Abstract
Purpose
To evaluate the dosimetric impact of four different radiotherapy techniques for a case of left-sided breast cancer with Internal Mammary lymph Nodes (IMN) involvement.
Materials and methods
To identify the best radiotherapy technique for this patient, four methods were compared: 3D Conformal Radiotherapy (3D-CRT), Volumetric Modulated Arc Therapy (VMAT), Tomotherapy (TOMO) and Intensity Modulated Proton Therapy (IMPT). Patient was treated using deep inspiration breath-hold (DIBH) technique. Prescribed dose was 40.05y in 15 fractions. Plan evaluation was performed on target coverage and dose to the organs-at-risk (OARs) using 3D-CRT as a baseline.
Results
TOMO has the most ideal Conformity Index (CI) at 1.139, followed by IMPT at 1.158, VMAT at 0.765, and 3D-CRT at 0.685. Using 3D-CRT as a baseline, VMAT, TOMO and IMPT all showed improved dose coverage. IMPT has the best dose coverage. TOMO has the most ideal homogeneity index (HI) and Conformity Number (CN). Mean heart dose (MHD) is lowest for IMPT at 0.55 Gy and highest for VMAT at 4.79 Gy. V20Gy of left lung is the lowest for IMPT at 11.11%, compared to 17.53% for TOMO, 18.19% for VMAT and 33.33% for 3D-CRT. V5Gy for the contralateral breast ranges from 0.01% in IMPT to 72.32% in TOMO.
Conclusion
3D-CRT compromising target coverage but achieving good OAR sparing for the contralateral right breast, left lung and right lung. Overall, IMPT performed best in terms of target coverage and OAR-sparing. Protons delivered superior target dose coverage and sparing of normal structures for this patient. As dose value parameters are expected to correlate with acute and chronic toxicities, proton therapy should be given due consideration as the preferred technique for the treatment of left-sided breast cancers with IMN involvement. Further studies with more patients can be done to evaluate the effectiveness of proton therapy on acute and chronic toxicities.
Keywords: Breast cancer, Internal Mammary Lymph nodes (IMN), TOMO, VMAT, IMPT, Proton
Introduction
Breast cancer is the most commonly diagnosed cancer and the leading cause of cancer death for females [1]. Main treatment is surgery, with most breast cancer patients then undergoing adjuvant radiotherapy to achieve long-term survival benefits [2].
Internal mammary chains are crucial metastases sites [3]. It has prognostic significance, indicating a more advanced cancer stage, worse distant disease-free survival and nearly 3-fold increase in mortality risk for node-positive patients [4]. Thus, IMN irradiation is done for survival benefits like a significant decrease in locoregional recurrence, overall recurrence and mortality for node-positive women [5]. According to National Comprehensive Cancer Network (NCCN)2, prophylactic treatment of IMN is recommended for N2 staging and above [6].
Traditionally, modified wide tangents (MWT) is used to treat the IMN [7]. However, a key disadvantage is more dose to the heart, ipsilateral and contralateral lung, and contralateral breast due to the bigger irradiation field compared to normal tangential fields, emphasising the need for OAR sparing and low doses to normal tissues.
Since radiotherapy is inevitable for such patients, this paper aims to decrease such adverse long-term side-effects by comparing the dosimetric differences between 3D-CRT, VMAT and PBT and provide clinical application references to investigate the most approximate customised radiotherapy treatment technique for such patients.
Materials and methods
Patient characteristics
In this report, the case of a 33-year-old Chinese female with cT3N2M0 left breast infiltrating ductal carcinoma (IDC) is discussed. The grade 3 tumour tested positive for progesterone and HER2, and was located at the lower outer quadrant in 5 o’clock position. Patient had neoadjuvant chemotherapy3 and Herceptin before surgery. Lympho-vascular invasion (LVI) was positive with 5 out of 19 nodes involved. Patient underwent wide excision, sentinel lymph node biopsy and axillary clearance. Radiotherapy of 3 fields was planned to the left breast, supraclavicular field (SCF) and IMN for 15 fractions with a breath-hold technique.
CT simulation
The treatment planning computed tomography (CT) images were obtained and pertinent structures were contoured. The patient was scanned supine on an inclined breast-board. Deep Inspiration Breath Hold (DIBH) was used to decrease cardiac doses. Breath-hold threshold was set at 80% of maximum inspiration capacity to ensure treatment reproducibility.
Planning objectives
Organs-At-Risk (OARs) including the heart, contralateral breast and both lungs were contoured by a senior radiation oncologist consultant according to Radiation Therapy Oncology Group (RTOG) atlas4 and double-checked by another senior consultant. Dose constraints adapted from Royal College of Radiologists (RCR) and Danish Breast Cancer Cooperative Group (DBCG) are listed in (Table 1) and strictly adhered to for each plan. Clinical Target Volume (CTV) consist of whole left breast including the subclinical spread of disease and IMN. Planning Target Volume (PTV) is the CTV with a 0.5 cm margin expansion.
Table 1.
OAR dose constraints.
| Organs-At-Risk (OARs) | Dose Constraints |
|---|---|
| Heart | V17Gy < 10% V35Gy < 5% Mean < 6 Gy |
| Ipsilateral Lung | V17Gy < 35% Mean < 16 Gy |
| Contralateral Lung | V5Gy < 10% |
| Contralateral Breast | Mean < 3.5 Gy |
Eclipse treatment planning system (Eclipse 13.7, Varian Medical Systems, Palo Alto, CA, USA) was used for 3D-CRT, VMAT and IMPT. Tomotherapy planning system was used for Tomotherapy. Dose calculation algorithms used were the AAA algorithm for 3D-CRT and VMAT, Superposition Convolution for Tomotherapy, and Pencil Beam algorithm for IMPT. All plans were designed by qualified dosimetrists with at least 6 years of planning experience.
3D-CRT
For 3D-CRT, 6MV modified wide tangents (MWT) with medial border extending over to the contralateral side to cover the IMN were used. A mono-isocentric technique was chosen with isocentre set at the inferior border of clavicle. Modified tangential breast fields and anterior supra-clavicular field were treated using asymmetrical jaws. Cardiac shielding and field-in-field are used for both tangential fields.
VMAT
For VMAT, 5 partial arcs were used to decrease doses to critical structures. Three clockwise (305 to 60°, 285–50°, 90–179°) and two counter-clockwise arcs (60–305°, 179–90°) were utilised with collimator rotation (350°, 355°, 20°, 10°, 340°) respectively for all arcs.
Tomotherapy
For Tomotherapy, a helical plan was used to treat the patient slice by slice from all 360° angles. Table pitch is set at 0.215 with field width of 5.0 cm. Leaf width is 0.625 cm. Blocking structures were created to limit the dose entry angles into the body from the contralateral side and control dose splashes in the body and contralateral lung.
IMPT
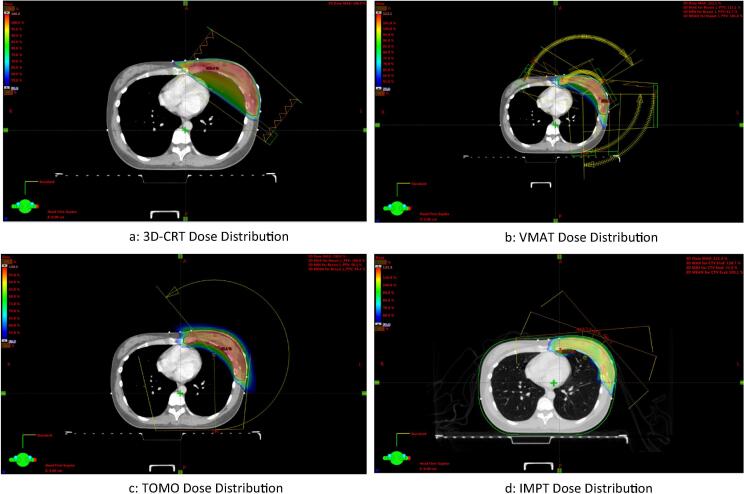
IMPT, a type of Proton Beam Therapy (PBT), uses Pencil Beam Scanning (PBS) with Multiple Field Optimisation (MFO). 3 en-face fields were used to treat the target volume. To account for range and setup uncertainty, robust optimization was used to optimize the spot pattern, giving the plan a robustness of 3.0% to CT calibration uncertainty and 3 mm of setup uncertainty. Nominal range and nominal SOBP width are both 10.75 cm. Beam energy used ranges from 129.7 MeV to 149.2 MeV. A range shifter of 4.5 cm Water Equivalent Thickness (WET) is used to extend the treatment range to superficial depths through reducing the residual range of the incident beam. Due to the fixed range shifter, air gap is different for each field depending on the anatomy and isocentre placement (see Fig. 1).
Fig. 1.
a–d. Dose distribution of 4 modalities.
Plan evaluation
Dosimetric parameters used for evaluating target coverage and OARs dose were extracted from the dose-volume histograms (DVHs). Below are formulas to evaluate target coverage.
Conformity Index (CI) measures dose conformity within PTV [8].
*Vri = volume of reference isodose, TV = target volume.
CI value of 1 represents ideal coverage or high conformity. CI greater than 1 means irradiated volume is more than Target Volume (TV). CI of less than 1 indicates a partially irradiated TV.
Homogeneity Index (HI) analyses dose distribution uniformity using the ratio between maximum and minimum dose in the TV [9].
HI value of 0 is ideal. HI increases as homogeneity decreases.
Conformity Number (CN) considers TV irradiation and healthy tissue irradiation [8].
*TVri = target volume covered by reference isodose.
CN ranges from 0 to 1 and the ideal value is 1. A value close to 0 indicates no conformation.
Results
Dosimetric comparisons for target coverage and OAR doses between 4 modalities are shown in Table 2, Table 3 respectively.
Table 2.
Target volume coverage & evaluation parameters.
| CTV coverage (Breast + Nodes) | 3D-CRT | VMAT | TOMO | IMPT |
|---|---|---|---|---|
| V95% (%) | 86.17% | 96.86% | 99.93% | 99.46% |
| Evaluation Parameters | 3D-CRT | VMAT | TOMO | IMPT |
| 0.685 | 0.765 | 1.139 | 1.158 | |
| 94.418 | 16.451 | 10.860 | 14.136 | |
| 0.438 | 0.655 | 0.721 | 0.604 | |
Table 3.
OAR doses.
| Heart | Achieved Values |
|||
|---|---|---|---|---|
| Modalities | 3D-CRT | VMAT | TOMO | IMPT |
| Dmax (Gy) | 37.50 Gy | 32.80 Gy | 39.40 Gy | 39.20 Gy |
| Dmin (Gy) | 0.10 Gy | 1.20 Gy | 0.80 Gy | 0.00 Gy |
| Dmean (Gy) | 1.81 Gy | 4.79 Gy | 3.95 Gy | 0.55 Gy |
| V5Gy (%) | 5.06% | 35.13% | 19.32% | 2.96% |
| Right Breast | Achieved Values | |||
| Modalities | 3D-CRT | VMAT | TOMO | IMPT |
| Dmax (Gy) | 13.90 Gy | 19.90 Gy | 19.60 Gy | 5.60 Gy |
| Dmin (Gy) | 0.00 Gy | 0.30 Gy | 1.60 Gy | 0.00 Gy |
| Dmean (Gy) | 0.33 Gy | 3.14 Gy | 7.16 Gy | 0.14 Gy |
| V5Gy (%) | 0.11% | 23.32% | 72.32% | 0.01% |
| Left Lung | Achieved Values | |||
| Modalities | 3D-CRT | VMAT | TOMO | IMPT |
| Dmax (Gy) | 40.60 Gy | 40.90 Gy | 42.90 Gy | 49.90 Gy |
| Dmin (Gy) | 0.10 Gy | 1.10 Gy | 0.80 Gy | 0.00 Gy |
| Dmean (Gy) | 13.63 Gy | 10.37 Gy | 10.14 Gy | 6.76 Gy |
| V5Gy (%) | 48.43% | 54.10% | 52.81% | 36.92% |
| V17Gy (%) | 34.81% | 22.26% | 20.92% | 14.16% |
| V20Gy (%) | 33.33% | 18.19% | 17.53% | 11.11% |
| Right Lung | Achieved Values | |||
| Modalities | 3D-CRT | VMAT | TOMO | IMPT |
| Dmax (Gy) | 1.80 Gy | 17.20 Gy | 17.10 Gy | 18.70 Gy |
| Dmin (Gy) | 0.00 Gy | 0.50 Gy | 0.20 Gy | 0.00 Gy |
| Dmean (Gy) | 0.21 Gy | 2.57 Gy | 2.96 Gy | 0.18 Gy |
| V5Gy (%) | 0.00% | 9.07% | 15.50% | 0.33% |
| V17Gy (%) | 0.00% | 0.00% | 0.00% | 0.00% |
| V20Gy (%) | 0.00% | 0.00% | 0.00% | 0.00% |
| Both Lungs | Achieved Values | |||
| Modalities | 3D-CRT | VMAT | TOMO | IMPT |
| Dmax (Gy) | 40.60 Gy | 40.90 Gy | 42.90 Gy | 49.90 Gy |
| Dmin (Gy) | 0.00 Gy | 0.50 Gy | 0.20 Gy | 0.00 Gy |
| Dmean (Gy) | 6.94 Gy | 6.48 Gy | 6.56 Gy | 3.48 Gy |
| V5Gy (%) | 24.30% | 31.66% | 34.22% | 18.69% |
| V17Gy (%) | 17.46% | 11.17% | 10.50% | 7.11% |
| V20Gy (%) | 16.72% | 9.13% | 8.79% | 5.58% |
Target volume
3D-CRT, VMAT, and TOMO were optimised on PTV. IMPT was robustly optimised on CTV. V95% dose coverage was best for IMPT at 99.46%, then TOMO at 97.68%, VMAT at 92.92% and 3D-CRT at 76.74%.
Homogeneity index, conformity index, conformity number
Best CI value arises from TOMO, then IMPT, VMAT, and 3D-CRT. HI was the best for TOMO, indicating TOMO has the best dose distribution uniformity. TOMO also has the best CN value, meaning it has the best conformation to target volume in terms of target coverage and dose overflow outside target volume. This was anticipated since Tomotherapy has a helical arc delivery and can create highly modulated plans. CN for IMPT was expectedly lower, because IMPT plans were robustly optimized on CTV, thus the optimizer would create a dose cloud around CTV to account for setup and range uncertainties.
OARs
IMPT performed the best in terms of target coverage and OAR sparing, making it most suitable for young patients requiring healthy tissue sparing due to its intrinsic Bragg Peak characteristics. 3D-CRT compromises target coverage but provide good OAR sparing due to MWT cutting off the doses. Both TOMO and VMAT performed better than 3D-CRT in terms of target coverage but are unable to surpass the degree of OAR sparing as shown by 3D-CRT for all OARs measured. TOMO is a helical therapy and doses come in from the contralateral side to patch up the areas of dose inadequacies, reflected in the significantly high value of 72.32% for V5Gy of contralateral breast. VMAT has worse target coverage compared to TOMO and significant low-dose spillage seen by V5Gy for doses to the heart and left lung. MHD for VMAT was also the highest amongst all modalities. However, VMAT doses to the right breast and right lung is lower than that of TOMO.
Discussion
Cardiotoxicity has been traditionally correlated with left breast cancer due to the heart’s proximity to the conventional tangential treatment fields for left breast cancer [10]. Breast radiotherapy may also result in radiation-induced secondary malignancies. This risk increases over the years, reaching its peak at 15 or more years after breast radiotherapy [11]. These patients are living longer due to improved therapy effectiveness, making minimisation of long-term adverse effects – risk of secondary malignancies, pulmonary toxicity and cardiotoxicity crucial.
It is generally believed that PBT will have more significant OAR sparing due to the phenomenon of Bragg Peak which allow protons to deposit maximum dose to tumour with minimal entry and exit doses. Both Taylor et al [12] and Ranger et al. [13] demonstrated that PBT is best able to minimise cardiac doses even with IMN involvement and lowest mean contralateral breast dose was delivered by PBT at <1 Gy. An apt summary of patients that can best benefit from PBT are those with MHD >5 Gy when using other modalities and below 50 years-old [11], [14]. Benefits from IMPT include a longer time without coronary heart disease manifestation for younger patients with higher baseline life expectancy compared to older patients.
More countries are adopting proton centres, making it relevant to research on the potential of PBT for breast cancer patients with IMN involvement for eligible patients to be treated locally. However, since PBT is relatively new, the cost might deter some patients from getting this treatment. Patients can consider using insurance to offset the high treatment cost if PBT is proven to be useful. Insurance help defray some expenses of PBT, with needy patients still being able to utilise government financial aid schemes. Regardless, careful patient selection and identification of subpopulations of each cancer type are required for PBT to be most cost-effective.
A cost-efficient alternative to PBT is DIBH when used in conjunction with 3D-CRT or VMAT. While this study did not compare free-breathing versus breath-hold, studies had concluded that breath-hold can minimise cardiac doses due to a larger separation between the heart and chest-wall during inspiration, thus significantly reducing doses to the heart and left anterior descending artery [15]. However, we must consider some patients with unacceptable heart doses during free-breathing might be unable to breath-hold due to advanced age or existing co-morbidities.
A major limitation of this study is that it is a case report of only one patient and thus unable to be generalised to the population. Further studies with bigger cohorts are required to investigate which treatment modality – 3D-CRT, VMAT, TOMO or IMPT with DIBH will deliver the best dosimetric outcome for patients with IMN involvement. A key point is no follow-up is done yet as patient had just completed radiotherapy. It is still too early for late radiotherapy side-effects to manifest – long-term follow-up is recommended to chart the radiotherapy adverse side-effects.
Conclusion
Overall, IMPT performed best in terms of target coverage and consistent ability to spare OARs due to the Bragg Peak. However, considering how expensive PBT is, this should be offered to younger patients that will stand to benefit most from PBT due to its OAR sparing ability. Techniques like DIBH can be incorporated into 3D-CRT or VMAT to improve dose distribution. TOMO and VMAT are alternatives if 3D-CRT is unable to meet the target coverage.
Although this study shows PBT can deliver better dosimetric outcomes compared to 3D-CRT, VMAT or TOMO, we cannot refute its inherent disadvantages. Due to high dependency on accurate modelling and prediction of Bragg peak range, PBT is particularly sensitive to anatomy changes. It also has inherent dose calculation and range uncertainties. In cases where patients have target volume changes, a re-plan might be required for PBT but may not be necessary for photons as they are more robust to anatomy changes.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
4 cycles of Cyclophosphamide (AC) and 12 cycles of Paclitaxel.
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018 doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG), Darby S., McGale P., Correa C., Taylor C., Arriagada R., Peto R. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet (London, England) 2011;378(9804):1707–1716. doi: 10.1016/S0140-6736(11)61629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang K., Zhang X., Zheng K., Yin X.-D., Xing L., Zhang A.-J. Predictors of internal mammary lymph nodes (IMLN) metastasis and disease-free survival comparison between IMLN-positive and IMLN-negative breast cancer patients: results from Western China Clinical Cooperation Group (WCCCG) database (CONSORT) Medicine. 2018;97(28):e11296. doi: 10.1097/MD.0000000000011296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yao M.S., Kurland B.F., Smith A.H., Schubert E.K., Dunnwald L.K., Byrd D.R. Internal mammary nodal chain drainage is a prognostic indicator in axillary node-positive breast cancer. Ann Surg Oncol. 2007;14(10):2985–2993. doi: 10.1245/s10434-007-9473-x. [DOI] [PubMed] [Google Scholar]
- 5.Poortmans P.M., Collette S., Kirkove C., Van Limbergen E., Budach V., Struikmans H. Internal mammary and medial supraclavicular irradiation in breast cancer. N Engl J Med. 2015;373(4):317–327. doi: 10.1056/NEJMoa1415369. [DOI] [PubMed] [Google Scholar]
- 6.National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: breast cancer; 2019. Retrieved from http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf.
- 7.Marks L.B., Hebert M.E., Bentel G., Spencer D.P., Sherouse G.W., Prosnitz L.R. To treat or not to treat the internal mammary nodes: a possible compromise. Int J Radiat Oncol Biol Phys. 1994;29(4):903–909. doi: 10.1016/0360-3016(94)90584-3. [DOI] [PubMed] [Google Scholar]
- 8.Feuvret L., Noël G., Mazeron J.-J., Bey P. Conformity index: a review. Int J Radiat Oncol Biol Phys. 2006;64(2):333–342. doi: 10.1016/j.ijrobp.2005.09.028. [DOI] [PubMed] [Google Scholar]
- 9.Kataria T., Sharma K., Subramani V., Karrthick K.P., Bisht S.S. Homogeneity Index: an objective tool for assessment of conformal radiation treatments. J Med Phys. 2012;37(4):207–213. doi: 10.4103/0971-6203.103606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bergom C., Currey A., Desai N., Tai A., Strauss J.B. Deep inspiration breath hold: techniques and advantages for cardiac sparing during breast cancer irradiation. Front Oncol. 2018;8:87. doi: 10.3389/fonc.2018.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silverman B.G., Lipshitz I., Keinan-Boker L. Second primary cancers after primary breast cancer diagnosis in Israeli women, 1992 to 2006. J Global Oncol. 2017;3(2):135–142. doi: 10.1200/JGO.2016.003699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taylor Carolyn W., Wang Zhe, Macaulay Elizabeth, Jagsi Reshma, Duane Frances, Darby Sarah C. Exposure of the heart in breast cancer radiation therapy: a systematic review of heart doses published during 2003 to 2013. Int J Radiat Oncol Biol Phys. 2015;93(4):845–853. doi: 10.1016/j.ijrobp.2015.07.2292. [DOI] [PubMed] [Google Scholar]
- 13.Ranger A., Dunlop A., Hutchinson K., Convery H., Maclennan M.K., Chantler H. A Dosimetric comparison of breast radiotherapy techniques to treat locoregional lymph nodes including the internal mammary chain. Clin Oncol. 2018;30(6):346–353. doi: 10.1016/j.clon.2018.01.017. [DOI] [PubMed] [Google Scholar]
- 14.Mailhot Vega R.B., Ishaq O., Raldow A., Perez C.A., Jimenez R., Scherrer-Crosbie M. Establishing cost-effective allocation of proton therapy for breast irradiation. Int J Radiat Oncol Biol Phys. 2016;95(1):11–18. doi: 10.1016/j.ijrobp.2016.02.031. [DOI] [PubMed] [Google Scholar]
- 15.Yeung R., Conroy L., Long K., Walrath D., Li H., Smith W. Cardiac dose reduction with deep inspiration breath hold for left-sided breast cancer radiotherapy patients with and without regional nodal irradiation. Radiat Oncol. 2015;10(1):200. doi: 10.1186/s13014-015-0511-8. [DOI] [PMC free article] [PubMed] [Google Scholar]