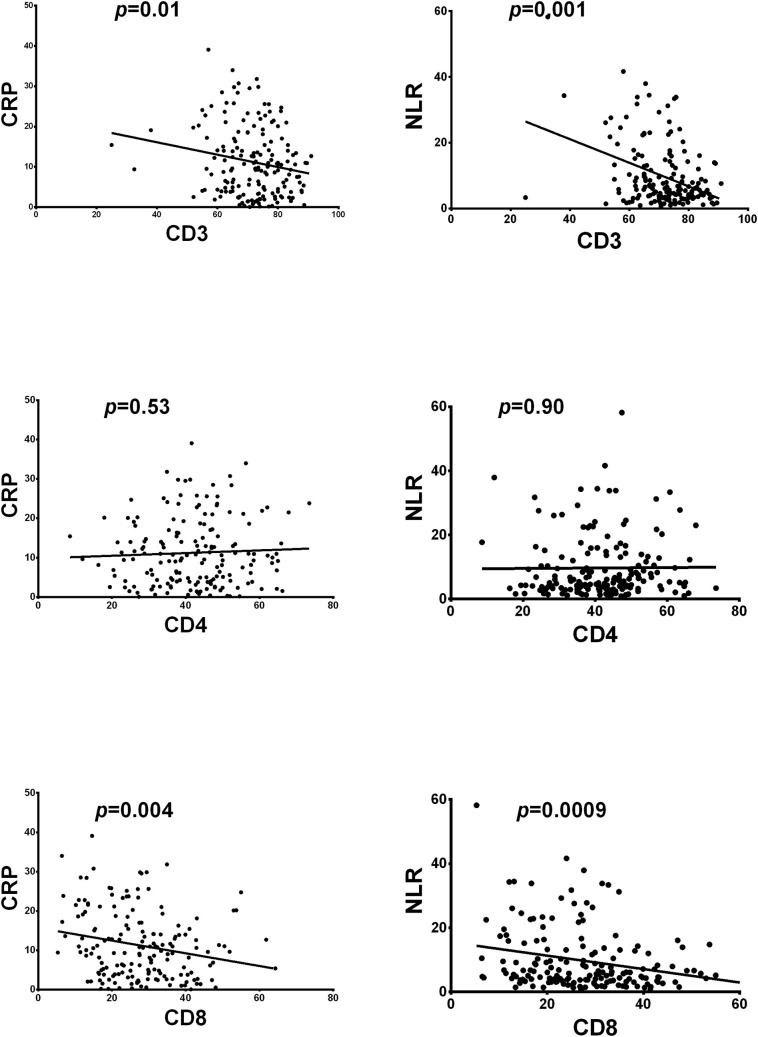
Inflammation-associated parameters such as CRP protein and NLR cell ratio in our study and in other previously published series [11] [12], were related with a worse prognosis of COVID-19. We analyzed in all patients included in our study the potential relationships between CD8 T-cell percentage decline and the previously cited parameters relating to inflammation. In this sense, Fig. 3 shows that there is a firm correlation between the highest values of inflammation indicators with the decrease in percentage of CD8 T-lymphocytes. This effect is not seen with CD4 T-cells. Linear regression values were significant for T-lymphocytes and for the CD8 T-lymphocyte subtype. The absence of correlation in CD4 T-lymphocytes allow us to deduce that the correlation observed in total T lymphocytes is mainly mediated by the effect on CD8 T-cells.
Fig. 3.
Linear regression between lymphocyte subpopulations CD3, CD4 and CD8 and the inflammation indicators C-reactive protein (CRP) and Neutrophil/ Lymphocyte ratio (NLR).
4. Discussion
Mortality from COVID-19 may be due to the fact that some patients develop severe pneumonia and acute respiratory distress syndrome requiring rapid admission to the ICU and invasive mechanical ventilation. Determining which patients progress to severe illness is very important in order to evaluate the prognosis of the disease and take early decisions about potential treatments.
In our study analyzed different comorbidities present in all patients, only the need for ICU care was associated with obesity (BMI, body mass index > 30). There is a clear association between obesity and basal inflammatory status characterized by higher circulating IL-6 and CRP levels. Adipose tissue in patients with obesity has a pro-inflammatory role, with increased expression of cytokines. Our result is in line with others in which obesity along with age has been presented as the major risk factors for worse disease progression in COVID-19. [13] [14]. The effect of obesity on the severity of COVID-19 will be influenced by the prevalence of obesity in the population.
Lymphopenia is a cellular factor associated with a poor prognosis in the evolution of the disease [15] [16]. Lymphocytes and their subsets play an important role in the maintenance of immune system function. CD8+ T cells are important for directly attacking and killing virus-infected cells, whereas CD4+ T cells are crucial to prime both CD8+ T cells and B cells. In patients with COVID-19, increased T cell exhaustion and reduced functional diversity predicted severe disease [17]. Virus infection can also lead to dysregulation in the levels of lymphocyte subsets [18]. Thus, it is important to clarify the characteristics of lymphocyte subsets in COVID-19, which could provide novel insights to explore the immune mechanism. To this end, we analyzed the differences between critical patients needing care in the ICU and patients with a lesser severity in relation to the subset of T-lymphocytes. One important finding in our study was that CD8+ T cells, but not CD4+ T cells, in circulation were reduced in ICU care patients compared to non-ICU patients. These findings indicate a more obvious change in CD8+ T cells than in other lymphocyte subsets after SARS-CoV-2 infection. Thus, the decline of lymphocytes, especially CD8+ T cells, might be a potential predictor for disease severity and clinical worsening in COVID-19. Lymphopenia might be caused by virus attachment or indirectly by immune injuries from inflammatory mediators. Moreover, pulmonary recruitment of immune cells from the blood and the infiltration of lymphocytes into the airways may explain the lymphopenia and increased neutrophil–lymphocyte ratio seen in around 80% of patients with SARS-CoV-2 infection. In most individuals, recruited cells clear the infection. However, in some patients, a dysfunctional immune response occurs, which triggers a cytokine storm that mediates widespread lung inflammation. It has been demonstrated in SARS-CoV an antigen-specific recall cytotoxic T lymphocyte response in cells of patients recovered from SARS, but not in the patients with critical SARS or died of SARS, suggesting that the latter apparently cannot generate sufficient protective immunity to eliminate SARS-CoV [19]. More recently, CD8+ T cells have been reported to be significantly decreased in peripheral blood in patients deaths from COVID-19 [20], and their levels partially recover after effective treatments [21].
Furthermore, in our work we showed that a decrease in CD8 T-lymphocyte values clearly correlated with increases in inflammation indicators. The presence of an insufficient adaptive response may favor the development of high rates of inflammation that ultimately lead to a poor disease progression. An inadequate CD8 T-cell response may be a factor suggesting a dysregulation of the adaptive immune system and consequently a greater severity in COVID-19 patients. These data indicated that progressive immune-associated injury and inadequate adaptive immune responses could be one possible mechanism by which SARS-CoV-2 causes severe illness and fatal outcomes.
In conclusion, obesity together with lymphopenia, especially whether they preferentially affects CD8 T lymphocytes, are factors that predict a poor prognosis in COVID-19 patients.
Ethical approval
Ethical approval for this study was obtained from Ethical committee of Hospital General Universitario de Ciudad Real.
Declaration of Competing Interest
The authors certify that they have NO affiliations with or involvement in any organization or entity with any financial interest in the subject matter or materials discussed in this manuscript.
Acknowledgements
This work was supported by Instituto de Salud Carlos III (ISCIII) co-founded by Fondo Europeo de Desarrollo Regional – FEDER for the Thematic Networks and Co-operative Research Centres: ARADyAL (RD 16/0006/0028).
References
- 1.Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W., Si H.-R., Zhu Y., Li B., Huang C.-L., Chen H.-D., Chen J., Luo Y., Guo H., Jiang R.-D., Liu M.-Q., Chen Y., Shen X.-R., Wang X., Zheng X.-S., Zhao K., Chen Q.-J., Deng F., Liu L.-L., Yan B., Zhan F.-X., Wang Y.-Y., Xiao G.-F., Shi Z.-L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.-H., Nitsche A., Müller M.A., Drosten C., Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tan L., Wang Q., Zhang D., Ding J., Huang Q., Tang Y.-Q., Wang Q., Miao H. Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study. Signal Transduct Target Ther. 2020;5:33. doi: 10.1038/s41392-020-0148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nicin L., Abplanalp W.T., Mellentin H., Kattih B., Tombor L., John D., Schmitto J.D., Heineke J., Emrich F., Arsalan M., Holubec T., Walther T., Zeiher A.M., Dimmeler S. Cell type-specific expression of the putative SARS-CoV-2 receptor ACE2 in human hearts. Eur. Heart J. 2020 doi: 10.1093/eurheartj/ehaa311. (n.d.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aggarwal S., Gollapudi S., Gupta S. Increased TNF-alpha-induced apoptosis in lymphocytes from aged humans: changes in TNF-alpha receptor expression and activation of caspases. J. Immunol. 1999;162:2154–2161. [PubMed] [Google Scholar]
- 6.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y., Zhao Y., Li Y., Wang X., Peng Z. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borges do Nascimento I.J., Cacic N., Abdulazeem H.M., von Groote T.C., Jayarajah U., Weerasekara I., Esfahani M.A., Civile V.T., Marusic A., Jeroncic A., Carvas Junior N., Pericic T.P., Zakarija-Grkovic I., Meirelles Guimarães S.M., Luigi Bragazzi N., Bjorklund M., Sofi-Mahmudi A., Altujjar M., Tian M., Arcani D.M.C., P D., Mathúna O., Marcolino M.S. Novel coronavirus infection (COVID-19) in humans: a scoping review and meta-analysis. J. Clin. Med. 2020;9 doi: 10.3390/jcm9040941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H., Wu Y., Zhang L., Yu Z., Fang M., Yu T., Wang Y., Pan S., Zou X., Yuan S., Shang Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir. Med. 2020 doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. HLH across speciality collaboration, UK, COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang Z., Fu Z., Huang W., Huang K. Prognostic value of neutrophil-to-lymphocyte ratio in sepsis: a meta-analysis. Am. J. Emerg. Med. 2019 doi: 10.1016/j.ajem.2019.10.023. [DOI] [PubMed] [Google Scholar]
- 11.Yang A.-P., Liu J.-P., Tao W.-Q., Li H.-M. The diagnostic and predictive role of NLR, d-NLR and PLR in COVID-19 patients. Int. Immunopharmacol. 2020;84:106504. doi: 10.1016/j.intimp.2020.106504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferrari D., Motta A., Strollo M., Banfi G., Locatelli M. Routine blood tests as a potential diagnostic tool for COVID-19. Clin. Chem. Lab. Med. 2020 doi: 10.1515/cclm-2020-0398. [DOI] [PubMed] [Google Scholar]
- 13.Naveed Sattar, McInnes Iain B., McMurray John J.V. Obesity a risk factor for severe COVID-19 infection: multiple potential mechanisms. Circulation. 2020 doi: 10.1161/CIRCULATIONAHA.120.047659. 0 (n.d.) [DOI] [PubMed] [Google Scholar]
- 14.Simonnet A., Chetboun M., Poissy J., Raverdy V., Noulette J., Duhamel A., Labreuche J., Mathieu D., Pattou F., Jourdain M., Lille Intensive Care COVID-19 and Obesity Study Group High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity (Silver Spring) 2020 doi: 10.1002/oby.22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H., Wu Y., Zhang L., Yu Z., Fang M., Yu T., Wang Y., Pan S., Zou X., Yuan S., Shang Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir. Med. 2020 doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruan Q., Yang K., Wang W., Jiang L., Song J. Correction to: clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020 doi: 10.1007/s00134-020-06028-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zheng H.-Y., Zhang M., Yang C.-X., Zhang N., Wang X.-C., Yang X.-P., Dong X.-Q., Zheng Y.-T. Elevated exhaustion levels and reduced functional diversity of T cells in peripheral blood may predict severe progression in COVID-19 patients. Cell. Mol. Immunol. 2020;17:541–543. doi: 10.1038/s41423-020-0401-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chan M.H.M., Wong V.W.S., Wong C.K., Chan P.K.S., Chu C.M., Hui D.S.C., Suen M.W.M., Sung J.J.Y., Chung S.S.C., Lam C.W.K. Serum LD1 isoenzyme and blood lymphocyte subsets as prognostic indicators for severe acute respiratory syndrome. J. Intern. Med. 2004;255:512–518. doi: 10.1111/j.1365-2796.2004.01323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen H., Hou J., Jiang X., Ma S., Meng M., Wang B., Zhang M., Zhang M., Tang X., Zhang F., Wan T., Li N., Yu Y., Hu H., Yang R., He W., Wang X., Cao X. Response of memory CD8+ T cells to severe acute respiratory syndrome (SARS) coronavirus in recovered SARS patients and healthy individuals. J. Immunol. 2005;175:591–598. doi: 10.4049/jimmunol.175.1.591. [DOI] [PubMed] [Google Scholar]
- 20.Du R.-H., Liang L.-R., Yang C.-Q., Wang W., Cao T.-Z., Li M., Guo G.-Y., Du J., Zheng C.-L., Zhu Q., Hu M., Li X.-Y., Peng P., Shi H.-Z. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: a prospective cohort study. Eur. Respir. J. 2020 doi: 10.1183/13993003.00524-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang F., Nie J., Wang H., Zhao Q., Xiong Y., Deng L., Song S., Ma Z., Mo P., Zhang Y. Characteristics of peripheral lymphocyte subset alteration in COVID-19 pneumonia. J. Infect. Dis. 2020 doi: 10.1093/infdis/jiaa150. [DOI] [PMC free article] [PubMed] [Google Scholar]