Abstract
A multiple sclerosis patient infected by SARS-CoV-2 during fingolimod therapy was hospitalized with moderate clinical features, and recovered in 15 days. High levels of CCL5 and CCL10 chemokines and of antibody-secreting B cells were detected, while the levels other B- and T-cell subsets were comparable to that of appropriate controls. However, CD4+ and CD8+ cells were oligoclonally expanded and prone to apoptosis when stimulated in vitro. This study suggests that fingolimod-immunosuppressed patients, despite the low circulating lymphocytes, may rapidly expand antibody-secreting cells and mount an effective immune response that favors COVID-19 recovery after drug discontinuation.
Keywords: COVID-19, Fingolimod, Multiple sclerosis
Graphical abstract
1. Introduction
The immune response is essential to control and eliminate all type of infections, with SARS-CoV-2 infection that makes no exception to this rule. Although the clinical characteristic of COVID-19 have stated to be defined, the knowledge of the immune response found in patients with COVID-2019 is only partial, and apparently limited to immunocompetent individuals. Patients admitted to intensive care units show high white blood cell and neutrophil counts (Lippi and Plebani, 2020). On the opposite, they display significant reduction of T lymphocytes, especially of CD8+ cells, but an increased percentage of activated T cells and antibody-secreting cells (ASC) as well as high interleukin 6 (IL-6), 10 (IL-10), 2 (IL-2), and interferon (IFN)-γ serum levels (Liu et al., 2020; Thevarajan et al., 2020).
Several multiple sclerosis (MS) therapies impair immune surveillance to various degrees and may predispose patients to develop community-acquired and opportunistic infections. Therefore, treated patients could be among those most at risk of severe COVID-19. Fingolimod, which regulates the trafficking between primary and secondary lymphoid organs (Sica et al., 2019), induces a redistribution of lymphocytes subsets causing an acquired lymphopenia that apparently do not compromise immunosurveillance, although cases of viral (especially varicella zoster) and bacterial infections have been described in real life settings (Grebenciucova and Pruitt, 2017).
This study describes the immune characterization of a fingolimod-treated MS patient with COVID-19, who was included in the Italian programme for COVID-19 infection in MS (Sormani et al., 2020).
2. Case report
2.1. Clinical characterization
A 45-years-old, obese (body mass index of 33.2) woman with relapsing remitting MS since 1996 was treated with IFN-beta 1a, glatiramer acetate, mitoxantrone, teriflunomide and, since 2017, with fingolimod. The patient (EDSS = 6.5 and Ambulation Index = 7) was taking fingolimod as prescribed and at the approved dose of 0.5 mg per day. During fingolimod therapy, lymphocytes fluctuated between 0.5 and 0.9 × 103/μL. On March 3, 2020 she developed fever (>38 °C) and asthenia, without cough or dyspnea; her respiratory rate was 18 breaths/min and oxygen saturation was 97% while breathing ambient air. On the same day, fingolimod was discontinued and the next day she was hospitalized. The day of hospitalization, chest radiography showed streaky opacities in both lung lower lobes, consistent with atypical pneumonia; C-reactive protein was 108 mg/mL, neutrophils and lymphocytes were 3.21 and 0.37 × 103/μL. Real-time RT-PCR revealed a positivity for SARS-CoV-2 on March 5; the patient was not aware of the source of COVID-19 exposure. Five days later, she was subjected to continuous positive airway pressure, as oxygen saturation dropped to 86%, and hemoglobin decreased from 14.7 to 11.7 g/dL; neutrophils were 4.85 × 103/μL and lymphocytes were 0.26 × 103/μL (they were 0.77 × 103/μL one month before hospitalization). During hospitalization period, the patient was treated with hydroxychloroquine, lopinavir/ritonavir, and with paracetamol, when needed. After 15 days, she become asymptomatic, without fever, cough or breathing difficulties. After discharge she started again IFN-beta 1a (44 μg) therapy, and she was not tested for the presence of anti SARS-CoV-2 antibodies, because in accordance with local directives, only healthcare personnel and a limited number of patients carried out the serological test.
The patient provided a written informed consent; the Ethics Committee approved the study (protocol NP4000 - CoronaLab).
2.2. Experimental procedures
Blood samples, collected in ethylenediaminetetraacetic acid tubes, were obtained from the COVID-19 patient and from appropriate controls (MS patients under fingolimod therapy and at the fingolimod washout). Cell surface staining was performed on fresh blood according to standard protocols using appropriate mixtures of the following fluorescent-labelled monoclonal antibodies (MoAb): anti-CD3-, anti-CD45RA- and anti-IgD-FITC; anti-CD8- and anti-CCR7-PE; anti-CD45-, anti CD127- and anti-CD38-PerCP-Cy5.5; anti-CD19- and anti-CD25-PE-Cy7; anti-CD27- and anti-CD4-APC; anti-CD8-, anti-CD20-, and anti-CD16-APC-H7; anti-CD56- and anti-IgM-Bv421; anti-CD3-V450; anti-CD45- and anti-HLAD- V500; anti-CD31- and anti-CD10-Bv480. All samples were acquired using a FACSCanto II flow cytometry. Analyses were performed using DIVA software focusing on T- and B-cell subsets. T-cell subsets were: naive (CD45RA+CCR7+), central memory (TCM; CD45RA-CCR7+), effector memory (TEM; CD45RA-CCR7-) and terminally differentiated (TEMRA; CD45RA+CCR7-); recent thymic emigrants (RTE) were defined as naive CD4+ cells expressing the CD31 molecule. Regulatory T cells were CD4+CD25+CD127low/- lymphocytes. B-cell subsets were: recent bone marrow emigrants (RBE; CD38++CD10+), naive (IgD+IgM+CD27-), unswitched memory (IgD+IgM+CD27+), switched memory (IgD-IgM-CD27+), and ASC (CD38++CD27+CD20-).
T- and B-cell production was also measured by quantifying T-cell receptor (TR) excision circles (TRECs) and K-deleting recombination excision circles (KRECs) with a digital droplet real-time PCR (Tessitore et al., 2017).
For proliferation assay, peripheral blood mononuclear cells (1.6 × 106/mL), were labelled with carboxyfluorescein succinimidyl ester (CFSE) for 20 min according to manufacturer protocol. CFSE-labelled or unlabeled cells were plated in 96-well culture plates and stimulated for 4 days at 37 °C with 6.25 μg/mL phytohemagglutinin (PHA) or anti-CD3 MoAb (5 μg/mL) with or without 600 U/mL of IL-2. After 3 days part of incubated cells were pulsed with [3H]thymidine and the radioactivity was measured after 18 h of incubation. The remaining cells were stained with anti-CD3 PerCP-Cy5.5, anti-CD4 APC, and anti-CD8 APC-H7 MoAb and then analyzed with FACSCanto II flow cytometry. T-cell proliferation was quantified using FlowJo software.
The diversity of TR beta variable (TRBV) subgroups was studied on CD4+ and CD4- lymphocytes, separated using magnetic beads, by spectratyping analysis after performing multiplex polymerase chain reactions (Chiarini et al., 2015). The length distributions of the obtained PCR products were used to calculate the distribution of fragment lengths, number of detectable peaks per TRBV element, and area under the curve. TRBV perturbations were estimated using the generalized Hamming distance method (Gorochov et al., 1998) in which the CDR3 length distribution of each TRBV of the patient was subtracted from the average Gaussian-like CDR3 length distribution of age-matched healthy controls.
Serum levels of IFN-γ–induced protein-10 (IP-10), IL-8 (IL-8), chemokine (C-C motif) ligand 5 (CCL5), chemokine (C-X-C motif) ligand 9 (CXCL9), and monocyte chemoattractant protein-1 (CCL2) were analyzed with Bead Array Human Chemokine Kit, following the manufacturer's instructions. Data were acquired on FACSCanto II flow-cytometer and data were analyzed by FCAP v3 array software.
2.3. Immunological characterization
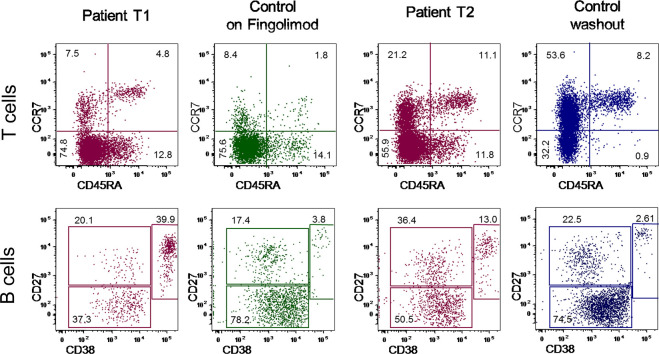
At six days from COVID-19 symptom onset, the patient presented with a marked reduction of newly generated T (TRECs: 158 vs 6687 ± 5996/mL in age-matched healthy controls) and B cells (KRECs: 1338 vs 18,488 ± 5518/mL), confirmed by the enumeration of recent thymic and bone marrow emigrants by flow cytometry (T1 of Table 1 ). T lymphocyte subsets expressing CCR7 (naïve and central memory), as well as regulatory T cells, were markedly decrease, but their values were comparable to that of non SARS-CoV-2-infected MS patients treated with fingolimod. B lymphocyte subsets were reduced as well, but characterized by a high percentage of ASC (Table 1). An immunophenotyping performed after 10 days (T2 of Table 1), revealed a significant and rapid increase of CCR7-expressing subsets, nearing values of patients at fingolimod washout, with the only specific feature of the COVID-19 patient being the high percentage of ASC.
Table 1.
T- and B-cell subset phenotyping.
| Patient T1 |
Controls SM (on fingolimod) |
Patient T2 |
Controls SM (washouta) |
Patient T1 |
Controls SM (on fingolimod) |
Patient T2 |
Controls SM (washout) |
|
|---|---|---|---|---|---|---|---|---|
| % | range (%) | % | range (%) | μL | range (μL) | μL | range (μL) | |
| CD3+ | 75.1 | 40.1–76.4 | 85.7 | 67.8–81.0 | 198 | 119–1090 | 568 | 746–1313 |
| CD3+CD4+ | 44.8 | 5.3–34.8 | 59.6 | 20.5–43.8 | 118 | 43–290 | 395 | 332–573 |
| CD3+CD4+CD45RA+CCR7+CD31+ (RTE)b | 2.6 | 0.2–3.6 | 6.8 | 1.7–8.2 | 3 | 0–3 | 27 | 7–47 |
| CD3+CD4+CD45RA+CCR7+ (naïve) | 4.8 | 0.5–6 | 11.1 | 5.6–13.3 | 6 | 0–10 | 44 | 23–76 |
| CD3+CD4+CD45RA-CCR7+ (TCM) | 7.5 | 1.8–17.7 | 21.2 | 53.6–62.4 | 9 | 1–25 | 84 | 191–307 |
| CD3+CD4+CD45RA-CCR7- (TEM) | 74.8 | 15.7–75.6 | 55.9 | 30.1–35.0 | 88 | 12–80 | 221 | 116–184 |
| CD3+CD4+HLA-DR+ | 2.9 | 2.8–9.4 | 11.1 | na | 3 | 2–12 | 44 | na |
| Regulatory T cells (CD3+CD4+CD25highCD127low/) | 2.2 | 1.1–9.1 | 5.4 | 8.3–10.5 | 3 | 1–8 | 21 | 30–51 |
| CD3+CD8+ | 24.7 | 12.5–65.1 | 22.2 | 21.9–56.9 | 65 | 37–928 | 147 | 242–922 |
| CD3+CD8+CD45RA+CCR7+ (naïve) | 4.1 | 0.3–2.8 | 9.3 | 4.0–12.0 | 3 | 1–4 | 14 | 18–50 |
| CD3+CD8+CD45RA-CCR7+ (TCM) | 2.2 | 0.2–2.6 | 2.9 | 9.9–10.9 | 1 | 0–5 | 4 | 24–91 |
| CD3+CD8+CD45RA-CCR7- (TEM) | 16.4 | 11.3–31.7 | 26.6 | 33.0–51.2 | 11 | 4–239 | 39 | 124–406 |
| CD3+CD8+CD45RA+CCR7- (TEMRA) | 77.3 | 63.8–85.7 | 61.3 | 31.4–44.2 | 50 | 100–683 | 90 | 76–388 |
| CD3+CD8+HLA-DR+ | 4.5 | 1.7–12.6 | 9.9 | na | 3 | 2–16 | 15 | na |
| CD4/CD8 ratio | 1.81 | 0.1–2.0 | 2.7 | 0.4–2.0 | – | – | – | – |
| CD3-CD56+CD3-CD16+ (NK cells) | 22.1 | 21.5–55.4 | 11 | 14.8–24.7 | 58 | 164–307 | 73 | 213–272 |
| CD19+ | 2.6 | 1.9–4.3 | 2.8 | 4.1–7.3 | 7 | 13–27 | 20 | 66–81 |
| CD38++CD10+ (RBE) | 8.8 | 9.5–39-7 | 9.6 | 2.9–34.4 | 1 | 1–11 | 2 | 2–27 |
| CD19+CD10-CD27-IgD+ (naive) | 28.5 | 32.1–52.3 | 40.9 | 40.1–48.4 | 2 | 6–11 | 8 | 29–37 |
| CD19+CD27+IgD+IgM+ (memory unswitched) | 10.6 | 2.9–16.1 | 14.2 | 7.5–8.8 | 1 | 1–4 | 3 | 5–7 |
| CD19+CD27+IgD-IgM- (memory switched) | 9.5 | 4.1–45.0 | 22.2 | 9.9–22.2 | 1 | 1–2 | 4 | 5–18 |
| CD19+CD27+CD38++CD20-(ASC) | 39.9 | 1.1–4.1 | 13.0 | 3.8–12.9 | 3 | 0–1 | 3 | 3–10 |
RBE: recent bone marrow emigrants; RTE: recent thymic emigrants; TCM: central memory T cells; TEM: effector memory T cells; TEMRA: terminally differentiated CD8+ cells; Treg: regulatory T cells; na: not available.
The number of MS controls on fingolimod and on fingolimod washout were 4 and 3, respectively.
The washout ranged from 14 to 29 days.
Values in healthy controls: RTE: 11.4–48.1% and 115–913/μL; RBE: 2.1–26.1% and 5–47 /μL; TEMRA: 5.2–63.5% and 22–467 μL; ASC: 0.2–8.1% and 0.3–22 μL; CD3 + CD4 + HLA-DR+: 1.6–12.2% and 15–123 μL; CD3 + CD8 + HLA-DR+: 2.7–31.7% and 17–346 μL.
Radioactive and CSFE assays revealed a lack of T-cell proliferation when peripheral blood mononuclear cells were incubated with PHA. Cells well proliferated to anti-CD3 MoAb and anti-CD3 plus IL-2 stimulation, although the proliferation to anti-CD3 alone was primarily sustained by CD4+ cells (final CD4:CD8 ratio 9:1).
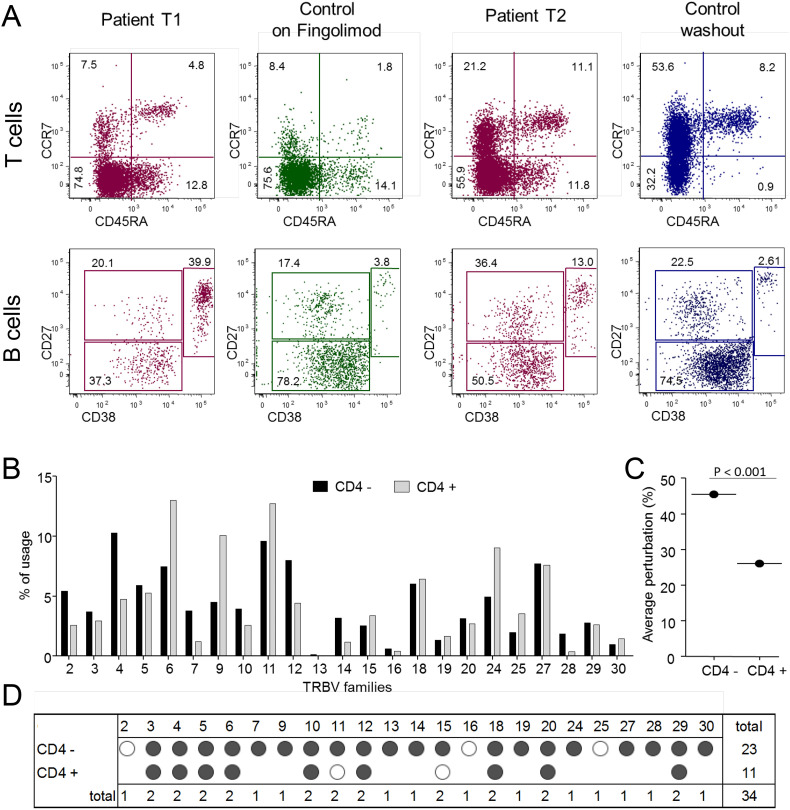
CDR3 spectratyping analysis of TR diversity, performed on separated CD4+ and CD4- lymphocytes, showed a different TRBV usage in the two populations, with TRBV4+ and TCBV12+ lymphocytes more expanded in CD4- subsets and TRBV6+, TRV9+, and TRV24+ lymphocytes n CD4+ subset (Fig. 1A). The percentage of perturbed TRBV chains was significantly higher in the CD4- lymphocytes (Fig. 1B) because 23 out 24 CD4- cells bearing TRBV were oligoclonally expanded (Fig. 1 C and D).
Fig. 1.
T- and B-cell immunophenotyping and TR repertoire analysis.
(A) Analysis of lymphocyte subsets in the COVID-19 patient after 5 and 15 days from hospitalization, compared with a representative MS patient on fingolimod (green) and a representative MS patient who discontinued fingolimod (14 days of washout; blue). CD4+ T cells and B cells are presented in the upper and lower row, respectively. (B) TRBV chain usage. (C) Average percentages of TRBV perturbations in CD4- and CD4+ populations. Dots represent the global average perturbation of the TRBV repertoire. (D) Map representing the CDR3 distribution perturbation at the single-TRBV. Black and white dots represent the TRBV families whose perturbations are respectively higher than the mean + 3SD and mean + 2SD of the value seen in the corresponding TRBV family calculated in 12 healthy controls. The number of these over-perturbed TRBV elements is indicated in the right column.
TRBV: T-cell receptor variable beta chain. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Among the tested chemokines, CCL5 was detected at the highest levels in COVID-19 patient (17,904 vs 3721 pg/mL in 3 controls), followed by CCL10 (6059 vs 448 pg/mL). The levels of CCL2 was slightly higher (172 vs 83 pg/mL), while those of CCL2 and IL-8 were comparable that of controls (178 vs 242 pg/mL and 11 vs 7.6 pg/mL).
3. Discussion
In this study, we demonstrate that the only relevant findings observed in the immunosuppressed COVID-19 MS patient were the increase of CCL5 and CCL10 chemokines and of ASC population. The detection of high levels of CCL5, which is involved in IFN-γ-dominant Th1 responses and it is a mediator in T-cell recruitment to the lung, suggests a potential role of this chemokine in the mobilization of T lymphocytes and monocytes to pulmonary tissues (Culley et al., 2006). The high levels of ASC could be crucial for controlling viral infection, as demonstrated during infection with influenza and Ebola viruses and after vaccination against influenza virus (McElroy et al., 2015; Ellebedy et al., 2016; Fujii et al., 2016). Most T-lymphocyte subset levels were low, but comparable to those generally detected in non-SARS-CoV-2 infected patients during and at discontinuation of fingolimod therapy. It could be hypothesized that antigen-specific T cells can be induced to exit lymph nodes by SARS-CoV-2, but this feature may not be not detected by immunophenotyping. It is also likely that the viral infection can result in a strong antigenic stimulation leading to increased number of HLA-DR+ cells in both CD4+ and CD8+ subsets. This can account for the oligoclonal T-cell repertoire and for the high propensity to apoptosis, as shown by the impaired response to PHA. Because of the immunological abnormalities observed in MS patients and of the changes induced by fingolimod (Sica et al., 2019), it is difficult to define which of the immunological features described in the COVID-19 patient are mainly attributable to SARS-CoV-2 infection or to inter-individual immune system heterogeneity (Brodin and Davis, 2017). However, this report shows that despite MS patients receiving immunosuppressive treatment may be at greatest risk of COVID-19 complications, the fingolimod-treated MS patient herein described, even in the presence of low circulating lymphocytes, was able to mount an effective immune response. Surprisingly, this patient survived COVID-19, had relative short duration of symptoms and well recovered, despite obesity, which is a main risk factor in COVID-19 patients (Stefan et al., 2020). It is important to mention that could be also concerns regarding stopping some MS therapies in setting of infections, especially fingolimod, because of the described rebounds, occurring in 5% of patients (Frau et al., 2018). During hospitalization, the COVID-19 patient remained without therapy for about one month and she did not developed MS symptoms during this period. However, it must be remembered that fingolimod induces a different immune reconstitution compared to other MS disease modifying therapies and that the immune response may be faster and more robust when fingolimod is stopped, since this drug just blocks lymphocyte egress from lymph nodes. Accordingly, there is a greater concern for drugs that deplete immune cells (Giovannoni et al., 2020).
Despite the several limitations of this study, which include the description of a single case only, unknown impact of stopping fingolimod versus continuing fingolimod and lack of sample to analyze from day of hospitalization, these results seem to be slightly reassuring and in line with what has already been reported on the management of MS treatments in Italy the time of the pandemic COVID-19 (Sormani et al., 2020).
In conclusion, this is an “immunological case report” from one of Italy's most severely affected pandemic areas. It would be important to characterize higher number of cases in the future and to complement the results those that will be obtained by the ongoing clinical trial aimed at determining the efficacy of fingolimod to prevent the development of acute respiratory distress syndrome associated with COVID-19 (https://clinicaltrials.gov/ct2/show / NCT04280588).
Ethical approval
The patient provided a written informed consent; the Ethics Committee approved the study (protocol NP4000 - CoronaLab).
Declaration of Competing Interest
M. Chiarini, S. Paghera, D. Moratto, M. Giacomelli, and R. Badolato report no disclosures relevant to the manuscript.
N. de Rossi received speaker honoraria from Biogen Idec, Genzyme, Novartis, Sanofi-Aventis, received funding for participation in advisory board to Novartis and Genzyme-Sanofi and for travel to scientific meetings from Biogen Idec, Teva, Sanofi-Genzyme, Roche, and Novartis.
R. Capra has received lecture fees and/or travel grants from Novartis, Biogen, Celgene, TEVA, Genzyme, and Sanofi-Aventis.
L. Imberti has received speaker honoraria from Biogen Idec, Meck-Serono, Novartis and Genzyme-Sanofi and has participated to scientific advisory board for Biogen Idec. She has received research funding from research support from Meck-Serono, and Genzyme-Sanofi.
Acknowledgment
Biogen Italia, Teva Italia, Roche Italia. Research funding from research support from Merck-Serono (ID EMR200136-555) and Genzyme-Sanofi (GZ402673).
References
- Brodin P., Davis M.M. Human immune system variation. Nat. Rev. Immunol. 2017;17:21–29. doi: 10.1038/nri.2016.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiarini M., Sottini A., Bertoli D., Serana F., Caimi L., Rasia S., et al. Newly produced T and B lymphocytes and T-cell receptor repertoire diversity are reduced in peripheral blood of fingolimod-treated multiple sclerosis patients. Mult. Scler. 2015;21:726–734. doi: 10.1177/1352458514551456. [DOI] [PubMed] [Google Scholar]
- Culley F.J., Pennycook A.M., Tregoning J.S., Dodd J.S., Walzl G., Wells T.N., et al. Role of CCL5 (RANTES) in viral lung disease. J. Virol. 2006;80:8151–8157. doi: 10.1128/JVI.00496-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellebedy A.H., Jackson K.J., Kissick H.T., Nakaya H.I., Davis C.W., Roskin K.M., et al. Defining antigen-specific plasmablast and memory B cell subsets in human blood after viral infection or vaccination. Nat. Immunol. 2016;17:1226–1234. doi: 10.1038/ni.3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frau J., Sormani M.P., Signori A., Realmuto S., Baroncini D., Annovazzi P., et al. Clinical activity after fingolimod cessation: disease reactivation or rebound? Eur. J. Neurol. 2018;25:1270–1275. doi: 10.1111/ene.13694. [DOI] [PubMed] [Google Scholar]
- Fujii C., Kondo T., Ochi H., Okada Y., Okada Y., Hashi Y., Adachi T., et al. Altered T cell phenotypes associated with clinical relapse of multiple sclerosis patients receiving fingolimod therapy. Sci. Rep. 2016;6 doi: 10.1038/srep35314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannoni G., Hawkes C., Lechner-Scott J., Levy M., Waubant E., Gold J. The COVID-19 pandemic and the use of MS disease-modifying therapies. Mult. Scler. Relat. Disord. 2020;39 doi: 10.1016/j.msard.2020.102073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorochov G., Neumann A.U., Kereveur A., Parizot C., Li T., Katlama C., et al. Perturbation of CD4+ and CD8+ T-cell repertoires during progression to AIDS and regulation of the CD4+ repertoire during antiviral therapy. Nat. Med. 1998;4:215–221. doi: 10.1038/nm0298-215. [DOI] [PubMed] [Google Scholar]
- Grebenciucova E., Pruitt A. Infections in patients receiving multiple sclerosis disease-modifying therapies. Curr. Neurol. Neurosci. Rep. 2017;17 doi: 10.1007/s11910-017-0800-8. [DOI] [PubMed] [Google Scholar]
- Lippi G., Plebani M. Laboratory abnormalities in patients with COVID-2019 infection. Clin. Chem. Lab. Med. 2020 doi: 10.1515/cclm-2020-0198. Mar 3. pii: /j/cclm.ahead-of-print/cclm-2020-0198/cclm-2020-0198.xml. [DOI] [PubMed] [Google Scholar]
- Liu J., Li S., Liu J., et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. medRxiv. 2020 doi: 10.1101/2020.02.16.20023671. preprint first posted online February 22, 2020. (Accessed February 26, 2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElroy A.K., Akondy R.S., Davis C.W., Ellebedy A.H., Mehta A.K., Kraft C.S., et al. Human Ebola virus infection results in substantial immune activation. Proc. Natl. Acad. Sci. U. S. A. 2015;112:4719–4724. doi: 10.1073/pnas.1502619112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sica F., Centonze D., Buttari F. Fingolimod immune effects beyond its sequestration ability. Neurol. Ther. 2019;8:231–240. doi: 10.1007/s40120-019-00162-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sormani MP on Behalf of the Italian Study Group on COVID-19 Infection in Multiple Sclerosis An Italian programme for COVID-19 infection in multiple sclerosis. Lancet Neurol. 2020 doi: 10.1016/S1474-4422(20)30147-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan N., Birkenfeld A.L., Schulze M.B., Ludwig D.S. Obesity and impaired metabolic health in patients with COVID-19. Nat. Rev. Endocrinol. 2020 doi: 10.1038/s41574-020-0364-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessitore M.V., Sottini A., Roccaro A.M., Ghidini C., Bernardi S., Martellosio G., et al. Detection of newly produced T and B lymphocytes by digital PCR in blood stored dry on nylon flocked swabs. J. Transl. Med. 2017;15 doi: 10.1186/s12967-017-1169-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thevarajan I., Nguyen T.H.O., Koutsakos M., Druce J., Caly L., Carolien van de Sandt E., et al. Breadth of concomitant immune responses prior to patient recovery: a case report of non-severe COVID-19. Nat. Med. 2020 doi: 10.1038/s41591-020-0819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]