The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and the resulting illness, coronavirus disease 2019 (COVID-19), developed in Wuhan, China, in late 2019.1 Within months, COVID-19 became a worldwide pandemic, with millions of cases reported.2 Nephrologists have been front and center, caring for infected dialysis patients while also treating hospitalized patients with COVID-19 and acute kidney injury (AKI). A controversial aspect of therapy in both settings has been the use of erythropoiesis-stimulating agents (ESAs) to treat anemia experienced by patients with COVID-19.
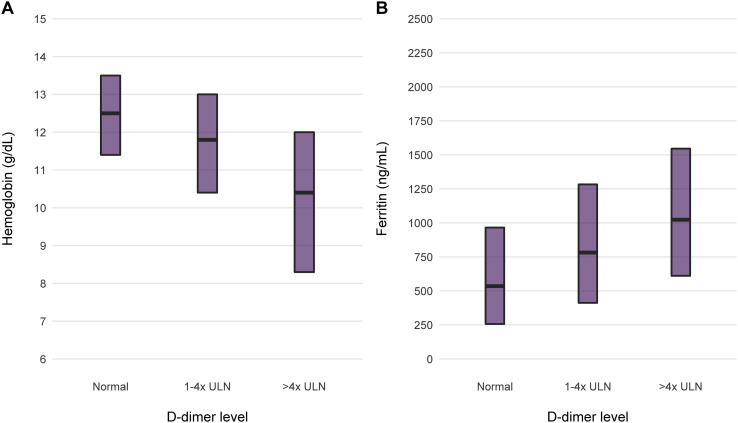
Both COVID-19 patients with AKI and maintenance dialysis patients infected with the virus have a high rate of anemia, often severe. The worst anemia in COVID-19 occurs in temporal relation with a burst of severe systemic inflammation that may occur during the course of the disease. We have witnessed this relationship in recent months, caring for patients hospitalized with COVID-19 in Northwell Health hospitals. Among 11,265 patients across 13 hospitals admitted between March 1 and April 27, 2020, we found that elevated D-dimer concentrations were associated with lesser hemoglobin and greater serum ferritin concentrations (Fig 1 ).
Figure 1.
Median (A) hemoglobin and (B) serum ferritin levels by ranges of D-dimer levels for patients treated in Northwell Health hospitals in New York. There were 11,265 patients across 13 hospitals, admitted between March 1 and April 27, 2020. Of these, 8,232 had at least 1 D-dimer and hemoglobin measurement and 7,994 had a D-dimer and ferritin measurement. Elevation in D-dimer level above normal in the 1-to-4-times and greater-than-4-times the upper limit of normal (ULN; 229 ng/mL) groups was associated with a stepwise lesser median hemoglobin level and a corresponding stepwise greater serum ferritin level.
The association of anemia and inflammation has long been recognized as the syndrome of anemia of chronic disease or the anemia of inflammation. The hepatic-derived protein hepcidin modulates the ferrokinetic response to inflammation.3 In the presence of inflammation, hepcidin restricts iron absorption from the intestines and iron release from storage tissues in the spleen and liver. The net effect is to limit iron entering circulation and tissues.3 Because many microbes are highly dependent on iron as a key nutrient, the teleology may be thought of as a protective action defending against infection by hepcidin-driven diminution in available iron supply. However, simultaneously, this lessens iron availability for host erythropoiesis, resulting in the development or worsening of anemia. In maintenance dialysis patients, inflammation-induced hepcidin production even without infection may be maladaptive, making anemia more difficult to treat. Clinical signs of hepcidin induction include reduced hemoglobin and elevated serum ferritin levels due to iron trapped in storage tissues, even with low circulating iron (low transferrin saturation).
In COVID-19, not much is known regarding the so-called “cytokine surge” and coincident hepcidin levels and iron kinetics. The severe anemia often seen in these patients may be due in part to hepcidin effects and dysregulated iron kinetics, though this has not yet been established. However, clearly, patients with severe COVID-19 usually have an intense inflammatory phase. In similar states of inflammation, ESAs have greatly limited efficacy.4 Thus, in COVID-19, patients with or without kidney disease but with anemia are unlikely to mount an effective response to ESAs due to the inflammation. Interestingly, the new class of hypoxia-inducible factor stabilizers are oral drugs that cause erythropoietin production and enhanced iron availability. Early study results hint at the fact that these drugs may be able to treat anemia more effectively in an inflammatory milieu than ESAs.5
Treatment with ESAs in patients with COVID-19 would not only have limited effectiveness but could also be potentially harmful. This is due to a remarkably prothrombotic state seen with severe COVID-196 and the tendency of ESAs to induce thromboses. Blood clots in patients with COVID-19 have been found frequently in both venous and arterial systems. Hemodialysis filters clot more frequently, particularly those used for continuous renal replacement therapies. Pulmonary embolism is an important problem as well, and in 1 study of 106 pulmonary computed tomography angiograms in patients with COVID-19, 32 patients (30%) had acute pulmonary emboli.7 Typically in critically ill patients, the rate is much lower.8 The cause of the prothrombotic state in COVID-19 is unclear. However, the intimate interplay of the inflammatory/immune system with coagulation is remarkable in these patients. As a result, a more aggressive thromboprophylaxis policy is in order for many patients hospitalized with COVID-19.
Although ESA treatment has been remarkably successful for treating anemia, reducing blood transfusions and possibly improving quality of life, thrombotic episodes have been frequently noted in treatment studies.9 This has been most clearly observed in studies in which ESAs have been used to increase hemoglobin concentrations to higher than the usual targets, particularly into the normal range.10 The prothrombotic effect of ESAs in concert with the prothrombotic milieu of COVID-19 could have an additive adverse effect.
When considering the probable reduced efficacy of ESAs in patients with COVID-19 inflammation and the potential for increasing thrombotic risk, the role for these agents should be significantly limited in these patients. Our recommendations are as follows.
-
1.
Patients with COVID-19 with or without AKI and anemia. We suggest avoiding ESA therapy in this setting. Hemoglobin concentrations will often be low and may require blood transfusion to maintain adequate systemic oxygen delivery. The risks of ESA treatment generally outweigh potential benefits.
-
2.
Maintenance dialysis patients with COVID-19 and anemia. This setting is different in that endogenous erythropoietin production is substantially reduced. As a result, patients have little ability to withstand the anemic effects of COVID-19. If the patient enters the hospital already receiving an ESA dose as an outpatient, we continue the dose in the hospital. However, we lower the hemoglobin target to attempt to mitigate thrombotic risk to the greatest extent possible. We only attempt to increase the hemoglobin level to 8 to 9 g/dL in these patients and do not escalate ESA doses if the goal cannot be achieved.
In conclusion, the anemia experienced by hospitalized patients with COVID-19 can be severe. Although the idea of using ESA treatment may be enticing, in most cases, the expected limited efficacy due to inflammation in this setting and potential risks limit the role of these agents.
Article Information
Authors’ Full Names and Academic Degrees
Steven Fishbane, MD, and Jamie S. Hirsch, MD.
Support
None.
Financial Disclosure
Dr Fishbane reports receipt of fees for research consulting from Astra Zeneca, Fibrogen, Akebia, and Megapro. Dr Hirsch declares that he has no relevant financial interests.
Peer Review
Received May 13, 2020, in response to an invitation from the journal. Direct editorial input from an Associate Editor and a Deputy Editor. Accepted in revised form May 20, 2020.
References
- 1.WHO Novel coronavirus – China. 2020. https://www.who.int/csr/don/12-january-2020-novel-coronavirus-china/en/
- 2.ArcGIS Dashboards. https://gisanddata.maps.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6
- 3.Weiss G., Ganz T., Goodnough L.T. Anemia of inflammation. Blood. 2019;133(1):40–50. doi: 10.1182/blood-2018-06-856500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chawla L.S., Krishnan M. Causes and consequences of inflammation on anemia management in hemodialysis patients. Hemodial Int. 2009;13(2):222–234. doi: 10.1111/j.1542-4758.2009.00352.x. [DOI] [PubMed] [Google Scholar]
- 5.Chen N., Hao C., Liu B.C. Roxadustat treatment for anemia in patients undergoing long-term dialysis. N Engl J Med. 2019;381(11):1011–1022. doi: 10.1056/NEJMoa1901713. [DOI] [PubMed] [Google Scholar]
- 6.Helms J., Tacquard C., Severac F., CRICS TRIGGERSEP Group (Clinical Research in Intensive Care and Sepsis Trial Group for Global Evaluation and Research in Sepsis) High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study [published online ahead of print May 4, 2020]. Intensive Care Med. https://doi.org/10.1007/s00134-020-06062-x [DOI] [PMC free article] [PubMed]
- 7.Leonard-Lorant I., Delabranche X., Severac F. Acute pulmonary embolism in COVID-19 patients on CT angiography and relationship to d-dimer levels [published online ahead of print April 23, 2020]. Radiology. https://doi.org/10.1148/radiol.2020201561 [DOI] [PMC free article] [PubMed]
- 8.Lim W., Meade M., Lauzier F. Failure of anticoagulant thromboprophylaxis: risk factors in medical-surgical critically ill patients. Crit Care Med. 2015;43:401–410. doi: 10.1097/CCM.0000000000000713. [DOI] [PubMed] [Google Scholar]
- 9.Pfeffer M.A., Burdmann E.A., Chen C.Y., TREAT Investigators A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med. 2009;361(21):2019–2032. doi: 10.1056/NEJMoa0907845. [DOI] [PubMed] [Google Scholar]
- 10.Palmer S.C., Navaneethan S.D., Craig J.C. Meta-analysis: erythropoiesis-stimulating agents in patients with chronic kidney disease. Ann Intern Med. 2010;153(1):23–33. doi: 10.7326/0003-4819-153-1-201007060-00252. [DOI] [PubMed] [Google Scholar]